Abstract
Proteomics has helped us to understand physiological functioning as well as pathological condition. Mesial temporal lobe epilepsy (MTLE), the most common type of focal epilepsy in humans, is frequently associated with hippocampal cell loss, cognitive deficit, and resistance to antiseizure medications (ASM). A major challenge in designing new treatments to control seizures or epileptogenesis is the sequence of events involved in the development of an epileptic focus. Neuroproteomics emerges as a powerful tool for the assessment of protein biomarkers in neurodegenerative diseases, including MTLE. In this chapter, we present the main techniques used in proteomics studies to determine differential expression of proteins in brain tissue or other biological samples from patients and experimental models of epilepsy. Data obtained with proteomics are very useful to understand the pathophysiology of MTLE and, in the future, may assist in finding target proteins for new therapies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Proteomics is a science or method developed to study the proteome. Proteome is a term used to refer to the amount of protein expressed in a cell or tissue of an organism. While the genome reflects the sum of genes, the proteome does not exhibit a fixed number of proteins. These vary according to the state of development, the tissue type, and the physiological conditions. A proteome, nevertheless, remains a direct product of a genome. The number of proteins in a proteome can exceed the number of genes as protein products expressed by alternative splicing or with different post-translational modifications are observed as separate molecules on a two-dimensional polyacrylamide gel electrophoresis (2D-PAGE) (Wilkins et al., 1995).
Proteomics allows us to find proteins changed by a cell, tissue, or organism’s response to internal states, external stimulations, or developmental changes, and to profile any differential protein expression (Mus-Veteau, 2002; Wang et al., 2010). Proteomics not only measures the amount of a given protein but also whether there are any modifications of a protein as phosphorylation, ubiqutination, palmitoylation, oxidation, and other post-translational modifications (PTMs) (Alzate, 2010). Proteomics is a multidisciplinary method that is based on principles of biochemical, biophysical, and bioinformatics to allow the distinguishing of healthy and diseased cellular processes at the protein level. Aslam et al. (2017) highlighted some aspects of a target organism’s proteome that can be assessed by proteomics, for example, protein identification, protein quantification, protein localization, post-translational modifications, functional proteomics, structural proteomics, and protein-protein interactions.
Currently, the proteomics technique has been applied in the investigation of biomarkers associated with disease (Liu et al., 2010). However, proteomics technology is not only applicable to study health or disease but also in agronomy researchers (i.e., corn, sugarcane, rice, wheat, etc.) or organisms with economic impact (i.e., Rhizhobium spp., which set nitrogen to legumes, or Xanthomonas spp., that cause diseases in bean or citrus cultivation) (Di Ciero & Bellato, 2003).
Proteomics technology applied to understanding the nervous system is called “neuroproteomics” or “neuromics”. The neuroproteomics enables to study proteome of brain fragments or single cell, in cultures or isolated, and this is important to determine the dynamics of subproteome under different conditions (i.e., oxidative stress, drugs, etc.). In a global analysis, complementary studies could contribute to the understanding of complex biological networks that include protein interactions, complexity of signal and metabolic pathways that can be applied to select potential targets for specific drug therapy, and to the development of diagnosis or prognosis for neurological disorders (Liu et al., 2010).
15.1.1 Techniques Used in Proteomics
In a broad view, the techniques applied to proteomic analysis can be classified as follows: 1 – Low-throughput analysis, which includes: (A) Antibody-based methods, ex. ELISA (enzyme-linked immunosorbent assay) and Western blot. These methods rely on the availability of antibodies targeted toward specific proteins or epitopes to identify proteins and quantify their expression levels. (B) Gel-based proteomics, the two-dimensional polyacrylamide gel electrophoresis (2D-PAGE or 2DE), the first proteomics technique developed. This technique uses an electric current to separate proteins differing in their isoelectric point in a gel (first dimension) and mass (second dimension). Differential gel electrophoresis (DIGE) is a modified form of 2DE that uses different fluorescent dyes to allow the simultaneous comparison of two to three protein samples on the same gel. These gel-based methods are used to separate proteins before further analysis by, e.g., mass spectrometry (MS), as well as for relative expression profiling. C) Chromatography-based methods: can be used to separate and purify proteins from complex biological mixtures such as cell lysates. For example, ion-exchange chromatography, size exclusion chromatography, and affinity chromatography. These methods can be used to purify and identify proteins of interest, as well as to prepare proteins for further analysis (Aslam et al., 2017).
2 – High-throughput analysis, including: (A) Analytical, functional, and reverse phase microarrays. Protein microarrays apply small amounts of lysed samples to a “chip” where antibodies can be immobilized and used to capture target proteins in a complex sample. This is termed an analytical protein microarray. Functional protein microarrays are used to characterize protein functions such as protein–RNA interactions and enzyme-substrate turnover. The protein arrays provide a “map” of the activated signaling network by the quantitative analysis of the phosphorylated, or activated, state of cell signaling proteins. Reverse-phase protein microarrays have been developed to generate a functional “map” of the cell signaling networks based directly on cellular analysis of tissue samples (Fig. 15.1). In a reverse-phase protein microarray, proteins from, e.g., healthy vs. diseased tissues or untreated vs. treated cells are bound to the chip, and the chip is then probed with antibodies against the target proteins (Aslam et al., 2017; Chandramouli & Qian, 2009).
Forward phase microarray (a) and reverse phase protein microarray (b). The forward phase microarray consists of the immobilization of antibodies on a solid surface of a chip, to capture proteins of interest, allowing direct comparison of different samples. In reverse-phase microarray proteins are immobilized on the chip surface, and the chip is probed with the antibodies. Reverse-phase array requires only one well-performing analyte detection reagent
(B) Mass-spectrometry-based proteomics. Gel-free methods for separating proteins would include isotope-coded affinity tag (ICAT), stable isotope labeling with amino acids in cell culture (SILAC) and isobaric tags for relative and absolute quantitation (iTRAQ). These techniques allow either quantitation or comparative/differential proteomics. Besides that, a less quantitative technique called multidimensional protein identification technology (MudPIT) can also be used. Other gel-free chromatographic techniques for protein separation include gas chromatography (GC) and liquid chromatography (LC).
The low-throughput gel-based proteomic analysis has four basic stages: extraction, purification, separation, and identification of proteins. The 2D-PAGE allows the separation of hundreds to thousands of proteins in a single experiment (Van den Bergh & Arckens, 2005; Kim et al., 2007) and mass spectrometry (MS) to detect either digested peptides or intact proteins (Li & Smit, 2008; Zetterberg et al., 2008). MS requires peptides or proteins to be studied as ions in the gas phase matrix-assisted laser desorption/ionization (MALDI) and electro-spray ionization (ESI) are most used for this purpose.
There are five types of mass analyzers commonly used for proteomics research, and they vary in their physical principles and analytical performance (see Liu et al., 2010). MALDI-TOF (time-of-flight, TOF) is generally used in proteomics studies to identify the protein from in-gel digestion of gel-separated protein bands by peptide mass fingerprinting due to its excellent mass accuracy, resolution, and sensitivity (Pappin et al., 1993). Nowadays, it is possible to conduct a study of proteomics analysis without the need for two-dimensional electrophoresis. Different methods enable the analysis without 2-DE gels, one being accomplished by methodology 2D-LC-MS, in which the proteins are labeled with a probe, trypsinized, and then analyzed by LC-MS, which makes the simple and reproducible method, but it is more expensive.
Other protein identification methods, like amino acid composition analysis, N-terminal sequencing, or immunochemistry, as well as column chromatography, can be used (Fountoulakis, 2001), or other biochemical techniques can be applied for protein enrichment (Fountoulakis & Takács, 2002).
15.1.2 Proteomics and Epilepsy
Epilepsy is a chronic neurological disease that affect approximately 50 million people worldwide, being characterized by spontaneous and recurrent seizures that occur in the absence of disease toxic-metabolic or fever (Beghi, 2019). Temporal lobe epilepsy (TLE) accounts for approximately 40% of all cases of epilepsy, being a common form of focal epilepsy in humans. There are two subtypes of TLE, namely, mesial temporal lobe epilepsy (MTLE) and neocortical temporal lobe epilepsy (NTL) (Pfänder et al., 2002). In most MTLE patients, seizures originate in the limbic areas and are focal, and may be perceptual or nonperceptive (Tatum, 2012). Hippocampal sclerosis (HS) is the most frequent histopathological feature present in many patients with MTLE, and this change can usually be detected by magnetic resonance imaging – MRI (Lewis et al., 2014; Blumcke et al., 2017; Bruxel et al., 2021). HS is characterized by neuronal loss in specific subregions of the hippocampus and glial scar, but the etiology and the pathogenesis of the cell death are not well understood (Blumcke et al., 2017). In addition, synaptic rearrangement and cell scattering in the granular layer of the dentate gyrus are frequently seen associated with HS in MTLE (Houser, 1990).
