Abstract
The increased need for natural products leads to the expansion of new techniques and creates the necessity for the implementation of these techniques to adequately respond to the increased interests. Plants of the genus Ocimum L. (basil) are natural sources of biologically active products with widespread use and wide utilization both traditionally and industrially. Numerous basil plants have been used for decades mainly because of their aromaticity, significant amount of essential oils, and biologically active phenolic compounds. Additionally, recent research indicates that isolated compounds from extracts of different basil cultivars possess significant biological effects important for human health. The in vitro plant culture (culture of plant cells, tissues, and organs) includes methods that are in recent years commonly used to produce secondary metabolites, primarily due to the efficient production of particular metabolites at significantly higher concentrations than ex vitro. The present chapter aims to summarize the crucial publications on the genus Ocimum, with a focus on research related to the production and diversity of biologically active secondary metabolites under tissue culture conditions. With a high content of biologically active secondary metabolites and with a multidisciplinary approach, different varieties of basil provide significant opportunities for in vitro manipulation and offer great opportunities for practical application.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The Ocimum (basil) genus includes the most cultivated species of the family Lamiaceae together with species of the genera Rosmarinus L., Thymus L., Salvia L., etc. The main characteristics of all representatives are the quadrangular stem, oppositely placed leaves, distinctly zygomorphic flowers, and the presence of highly aromatic essential oils. The first taxonomic description of the genus was done in 1753 by Carl von Linné, who included five species within the genus Ocimum, but since then the classification of this genus has changed several times. Pushpangadan (1974) is the author of the infrageneric classification according to which two groups are distinguished: the Basilicum group (herbaceous annual, rarely perennial plants with strongly mucinous black, ellipsoid seeds, and the basic number of chromosomes x = 12) and the Sanctum group (bushy perennial forms with spherical, brown seeds, without or with very little mucus and basic number of chromosomes x = 8). Based on this classification, the section Ocimum and subsection Ocimum together with the most widespread taxa (var. basilicum, var. difforme, var. purpurascens) are included in the Basilicum group, while all other representatives are classified in the Sanctum group. Although this type of classification is most often used in scientific and popular literature, the main problem with this type of infrageneric classification is noncompliance with the standards of the International Code of Botanical Nomenclature (Paton et al. 1999; Carović-Stanko et al. 2011). Today, the genus Ocimum includes about 150 annual and perennial species of herbaceous plants, rarely shrubs, native to subtropical and tropical parts of Asia, central parts of South America, and Africa (Labra et al. 2004). The most numerous are annual, shallow-rooted herbaceous plants, and less often representatives have a solid bushy structure. They are characterized by branched quadrangular stems on which are oppositely arranged leaves on long stalks, egg-shaped, with a flat or toothed edge. The inflorescences (with flowers located on a short flower stem) are at the top of the stem.
The cropping of economically significant representatives of the genus Ocimum has intensified worldwide, primarily due to their nutritional and pharmaceutical importance, and their adaptability to different climatic conditions and various soil types. Long-term traditional use together with wide distribution, selection, and breeding has significantly contributed to the variability among the genotypes. Nowadays agronomists mainly distinguish different basil genotypes based on the color of the leaves and leaf area (green to dark purple; small to large leaf), flower color (white to purple), and other characteristics such as shoot shape, flowering period, height, and aroma (Carović-Stanko et al. 2010). However, the criteria for the characterization of taxa within the genus Ocimum are far more complex. Characteristics used for decades in the taxonomic classification of representatives of the genus Ocimum because the intensive cultivation, intraspecific hybridization, and polyploidy show a high degree of variability, including chemical composition and characteristics such as the color, shape, and size of the flower, leaf, or stem (Patel et al. 2016). Recent taxonomic studies, in order to achieve better intra- and interspecies classification, are based on different approaches, including