Abstract
Dendritic spines, key sites for neural plasticity, are influenced by gonadal steroids. In this chapter, we review the effects of gonadal steroids on dendritic spine density in areas important to cognitive function, the hippocampus, and prefrontal cortex. Most of these animal model studies investigated the effects of estrogen in females, but we also include more recent data on androgen effects in both males and females. The underlying genomic and non-genomic mechanisms related to gonadal steroid-induced spinogenesis are also reviewed. Subsequently, we discuss possible reasons for the observed sex differences in many neuropsychiatric diseases, which appear to be caused, in part, by aberrant synaptic connections that may involve dendritic spine pathology. Overall, knowledge concerning the regulation of dendritic spines by gonadal hormones has grown since the initial discoveries in the 1990s, and current research points to a potential role for aberrant spine functioning in many neuropsychiatric disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Dendritic spines are an important site of neural plasticity. As such, many factors, including gonadal steroid hormones, which are the primary focus of this chapter, influence spine density. Most of the work reviewed here focuses on the effects of estrogens on dendritic spine plasticity in the context of mediating cognition. Although less well studied, androgens have also been shown to alter spine density and impact cognition, and therefore, they will be reviewed as well. In addition, it is becoming increasingly evident that dendritic spines play a role in neuropsychiatric disorders, and given that gonadal steroids influence spine plasticity, we will speculate on the potential role that gonadal steroids may play in mediating neural dysfunction.
8.2 Dendritic Spines
In general, dendrites are covered extensively by dendritic spines, which, as they are sites for synaptic contact, have a prominent post-synaptic density that contains actin and scaffolding proteins that are activated or deactivated depending on physiological state (Calabrese et al. 2006; Chidambaram et al. 2019). The number of dendritic spines increases with development to a critical point (Urbanska et al. 2012), and following the establishment of connectivity between neurons, dendritic spine turnover actively continues until adulthood when spines achieve relative stability and less turnover (Koleske 2013). In the adult, several distinct dendritic spine subtypes have been described, with thin filopodial types presumed to be immature spines capable of plasticity, and larger, mushroom-shaped spines that are more stable and are the sites of functioning synapses (Bourne and Harris 2007; Von Bohlen Und Halbach 2009).
Although relatively stable in adulthood as compared to development, dendritic spines do exhibit plasticity, including alterations in number and spine subtype, in adult mammals in response to varied stimuli, including denervation/reinnervation (Deller et al. 2006; Parnavelas et al. 1974), hormonal changes (Luine and Frankfurt 2020b; Frankfurt and Luine 2015), drug exposure (Frankfurt et al. 2011; Robinson et al. 2001; Kolb and Gibb 2015), environmental stimuli (Kolb et al. 2003), learning and memory (Luine and Frankfurt 2020c; Kasai et al. 2010a), and stress (Watanabe et al. 1992). Notably, spine plasticity varies during the lifespan. During adolescence, pruning of dendritic spines occurs in the neocortex (Kolb et al. 2012; Holtmaat et al. 2005; Khanal and Hotulainen 2021). Pruning at this stage suggests a refinement of synapses such that weaker connections are eliminated, and stronger ones are maintained. In the aging brain, dendritic spines and synapse density decrease. There are decreases in dendritic spine density in the cortex (Dickstein et al. 2013; Dumitriu et al. 2010) and dendritic spines and axospinous synapses in the hippocampus with aging (Geinisman et al. 1992; Von Bohlen Und Halbach et al. 2006). Given that during adolescence and aging there is significant change in steroid hormone levels and function, dendritic spine plasticity during these times may be more susceptible to hormonal influences.
Dendritic spine plasticity is essential for learning and memory (Koleske 2013; Chidambaram et al. 2019; Khanal and Hotulainen 2021), which has also been demonstrated to be influenced by gonadal steroids (Luine and Frankfurt 2020a, c; Luine et al. 2018, 2022). The hippocampus and the medial prefrontal cortex (mPFC) are integral to learning and memory (Churchwell and Kesner 2011; Churchwell et al. 2010) and changes in dendritic spine density in these areas play a critical role in these cognitive processes (Jedlicka et al. 2008; Leuner et al. 2003). For this reason, alterations in spine density in the hippocampus and mPFC have been studied more than in other brain regions (Fig. 8.1). Many studies have demonstrated estrogen-dependent enhancements in learning and memory, and these enhancements are associated with increases in spine density on apical and basal dendrites in pyramidal cells in the CA1 region of the hippocampus (CA1) and mPFC in rodents (Luine and Frankfurt 2012, 2013, 2020a, b; Luine 2015, 2016). Therefore, estrogen-induced dendritic spine plasticity has been more extensively studied in the mPFC and CA1 than in other brain regions.
8.2.1 Steroids and Dendritic Spine Plasticity: Estrogens
Early studies demonstrated that dendritic spine density on pyramidal cells in CA1 in gonadally intact female rats fluctuated over the estrous cycle (Woolley et al. 1990; Woolley and McEwen 1992) with the highest levels in proestrus when estrogen levels are also highest. Initial results in the hippocampus were supported by later studies (Kinsley et al. 2006; Gonzalez-Burgos et al. 2005). Alterations in spine density during the estrous cycle have also been demonstrated in other brain regions including the ventromedial nucleus of hypothalamus (Frankfurt et al. 1990; Gonzalez-Burgos et al. 2015), the amygdala (Rasia-Filho et al. 2012), and pyramidal cells in layers III and V of the sensorimotor cortex (Chen et al. 2009). In general, spine density was greatest when estrogen levels were highest apart from the medial nucleus of the amygdala where spine density was lowest on neurons when estrogen levels were high (Rasia-Filho et al. 2012). Alterations in spine density during the estrous cycle in these regions may underlie lordosis and other reproductive behaviors.