Seizures are often frequent, and about 67–89% of patients with MTLE do not respond to antiseizure medications (Chipaux et al., 2016; Do Canto et al., 2021). According to the International League Against Epilepsy (ILAE), the drug resistance epilepsy (DRE) occurs as the failure of adequate trials of two tolerated, appropriately chosen, and used antiseizure medications, whether as monotherapy or in combination, to achieve sustained seizure freedom (Kwan et al., 2010). The concept of DRE not only implies intractable seizures, but the underlying pathogenesis is responsible for structural and neurobiochemical changes that also cause cognitive and neuropsychiatric disturbances and psychosocial dysfunction in patients with TLE (Kwan & Brodie, 2002; Fattorusso et al., 2021). Therefore, DRE represents a spectrum of different clinical and neurobiological pictures rather than a group of patients with the same disease, requiring a complex approach to several issues that we will further try to focus on.
Until now, there is no antiseizure medications able to prevent seizures in patients with TLE that are efficient in preventing epileptogenesis (Temkin, 2009). Thus, the question is whether the epileptogenic process could be explained by common molecular and network events that would be applied in new therapeutics. In this way, proteomics has been a powerful tool for protein profiling because it allows comparing proteomes of cells and tissues in normal and pathological conditions. Since the expression of proteins is determined, the transcriptional level can be examined to find the underlying mechanism for the reduction or increase of certain gene products. For this reason, proteomics has been widely used in clinical research to identify biomarkers associated with disease.
Neurosciences have benefited greatly from the increased use of the technique proteomics in recent years. Despite this, studies of epilepsies are still modest with the application of proteomics. Experimental models that reliably reproduce the main symptoms of diseases have aroused the interest of researchers in the search for biomarkers. Proteomics has been used in epilepsy models and brain tissue or cerebrospinal fluid (CSF), obtained from patients with refractory seizures, to identify biological markers, enabling new treatments. In the next sections, we will briefly review these results.
Despite technological advances applied to neurosciences, little is known about the cellular and molecular phenomena related to the epileptogenic process (Silva & Cabral, 2008).
15.1.2.1 Proteomics Profile of Epilepsy Models
The use of experimental animal models of epilepsy has contributed greatly to the knowledge of different pathological mechanisms that are difficult to study directly in humans due to the limitations of human tissue availability (Becker, 2018). These models approach the reality of the complex and heterogeneous physiological processes involved, enabling the creation of therapeutic strategies for the disease. The most common acquired epilepsy model of MTLE in rats and mice are pilocarpine, kainic acid, and electrical stimulation. Some genetic models have also been largely studied due to the pathophysiological characteristics they present, and they are, Wistar Albino Glaxo/Rijswijk (WAG/Rij) rat, BS/Orl and BR/Orl mouse strains and Genetic Absence Epilepsy from Strasbourg (GAERS) (Becker, 2018). Each model produces different mechanisms that lead to epileptogenesis; therefore, not all have the same proteomic profile, although there are similarities in the immune response and inflammatory pathways (Pitkänen et al., 2015).
Although these findings are interesting and enable us to obtain clues about the mechanisms involved with intractable epilepsy, we must bear in mind that these clues refer to mechanisms already established and irreversible, such as cell loss, sprouting, cell dispersion, glial scar, metabolic changes, etc. (Pitkänen et al., 2015). Studies employing human tissue are limited by the low amount obtained by surgical procedures and for ethical reasons. The use of the experimental model of epilepsy can expand our knowledge regarding these mechanisms involved in epileptogenesis, allowing interference with or preventing the onset of spontaneous seizures.
Chemical convulsants such as pilocarpine (Cavalheiro et al., 1991; Cavalheiro, 1995) and kainic acid (Ben-Ari, 1985) can initiate status epilepticus in rodents and cause hippocampal sclerosis, memory impairment and spontaneous and recurrent seizures (Cavalheiro et al., 1994).
Using the proteomics method, we studied the differentially expressed proteins in the hippocampal samples of rats subjected to pilocarpine-induced MTLE. In the hippocampal samples from rats studied 90 days following status epilepticus, we found 40 proteins differentially expressed when compared to control animals. Among them, 37 proteins were successfully identified, as shown in Table 15.1. The protein profile revealed that 29 proteins were upregulated, 6 were downregulated and 2 were expressed only in control animals.
Among the downregulated proteins, we found enzymes related to carbohydrate metabolism and ATP synthesis, reflecting disturbances in the energetic metabolism. These data are in line with findings reported by other authors (Greenberg et al., 2005; Masino et al., 2009). The gene encoding the malate dehydrogenase was reported as a factor involved in the generation of generalized idiopathic epilepsy (Greenberg et al., 2005). Altered proteins such as phospholipase A2, fructose-bisphosphate aldolase, and enolase, have been reported by other authors associated with neuropsychiatric mechanisms (Martins-de-Souza et al., 2009; Ross et al., 1997; Adibhatla & Hatcher, 2008). However, phospholipase A2 can also participate in neurogenesis processes (Talib et al., 2008).
The guanine nucleotide-binding protein (G proteins) was downregulated in the hippocampi of pilocarpine-induced epilepsy. This is an important finding considering the wide role of this protein in the signal transduction by hormones, neurotransmitters, chemokines, and autocrine and paracrine factors (Neves et al., 2002).
In another study using the lithium-pilocarpine model, we identified 24 proteins in the hippocampal samples of rats, but only 7 were differentially expressed compared to control rats; namely, 4 were upregulated and 3 were downregulated (Marques-Carneiro et al., 2017). The interactome analysis revealed that the proteins are mainly related to glycolysis (14%) and to inflammation processes mediated by chemokine and cytokine signaling (5%). We also found proteins associated with Huntington’s (5%) and Parkinson’s disease (5%) and associated with fructose and galactose metabolism (4.80%). In addition, minor changes (2%) were also observed in several other pathways (Marques-Carneiro et al., 2017).
Two genetic models of absence epilepsy, GAERS and WAG/Rij, are resistant to the progression of partial seizures induced by electrical stimulation of the amygdala (Aker et al., 2010; Onat et al., 2007), hippocampus (Akman et al., 2008) or perirhinal cortex (Akman et al., 2010). Considering that absence seizures start in the thalamocortical circuitry, these data suggest an interaction between thalamocortical loop and limbic circuitry (Danober et al., 1998). Danış et al. (2011) performed a 2D-PAGE to compare GAERS to Non-Epileptic Control (NEC) rats and showed six differentially expressed proteins, two in the parietal cortex (ATP synthase subunit delta and the 14-3-3 zeta isoform), two in the thalamus (myelin basic protein – MBP and macrophage migration inhibitory factor – MIF), and two in the hippocampus (MIF and 0-beta 2 globulin). Almost all proteins were upregulated in GAERS compared to NEC, except 0-beta globulin, a protein from hemoglobin complex predicted to be involved in oxygen transport (https://www.ncbi.nlm.nih.gov/protein/CAA47877.1/; GenBank: CAA47877.1). In line with these authors, MIF was also found upregulated in the frontal cortex and in the hippocampus of rats subjected to kainic acid-induced epilepsy (Lo et al., 2010). MIF, a pro-inflammatory cytokine released in response to inflammatory stimuli, is highly expressed in immune and nonimmune cells, including those in the brain. A study by Chai et al. (2020) showed that MIF is important to the process of hippocampal neurogenesis, affecting cell proliferation in the dentate gyrus.
15.1.2.2 Proteomics Profile of the Patients with Epilepsy
The study of biomarkers using proteomics in patients with epilepsy is a challenge. Access to the brain tissue consists of an essential approach to measure molecular dynamics at a determined point in the time course of the disease. However, in humans, the availability of brain tissue is very difficult; in epilepsy the brain is only available postmortem or from surgery performed on patients with focal epilepsy who do not respond to the pharmacological treatment (Do Canto et al., 2021). There are few studies using proteomics in humans, but compared to the last 10 years, we have seen a growing increase in data obtained from brain tissue and plasma and cerebrospinal fluid.
Some authors employed proteomics analysis to identify proteins differentially expressed in the hippocampi of patients with MTLE compared to control tissue obtained at autopsy (Yang et al., 2004). They found a reduction in the expression of the cytosolic enzyme acyl-CoA thioester hydrolase, known for its role in energy production through beta-oxidation in the mitochondria and peroxisomes, signal transduction, and protein kinase C activation, has been reported in patients with MTLE (Yang et al., 2004). In a subsequent study, the authors observed a reduction in the expression of 18 proteins playing different roles in the brain (Yang et al., 2006). Proteins with different roles as chaperone (TCP-1-alpha and HSP70), cell signaling (MAPKK), transcriptional signaling (NAD-dependent deacetylase sirtui-2), or which are components of synaptosomes (synaptotagmin I and alpha-synuclein) and cytoskeleton (tubulins, vinculin, and profiling) are among them. On the other hand, increased expression of proteins associated with antioxidant function (peroxiredoxin 6), gliosis, and increased microvascular endothelial cells (apo A-I) was also reported by the same authors (Yang et al., 2005, 2006).