geographical origin, morphology, karyotype, and chemical composition. Based on the geographical origin, Hiltunen and Holm (2003) classified basil chemotaxonomically into four chemotypes: reunion (Egyptian) chemotype with methyl chavicol (about 80%) as the main component; European chemotype with the highest-quality aroma and high content of linalool (from 35% to 50%) and methyl chavicol (from 15% to 25%); tropical chemotype where methyl cinnamate is the main component; Java (eugenol) chemotype with a high content of eugenol. According to Grayer et al. (1996), basil chemotype is defined by the component that has more than 20% of the total essential oils content, and by applying this classification system, methyl chavicol, linalool, methyl eugenol, eugenol, and geraniol type can be distinguished within the most cultivated basil species Ocimum basilicum L. Nevertheless, the classification based on chemotypes has its weaknesses, considering that one taxon can include two or more components that are almost equally represented. This is why various plant breeding, tissue culture, and metabolic and transgenic engineering strategies are being investigated to improve specific chemotype profiles and essential oils yields in Ocimum species (Gurav et al. 2021). Additionally, it has been shown that the quantitative-qualitative content of basil essential oils varies depending on numerous abiotic factors (Daneshian et al. 2009). According to Pushpangadan and Bradu (1995), the Ocimum genus counts about 160 species; however, about 65 species are native to Ocimum, while the rest should be taken into account as synonyms (Chowdhury et al. 2017). Sipos et al. (2021) pointed to seven different morphotypes including tall type, bush (dwarf) type, large-leafed (Italian) type, compact (Thai) type, purple and purpurascens types, and flavored citriodorum type. The standardized list for the identification of basil cultivars based on morphological characteristics is developed by the International Union for the Protection of New Varieties of Plants (UPOV). Today, more than 70 basil taxa possess accepted scientific names (www.theplantlist.org). Considerable progress has been made in basil classification using DNA analyses that allow phylogenetic and taxonomic studies and cultivar identification by comparing genotypes independently of phenotypes (Labra et al. 2004). Therefore, to better identify different Ocimum genotypes, existing evaluation techniques and chemotaxonomy should be integrated with environmentally independent molecular markers (Chowdhury et al. 2017). No matter of classification and geographical origin O. basilicum var. basilicum (cultivars ‘sweet basil’ and ‘Genovese’), O. basilicum var. purpurascens (cultivars ‘purple ruffle’ and ‘dark opal’), O. basilicum var. thyrsiflorum, O. basilicum var. difforme, O. minimum (syn. O. basilicum var. minimum), O. gratisimum, and O. sanctum (syn. O. tenuiflorum) are most commonly grown worldwide. Figure 1 shows the most cultivated Ocimum taxa where different morphology of aboveground plant parts can be seen.
In recent decades basil cultivation has increased globally, mainly because of the fragrance and nutritional properties, but at the same time also due to the high harvest index and profitability margin (Polyakova et al. 2015). For instance, it is estimated that the market-available essential oils from basil originating from India typically cost between 40 and 45 euros per 1 kg (Lubbe and Verpoorte 2011). Sipos et al. (2021) pointed out that according to the research report of the global basil leaves market growth is expected from 57 million USD to 62 million USD due to the worldwide increasing consumption of basil. Basil plants are mostly produced in an open field under the traditional production method as fragrant plants for fresh herbs. However, it is impossible to manage environmental factors such as light or temperature under these cultivation conditions (Bączek et al. 2019). Additionally, it has been demonstrated that better basil production can be obtained under soilless systems compared to traditional ones, with two times reduced production costs (Saha et al. 2016; Khater et al. 2021). In the food industry, basil plants are commonly used fresh for culinary purposes but also can be preserved by freezing, drying, or extracting (Raimondi et al. 2006). In recent decades cultivation of basil plants is enhanced due to increased demand for biologically active natural compounds since various basil genotypes are significant sources of phenolic compounds (PC) and essential oils with antimicrobial, antioxidant, anticancer, cytotoxic, antiproliferative, bioherbicidal, and insecticidal activities (Dudai et al. 1999; Grayer et al. 2004; Nguyen and Niemeyer 2008; Telci et al. 2009; Carović-Stanko et al. 2010; Imen et al. 2012; Tang et al. 2013; Zabka et al. 2014; Flanigan and Niemeyer 2014; Elansary and Mahmoud 2015; Jakovljević et al. 2019, 2021; Zagoto et al. 2021).