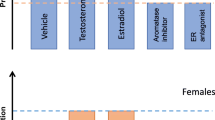
The data on intact cycling rats are supported by studies that show a decrease in spine density in ovariectomized (OVX) rats in CA1 (Gould et al. 1990b) that was subsequently restored by administration of estrogen for different time periods (Fig. 8.2), acute (<2 h) to subchronic (2–7 days). In initial studies, subchronic estrogen was shown to reverse the OVX-induced decrease in spine density on pyramidal cells in CA1 (Gould et al. 1990b; Luine and Frankfurt 2013). Spine synapses in the hippocampi of OVX monkeys (Leranth et al. 2002) and rats (Woolley and McEwen 1992) are also restored after subchronic estrogen administration. More recently, acute estradiol or estrogen agonists, given for less than 2 h, have been found to induce rapid increases in spine density in gonadectomized female (Inagaki et al. 2012; Luine and Frankfurt 2020a; Phan et al. 2012; Phan et al. 2011; Phan et al. 2015) and male rats (Jacome et al. 2016). A decrease in spine density after OVX has also been shown in CA1 and the mPFC (Wallace et al. 2006). Dendritic spines are decreased in both CA1 and the mPFC in aged females that have lower levels of estrogen (Wallace et al. 2007; Luine et al. 2011). Moreover, when OVX rats are fed a diet low in phytoestrogens, spine density in both CA1 and the mPFC is lower than those fed a high phytoestrogen diet (Luine et al. 2006). Finally, chronic exposure to high levels of estrogen during and after pregnancy increases spine density in CA1 pyramidal cells in rats (Kinsley et al. 2006).
As with the estrous cycle, most studies have been done in the hippocampus, but dendritic spine density in other brain regions is also altered when estrogen fluctuates. In the rat, OVX-induced decreases in spine density in the ventromedial nucleus of the hypothalamus (Frankfurt et al. 1990), amygdala (Rasia-Filho et al. 2012), and layers III and V of the somatosensory cortex (Chen et al. 2009) are reversed by estradiol administration (Fig. 8.2). Ye et al. (2019) found that pyramidal cells in layer V of the frontal, motor, and somatosensory cortex in the OVX mouse have decreased spine density that is also reversed when estradiol is given (Ye et al. 2019). The fact that spine density is altered in many brain areas by estrogen illustrates that many neurons are probably sensitive to hormonal alterations, and this understanding may shed light on the observation of sex differences in many neuropsychiatric diseases.
8.2.2 Gonadal Steroids and Dendritic Spine Plasticity: Androgens
Many neurons in the central nervous system are also sensitive to circulating androgens. Although far fewer studies have addressed the interaction between androgens and dendritic spines, it has been clearly demonstrated that various androgens and several androgenic metabolites function similarly to estrogens in terms of their ability to increase spine synapse and dendritic spine density. In gonadectomized male and female rats, both testosterone propionate (TP) and dehydroepiandrosterone (DHEA) increased dendritic spine density on pyramidal cells in CA1 and the mPFC (Luine et al. 2022; Jacome et al. 2016). Similarly, spine synapse density decreases in CA1 after gonadectomy are reversed in rats of both sexes after TP, dihydrotestosterone (DHT), and DHEA administration (Hajszan et al. 2004; Maclusky et al. 2004; Leranth et al. 2004; Atwi et al. 2016). In adult male mouse hippocampus, testosterone (T) increased spine density (Li et al. 2012), and in rats, castration (CAS) reduced, while administration of DHT or estradiol increased spine synapse density in the mPFC (Hajszan et al. 2007). Neurons in the brains of females are also sensitive to androgens as subchronic TP, and DHEA increased spine density on pyramidal cells in the mPFC and CA1 of adult OVX female rats (Luine et al. 2022). Again, as with estrogens, most studies use subchronic treatments, but rapid effects of androgens have also been shown. Acute administration of both T and DHT increases spine density in CA1 in gonadectomized male and female rats (Jacome et al. 2016; Luine et al. 2022) and in hippocampal slices taken from male rats (Murakami et al. 2018). Thus, both spine synapses and synapse density fluctuate with changing androgen levels (Fig. 8.2).
Consistent with the estrogen studies, there are reports of androgens increasing spine density in brain regions other than the hippocampus and mPFC. Syrian hamsters had decreased dendritic spine density in the medial preoptic area 9 weeks after gonadectomy compared to intact male hamsters and gonadectomized hamsters treated with T for 9 weeks (Garelick and Swann 2014). Gonadectomy decreased spine density in the medial preoptic nucleus and medial amygdala, and this effect was reversed by DHT 24 h after injection (Huijgens et al. 2021). Androgen-induced alterations in spine density in these regions may underlie regulation of male sexual behavior.
8.3 Mechanism of Gonadal Steroid Action on Dendritic Spines
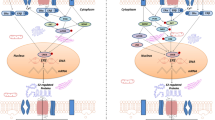
Gonadal steroids exert their effects via receptors that mediate both nuclear, genomic, and membrane, non-genomic, mechanisms (Fig. 8.3). Estrogen receptors (ER) α and β are found within cell nuclei and on cell membranes in neurons in many brains regions, while the more recently described G-protein-linked estrogen receptor (GPER) is found on membranes of both neurons and glia (Korol and Pisani 2015; Waters et al. 2015; Torres-Revereron et al. 2020). Chronic effects of steroids are mediated mainly through nuclear receptors and genomic mechanisms, while binding to membrane receptors mediates the rapid effects through the activation of numerous signaling pathways. Recent studies indicate that interaction between nuclear and membrane receptors may also mediate some steroid effects (Arevalo et al. 2015; Kramár et al. 2013; Luine and Frankfurt 2012). As a membrane receptor, GPER also activates signaling pathways. Which receptors are involved in mediating spine dynamics in neurons and whether the different receptors have an additive effect on steroid-mediated spinogenesis remain to be determined.