With the aim of obtaining biomarkers for TLE, Xiao et al. (2009) analyzed the cerebrospinal fluid (CSF) of patients using proteomics. The authors found five differentially expressed proteins in TLE patients compared to control, and six6 expressed only in patients with epilepsy. Vitamin D-binding protein (DBP) was increased, whereas cathepsin D, apolipoprotein J, Fam3c, and superoxide dismutase 1 (SOD1) were decreased in the TLE samples compared to the control. The six proteins found only in the patients were: tetranectin (TN), talin-2, apolipoprotein E, immunoglobulin lambda light chain (IGLc), immunoglobulin kappa variable light chain 1–5 (IGKV1–5), and procollagen C-endopeptidase enhancer 1 (PCOLCE). Table 15.2 shows the main functions of these proteins. Abnormal expression of some of these proteins, such as cathepsin D and SOD1, for example, has been reported in a study with proteomics using brain tissue (Eun et al., 2004), indicating that low levels of these proteins in the CSF may reflect deficiency in the brain (Xiao et al., 2009).
Despite most biomarkers to predict DR-TLE having been obtained mainly by samples of patients, the CSF has emerged as a promising source for the identification of brain biomarkers, since it is the body fluid with the closest anatomical contact with the changes that occur in the brain. Xiao et al. (2009) reported that the four proteins downregulated in the CSF of patients with DRE are proteins involved in anti-inflammatory mechanisms, anti-oxidative, and neuroprotection, suggesting a possible role in the epileptogenesis (Table 15.2).
Cathepsin D belongs to the pepsin family of proteases and is one of the most studied aspartic proteases. This lysosomal protease is involved in proteolytic degradation, cell invasion, and apoptosis. Cathepsin D participates in the mechanism of autophagy, a cellular process undertaken by neurons in the central nervous system to transport unneeded constituents to lysosomes (Uchiyama et al., 2009). This process is essential for the maintenance of cellular metabolism under physiological conditions. Processes that cause the reduction of lysosomal proteinases such as cathepsin D, B, and L, can induce neurodegeneration and be involved in epilepsy (Uchiyama et al., 2009).
Besides cathepsin D, downregulation of the antiapoptotic clusterin (apolipoprotein J) was also observed in the CSF of the patients with DRE compared to the controls (Xiao et al., 2009). These data are in line with those of the other authors, which showed reduced levels of CSF-clusterin in patients with drug-resistant and drug-responsive TLE compared to the controls, suggesting a pro-epileptogenic role (Yu et al., 2014). The clusterin, described as a glycoprotein, is presented as a nuclear form (nCLU) and a secretory form (sCLU) and perform dual role on apoptosis. nCLU is proapoptotic (Kim et al., 2012) and sCLU is antiapoptotic (Zhang et al., 2005). Clusterin is involved in several neurological diseases including Alzheimer’s disease, Parkinson’s disease (Milinkeviciute & Green, 2023; Charnay et al., 2012), and seizure-induced neuronal loss (Yu et al., 2014; Kim et al., 2012). Moreover, clusterin was markedly decreased in the CSF of patients with DRE rather than in patients with drug-responsive epilepsy (Xiao et al., 2009). The decreased level of CSF-clusterin in patients with DRE suggests that the cytoprotective effect of sCLU was attenuated in response to seizures. According to Yu et al. (2014), patients with DRE usually have a significant longer duration of disease compared to patients with drug-responsive epilepsy, suggesting that CSF-clusterin concentration could also be correlated with seizure and disease duration. Furthermore, time-dependent alteration of clusterin was also observed in the epilepsy model induced by kainic acid in rats (Kim et al., 2012).
Superoxide dismutase (SOD), a key antioxidant enzyme, cleans the superoxide radical and forms the first line of defense against oxidative stress and its subsequent effects (Costello & Delanty, 2004). Prolonged seizures may result in mitochondrial dysfunction, increased production of reactive oxygen species and nitric oxide, and neuronal cell death. Moreover, chronic mitochondrial oxidative stress and the resultant cellular dysfunction can render the brain more susceptible to epileptic seizures (Costello & Delanty, 2004). Thus, mitochondrial oxidative stress is considered a key factor in epileptogenesis. As a response to seizures, the synthesis of SOD1 protein protects against neuronal degeneration induced by oxidative stress, but hippocampal CA1 and CA3 neurons in an epileptic rat model no longer have SOD1 immunoreactivity (Kim et al., 2000). However, a reduced level of SOD was observed in the cortex of patients with TLE who had received surgical treatment (Eun et al., 2004) and in a rat model of electroconvulsive shock and pilocarpine (Erakovic et al., 2000; Bellissimo et al., 2001). Taking this into account, a lower level of SOD1 in the CSF of TLE patients described by Xiao et al. (2009) may reflect deficiency of antioxidant enzymes in the central nervous system in this pathological state.
FAM3C is a member of a cytokine-like gene family. Fam3c, also known as interleukin-like epithelial–mesenchymal transition inducer (ILEI), is a secreted protein found to be ubiquitously expressed in tissues, including bone, muscle, and brain whose function has not been well known (Kraya et al., 2015). Studies have shown that the level of secreted Fam3c is markedly decreased in the brains of AD patients, whereas this protein destabilizes the β-secretase-cleaved APP carboxy-terminal fragment (Hasegawa et al., 2014). Overexpression of Fam3C significantly reduces the brain Aβ burden and ameliorates the memory deficit in AD model mice. Fam3c may be a plausible target for the development of disease-modifying therapies (Hasegawa et al., 2014). Otherwise, Fam3c has been reported as a member of a group of secreted proteins involved with tumor formation and metastasis (Kraya et al., 2015). High level of Fam3c has been detected in melanoma cell-conditioned media and patient, suggesting high level of autophagy associated with malignant melanoma’s progression and aggressiveness (Kraya et al., 2015). On the other hand, the function of Fam3c in epilepsy has not been well determined.
The six proteins detected only in the CSF of patients with DRE might be involved in epileptogenesis (Xiao et al., 2009). Tetranectin is a glycoprotein which is encoded by the CLEC3B gene (C-type lectin domain family 3, member B) in human (Clemmensen et al., 1986). This protein is expressed in a variety of cells including monocytes, neutrophils, fibroblasts, and osteoblasts, and has been associated with the regulation of fibrinolysis, tissue remodeling, and proteolytic processes (Wang et al., 2010; Dahiya et al., 2017). Studies have shown that tetranectin has a defined role in the pathophysiology of epilepsy (Dahiya et al., 2017). Tetranectin levels were significantly downregulated in patients with DR epilepsy; the lower plasma tetranectin level was correlated with frequency of seizures and disease advances.
Talin-2 is another protein detected in CSF of patients with DRE. Talin-2 is a cytoskeletal protein found in high concentrations in the synapses. This protein plays an important role in the synaptic vesicle endocytosis, as in cell adhesion and in the recycling of synaptic vesicles (Xiao et al., 2010). To maintain continuous and reliable neurotransmitter release, synaptic vesicles must be rapidly recycled. A predominant mechanism responsible for synaptic recycling is clathrin-mediated endocytosis. Reduced levels of Talim-2 in the CSF may indicate impairment in the clathrin-mediated synaptic vesicle endocytosis, which can disturb neurotransmitter release and contribute to the epileptogenesis (Zheng et al., 2010).
Apolipoproteins play a well-established role in the transport and metabolism of lipids within the CNS, with a fundamental role in the maintenance and repair of neuron cell membranes (Mahley, 1988). The ApoE gene is polymorphic, and three isoforms have been identified ɛ2, ɛ3, and ɛ4, showing different functions in the CNS (Morrow et al., 2000). Previous studies investigated the role of ApoE variants in TLE (Briellmann et al., 2000, Xu et al., 2021, Salzmann et al., 2008). Specifically, the results concerning the role of the ApoE ɛ4 isoform as a modifier of the age of onset of epilepsy have been controversial (Kilpatrick et al., 1996, Tan et al., 2004). Kauffman et al. (2010) performed a molecular epidemiology study, a systematic review, and a meta-analysis to study the role of ApoE ɛ4 isoform as a modifier of the age at onset of TLE. The authors showed that the ApoE ɛ4 allele is associated with an earlier onset of TLE. These results were also confirmed by a meta-analysis study inasmuch all the populations analyzed showed a trend for a lower age at the onset of epilepsy in ApoE ɛ4 carriers compared with noncarriers (Kauffman et al., 2010). TLE, the most common form of partial epilepsy, is a polygenic and complex disease. There are several factors that might influence the age at the onset of TLE, and the genetic factor ApoE ɛ4 seems to participate in some of them, such as, for example, disruption of the cytoskeleton, potentiation of apoptosis and increase in the deposit of β amyloid. Therefore, the findings highlight the ApoE gene as a candidate to influence the epileptogenic processes occurring in TLE. However, the authors reported that other variables that were not fully analyzed such as age, ethnicity, gender, and epilepsy etiology may be a source of bias in the results.
The immunoglobulin kappa-free light chains (IGKV1-5) and lambda-free light chains (IGLc) were also found to be expressed in the CSF of patients with DR-TLE but not in the CSF of control (Xiao et al., 2009). Since the discovery that autoimmune mechanisms were able to induce epilepsy in animal models and that gamma globulin could be used to treat epilepsy encephalopathy, subclasses of immunoglobulins have been investigated in the epileptic process. Increased kappa/lambda ratio has been reported in the serum and in cerebrospinal fluid of patients with various neurological diseases such as multiple sclerosis, amyotrophic lateral sclerosis, polyneuropathy, viral encephalitis, and intractable epilepsy, suggesting that the examination of light immunoglobulin chains is a good tool for diagnosing DR epilepsy in childhood (Bollengier et al., 1978; Lischka et al., 1994). In contrast, Haraldsson et al. (1992) observed a decreased serum total kappa/lambda ratio suggesting disturbed immunological mechanisms in children with DR epilepsy resulting from a developmental delay in immunopathophysiological and neuropathophysiological mechanisms in childhood epilepsies.