2 Secondary Metabolism in Basil Plants
By reviewing earlier studies that include different varieties of basil, it can be seen that the majority of research was conducted to examine morphological and anatomical differences, as well as differences in biological properties of herbs originating from different genotypes of basil. When it comes to secondary metabolism, investigations have mainly included differences in terms of the quantity of essential oils or identifications of chemotypes based on the qualitative characteristics of essential oils (Grayer et al. 1996; Lee et al. 2005; Anandjiwala et al. 2006; Politeo et al. 2007; Hussain et al. 2016). The obtained data indicate significant variations in the quantity and quality of secondary metabolites, primarily concerning the origin of the tested samples but also due to cultivation conditions and environmental factors. Basil, like most representatives of the Lamiaceae family, is characterized by a high concentration of secondary metabolites, particularly from the PC group, but their composition and characteristics are variable depending on numerous factors (Jakovljević et al. 2019). It was shown that extracts from different varieties of basil are characterized by the presence of various PC from the group of phenolic acids (primarily rosmarinic, caffeic, caftaric, and cichoric acids), which significantly contribute to antioxidant properties. In addition to phenolic acids, PC from the flavonoid group are also presented (Javanmardi et al. 2002; Lee and Scagel 2009; Kwee and Niemeyer 2011; Flanigan and Niemeyer 2014; Ghasemzadeh et al. 2016; Jakovljević et al. 2022).
Aromatic amino acid phenylalanine overall represents the central molecules of plant metabolism because, in addition to being an essential component in protein building, it is a significant precursor for a wide range of secondary metabolites, in particular aromatic metabolites with numerous biological activities. The synthesis of phenylalanine takes place through the shikimic acid pathway and is restricted exclusively to the plastid stroma, and the complexity of this process was explored by Tegeder and Weber (2006) and Vogt (2010). The first step in the synthesis of phenylalanine is catalyzed by the enzyme chorismite-mutase, which converts chorismate to prephenate. Through further transamination, arogenate is produced, while the removal of hydroxyl and carboxyl groups by arogenate-dehydrogenase leads to the production of phenylalanine. The phenylalanine ammonia-lyase (PAL) catalyzes non-oxidative deamination of phenylalanine to trans-cinnamate (Tzin and Galili 2010; Fraser and Chapple 2011). The produced trans-cinnamate is further transformed into numerous PC. Phenylpropanoid metabolism is a necessary metabolic pathway ending with different aromatic products and building components essential for structural support and vascular integrity and plant defense against various biotic and abiotic factors. The expression of the genes which are responsible for PAL enzyme activity is controlled developmentally and spatially, and changes in activity can additionally occur due to abiotic and biotic factors (Vogt 2010; Fraser and Chapple 2011; Zhang and Liu 2015).
For the initial steps catalyzed by PAL, cinnamate 4-hydroxylase (C4H) and 4-coumarate-coenzyme A ligase (4CL) are necessary components creating the basis of further metabolite synthesis of various aromatic components, i.e., polyphenols (Fig. 2). About 8000 aromatic metabolites are formed in this way, and these metabolites are further classified into different subclasses (or classes), including phenolic acids, flavonoids, anthocyanins, coumarins, stilbenes, lignins, and others. During the process of growth and development, PAL activity is induced, but the activity can also be induced by many environmental factors such as UV radiation, pathogen attack, lack of nutrients, and other processes in which synthetized aromatic components protect plants (Baque et al. 2010; Jakovljević et al. 2019). Control of PAL activity takes place through various mechanisms, including transcriptional and translational regulation, product inhibition, posttranslational inactivation, subcellular compartmentation, and metabolic feedback regulation. Understanding the mechanisms of synthesis of secondary metabolites and their quantity and quality under various environmental conditions continues to be a research priority (Lattanzio et al. 2009; Zhang and Liu 2015).