Schematic illustrating the mechanisms underlying estradiol (E2)-induced spinogenesis via both genomic and non-genomic means. E2 diffuses across the cell membrane to bind to cytosolic ERs, which then enter the nucleus and bind to the estrogen response element (ERE) inducing the synthesis of synaptic and other proteins. E2 also binds several membrane receptors, which then alters second messenger systems that result in the polymerization of actin, which increases the number of dendritic spines. Genomic and non-genomic mechanisms may have some degree of interaction in mediating these effects
Although some differences in effects have been reported, agonists for both ERα and ERβ alter spine density (Murakami et al. 2006; Phan et al. 2011). Studies in OVX mice showed that propyl pyrazole triol (PPT), an ERα agonist, increased dendritic spine density in the stratum radiatum and lacunosum-moleculare of CA1 within 1 h, whereas diarylpropionitrile (DPN), an ERβ agonist, decreased spine density in the lacunosum-moleculare of CA1 (Phan et al. 2011). In the PFC, agonists of the GPER, but not ERα/β receptors, rapidly increased spine density, and the opposite selectivity was found in CA1 (Ye et al. 2019).
Dendritic spine plasticity implies cycling of immature spines to mature spines that make synaptic contact (Ziv and Smith 1996) and changes that existing spines may undergo after exposure to different stimuli (Kasai et al. 2010b; Koleske 2013; Sehgal et al. 2013). This process requires mobilization of many proteins, particularly actin and associated proteins (Penzes and Rafalovich 2012; Hokenson et al. 2021; Koleske 2013). The cycling between filamentous and globular actin is an essential part of spine plasticity and requires interaction with other proteins, including several actin-binding proteins such as cofilin and profilin, which regulate actin polymerization (Basu and Lamprecht 2018; Borovac et al. 2018).
Since spine plasticity is dependent on mobilization of actin and synaptic proteins, it is notable that these proteins have also been shown to be altered by gonadal steroids. Estradiol inactivates cofilin, which is responsible for the disassembly of actin (Kramár et al. 2009). In addition, OVX decreased spine density in the CA1 region of mice in which the expression of cofilin was increased and profilin, which promotes actin polymerization, decreased (Lan et al. 2021). These results may explain how estradiol promotes filamentous actin and spine assembly (Kramár et al. 2009). Estrogen also increases other proteins that are found in the synapses, such as PSD95 and spinophilin (Tang et al. 2004; Maclusky et al. 2005; Lee et al. 2004). Estrogen-induced increases in dendritic spine density have been demonstrated to involve the activation of multiple cell signaling pathways, such as ERK, mTOR (Tuscher et al. 2016), CREB, and PI3, that promote the assembly of actin and protein synthesis and other proteins involved in spine dynamics (Sheppard et al. 2019; Frankfurt and Luine 2015; Fortress et al. 2013; Luine and Frankfurt 2013; Bethea and Reddy 2010; Hansberg-Pastor et al. 2015). Overall, it appears that estrogen acting via multiple pathways influences the assembly of actin and synaptic proteins, which, in turn, increases the number or the maturity of existing spines (Fig. 8.3).
The mechanisms by which androgens influence dendritic spines have been less well studied than estrogens, but the presence of both nuclear and membrane receptors on neurons has also been described for androgens (Atwi et al. 2016; Chen et al. 2022). Studies to date show that androgens exert similar effects to estrogens in terms of altering cytoskeletal and other proteins in dendritic spines. For example, orchiectomy decreases spine density, actin polymerization, and post-synaptic density thickness in adult male mice (Zhao et al. 2018). In addition, Chen et al. (2022) demonstrated that, in cultured hippocampal neurons from male mice, T promoted the maturation of immature spines and increased synaptic markers PSD 95 and synapsin.
Taken together, the data suggest that steroids can alter dendritic spine density by binding with steroid receptors on neurons and then initiating a series of intracellular events that promote the proteins, which increase the number of dendritic spines. Although most of the studies described here are related to rapid membrane-mediated effects of gonadal steroids, it is interesting to note that both types of receptors appear to mediate similar effects on synaptic proteins. Using antagonists to both nuclear and membrane estrogen receptors, Xing et al. (2018) found that receptor antagonists to ERα, Erβ, and GPER administered to mice decreased PSD-95, spinophilin, spine density, and synaptic density. These results suggest that both genomic and non-genomic receptors play a role in estrogen-induced reorganization of the actin cytoskeleton. There is some evidence for cross talk between the genomic and membrane estrogen receptors, especially given that binding of estrogens to nuclear ERα and ERβ in some circumstances results in alterations in rapid signaling pathways (Kramár et al. 2013; Arevalo et al. 2015; Luine and Frankfurt 2012).
8.4 Dendritic Spine Plasticity and Gonadal Steroids: Potential Clinical Importance
There are clear sex differences in the incidence of some neuropsychiatric diseases (Bangasser and Cuarenta 2021; Seney et al. 2022; Bangasser and Valentino 2014; Schulte Holthausen and Habel 2018; Vegeto et al. 2020). Alzheimer’s disease is more prevalent in women, and Parkinson’s disease occurs more often in men (Vegeto et al. 2020). Psychiatric disorders such as major depressive disorder and anxiety are more prevalent in women (Bangasser and Cuarenta 2021; Seney et al. 2022; Bangasser and Valentino 2014). Personality disorders, such as paranoid, schizotypal, and narcissistic disorders, are diagnosed more often in men, and borderline histrionic disorders are more common in women (Schulte Holthausen and Habel 2018).
Thus, it is interesting to speculate on the possible clinical importance of gonadal steroid interactions with spines in neural and psychiatric diseases because this information may provide insights into the etiologies and possible treatments for these conditions.
8.4.1 Sex Differences in the Brain
Results of preclinical, clinical, and anatomical studies provide some basis for the sex differences in neuropsychiatric disease. There are reports of sex differences in neural structure in rats (McEwen and Milner 2017; Brandt et al. 2020; Scharfman and Maclusky 2017; Yagi and Galea 2019; Marrocco and McEwen 2016). Nevertheless, sex differences in spine density reports are inconclusive. Female rats in proestrus were found to have greater spine density in CA1 than male rats (Woolley et al. 1990; Shors et al. 2001), and male rats have more thorny excrescences in hippocampal CA3 neurons than female rats (Gould et al. 1990a). However, in other studies no sex differences in spine density were seen in CA1 or the mPFC (Bowman et al. 2015; Gould et al. 1990a). The latter two studies did not consider the estrous stage of the females, and this may account for the different findings among studies. Thus, while sex differences in brain structure exist, data regarding spine density differences are limited and further research is required to determine possible relationship(s) to clinically observed sex differences in diseases.