The final protein found by Xiao et al. (2009) expressed only in the CSF of patients with DR-TLE was the PCOLCE, an extracellular matrix protein that enhances the activities of procollagen C-proteinases (Table 15.2). This protein has also been found to be expressed in brain tissues (Takahara et al., 1994). PCOLCE is critical for collagen deposition. Studies in animal models have shown that, due to the chemoattractant property of collagen, this protein may play a role in cell migration and seizure activity in patients with TLE (Veznedaroglu et al., 2002). Therefore, Xiao et al. (2009) proposed that the presence of PCOLCE in the CSF of patients with TLE indicates cellular pathological changes in collagen metabolism in the epileptic brain.
A recent study performed to analyze the protein profile of patients with focal seizures (FS) due to TLE and with psychogenic nonepileptic seizures (PNES) showed that blood-brain barrier (BBB) damage is the main event that distinguishes patients with PNES from patients with FS (Hamrah et al., 2020). Under this condition of increased permeability of the BBB, molecules that are normally expected to be found only in the central nervous system, may diffuse into peripheral blood as well as serum proteins may reach the brain parenchyma. The altered proteins found in the serum following focal seizures were S-100B, ceruloplasmin, alpha 1-acilglycoprotein 1, and malate dehydrogenase 2, and they can be used as markers to differentiate seizures (Hamrah et al., 2020).
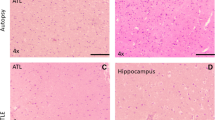
A study carried out by our group also showed the presence of serum albumin in hippocampal samples from patients with MTLE (Persike et al., 2018). Using proteomics (2D-PAGE coupled to LC-ESI-MS/MS) we have shown that the total number of spots was noticeably smaller in the hippocampus of MTLE patients with DRE than in control tissue (Fig. 15.2). A total of 16 proteins were differentially expressed in the hippocampal samples of patients with MTLE compared to control, but only 9 proteins were identified by NCBI database, as shown in Table 15.3. Among the nine identified proteins, six were upregulated, and one was downregulated in the hippocampal samples of MTLE group compared to control; two proteins were expressed only in the 2D-PAGE of patients with epilepsy. The identified proteins were: isoform 1 of serum albumin (ALB1), HSP70, dihydropyrimidinase-related protein 2 (DPYSL2), isoforms of myelin basic protein-1 (MBP1), isoform 3 of spectrin alpha chain (SPTAN1), proton ATPase catalytic subunit A (ATP6V1A), glutathione S-transferase P (GSTP1), protein DJ-1 (PARK7), and dihydrolipoyllysine-residue acetyltransferase component of pyruvate dehydrogenase complex (DLAT). Glutathione S-transferase P and PARK7 were detected only in hippocampal samples of patients with MTLE. The expression of spectrin was downregulated in the hippocampi of the patients. All other proteins were upregulated in epilepsy. The expression of HSP-70, ATP6V1A and GSTP1 was validated by Western blot (Persike et al., 2018).
Representative 2D-PAGE image of hippocampal protein extracts from control (a) and patients with mesial temporal lobe epilepsy (b). An amount (0.5 mg) of total protein of each sample was separated by isoelectrofocusing on a pH 3–10 linear gradient followed by the second dimension run on SDS-PAGE. Gels were stained with Coomassie brilliant blue. The peptides obtained from protein digestion of spots differentially expressed were analyzed by LC-ESI-MS/MS
Most of the identified proteins do not possess a well-defined function in the epileptic process. However, some authors suggest that the increase in the expression of myelin basic protein and albumin, may, for example, be indicative of alteration in the permeability of the blood-brain barrier and in myelination processes in experimental model of epilepsy (Huang et al., 2008; Marchi et al., 2010). Our data corroborate those of authors who show increased barrier permeability as a factor that contributes to epileptogenesis by facilitating the exposure of neurons to pro-inflammatory cytokines and predisposing them to drug resistance epilepsy.
The vacuolar H+ATPase, an evolutionarily ancient enzyme involved in neurotransmitter release mechanism (Wilkens et al., 2005) and acidification of synaptic vesicles after exocytosis (Li et al., 2005), was found to be upregulated in patients with MTLE compared to control. Likewise, the increased expression of HSP70 in hippocampal samples of the patients with epilepsy may represent a compensatory mechanism since HSP70 is a chaperone involved in the folding of new proteins (Mayer & Bukau, 2005).
The dihydropyrimidinase-related protein 2 (DPYSL2) is a member of cytosolic phosphoproteins, and is involved with neuronal migration, axon growth, and guidance (Morimura et al., 2013). Previous studies have reported an upregulation of DPYSL2 in hippocampal samples of patients with TLE (Persike et al., 2018) and in experimental model of epilepsy induced by pilocarpine (Marques-Carneiro et al., 2017). Increased expression of this protein has been associated with susceptibility to psychiatric disorders, such as schizophrenia (Ujike et al., 2006) or in diseases in which the psychiatric disorders appear as comorbidity such as Alzheimer’s disease (Castegna et al., 2002). Upregulation of DPYSL2 was also observed in the hippocampi of patients with MTLE and considering its role in axonal growth and guidance, Persike et al. (2018) suggested that DPYSL2 can be involved in neuronal sprouting and seizures generation.
In contrast, the authors found downregulation of the spectrin protein in hippocampal samples of patients with MTLE (Persike et al., 2018). This protein is responsible for anchoring the NMDA receptors (NR2A, NR2B, and NR1) to the actin cytoskeleton in the membrane, and it plays an important role in synaptic plasticity and long-term potentiation (LTP) (Wechsler & Teichberg, 1998). Thus, changes in spectrin protein expression can cause LTP impairment and cognitive deficit.
The glutathione S-transferase P (GSTP1) and PARK-7 were expressed only in the hippocampal samples of patients with MTLE. Previous study showed that these proteins play an important role as antioxidants following brain injury (Sharma et al., 2004), in addition to playing an important role in detoxification. Besides, GSTP1 has been associated with the inactivation of antiepileptic drugs in the liver (Shang et al., 2008), and it may be responsible for the poor penetration of antiseizure medications. This protein may represent an important target for studies related to drug resistance frequently associated with MTLE. On the other hand, upregulation of PARK-7 indicates the presence of neuroprotective mechanisms associated to MTLE (Persike et al., 2018).
Mériaux et al. (2014), using MALDI mass spectrometry imaging (MSI) coupled with proteomics found seven neuropeptides differentially expressed in the layer of dentate gyrus (DG) of patients with MTLE. The identified proteins exert different roles being associated with axons regeneration (neurotrophin), extracellular matrix proteins, cell surface proteins, membrane proteins, G proteins, cytoskeleton proteins, and tumor suppressors (TS). The Leucine-rich glioma inactivated 1 (LGI1) protein was found in the hippocampus of MTLE and has been related to the heritability of TLE.
A proteomic study performed by Keren-Aviram et al. (2018) investigated proteins differentially expressed in the neocortex of patients with DRE, at high-frequency spikes. The authors identified eight upregulated proteins (SNCA, STMN1, UGP2, DSP, CA1, PRDX2, SYN2, and DPYSL2), and ten downregulated (GFAP, HNRNPK, CPNE6, CRYAB, GNAO1, PHYHIP, HNRPDL, ALDH2, GAPDH, and LASP1). In their analysis, the authors associated proteins with vascular modifications and the decreased expression of GFAPα as being related to metabolic changes and increased cortical activity.
Proteomics studies using plasma are very scarce. Banote et al. (2021) identified 41 proteins differentially expressed compared to the control group. Some of them have previously been reported to be associated with epilepsy, including Pentraxin-related protein (PTX3), von Willebrand factor (VWF), Sonic hedgehog (SHH), MAPK14, a member of mitogen-activated protein kinases, Apolipoprotein E (APOE), C–C motif chemokine 5 (CCL5), Laminin subunit beta-2 (LAMB2), and FYN-binding protein 1 (FYB1). Many of these proteins are involved in epileptogenic processes, such as inflammation, calcium ion binding, lipid binding activity, metal ion binding, and identical protein binding activity.
The presence of the chemokine CCL5 has also been reported by Toledo et al. (2021) in the cerebral cortex of patients with drug-resistant TLE (DR-TLE) compared to patients with TLE responders to treatment and healthy controls. According to the authors, the presence of CCL5 together with other cytokines (IFN-gamma and IL-17) in the cerebral cortex of the patients indicates involvement in lymphocyte recruitment, suggesting communication between central and peripheral inflammatory markers (Toledo et al., 2021).