Pathway of phenylpropanoid biosynthesis and phenylalanine ammonia-lyase (PAL) as an entry point enzyme of the general phenylpropanoid pathway (according to Zhang and Liu (2015) with minor modifications). 4CL 4-coumarate-coenzyme A ligase, C4H cinnamate 4-hydroxylase, CHS chalcone synthase, FLS flavonol synthase, HCT hydroxycinnamoyl- coenzyme A:shikimate hydroxycinnamoyltransferase, UGT78D1 flavonol 3-O-rhamnosyltransferase, UGT78D2 flavonoid 3-O-glucosyl- transferase
Although the PAL enzyme cannot be regarded as a component of the plant antioxidant system, the compounds synthesized through the activity of this enzyme significantly contribute to the overall antioxidant capacity of plants. Considering that PAL enzyme activity is induced during the process of growth and development but also during irradiation, nutritional deprivation, and many other processes during which aromatic components protect plant compartments (Baque et al. 2010; Jakovljević et al. 2017, 2019), understanding the mechanisms that control quantitative and qualitative traits of synthesized compounds have become a priority in recent investigations of secondary metabolites (Lattanzio et al. 2009; Zhang and Liu 2015). Besides the fact that the identification of metabolic differences between organs, tissues, and cells is an important component of understanding plant metabolism (de Miguel et al. 2016), there is obscure information about the secondary metabolite content and PAL enzyme activity correlation in plant cells and tissues of different basil genotypes. In a recent study conducted to investigate the possibilities of induced synthesis of secondary metabolites in different basil cultivars under tissue culture conditions (Jakovljević et al. 2019), it was demonstrated that the increased activity of PAL and accordingly high content of PC and better antioxidant capacity of three basil cultivars (‘Genovese’, ‘small leaved’, and ‘lemon basil’) can be obtained under nutrient deficiency conditions. Additionally, for the purple basil cultivar ‘dark opal’, reduced PAL activity was correlated with the lower amount of PC under nutrient deprivation. These types of research involving PAL enzyme activity may be of particular interest when considering the instability in the production of secondary metabolites of certain basil varieties. For example, the dark purple color of the O. basilicum var. purpurascens originates from anthocyanins, which are localized in the epidermal layer in the leaves and flowers, while in the stem, they are also present in the inner layers. Although the purple color and the presence of anthocyanin increase the utility value of this variety, the instability of the cultivars of this basil variety in the amount of anthocyanin is one of the main problems during cultivation. The genes responsible for anthocyanin synthesis are extremely unstable, so even vegetatively propagated shoots can lose their purple color. In the ‘dark opal’, depigmentation is accompanied by a uniform loss of anthocyanins throughout the shoot, while in the ‘purple ruffles’, the loss of pigments occurs in individual parts of the shoot (Phippen and Simon 2000). Therefore, investigations of PAL activity and synthesis of basil PC under controlled conditions can have significant applications in the future.
3 Basil Tissue Culture
The plant cell, tissue, and organ culture, i.e., aseptic culture of plants in vitro, mean the cultivation and multiplication of cells, tissues, or organs of plants on a liquid or solid medium, under strictly controlled conditions. Based on the totipotency, i.e., the principle of pluripotency of plant cells, in vitro culture can be applied for almost any plant species, and it is commonly used for mass production of uniform plant material, preservation of germplasm, studies of growth and development regulation, synthesis of metabolites, etc. The tissue culture method can provide pathogen-free plants, rapid preservation, and cloning of the genotypes of interest, and all this can be achieved in a short period of time. Additionally, for certain plant species, conventional techniques of propagation may require large financial investments and a longer period. Therefore, for these plant species, plant cell, tissue, or organ culture are of multiple importance (Máthé et al. 2015; da Silva et al. 2017). Today, in vitro culture of plants is established routinely under aseptic conditions, and in recent years it has been intensified for medicinal and aromatic plants, mainly because of the efficient production of secondary metabolites under in vitro controlled conditions. An additional advantage of the established in vitro system in comparison to the plantation method of plant growing is the use of aseptic media with a clearly defined composition, without the need for nutritional supplements, and with less physical space. All these advantages enable easier material processing with an effective screening of the obtained plant material. However, difficulties and failures may arise in the establishment of tissue cultures with the aim of the production of secondary metabolites mainly due to the insufficiently clarified pathways of the synthesis of particular metabolites (Karuppusamy 2009; Srivastava et al. 2014).