What about sex differences in brain structure in humans? Imaging studies have shown that men have larger brains, more cortical surface area, and more white matter (except for the corpus callosum) than women, and women have denser gray matter than men (Salminen et al. 2022). Male brains have been shown to have greater ipsilateral connectivity, while female brains have greater commissural connectivity (Ingalhalikar et al. 2014). In the hippocampus, there are sex differences in the size of different hippocampal subregions (Van Eijk et al. 2020). These studies are not conclusive, and it should be noted that there is controversy regarding how real these differences are after being corrected for men’s larger brain sizes, sample sizes, and general differences in analysis (for reviews see Hines (2020), Salminen et al. (2022), and Hoggetts and Hausman (2023)).
Therefore, it appears that sex differences in the brain are more subtle than straightforward sexual dimorphisms and may be the result of ongoing developmental exposure during critical periods in the lifespan. One must consider that hormone-induced effects on neurons in adults are the result of multiple effects of hormones at different life stages. These include organizational exposure to gonadal steroids during development and activational exposure starting with adolescence, which may be further influenced by environmental factors (Fig. 8.4), rendering it challenging to correlate levels of steroid hormones with disease (McEwen and Milner 2017). Finally, there is a great deal of variability in preclinical studies with respect to time from gonadectomy to steroid replacement, dose used, length of steroid administration, and age of the animals during the experiment and animal strain, all of which could impact the results. Sex differences in response to stress (see below) are an example of the interaction of factors that may occur when sex differences in disease are manifested. Thus, neural networks seem more important for function than individual differences. Neural networks are connected by spines and synapses, which make them important to study. In the next section, we will review the intersection between spine pathology and gonadal steroids in a few examples to address potential mechanisms that may underlie the sex differences observed clinically.
8.5 Dendritic Spine Plasticity, Gonadal Steroids, and Neuropsychiatric Disorders
8.5.1 Depression
Depression-related alterations in neural plasticity have been studied extensively in animal models subjected to stress because stressed animals exhibit depression-like disturbances, such as anhedonia and alterations in dendritic spines and synapses in the hippocampus and PFC (Leuner and Shors 2013; Yang et al. 2020; Licznerski and Duman 2013). Therefore, stress-induced plasticity in rodents is thought to model what occurs in the human brain with depression.
The brain regions involved in mediating stress-induced responses include the PFC, hippocampus, and amygdala, which have extensive interconnections. Chronic restraint stress has been shown to decrease dendritic spine density in the hippocampus and PFC and increase it in the amygdala (Qiao et al. 2016). Most studies have only been done in male animals, and unfortunately, there are little data from female animals. However, 21 days of chronic restraint stress causes retraction of apical dendrites in the CA3 region of the hippocampus in male, but not female, rats (Galea et al. 1997). In a mouse model in which animals were stressed for 1 h for 6 days using different stressors, only OVX female mice were susceptible to the stress (Iqbal and Ma 2020). These authors found that OVX female mice had significantly higher corticosterone levels, increased spine density on PFC neurons, increased immobility time of several behavioral tests, and decreased sucrose consumption, which is consistent with anhedonia, in comparison with intact males and sham-operated females. Interestingly, sex differences in behavioral responses to stress have been clearly demonstrated in rats. Chronic restraint stress, 6 h for 21 consecutive days, impairs male performance on several behavioral cognitive tasks and either enhances or has no effect on female cognitive function (Luine et al. 2017; Bowman et al. 2022). In terms of spine density, Shors et al. (2001) found that 24 h after 30 minutes of intermittent stress, spine density in CA1 pyramidal cells was increased in male but decreased in female rats. In the lateral hypothalamic area, there is a sex difference in spine density on putative orexin neurons, and males have less spines than females (Grafe et al. 2019). Following 5 days of 30-minute restraint stress, this sex difference was no longer present, meaning that stress decreased spine density in females only. Following a paradigm of 30 minutes of restraint stress for 5 days, male rats were able to habituate to the stress but female rats did not, and females had significantly higher levels of corticosterone compared to males (Grafe and Bhatnagar 2020). These different stress studies may yield inconsistent results because of the different stress paradigms and behavioral assessments used, but the results do suggest that neural networks related to depression are differentially affected during stress and these changes may help explain sex differences in the incidence of depression.
8.5.2 Schizophrenia
Spine density alterations have also been shown in other diseases (Khanal and Hotulainen 2021). Postmortem Golgi studies have found a decrease in spine density in the dorsolateral prefrontal cortex (DLPFC) and the superior temporal gyrus (Glausier and Lewis 2013; Penzes et al. 2011) of schizophrenic patients, which implies decreased connectivity in regions known to be critical to cognitive function. These authors speculate that dendritic spine plasticity/pruning may be altered during early development and adolescence in schizophrenic patients, time periods in which gonadal steroids influence dendritic spine turnover. Although direct comparisons to the PFC in rodents are difficult, a preclinical study in rats subjected to repeated variable perinatal stress demonstrated a sex difference in the pattern of dendritic development in the PFC (Markham et al. 2013). Dendritic connectivity in both sexes in layer III pyramidal cells of the PFC during adolescence was increased, but maximal growth occurred earlier in female rats and lasted later, into adulthood. Increased spine density was seen in both sexes before puberty, but only females showed pruning of spines in late adolescence. These preliminary results support potential network alterations during a period of gonadal hormone secretions that may explain the observed sex difference in schizophrenia.