The endogenous level of CCL5 is very low in the plasma of healthy individuals, but it increases dramatically in both peripheral and CNS in pathological conditions, i.e., in autoimmune diseases (encephalomyelitis and multiple sclerosis), and in DR-TLE (Pittaluga, 2017; Toledo et al., 2021; Wolinski et al., 2022). There are two mechanisms by which the CCL5 can become bioavailable in the CNS, (i) by the permeabilization of the blood-brain barrier during inflammation, which favors the entry of CCL5 from the periphery into the brain, (ii) by concomitant massive local production of CCL5 from activated astrocytes and, to a lesser extent, from microglial cells triggered by pro-inflammatory cytokines (Pittaluga, 2017). Once released in the synaptic cleft, CCL5 regulates the function of glial cells (microglia and astrocytes) themselves through autocrine processes by means of CCR5 receptors, and to a less extent, of CCR1 and CCR3 receptors (Pittaluga, 2017). In physiological conditions, the expression of CCR5 in astrocytes is low but rapidly increases upon stimulation by cytokines released by microglial cells contributing to higher expression of CCR proteins (Sørensen et al., 1999). Thus, this cascade is an event related to neuroinflammation.
In CNS, CCL5 is involved with Ca++ ions mobilization, suggesting that this chemokine could modulate glutamate exocytosis in the CNS (Meucci et al., 1998; Klein et al., 1999; Musante et al., 2008; Pittaluga, 2017). However, some authors have shown that the CCL5-induced changes to glutamate release are area specific and involve different receptor repertoires, indicating heterogeneity in the effect of this chemokine in the CNS (Di Prisco et al., 2012). CCL5 plays a role in coupling inflammation and synaptic excitability in CNS diseases secondary to viral infections, such as acquired immune deficiency syndrome-related dementia, or involving neuroinflammatory processes, such as MS and Alzheimer’s dementia (Pittaluga, 2017).
When we compared the proteins differentially expressed in the hippocampus of patients with MTLE and from rats subjected to pilocarpine compared to their respective controls, we identified three proteins in common, Park-7, DPYSL2, and ATP6V1A (Table 15.3). The proteins DPYSL2 and ATP6V1a were differentially increased in both patients, and rat hippocampi, while Park-7 was differentially expressed in rat (increased expression compared to control) and found exclusively expressed in the samples of MTLE patients. Increased expression of these proteins may be involved in neuronal development and plasticity (DPYSL2), neuroprotection mechanisms (Park-7), and neuronal excitability (ATP6V1a). The ATP6V1a is a protein involved with releasing neurotransmitters and acidifying synaptic vesicles after exocytosis for recycling (Li et al., 2005; Wilkens et al., 2005). The upregulation of this protein can reflect an increase in the dynamics of synthesis, storage, and release of neurotransmitters present in epileptic tissue and therefore increased excitation (Marques-Carneiro et al., 2017; Persike et al., 2018) (Table 15.4).
15.2 Conclusions
Proteomics has contributed to improve knowledge about altered mechanisms in the hippocampus (the main area affected in this disease) of patients with TLE, and experimental models of TLE. Proteomics, a powerful methodology that combines ancient techniques (i.e., two-dimensional electrophoresis and amino acid analysis) with advanced technologies (mass spectrometry), emerges as a powerful alternative in the search for target proteins to treat or perhaps prevent the occurrence of seizures. Proteomics allows not only detects the differential expression of proteins in the hippocampi of patients with epilepsy in relation to the control tissue but also provides information about the protein class to which they belong, the cellular compartment where they are found, and their molecular and biological functions, through qualitative analysis using specific software (GENEmania system, Metacore software) (Marques-Carneiro et al., 2017; Persike et al., 2018; Canto et al., 2022).
Data from studies with proteomics in the hippocampus of patients with MTLE and from experimental models of MTLE show common mechanisms involved with synaptic plasticity, neurotransmission, and neuroprotection. The proteins involved in these mechanisms may be important biomarkers for studies of these alterations associated with the epileptic condition.
Considering the complex physiology of the central nervous system, it may be convenient to combine simultaneous analyzes of material collected by surgical procedure from patients with MTLE. Patients with adequate clinical follow-up undergoing surgical treatment of epilepsy can provide important material for the research of biomarkers using proteomics.
Unbiased big data mining has emerged to generate patterns of genes, proteins, and metabolites, and signaling pathways, from brain tissue samples from MTLE patients (Kirchner et al., 2020). The generated data set can be organized into a multivariate interactome, together with clinical data, in the search for biomarkers or therapeutic targets employing experimental models or in vitro preparations in reverse translation. Currently, proteomics studies have been greatly relying on the mass spectrum (MS). Despite the high sensitivity, molecules with low concentrations in the tissue may not be detected. Thus, neuroscience still needs more sensitive methods that can directly read entire protein sequences without having to resort to databases to identify theoretical proteins.
References
Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Lipids Health Dis. 2008:241–68. https://doi.org/10.1007/978-1-4020-8831-5_9.
Aker RG, Yananli HR, Gurbanova AA, Ozkaynakci AE, Ates N, Luijtelaar G, Onat FY. Amygdala kindling in the WAG/Rij rat model of absence epilepsy. Epilepsia. 2010;47(1):33–40. https://doi.org/10.1111/j.1528-1167.2006.00367.x.
Akman O, Karson A, Aker RG, Ates N, Onat FY. Hippocampal kindling in rats with absence epilepsy resembles amygdaloid kindling. Epilepsy Res. 2008;81(2–3):211–9. https://doi.org/10.1016/j.eplepsyres.2008.06.004.
Akman O, Karson A, Aker RG, Ates N, Onat FY. Perirhinal cortical kindling in rats with genetic absence epilepsy. Neurosci Lett. 2010;479(1):74–8. https://doi.org/10.1016/j.neulet.2010.05.034.
Alzate O. Neuroproteomics. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010; Chapter 1. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56022/.
Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics:technologies and their applications. J Chromatogr Sci. 2017;55(2):182–96. https://doi.org/10.1093/chromsci/bmw167.
Banote RK, Larsson D, Berger E, Kumlien E, Zelano J. Quantitative proteomic analysis to identify differentially expressed proteins in patients with epilepsy. Epilepsy Res. 2021;174:106674. https://doi.org/10.1016/j.eplepsyres.2021.106674. Epub 2021 May 15. PMID: 34029912.
Becker AJ. Review: animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathol Appl Neurobiol. 2018;44(1):112–29. https://doi.org/10.1111/nan.12451. PMID: 29130506.
Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2019;54:1–7. https://doi.org/10.1159/000503831.
Bellissimo MI, Amado D, Abdalla DS, Ferreira EC, Cavalheiro EA, Naffah-Mazzacoratti MG. Superoxide dismutase, glutathione peroxidase activities and the hydroperoxide concentration are modified in the hippocampus of epileptic rats. Epilepsy Res. 2001;46:121–8.
Ben-Ari Y. Limbic seizures and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience. 1985;14(2):375–403. https://doi.org/10.1016/0306-4522(85)90299-4. PMID: 2859548.
Blumcke I, Spreafico R, Haaker G, Coras R, Kobow K, Bien CG, et al. Histopathological findings in brain tissue obtained during epilepsy surgery. N Engl J Med. 2017;377(17):1648–56. https://doi.org/10.1056/nejmoa1703784.
Bollengier F, Rabinovitch N, Lowenthal A. Oligoclonal immunoglobulins, light chain ratios and free light chain ratios in cerebrospinal fluid and serum from patients affected with various neurological diseases. J Clin Chem Clin Biochem. 1978;1:165–73.
Briellmann RS, Torn-Broers Y, Busuttil BE, Major BJ, Kalnins RM, Olsen M, Jackson GD, Frauman AG, Berkovic SF. APOE epsilon4 genotype is associated with an earlier onset of chronic temporal lobe epilepsy. Neurology 2000;55(3):435–7. https://doi.org/10.1212/wnl.55.3.435
Bruxel EM, do Canto AM, Bruno DCF, Geraldis JC, Lopes-Cendes I. Multi-omic strategies applied to the study of pharmacoresistance in mesial temporal lobe epilepsy. Epilepsia Open. 2021;7 https://doi.org/10.1002/epi4.12536.
Canto AM, Godoi AB, Matos AHB, Geraldis JC, Rogerio F, Alvim MKM, Yasuda CL, Ghizoni E, Tedeschi H, Veiga DFT, Henning B, Souza W, Rocha CS, Vieira AS, Dias EV, Carvalho BS, Gilioli R, Arul AB, Robinson RAS, Cendes F, Lopes-Cendes I. Benchmarking the proteomic profile of animal models of mesial temporal epilepsy. Ann Clin Transl Neurol. 2022;9(4):454–67. https://doi.org/10.1002/acn3.51533. Epub 2022 Mar 3. PMID: 35238489; PMCID: PMC8994989
Cardenas-Rodriguez N, Huerta-Gertrudis B, Rivera-Espinosa L, Montesinos-Correa H, Bandala C, Carmona-Aparicio L, Coballase-Urrutia E. Role of oxidative stress in refractory epilepsy: evidence in patients and experimental models. Int J Mol Sci. 2013;14:1455–76. https://doi.org/10.3390/ijms14011455.
Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer’s disease brain. Part II: dihydropyrimidinase-related protein 2, α-enolase and heat shock cognate 71. J Neurochem. 2002;82(6):1524–32. https://doi.org/10.1046/j.1471-4159.2002.01103.x.