In vitro cultures, although were primarily developed for basic research on morphogenesis and differentiation of cells and tissues, today is considered one of the most prominent techniques of plant manipulation and sustainable plant biotechnology. Manipulation of plant explants in aseptic conditions, combined with growth and differentiation under the defined temperature, humidity, and light intensity, allows maintaining of uniformity and the desired plant characteristics, while the production of biologically active secondary metabolites in the mentioned conditions enables the rational use of natural resources and conservation (Jakovljević and Stanković 2020; Jakovljević et al. 2022). Different varieties of basil, when considering their multiple importance, have been insufficiently studied under in vitro conditions. The most of investigations are related to propagation through different explants and on different substrates. Still, investigations from the last decade indicate increased production of secondary metabolites in basil plants under in vitro conditions, particularly through the callus, cell suspension, and hairy root culture. The most commonly identified compounds and their chemical structure are presented in Table 1. With the selection of an adequate type of tissue culture, the composition and concentration of the medium, and the application of exogenous elicitors, the synthesis of metabolites of interest can be induced in significantly higher concentrations than when growing the same basil genotype using standard cultivation methods (Jakovljević 2018; Jakovljević et al. 2022).
Through the callus culture, the undifferentiated plant cells can be maintained for a long period on a solid medium with a unique growth pattern of callus tissue established after callus induction and further cell division. The changes in the callus structure, the size of cells and tissues, and in cell metabolism can be seen through the different developmental stages of callus culture, whereas the placement of the callus on media and conditions which cause the increased synthesis of metabolites of interest can be of great importance in the production of bioactive components originating from plant sources. The great practical importance can be seen in successfully established callus culture since the growing tissue can have different metabolism compared to the plant from which callus culture is established. When it comes to the callus culture of basil with the aim of producing secondary metabolites, previous reports demonstrated high content of total PC (including flavonoids and phenolic acids) in O. basilicum (Guirgis et al. 2007; Abdel Rahman et al. 2015; Pandey et al. 2015; Duran et al. 2019; Nadeem et al. 2019), O. basilicum var. purpurascens (Nazir et al. 2019, 2020a, b), O. basilicum cv. “Thai basil” (Nazir et al. 2020c, 2021a, b), O. sanctum (Hakkim et al. 2007; Pandey et al. 2015), and O. kilimandsharicum (Pandey et al. 2015).
Cell suspension culture consists of cells and cell aggregates in a mobile liquid medium. During the incubation of cells in a liquid medium and in the initial stages of cell suspension, the amount of plant material is constantly increasing. This increase is time-limited, given that the cell suspension reaches the maximum amount of plant material at one point, so the progressive growth of the cell suspension is followed by a stationary phase. If the cell suspension is subcultured, i.e., placed in the initial conditions with the same cell content and the same medium concentration, it can be expected that in the following period, the cell suspension will go through the same or a similar growth pattern and yield a similar amount of plant material. In this way, the cell suspension can be propagated by successive passages for a long period. For the secondary metabolite production in cell suspension culture, the high metabolic rate, rapid cell mass proliferation, and rapid response to various stimulants are the most important advantages (Jakovljević et al. 2022). This type of tissue culture is used for the production of PC in O. basilicum (Kintzios et al. 2003, 2004; Pandey et al. 2019; Açıkgöz 2020, 2021), O. basilicum cv. ‘dark opal’ (Strazzer et al. 2011), and O. sanctum (Hakkim et al. 2011a, b).
Hairy root culture implies the cultures of genetically transformed plant roots caused by the infection with Agrobacterium rhizogenes. The hairy roots produced in this way possess a high growth rate and can be characterized by higher levels of secondary metabolites compared to untransformed (intact) plants (Giri and Narasu 2000). Due to fast and stable growth, growth on media free of hormones, genetic stability, and the production of secondary metabolites in concentrations higher than in untransformed roots, transgenic cultures of hairy roots represent an effective biotechnological approach for the secondary metabolites production (Bais et al. 2002; Sharan et al. 2019). Hairy root culture was established for O. basilicum to produce phenolic acids (Tada et al. 1996; Bais et al. 2002; Marzouk 2009; Srivastava et al. 2016; Kwon et al. 2021) and for O. tenuiflorum to produce various PC (Vyas and Mukhopadhyay 2014; Sharan et al. 2019).