8.5.3 Alzheimer’s Disease
The incidence of neurodegenerative diseases increases with aging. With aging, there are also decreases in spine density (Young et al. 2014; Dumitriu et al. 2010; Walker and Herskowitz 2021; Wallace et al. 2007; Luine et al. 2011). Whether the decreases in spine density in these regions are due to an overall decrease in neuronal number is unclear. However, these changes may be related to the increased incidence of neurodegenerative diseases that is seen with aging. Spine abnormalities, including decreases in number and alterations in spine subtype, have been reported for numerous neurodegenerative diseases that have a cognitive component, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease (for review, see Herms and Dosostkar (2016), and Walker and Herskowitz (2021)). The accumulation of extracellular proteins in Alzheimer’s disease appears to interfere with dendritic spines, leading to synaptic loss in both the hippocampus and cortex of patients with Alzheimer’s disease (Chidambaram et al. 2019). Given the importance of spine plasticity to the process of learning and memory, it is not surprising that in Alzheimer’s disease there are alterations in dendritic spine density (Walker and Herskowitz 2021). Interestingly, Walker and Hershowitz (2021) review the literature that demonstrates that patients with preclinical Alzheimer’s disease, who have some signs of the disease but have normal cognitive functions, have higher levels of dendritic spines and synaptic proteins in the hippocampus and PFC than patients who were known to have impaired cognition. This finding implies that dendritic spines may confer resilience to cognitive decline and that decreases in spine density are related to impaired cognition, which is consistent with previous animal studies (Luine and Frankfurt 2020c; Frankfurt and Luine 2015).
8.6 Conclusion
Gonadal steroids exert acute and chronic effects on dendritic spines in pyramidal neurons across the lifespan in both males and females. These effects are mediated by both genomic and non-genomic mechanisms, which influence the assembly of actin and synaptic proteins to promote spinogenesis. Although gonadal steroids have been shown to influence spine density in many brain areas, sex differences have not been adequately investigated, and therefore, it is a challenge to relate differences observed in neuropsychiatric disorders to the basic and clinical data on sex differences to date. This apparent discrepancy may be due to the multifactorial processes and timing during hormonal exposure. Given that there are also spine density changes reported for these disorders, it may become important to consider potential differences in treatment based on sex. However, local alterations in spine density under different conditions imply that alterations in the neural networks may be a critical underlying issue and should be further investigated in relation to potential sex differences.
References
Arevalo MA, Azcoitia I, Gonzalez-Burgos I, Garcia-Segura LM (2015) Signaling mechanisms mediating the regulation of synaptic plasticity and memory by estradiol. Horm Behav 74:19–27
Atwi S, Mcmahon D, Scharfman H, Maclusky NJ (2016) Androgen modulation of hippocampal structure and function. Neuroscientist 22:46–60
Bangasser DA, Cuarenta A (2021) Sex differences in anxiety and depression: circuits and mechanisms. Nat Rev Neurosci 22:674–684
Bangasser DA, Valentino RJ (2014) Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol 35:303–319
Basu S, Lamprecht R (2018) The role of actin cytoskeleton in dendritic spines in the maintenance of long-term memory. Front Mol Neurosci 11:143
Bethea CL, Reddy AP (2010) Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol Psychiatry 15:1034–1044
Borovac J, Bosch M, Okamoto K (2018) Regulation of actin dynamics during structural plasticity of dendritic spines: signaling messengers and actin-binding proteins. Mol Cell Neurosci 91:122–130
Bourne J, Harris KM (2007) Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol 17:381–386
Bowman RE, Luine V, Diaz Weinstein S, Khandaker H, Dewolf S, Frankfurt M (2015) Bisphenol-A exposure during adolescence leads to enduring alterations in cognition and dendritic spine density in adult male and female rats. Horm Behav 69:89–97
Bowman R, Frankfurt M, Luine V (2022) Sex differences in cognition following variations in endocrine status. Learn Mem 29:234–245
Brandt N, Löffler T, Fester L, Rune GM (2020) Sex-specific features of spine densities in the hippocampus. Sci Rep 10:11405
Calabrese B, Wilson MS, Halpain S (2006) Development and regulation of dendritic spine synapses. Physiology (Bethesda) 21:38–47
Chen JR, Yan YT, Wang TJ, Chen LJ, Wang YJ, Tseng GF (2009) Gonadal hormones modulate the dendritic spine densities of primary cortical pyramidal neurons in adult female rat. Cereb Cortex 19:2719–2727
Chen H, Qiao D, Si Y, He Z, Zhang B, Wang C, Zhang Y, Wang X, Shi Y, Cui C, Cui H, Li S (2022) Effects of membrane androgen receptor binding on synaptic plasticity in primary hippocampal neurons. Mol Cell Endocrinol 554:111711
Chidambaram SB, Rathipriya AG, Bolla SR, Bhat A, Ray B, Mahalakshmi AM, Manivasagam T, Thenmozhi AJ, Essa MM, Guillemin GJ, Chandra R, Sakharkar MK (2019) Dendritic spines: revisiting the physiological role. Prog Neuro-Psychopharmacol Biol Psychiatry 92:161–193
Churchwell JC, Kesner RP (2011) Hippocampal-prefrontal dynamics in spatial working memory: interactions and independent parallel processing. Behav Brain Res 225:389–395
Churchwell JC, Morris AM, Musso ND, Kesner RP (2010) Prefrontal and hippocampal contributions to encoding and retrieval of spatial memory. Neurobiol Learn Mem 93:415–421
Deller T, Bas Orth C, Vlachos A, Merten T, Del Turco D, Dehn D, Mundel P, Frotscher M (2006) Plasticity of synaptopodin and the spine apparatus organelle in the rat fascia dentata following entorhinal cortex lesion. J Comp Neurol 499:471–484