Cavalheiro EA. The pilocarpine model of epilepsy. Ital J Neurol Sci. 1995;16(1–2):33–7. https://doi.org/10.1007/bf02229072.
Cavalheiro EA, Leite JP, Bortolotto ZA, Turski WA, Ikonomidou C, Turski L. Long-term effects of pilocarpine in rats: structural damage of the brain triggers kindling and spontaneously recurrent seizures. Epilepsia. 1991;6:778–82. https://doi.org/10.1111/j.1528-1157.1991.tb05533.x.
Cavalheiro EA, Fernandes MJ, Turski L, Naffah-Mazzacoratti MG. Spontaneous recurrent seizures in rats: amino acid and monoamine determination in the hippocampus. Epilepsia. 1994;35(1):1–11. https://doi.org/10.1111/j.1528-1157.1994.tb02905.x.
Chai X, Zhang W, Li L, Wu Y, Zhu X, Zhao S. Profile of MIF in developing hippocampus: association with cell proliferation and neurite outgrowth. Front Mol Neurosci. 2020;12(13):147. https://doi.org/10.3389/fnmol.2020.00147. PMID: 32903462; PMCID: PMC7434973.
Chandramouli K, Qian PY. Proteomics: challenges, techniques, and possibilities to overcome biological sample complexity. Hum Genomics Proteomics. 2009;1:239204. https://doi.org/10.4061/2009/239204.
Charnay Y, Imhof A, Vallet PG, Kovari E, Bouras C, Giannakopoulos P. Clusterin in neurological disorders: molecular perspectives and clinical relevance. Brain Res Bull. 2012;88(5):434–43. https://doi.org/10.1016/j.brainresbull.2012.05.006.
Chipaux M, Szurhaj W, Vercueil L, Milh M, Villeneuve N, Cances C, Auvin S, Chassagnon S, Napuri S, Allaire C, Derambure P, Marchal C, Caubel I, Ricard-Mousnier B, N’Guyen The Tich S, Pinard JM, Bahi-Buisson N, de Baracé C, Kahane P, Gautier A, Hamelin S, Coste-Zeitoun D, Rosenberg SD, Clerson P, Nabbout R, Kuchenbuch M, Picot MC, Kaminska A. Epilepsy diagnostic and treatment needs identified with a collaborative database involving tertiary centers in France. Epilepsia. 2016;57(5):757–69. https://doi.org/10.1111/epi.13368. Epub 2016 Apr 1. PMID: 27037674.
Clemmensen I, Petersen LC, Kluft C. Purification, and characterization of a novel, oligomeric, plasminogen kringle 4 binding protein from human plasma: tetranectin. Eur J Biochem. 1986;156(2):327–33.
Costello DJ, Delanty N. Oxidative injury in epilepsy: potential for antioxidant therapy? Expert Rev Neurother. 2004;4(3):541–53.
Dahiya ES, Mehndiratta MM, Pillai KK. Plasma tetranectin as a potential clinical biomarker for epilepsy and correlation with clinical and social characteristics. Int J Epilepsy. 2017;4(1):2–5. https://doi.org/10.1016/j.ijep.2016.12.003.
Danış O, Demir S, Günel A, Aker RG, Gülçebi M, Onat F, Ogan A. Changes in intracellular protein expression in cortex, thalamus and hippocampus in a genetic rat model of absence epilepsy. Brain Res Bull. 2011;84(6):381–8. https://doi.org/10.1016/j.brainresbull.2011.02.002. Epub 2011 Feb 15. PMID: 21310218.
Danober L, Deransart C, Depaulis A, Vergnes M, Marescaux C. Pathophysiological mechanisms of genetic absence epilepsy in the rat. Prog Neurobiol. 1998;55(1):27–57. https://doi.org/10.1016/s0301-0082(97)00091-9. PMID: 9602499.
Di Ciero L, Bellato CM. Proteoma: avanços recentes em técnicas de eletroforese bidimensional eespectrometria de massa. Biotecnol Clin Desenvolv. 2003;29:158–64. http://www.biotecnolo-gia.com.br/revista/bio29/proteoma.pdf
Di Prisco S, Summa M, Chellackudam V, Rossi PI, Pittaluga A. RANTES-mediated control of excitatory amino acid release in mouse spinal cord. J Neurochem. 2012;121:428–37. https://doi.org/10.1111/j.1471-4159.2012.07720.x.
Do Canto AM, Donatti A, Geraldis JC, Godoi AB, da Rosa DC, Lopes-Cendes I. Neuroproteomics in epilepsy: what do we know so far? Front Mol Neurosci. 2021;13:604158. https://doi.org/10.3389/fnmol.2020.604158. PMID: 33488359; PMCID: PMC7817846.
Dragunow M, Preston K, Dodd J, Young D, Lawlor P. Christie D Clusterin accumulates in dying neurons following status epilepticus. Brain Res Mol Brain Res. 1995;2:279–90.
Erakovic V, Zupan G, Varljen J, Radosevic S, Simonic A. Electroconvulsive shock in rats: changes in superoxide dismutase and glutathione peroxidase activity. Brain Res Mol Brain Res. 2000;76:266–74.
Eun JP, Choi HY, Kwak YG. Proteomic analysis of human cerebral cortex in epileptic patients. Exp Mol Med. 2004;36:185–91.
Fattorusso A, Matricardi S, Mencaroni E, Dell’Isola GB, Striano P, Verrotti A. The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front Neurol. 2021;12:674483. https://doi.org/10.3389/fneur.2021.674483.
Fountoulakis M. Proteomics: current technologies and applications in neurological disorders and toxicology. Amino Acids. 2001;21(4):363–81. https://doi.org/10.1007/s007260170002.
Fountoulakis M, Takács B. Enrichment and proteomic analysis of low-abundance bacterial proteins. Methods Enzymol. 2002;358:288–306. https://doi.org/10.1016/S0076-6879(02)58096-4.
Fritz IB, Burdzy K, Setchell B, Blaschuk O. Ram rete fluid contains a protein (clusterin) which influences cell–cell interactions in vitro. Biol Reprod. 1983;28:1173–88.
Greenberg DA, Cayanis E, Strug L, Sudhir M, Durner M, Pal DK, Alvin GB, Klotz I, Dicker E, Shinnar S, Bromfield EB, Resor S, Cohen J, Moshe SL, Harden C, Kang H. Malic Enzyme 2 underline susceptibility to adolescent-onset idiopatic generalized epilepsy. Am J Hum Genet. 2005;76(1):139–46. https://doi.org/10.1086/426735.
Hamrah MP, Tavirani MR, Movahedi M, Karvigh SA. Identification of serum biomarkers for differentiating epileptic seizures from psychogenic attacks using a pro- teomic approach; a comparative study. Arch Acad Emerg Med. 2020;8(1):e87. PMID: 33244522; PMCID: PMC7682629.
Haraldsson A, van Engelen BGM, Renier W, Bakkeren JAJM, Weemaes CMR. Light chain ratios and concentrations of serum immunoglobulins in children with epilepsy. Epilepsy Res. 1992;13:255–60.
Hasegawa H, Liu L, Tooyama I, Murayama S, Nishimura M. The FAM3 superfamily member ILEI ameliorates Alzheimer’s disease-like pathology by destabilizing the penultimate amyloid-β precursor. Nat Commun. 2014;5:3917. https://doi.org/10.1038/ncomms4917.
Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535(2):195–204. https://doi.org/10.1016/0006-8993(90)91601-c.
Huang ZL, Zhou Y, Xiao B, Wu J, Wu XM, Yang P, Wu LY. Proteomic screening of postsynaptic density proteins related with temporal lobe epilepsy. Zhonghua Yi Xue Za Zhi. 2008;88(45):3205–9. Chinese. PMID: 19171095.
Kauffman MA, Consalvo D, Morona DG, Lereis VP, Kochen S. ApoE ɛ4 genotype and the age at onset of temporal lobe epilepsy: a case–control study and meta-analysis. Epilepsy Res. 2010;9(3):234–9. https://doi.org/10.1016/j.eplepsyres.2010.05.007.
Keren-Aviram G, Dachet F, Bagla S, Balan K, Loeb JA, Dratz EA. Proteomic analysis of human epileptic neocortex predicts vascular and glial changes in epileptic regions. PLoS One. 2018;13(4):e0195639. https://doi.org/10.1371/journal.pone.0195639.
Kilpatrick ES, Jagger CE, Spooner, RJ, Brodie, MJ, 1996. Apolipoprotein E and epilepsy. Ann Clin Biochem. 1996;33(Pt2):146–47. https://doi.org/10.1177/000456329603300208
Kim HC, Bing G, Jhoo WK, Ko KH, Kim WK, Suh JH, Kim SJ, Kato K, Hong JS. Changes of hippocampal Cu/Zn-superoxide dismutase after kainate treatment in the rat. Brain Research. 2000;853(2):215–26. https://doi.org/10.1016/S0006-8993(99)02254-4
Kim N, Choi WS. Proapoptotic role of nuclear clusterin in brain. Anat Cell Biol. 2011;44:169–75. https://doi.org/10.5115/acb.2011.44.3.169
Kim H, Eliuk S, Deshane J, Meleth S, Sanderson T, Pinner A, Robinson G, Wilson L, Kirk M, Barnes S. 2D gel proteomics: an approach to study age-related differences in protein abundance or isoform complexity in biological samples. Methods Mol Biol. 2007;371:349–91. https://doi.org/10.1007/978-1-59745-361-5_24.