4 Phytochemical Diversity of Basil Secondary Metabolites Produced In Vitro
Plant secondary metabolites from the group of PC are among the largest and most comprehensive products of plant secondary metabolism ubiquitously present in the plant kingdom but uncommon in algae, fungi, and bacteria. PC has received a lot of attention in the last few years since it is found that reduced risk of various disease development may be related to the intake of juices, brews, and vegetables with a high level of these aromatic compounds. Therefore, PC are applicable for the preparation of nutraceuticals, ingredients of functional food, dietary supplements, or cosmeceuticals (Lattanzio et al. 2008). Today the antioxidant ability of PC is well known. These natural antioxidants, mainly presented in medicinal and aromatic herbs, make an increasing interest because of their ability to protect cell membranes from induced oxidative stress and free-radical-induced damage. These antioxidant properties of PC are a consequence of their ability to act as electron donors, as well as their ability to chelate metal ions (Filip et al. 2017; Kruk et al. 2022). Due to the carcinogenic, mutagenic, and overall toxic properties that synthetic antioxidants like BHA (butylated hydroxyanisole) and BHT (butylated hydroxytoluene) demonstrated in terms of human health, the usage of these compounds has been restricted in recent years (Cooper and Grover 2012). On the other hand, substances belonging to PC show flavor, color, and harshness – characteristics that are typically related to the organoleptic properties of foods. Additionally, the PC presented in a food matrix has significant effects on its availability, extractability, solubility, and biological activity, making them widely used in the industry of food (Karaś et al. 2017). Moreover, PC are linked with many functions like germination, pollination, resistance to predators and pests, seed development, reproduction, and sensorial properties. As it is mentioned, more than 8000 different PC synthesized by higher plants have been identified, and the amount is continually growing (Kabera et al. 2014).
Plant cells and tissues contain numerous compounds including flavonoids, proanthocyanidins, amides, esters, glycosides of hydroxycinnamic acids, and polymers with a phenolic structure such as pollen sporopollenin, suberin, and lignin. Several soluble PC, e.g., chlorogenic acid, are broadly widespread, while for diverse compounds, the distribution is restricted to specific species, genera, or families, which make them appropriate biomarkers in studies of plant taxonomic (da Silva et al. 2022). There are different ways of PC classification – from highly polymerized compounds to simple molecules. Still, there is no consensus regarding a classification system for phenolics in general. Santana-Gálvez and Jacobo-Velázquez (2017) divided PC from the most general to the most specific as follows: (1) flavonoids and non-flavonoids, (2) PC classified by the number of aromatic rings, (3) PC classified by carbon skeleton, and (4) PC classified by basic chemical composition. Kabera et al. (2014) presented a different approach to phenolic classification. Firstly, PC classification can be based on a number of hydroxyl groups. According to this classification, polyphenols are PC which contain more than one OH-group in the aromatic ring. Secondly, PC can be classified into mono-, di, oligo-, and polyphenols, and this classification is based on chemical structure. Thirdly, PC can be classified based on substitutes in the carbon skeleton, a number of carbon atoms, and aromatic rings in the side chain. Based on the third principle, PC can be further classified into four subgroups: (i) phenolics with one or (ii) two aromatic rings, (iii) quinones, and (iv) polymers. Numerous compounds possess one aromatic ring, including simple phenols (C6); phenols with one aromatic ring and one carbon atom attached (C6-C1), e.g., gallic acid and salicylic acid; phenols with one aromatic ring and with two carbon atoms (C6-C2), e.g., phenylpropanoids; and phenols with three carbon atoms attached (C6-C3), e.g., hydroxycinnamic acid, ferulic acid, and sinapic acid. Benzoquinones and xanthones (C6-C1-C6) are included in the group of PC which contains two aromatic rings linked with two atoms of carbon. Flavonoids contain three carbon atoms (C6-C3-C6).