Dickstein DL, Weaver CM, Luebke JI, Hof PR (2013) Dendritic spine changes associated with normal aging. Neuroscience 251:21–32
Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH (2010) Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci 30:7507–7515
Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM (2013) Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem 20:147–155
Frankfurt M, Luine V (2015) The evolving role of dendritic spines and memory: interaction(s) with estradiol. Horm Behav 74:28–36
Frankfurt M, Gould E, Woolley CS, McEwen BS (1990) Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology 51:530–535
Frankfurt M, Salas-Ramirez K, Friedman E, Luine V (2011) Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse 65:955–961
Garelick T, Swann J (2014) Testosterone regulates the density of dendritic spines in the male preoptic area. Horm Behav 65:249–253
Geinisman Y, Detoledo-Morrell L, Morrell F, Persina IS, Rossi M (1992) Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus 2:437–444
Glausier JR, Lewis DA (2013) Dendritic spine pathology in schizophrenia. Neuroscience 251:90–107
Gonzalez-Burgos I, Alejandre-Gomez M, Cervantes M (2005) Spine-type densities of hippocampal Ca1 neurons vary in proestrus and estrus rats. Neurosci Lett 379:52–54
Gonzalez-Burgos I, Velazquez-Zamora DA, Gonzalez-Tapia D, Cervantes M (2015) A Golgi study of the plasticity of dendritic spines in the hypothalamic ventromedial nucleus during the estrous cycle of female rats. Neuroscience 298:74–80
Gould E, Westlind-Danielsson A, Frankfurt M, McEwen BS (1990a) Sex differences and thyroid hormone sensitivity of hippocampal pyramidal cells. J Neurosci 10:996–1003
Gould E, Woolley CS, Frankfurt M, McEwen BS (1990b) Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci 10:1286–1291
Grafe LA, Bhatnagar S (2020) The contribution of orexins to sex differences in the stress response. Brain Res 1731:145893
Grafe LA, Geng E, Corbett B, Urban K, Bhatnagar S (2019) Sex- and stress-dependent effects on dendritic morphology and spine densities in putative orexin neurons. Neuroscience 418:266–278
Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS (1997) Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience 81:689-697
Hajszan T, Maclusky NJ, Leranth C (2004) Dehydroepiandrosterone increases hippocampal spine synapse density in ovariectomized female rats. Endocrinology 145:1042–1045
Hajszan T, Maclusky NJ, Johansen JA, Jordan CL, Leranth C (2007) Effects of androgens and estradiol on spine synapse formation in the prefrontal cortex of normal and testicular feminization mutant male rats. Endocrinology 148:1963–1967
Hansberg-Pastor V, González-Arenas A, Piña-Medina AG, Camacho-Arroyo I (2015) Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front Psych 6:165
Herms J, Dosostkar MM (2016) Dendritic spine pathology in neurodegenerative diseases. Ann Rev Pathol 11:221–250
Hines M (2020) Neuroscience and Sex/Gender: Looking Back and Forward. J Neurosci 40:37–43
Hodgetts S, Hausmann M (2023) Sex/gender differences in brain lateralisation and connectivity. Curr Top Behav Neurosci 62:71–99
Hokenson RE, Short AK, Chen Y, Pham AL, Adams ET, Bolton JL, Swarup V, Gall CM, Baram TZ (2021) Unexpected role of physiological estrogen in acute stress-induced memory deficits. J Neurosci 41:648–662
Holtmaat AJ, Trachtenberg JT, Wilbrecht L, Shepherd GM, Zhang X, Knott GW, Svoboda K (2005) Transient and persistent dendritic spines in the neocortex in vivo. Neuron 45:279–291
Huijgens PT, Snoeren EMS, Meisel RL, Mermelstein PG (2021) Effects of gonadectomy and dihydrotestosterone on neuronal plasticity in motivation and reward related brain regions in the male rat. J Neuroendocrinol 33:E12918
Inagaki T, Frankfurt M, Luine V (2012) Estrogen-induced memory enhancements are blocked by acute bisphenol a in adult female rats: role of dendritic spines. Endocrinology 153:3357–3367
Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R (2014) Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A 111:823–828
Iqbal J, Ma XM (2020) Impact of subchronic variable stress on ovariectomy and dendritic spine density in prefrontal cortex in mice. Neuroreport 31:213–219
Jacome LF, Barateli K, Buitrago D, Lema F, Frankfurt M, Luine VN (2016) Gonadal hormones rapidly enhance spatial memory and increase hippocampal spine density in male rats. Endocrinology 157:1357–1362
Jedlicka P, Vlachos A, Schwarzacher SW, Deller T (2008) A role for the spine apparatus in LTP and spatial learning. Behav Brain Res 192:12–19
Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J (2010a) Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci 33:121–129
Kasai H, Hayama T, Ishikawa M, Watanabe S, Yagishita S, Noguchi J (2010b) Learning rules and persistence of dendritic spines. Eur J Neurosci 32:241–249
Khanal P, Hotulainen P (2021) Dendritic spine initiation in brain development, learning and diseases and impact of bar-domain proteins. Cells 10:2392
Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG (2006) Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav 49:131–142
Kolb B, Gibb R (2015) Plasticity in the prefrontal cortex of adult rats. Front Cell Neurosci 9:15
Kolb B, Gorny G, Soderpalm AH, Robinson TE (2003) Environmental complexity has different effects on the structure of neurons in the prefrontal cortex versus the parietal cortex or nucleus accumbens. Synapse 48:149–153
Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R (2012) Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A 109(Suppl 2):17186–17193
Koleske AJ (2013) Molecular mechanisms of dendrite stability. Nat Rev Neurosci 14:536–550
Korol DL, Pisani SL (2015) Estrogens and cognition: friends or foes?: an evaluation of the opposing effects of estrogens on learning and memory. Horm Behav 74:105–115
Kramár EA, Chen LY, Brandon NJ, Rex CS, Liu F, Gall CM, Lynch G (2009) Cytoskeletal changes underlie estrogen’s acute effects on synaptic transmission and plasticity. J Neurosci 29:12982–12993