Kim YS, Choi MY, Ryu JH, Lee DH, Jeon BT, Roh GS, Kang SS, Kim HJ, Cho GJ, Choi WS. Clusterin interaction with Bcl-xL is associated with seizure-induced neuronal death. Epilepsy Res. 2012;99:240–51. https://doi.org/10.1016/j.eplepsyres.2011.12.002
Kirchner A, Dachet F, Loeb JA. Identifying targets for preventing epilepsy using systems biology of the human brain. Neuropharmacology. 2020;168:107757. https://doi.org/10.1016/j.neuropharm.2019.107757.
Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, et al. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–46. https://doi.org/10.1002/glia.10181.
Kraya AA, Piao S, Xu X, Zhang G, Herlyn M, Gimotty P, Levine B, Amaravadi RK, Speicher DW. Identification of secreted proteins that reflect autophagy dynamics within tumor cells. Autophagy. 2015;11(1):60–74.
Kwan P, Brodie MJ. Refractory epilepsy: a progressive, intractable but preventable condition? Seizure. 2002;11:77–84. https://doi.org/10.1053/seiz.2002.0593.
Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshé SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–77. https://doi.org/10.1111/j.1528-1167.2009.02397.x.
Lewis DV, Shinnar S, Hesdorffer DC, Bagiella E, Bello JA, Chan S, Xu Y, MacFall J, Gomes WA, Moshé SL, Mathern GW, Pellock JM, Nordli DR, Frank LM, Provenzale J, Shinnar RC, Epstein LG, Masur D, Litherland C, Sun S, FEBSTAT Study Team. Hippocampal sclerosis after febrile status epilepticus: the FEBSTAT study. Ann Neurol. 2014;75(2):178–85. https://doi.org/10.1002/ana.24081. Epub 2014 Mar 1. PMID: 24318290; PMCID: PMC3980500.
Li KW, Smit AB. Subcellular proteomics in neuroscience. Front Biosci. 2008;13:4416–25. https://doi.org/10.2741/3014.
Li Z, Burrone J, Tyler WJ, Hartman KN, Albeanu DF, Murthy VN. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc Natl Acad Sci U S A. 2005;102(17):6131–6. https://doi.org/10.1073/pnas.0501145102. Epub 2005 Apr 18. PMID: 15837917; PMCID: PMC1087931.
Lischka A, Graf M, Hauser E, Herkner KR. Kappa/lambda ratio as parameter for evaluation of therapy-resistant epilepsy of childhood. Eur Neurol. 1994;34(1):74–8.
Liu X, Wen F, Yang J, Chen L, Wei YQ. A review of current applications of mass spectrometry for neuroproteomics in epilepsy. Mass Spectrom Rev. 2010;29:197–246. https://doi.org/10.1002/mas.20243.
Lo WY, Tsai FJ, Liu CH, Tang NY, Su SY, Lin SZ, Chen CC, Shyu WC, Hsieh CL. Uncaria rhynchophylla upergulates the expression of MIF and cyclophilin a in kainic acid-induced epilepsy rats: a proteomic analysis. Am J Chin Med. 2010;38:745–59. https://doi.org/10.1142/S0192415X10008214.
Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–30. https://doi.org/10.1126/science.328393
Marchi N, Teng Q, Ghosh C, Fan Q, Nguyen MT, Desai NK, Bawa H, Rasmussen P, Masaryk TK, Janigro D. Blood-brain barrier damage, but not parenchymal white blood cells, is a hallmark of seizure activity. Brain Res. 2010;24(1353):176–86. https://doi.org/10.1016/j.brainres.2010.06.051.
Marques-Carneiro JE, Persike DS, Litzahn JJ, Cassel J-C, Nehlig A, Fernandes MJ d S. Hippocampal proteome of rats subjected to the Li-pilocarpine epilepsy model and the effect of carisbamate treatment. Pharmaceuticals. 2017;10(4):67. https://doi.org/10.3390/ph10030067.
Martins-de-Souza D, Gattaz WF, Schmitt A, Novello JC, Marangoni S, Turck CW, Dias-Neto E. Proteome analysis of schizophrenia patients Wernicke’s area reveals an energy metabolism dysregulation. BMC Psychiatry. 2009;9:17. https://doi.org/10.1186/1471-244X-9-17.
Masino SA, Kawamura M, Wasser CD, Pomeroy LT, Ruskin DN. Adenosine, ketogenic diet and epilepsy: the emerging therapeutic relationship between metabolism and brain activity. Curr Neuropharmacol. 2009;7(3):257–68. https://doi.org/10.2174/157015909789152164.
May PC, Finch CE. Sulfated glycoprotein 2: new relationship of this multifunctional protein to neurodegeneration. Trends Neurosci. 1992;15:391–6.
Mayer MP, Bukau B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol Life Sci. 2005;62(6):670–84. https://doi.org/10.1007/s00018-004-4464-6.
Mériaux C, Franck J, Park DB, Quanico J, Kim YH, Chung CK, Park Y, Steinbusch H, Salzet M, Fournier I. Human temporal lobe epilepsy analyses by tissue proteomics. Hippocampus. 2014;24:628–42. https://doi.org/10.1002/hipo.22246.
Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95:14500–5. https://doi.org/10.1073/pnas.95.24.14500.
Milinkeviciute G, Green KN. Clusterin/apolipoprotein J, its isoforms and Alzheimer’s disease. Front Aging Neurosci. 2023;15. https://doi.org/10.3389/fnagi.2023.1167886.
Morimura R, Nozawa K, Tanaka H, Ohshima T. Phosphorylation of Dpsyl2 (CRMP2) and Dpsyl3 (CRMP4) is required for positioning of caudal primary motor neurons in the zebrafish spinal cord. Dev Neurobiol. 2013;73:911–20. https://doi.org/10.1002/dneu.22117.
Morrow JA, Segall ML, Lund-Katz S, Phillips MC, Knapp M, Rupp B, Weisgraber KH. Differences in stability among the human apolipoprotein E isoforms determined by the amino-terminal domain. Biochemistry. 2000;39(38):11657–66. https://doi.org/10.1021/bi000099m.
Musante V, Longordo F, Neri E, Pedrazzi M, Kalfas F, Severi P, et al. RANTES modulates the release of glutamate in human neocortex. J Neurosci. 2008;28:12231–40. https://doi.org/10.1523/JNEUROSCI.3212-08.2008.
Mus-Veteau I. Heterologous expression and purification systems for structural proteomics of mammalian membrane proteins. Comp Funct Genomics. 2002;3:511–7. https://doi.org/10.1002/cfg.218.
Neves SR, Ram PT, Iyengar R. G protein pathways. Nat. 2002;296(5573):1636–9. https://doi.org/10.1126/science.1071550.
Onat FY, Aker RG, Gurbanova AA, Ates N, Luijtelaar G. The effect of generalized absence seizures on the progression of kindling in the rat. Epilepsia. 2007;5:150–6. https://doi.org/10.1111/j.1528-1167.2007.01303.x.
Pappin DJ, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Sci Direct. 1993;3(6):327–32. https://doi.org/10.1016/0960-9822(93)90195-T.
Persike DS, Lima M, Amorim R, Cavalheiro E, Yacubian E, Centeno R, Carrete H, Shenkman S, Canzian M, Fernandes MJS. Hippocampal proteomic profile in temporal lobe epilepsy. J Epilepsy Clin Neurophysiol. 2012;18(2):53–6. https://doi.org/10.1590/s1676-26492012000200007.
Persike DS, Marques-Carneiro JE, Stein MLL, Yacubian EMT, Centeno R, Canzian M, Fernandes MJS. Altered proteins in the hippocampus of patients with mesial temporal lobe epilepsy. Pharmaceuticals (Basels). 2018;11(4):95. https://doi.org/10.3390/ph11040095.
Pfänder M, Arnold S, Henkel A, Weil S, Noachtar S. Clinical features and EEG findings differentiating mesial from neocortical temporal lobe epilepsy. Epileptic Disord. 2002;4(3):189–95. PMID: 12446221.
Pitkänen A, Lukasiuk K, Dudek FE, Staley KJ. Epileptogenesis. Cold Spring Harb Perspect Med. 2015;5(10):1–17. https://doi.org/10.1101/cshperspect.a022822.
Pittaluga A. CCL5–glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front Immunol. 2017;8:1079. https://doi.org/10.3389/fimmu.2017.01079.
Ross BM, Hudson C, Erlich J, Warsh JJ, Kish SJ. Increased phospholipid breakdown in schizophrenia. Evidence for the involvement of a calcium independent phospholipase A2. Arch Gen Psychiatry. 1997;54(5):487–94. https://doi.org/10.1001/archpsyc.1997.01830170113015.