Kramár EA, Babayan AH, Gall CM, Lynch G (2013) Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience 239:3–16
Lan Z, Meng Z, Lian B, Liu M, Sun T, Sun H, Liu Z, Hu Z, Guo Q, Zhang J (2021) Hippocampal aromatase knockdown aggravates ovariectomy-induced spatial memory impairment, abeta accumulation and neural plasticity deficiency in adult female mice. Neurochem Res 46:1188–1202
Lee SJ, Romeo RD, Svenningsson P, Campomanes CR, Allen PB, Greengard P, McEwen BS (2004) Estradiol affects spinophilin protein differently in gonadectomized males and females. Neuroscience 127:983–988
Leranth C, Shanabrough M, Redmond Jr, DE (2002) Gonadal hormones are responsible for maintaining theintegrity of spine synapses in the CA1 hippocampal subfield of female nonhuman primates. J Comp Neurol 447:34–42
Leranth C, Hajszan T, Maclusky NJ (2004) Androgens increase spine synapse density in the Ca1 hippocampal subfield of ovariectomized female rats. J Neurosci 24:495–499
Leuner B, Shors TJ (2013) Stress, anxiety, and dendritic spines: what are the connections? Neuroscience 251:108–119
Leuner B, Falduto J, Shors TJ (2003) Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci 23:659–665
Li M, Masugi-Tokita M, Takanami K, Yamada S, Kawata M (2012) Testosterone has sublayer-specific effects on dendritic spine maturation mediated by Bdnf and Psd-95 in pyramidal neurons in the hippocampus Ca1 area. Brain Res 1484:76–84
Licznerski P, Duman RS (2013) Remodeling of axo-spinous synapses in the pathophysiology and treatment of depression. Neuroscience 251:33–50
Luine V (2015) Recognition memory tasks in neuroendocrine research. Behav Brain Res 285:158–164
Luine V (2016) Estradiol: mediator of memories, spine density and cognitive resilience to stress in female rodents. J Steroid Biochem Mol Biol 160:189–195
Luine VN, Frankfurt M (2012) Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol 33:388–402
Luine V, Frankfurt M (2013) Interactions between estradiol, Bdnf and dendritic spines in promoting memory. Neuroscience 239:34–45
Luine V, Frankfurt M (2020a) Estrogenic regulation of memory: the first 50 years. Horm Behav 121:104711
Luine V, Frankfurt M (2020b) Estrogenic regulation of object memory, spatial memory, and spinogenesis. In: Frick KM (ed) Estrogens and memory: basic research and clinical implications. Oxford University Press, New York
Luine VN, Frankfurt M (2020c) Estrogenic regulation of recognition memory and spinogenesis. In: Frick KM (ed) Estrogens and memory. Oxford University Press, New York
Luine V, Attalla S, Mohan G, Costa A, Frankfurt M (2006) Dietary phytoestrogens enhance spatial memory and spine density in the hippocampus and prefrontal cortex of ovariectomized rats. Brain Res 1126:183–187
Luine VN, Wallace ME, Frankfurt M (2011) Age-related deficits in spatial memory and hippocampal spines in virgin, female Fischer 344 rats. Curr Gerontol Geriatr Res 2011:316386
Luine V, Gomez J, Beck K, Bowman R (2017) Sex differences in chronic stress effects on cognition in rodents. Pharmacol Biochem Behav 152:13–19
Luine V, Serrano P, Frankfurt M (2018) Rapid effects on memory consolidation and spine morphology by estradiol in female and male rodents. Horm Behav 104:111–118
Luine V, Mohan G, Attalla S, Jacome L, Frankfurt M (2022) Androgens enhance recognition memory and dendritic spine density in the hippocampus and prefrontal cortex of ovariectomized female rats. Neuroscience. https://doi.org/10.1016/j.neuroscience.2022.06.002
Maclusky NJ, Hajszan T, Leranth C (2004) Effects of dehydroepiandrosterone and flutamide on hippocampal Ca1 spine synapse density in male and female rats: implications for the role of androgens in maintenance of hippocampal structure. Endocrinology 145:4154–4161
Maclusky NJ, Luine VN, Hajszan T, Leranth C (2005) The 17alpha and 17beta isomers of estradiol both induce rapid spine synapse formation in the Ca1 hippocampal subfield of ovariectomized female rats. Endocrinology 146:287–293
Markham JA, Mullins SE, Koenig JI (2013) Periadolescent maturation of the prefrontal cortex is sex-specific and is disrupted by prenatal stress. J Comp Neurol 521:1828–1843
Marrocco J, McEwen BS (2016) Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci 18:373–383
McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95:24–39
Murakami G, Tsurugizawa T, Hatanaka Y, Komatsuzaki Y, Tanabe N, Mukai H, Hojo Y, Kominami S, Yamazaki T, Kimoto T, Kawato S (2006) Comparison between basal and apical dendritic spines in estrogen-induced rapid spinogenesis of Ca1 principal neurons in the adult hippocampus. Biochem Biophys Res Commun 351:553–558
Murakami G, Hojo Y, Kato A, Komatsuzaki Y, Horie S, Soma M, Kim J, Kawato S (2018) Rapid nongenomic modulation by neurosteroids of dendritic spines in the hippocampus: androgen, oestrogen and corticosteroid. J Neuroendocrinol 30. https://doi.org/10.1111/jne.12561
Parnavelas JG, Lynch G, Brecha N, Cotman CW, Globus A (1974) Spine loss and regrowth in hippocampus following deafferentation. Nature 248:71–73
Penzes P, Rafalovich I (2012) Regulation of the actin cytoskeleton in dendritic spines. Adv Exp Med Biol 970:81–95
Penzes P, Cahill ME, Jones KA, Vanleeuwen JE, Woolfrey KM (2011) Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci 14:285–293
Phan A, Lancaster KE, Armstrong JN, Maclusky NJ, Choleris E (2011) Rapid effects of estrogen receptor alpha and Beta selective agonists on learning and dendritic spines in female mice. Endocrinology 152:1492–1502
Phan A, Gabor CS, Favaro KJ, Kaschack S, Armstrong JN, Maclusky NJ, Choleris E (2012) Low doses of 17β-estradiol rapidly improve learning and increase hippocampal dendritic spines. Neuropsychopharmacology 37:2299–2309
Phan A, Suschkov S, Molinaro L, Reynolds K, Lymer JM, Bailey CD, Kow LM, Maclusky NJ, Pfaff DW, Choleris E (2015) Rapid increases in immature synapses parallel estrogen-induced hippocampal learning enhancements. Proc Natl Acad Sci U S A 112:16018–16023
Qiao H, Li MX, Xu C, Chen HB, An SC, Ma XM (2016) Dendritic spines in depression: what we learned from animal models. Neural Plast 2016:8056370
Rasia-Filho AA, Dalpian F, Menezes IC, Brusco J, Moreira JE, Cohen RS (2012) Dendritic spines of the medial amygdala: plasticity, density, shape, and subcellular modulation by sex steroids. Histol Histopathol 27:985–1011
Robinson TE, Gorny G, Mitton E, Kolb B (2001) Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse 39:257–266
Salminen LE, Tubi MA, Bright J, Thomopoulos SI, Wieand A, Thompson PM (2022) Sex is a defining feature of neuroimaging phenotypes in major brain disorders. Hum Brain Mapp 43:500–542
Scharfman HE, Maclusky NJ (2017) Sex differences in hippocampal area Ca3 pyramidal cells. J Neurosci Res 95:563–575
Schulte Holthausen B, Habel U (2018) Sex differences in personality disorders. Curr Psychiatry Rep 20:107
Sehgal M, Song C, Ehlers VL, Moyer JR Jr (2013) Learning to learn – intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol Learn Mem 105:186–199
Seney ML, Glausier J, Sibille E (2022) Large-scale transcriptomics studies provide insight into sex differences in depression. Biol Psychiatry 91:14–24
Sheppard PAS, Choleris E, Galea LAM (2019) Structural plasticity of the hippocampus in response to estrogens in female rodents. Mol Brain 12:22
Shors TJ, Chua C, Falduto J (2001) Sex differences and opposite effects of stress on dendritic spine density in the male versus female hippocampus. J Neurosci 21:6292–6297
Tang Y, Janssen WG, Hao J, Roberts JA, Mckay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH (2004) Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex 14:215–223
Torres-Revereron A, Brake W, Milner TA (2020) Estrogen receptor distribution in the hippocampus and prefrontal cortex. In: Frick KM (ed) Estrogens and memory: basic research clinical implications. Oxford University Press, New York
Tuscher JJ, Luine V, Frankfurt M, Frick KM (2016) Estradiol-mediated spine changes in the dorsal hippocampus and medial prefrontal cortex of ovariectomized female mice depend on ERK and mTOR activation in the dorsal hippocampus. J Neurosci 36:1483–1489
Urbanska M, Swiech L, Jaworski J (2012) Developmental plasticity of the dendritic compartment: focus on the cytoskeleton. Adv Exp Med Biol 970:265–284
Van Eijk L, Hansell NK, Strike LT, Couvy-Duchesne B, De Zubicaray GI, Thompson PM, Mcmahon KL, Zietsch BP, Wright MJ (2020) Region-specific sex differences in the hippocampus. NeuroImage 215:116781
Vegeto E, Villa A, Della Torre S, Crippa V, Rusmini P, Cristofani R, Galbiati M, Maggi A, Poletti A (2020) The role of sex and sex hormones in neurodegenerative diseases. Endocr Rev 41:273–319
Von Bohlen Und Halbach O (2009) Structure and function of dendritic spines within the hippocampus. Ann Anat 191:518–531
Von Bohlen Und Halbach O, Zacher C, Gass P, Unsicker K (2006) Age-related alterations in hippocampal spines and deficiencies in spatial memory in mice. J Neurosci Res 83:525–531
Walker CK, Herskowitz JH (2021) Dendritic spines: mediators of cognitive resilience in aging and Alzheimer’s disease. Neuroscientist 27:487–505
Wallace M, Luine V, Arellanos A, Frankfurt M (2006) Ovariectomized rats show decreased recognition memory and spine density in the hippocampus and prefrontal cortex. Brain Res 1126:176–182
Wallace M, Frankfurt M, Arellanos A, Inagaki T, Luine V (2007) Impaired recognition memory and decreased prefrontal cortex spine density in aged female rats. Ann N Y Acad Sci 1097:54–57
Watanabe Y, Gould E, McEwen BS (1992) Stress induces atrophy of apical dendrites of hippocampal Ca3 pyramidal neurons. Brain Res 588:341–345
Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA (2015) G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci 35:2384–2397
Woolley CS, McEwen BS (1992) Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci 12:2549–2554
Woolley CS, Gould E, Frankfurt M, McEwen BS (1990) Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci 10:4035–4039
Xing FZ, Zhao YG, Zhang YY, He L, Zhao JK, Liu MY, Liu Y, Zhang JQ (2018) Nuclear and membrane estrogen receptor antagonists induce similar mTORC2 activation-reversible changes in synaptic protein expression and actin polymerization in the mouse hippocampus. CNS Neurosci Ther 24:495–507
Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44:200–213
Yang M, Luo CH, Zhu YQ, Liu YC, An YJ, Iqbal J, Wang ZZ, Ma XM (2020) 7, 8-Dihydroxy-4-methylcoumarin reverses depression model-induced depression-like behaviors and alteration of dendritic spines in the mood circuits. Psychoneuroendocrinology 119:104767
Ye Z, Cudmore RH, Linden DJ (2019) Estrogen-dependent functional spine dynamics in neocortical pyramidal neurons of the mouse. J Neurosci 39:4874–4888
Young ME, Ohm DT, Dumitriu D, Rapp PR, Morrison JH (2014) Differential effects of aging on dendritic spines in visual cortex and prefrontal cortex of the rhesus monkey. Neuroscience 274:33–43
Zhao J, Bian C, Liu M, Zhao Y, Sun T, Xing F, Zhang J (2018) Orchiectomy and letrozole differentially regulate synaptic plasticity and spatial memory in a manner that is mediated by Src-1 in the hippocampus of male mice. J Steroid Biochem Mol Biol 178:354–368
Ziv NE, Smith SJ (1996) Evidence for a role of dendritic filopodia in synaptogenesis and spine formation. Neuron 17:91–102
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Frankfurt, M., Nassrallah, Z., Luine, V. (2023). Steroid Hormone Interaction with Dendritic Spines: Implications for Neuropsychiatric Disease. In: Rasia-Filho, A.A., Calcagnotto, M.E., von Bohlen und Halbach, O. (eds) Dendritic Spines. Advances in Neurobiology, vol 34. Springer, Cham. https://doi.org/10.1007/978-3-031-36159-3_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-36159-3_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-36158-6
Online ISBN: 978-3-031-36159-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)