Salzmann A, Perroud N, Crespel A, Lambercy C, Malafosse A. Candidate genes for temporal lobe epilepsy: a replication study. Neurol Sci. 2008;29:397–403. https://doi.org/10.1007/s10072-008-1060-9.
Shang W, Liu WH, Zhao XH, Sun QJ, Bi JZ, Chi ZF. Expressions of glutathione S-transferase alpha, mu, and pi in brains of medically intractable epileptic patients. BMC Neurosci. 2008;9:67:1–5. https://doi.org/10.1186/1471-2202-9-67.
Sharma R, Yang Y, Sharma A, Awasthi S, Awasthi YC. Antioxidant role of glutathione S-transferases: protection against oxidant toxicity and regulation of stress-mediated apoptosis. Antioxid Redox Signal. 2004;6(2):289–300. https://doi.org/10.1089/152308604322899350.
Silva AV, Cabral FR. Ictogênese, Epileptogênese e Mecanismo de Ação das Drogas na Profilaxia e Tratamento da Epilepsia. J Epilepsy Clin Neurophysiol. 2008;14(2):39–45. ISSN 1676-2649
Sørensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, et al. Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest. 1999;103:807–15. https://doi.org/10.1172/JCI5150.
Takahara K, Kessler E, Biniaminov L, Brusel M, Eddy RL, Jani-Saitfl S, Shows TB, Greenspan, DS. Type I Procollagen COOH-terminal proteinase enhancer protein: identification, primary structure, and chromosomal localization of the cognate human gene (PCOLCE). J of Biol Chem 1994;269(42):26280–85.
Talib LL, Yassuda MS, Diniz BSO, Forlenza OV, Gattaz WF. Cognitive training increases platelet PLA2 activity in healthy elderly subjects. Prostaglandins Leukot Essent Fat Acids. 2008;78:265–9. https://doi.org/10.1016/j.plefa.2008.03.002.
Tan NC, Mulley JC, Berkovic SF. Genetic association studies in epilepsy: “the truth is out there”. Epilepsia. 2004;45(11):1429–42. https://doi.org/10.1111/j.0013-9580.2004.22904.x.
Tatum WO. Mesial temporal lobe epilepsy. J Clin Neurophysiol. 2012;29(5):356–65. https://doi.org/10.1097/WNP.0b013e31826b3ab7.
Temkin NR. Preventing and treating posttraumatic seizures: the human experience. Epilepsia. 2009;50(2):10–3. https://doi.org/10.1111/j.1528-1167.2008.02005.x.
Toledo A, Orozco-Suarez S, Rosetti M, Maldonado L, Bautista SI, Flores X, Arellano A, Moreno S, Alonso M, Martínez-Juárez IE, Fragoso G, Sciutto E, Fleury A. Temporal lobe epilepsy: evaluation of central and systemic immune-inflammatory features associated with drug resistance. Seizure. 2021;91:447–55. https://doi.org/10.1016/j.seizure.2021.07.028.
Uchiyama Y, Koike M, Shibata M, Sasaki M. Autophagic neuron death. Methods Enzymol. 2009;453:33–51.
Ujike H, Sakai A, Tanaka Y, Kodaka T, Okahisa Y, Harano M, Inada T, Yamada M, Komivama T, Hori T, Sekine Y, Iwata N, Sora I, Ivo M, Ozaki N, Kuroda S. Association study of the dihydropyrimidinase-related protein 2 gene and methamphetamine psychosis. Ann N Y Acad Sci. 2006;1074:90–6. https://doi.org/10.1196/annals.1369.008.
Van den Bergh G, Arckens L. Recent advances in 2D electrophoresis: an array of possibilities. Expert Rev Proteomics. 2005;2:243–52. https://doi.org/10.1586/14789450.2.2.243.
Veznedaroglu E, Van-Bockstaele EJ, O’Connor MJ. Extravascular collagen in the human epileptic brain: a potential substrate for aberrant cell migration in cases of temporal lobe epilepsy. J Neurosurg. 2002;97:1125–30.
Wang YY, Smith P, Murphy M, Cook M. Global expression profiling in epileptogenesis: does it add to the confusion? Brain Pathol. 2010;20:1–16. https://doi.org/10.1111/j.1750-3639.2008.00254.x.
Wechsler A, Teichberg VI. Brain spectrin binding to the NMDA receptor is regulated by phosphorylation, calcium and calmodulin. EMBO J. 1998;17(14):3931–9. https://doi.org/10.1093/emboj/17.14.3931.
Wiggins AK, Shen PJ, Gundlach AL. Delayed, but prolonged increases in astrocytic clusterin (ApoJ)mRNA expression following acute cortical spreading depression in the rat: evidence for a role of clusterin in ischemic tolerance. Brain Res Mol Brain Res. 2003;114:20–30.
Wilkens S, Zhang Z, Zheng Y. A structural model of the vacuolar ATPase from transmission electron microscopy. Micron. 2005;36:109–26. https://doi.org/10.1016/j.micron.2004.10.002.
Wilkins MR, Sanchez IC, Gooley AA, Appel RD, Humphery-Smith I, Hochstrasser DF, Willians KL. Progress with proteome projects: why all proteins expressed by a genome should be identified and how to do it. Biotechnol Genet Eng Rev. 1995;13:19–50. https://doi.org/10.1080/02648725.1996.10647923.
Wolinski P, Ksiazek-Winiarek D, Glabinski A. Cytokines and neurodegeneration in epileptogenesis. Brain Sci. 2022;12:380. https://doi.org/10.3390/brainsci12030380.
Xiao F, Chen D, Lu Y, Xiao Z, Guan LF, Yuan J, Wang L, Xi ZQ, Wang XF. Proteomic analysis of cerebrospinal fluid from patients with idiopathic temporal lobe epilepsy. Brain Res. 2009;1255:180–9. https://doi.org/10.1016/j.brainres.2008.12.008.
Xiao Z, Shen L, Chen D, Wang L, Xi Z, Xiao F, Wang X. Talin 2 concentrations in cerebrospinal fluid in patients with epilepsy. Clin Biochem. 2010;43(13–14):1129–32. https://doi.org/10.1016/j.clinbiochem.2010.06.015.
Xu T, Zhang H, Qiu X, Meng Y. Genetic influence of Apolipoprotein E gene ε2/ε3/ε4 isoforms on odds of mesial temporal lobe epilepsy. Afr Health Sci. 2021;21(2):866–74. https://doi.org/10.4314/ahs.v21i2.48.
Yang JW, Czech T, Yamada J, Csaszar Z, Baumgartner C, Slavc I, Lubec G. Aberrant cytosolic acyl-CoA thioester hydrolase in hippocampus of patients with mesial temporal lobe epilepsy. Amino Acids. 2004;27:269–75. https://doi.org/10.1007/s00726-004-0138-9.
Yang JW, Czech T, Gelpi E, Lubec G. Extravasation of plasma proteins can confound interpretation of proteomic studies of brain: a lesson from apo A-I in mesial temporal lobe epilepsy. Mol Brain Res. 2005;139:348–56. https://doi.org/10.1016/j.molbrainres.2005.06.010.
Yang JW, Czech T, Felizardio M, Baumgartner C, Lubec G. Aberrant expression of cytoskeleton proteins in hippocampus from patients with mesial temporal lobe epilepsy. Amino Acids. 2006;30:477–93. https://doi.org/10.1007/s00726-005-0281-y.
Yu W, Dan CD, Wang Z, Zhou C, Luo J, Xu Y, Shen L, Yin H, Tao S, Xiao Z, Xiao F, Lü Y, Wang X. Time-dependent decrease of clusterin as a potential cerebrospinal fluid biomarker for drug-resistant epilepsy. J Mol Neurosci. 2014;54:1–9. https://doi.org/10.1007/s12031-014-0237-3.
Zetterberg FL, Ruetschi U, Portelius E, Brinkmalm G, Andreasson U, Blennow K, Brinkmalm A. Clinical proteomics in neurodegenerative disorders. Acta Neurol Scand. 2008;118:1–11. https://doi.org/10.1111/j.1600-0404.2007.00985.x.
Zhang H, Kim JK, Edwards CA, Xu Z, Taichman R, Wang CY. Clusterin inhibits apoptosis by interacting with activated Bax. Nat Cell Biol. 2005;7:909–15. https://doi.org/10.1038/ncb1291.
Zheng X, Lan S, Dan C, Liang W, Zhiqin X, Fei X, Xuefeng W. Talin 2 concentrations in cerebrospinal fluid in patients with epilepsy. Clinical Biochemistry, 2010;43(13–14):1129–32. https://doi.org/10.1016/j.clinbiochem.2010.06.015.
Acknowledgments
The authors thank Fapesp, CNPq, and CAPES for financial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fernandes, M.J.d.S., Pereira, A.S., Jaures, C.C.P.R., Prudencio, M.B., Persike, D.S. (2023). Application of Proteomics in the Study of Molecular Markers in Epilepsy. In: Rocha, L.L., Lazarowski, A., Cavalheiro, E.A. (eds) Pharmacoresistance in Epilepsy. Springer, Cham. https://doi.org/10.1007/978-3-031-36526-3_15
Download citation
DOI: https://doi.org/10.1007/978-3-031-36526-3_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36525-6
Online ISBN: 978-3-031-36526-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)