The first group of polyphenolics, the largest among PC, belongs to the flavonoids. These pigments are water-soluble and widely distributed in nature. In plant cells, flavonoids are located in the vacuoles. More than 5000 flavonoids have been identified among plants, consistently present along with carotenoids and chlorophylls (Stalikas 2007). The definition of flavonoid is commonly used to describe phenolic with a chemical structure consisting of a C6-C3-C6 carbon skeleton, more specifically, an aromatic ring attached to a benzopyran moiety. In this structure, A ring is called an aromatic in the benzopyran group; the B ring is also aromatic but attached to the benzopyran group, whereas the C ring is with the O-heterocyclic. Flavonoids can be divided into three groups based on the position of the B ring: isoflavonoids (3-phenylbenzopyrans), neoflavonoids (4-phenylbenzopyrans), and flavonoids (2-phenylbenzopyrans) (Marais et al. 2007). Additionally, flavonoids can be classified into the following groups: flavonols, flavones, and anthocyanins. The most common forms of flavonoids occurring in the vacuolar juice of plant cells are either aglycons or glycosides. One flavone C-glycoside, six flavone O-glycosides, and seven flavonol O-glycosides were identified in species of Ocimum by Grayer et al. (2002). In higher plants, flavonoids are included in numerous functions including symbiotic nitrogen fixation, ultraviolet filtration, and flowers coloration related to the pollinator animal attractions. Additionally, flavonoids are cell cycle inhibitors, chemical messengers, and physiological regulators or may possess inhibitory activities against plant pathogens, e.g., Fusarium oxysporum. In recent years there is an increasing interest in flavonoids originating from plants, mainly due to their benefits related to human health, including antioxidant, anti-allergic, antiviral, anti-inflammatory, and anticancer activities, whereas sinusitis, asthma, eczema, and high fever can be relieved by flavonoid quercetin.
The other group of PC are tannins, commonly classified into condensed tannins and hydrolyzable tannins. Because of the various possibilities to form oxidative linkage, tannins show considerable structure diversity (Hassanpour et al. 2011). A large number of oligomeric compounds with molecular weights between 2000 and 5000 Daltons are produced via intermolecular oxidation processes. Flavan-3-oligomers or polymers known as condensed tannins are connected by an interflavan carbon bond. They are also known as proanthocyanidins because, when heated in acidic alcohol solutions, they undergo an oxidation reaction that is catalyzed by acid, converting them to anthocyanidins.
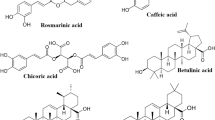
The primary PC in basil plants are flavonol-glycosides and phenolic acids (Kividompolo and Hyotylainen 2007; Castronuovo et al. 2019). Gallic, coumaric, caffeic, and ferulic acids are examples of acids that fall under the two categories of benzoic and cinnamic acid derivatives, respectively. Additionally, phenolic acids can be found in food plants as glycosides or esters that are conjugated to other organic substances such as glucosides, flavonoids, hydroxy fatty acids, sterols, and alcohols. The most common esterification of caffeic acid with quinic acid results in chlorogenic acid, which is the main PC in coffee. Caffeic acid is the most frequent phenolic acid in numerous fruits and vegetables. Ferulic acid (esterified to hemicelluloses in the cell wall) is another phenolic acid commonly found in cereals (D’Archivio et al. 2007). One of the most significant caffeic acid esters found in Ocimum spp. is rosmarinic acid. This phenolic acid provides therapeutic and pharmacological benefits for the food industries or cosmetic sectors. These benefits include antibacterial, antiviral, antioxidant, and anti-inflammatory activities (Mastaneh et al. 2014; Nazir et al. 2020b).
Diverse types of chemicals, including essential oils, simple phenolics, coumarins, flavonoids, anthocyanins, terpenoids, fixed oils, and steroids, have been found in various Ocimum species which make them a fragrant herb used both in cooking and in medicinal (Kasem 2017; Zahran et al. 2020). Among these chemicals, rosmarinic acid, caffeic acid, chlorogenic and p-hydroxybenzoic acids, quercetin, rutin, and apigenin, as well as essential oil components methyl chavicol, linalool, eugenol, α-pinene, ocimene, β-pinene, 1,8-cineole, geraniol, borneol, B-caryophyllene, and n-cinnamate, are basil’s most significant components regarding the antioxidant activity (Teofilović et al. 2017; Shahrajabian et al. 2020; Avetisyan et al. 2017). Gang et al. (2001) emphasize that on the surface of basil leaves, both capitate and peltate glandular trichomes can be found. Still, single or two distinct phenylpropene molecules are present in the essential oils they generate. Though, because of biochemical and genetic heterogeneity within the Lamiaceae family, and following individual variability, there is a significant challenge in the use of Lamiaceae species for medicinal reasons. Therefore, using in vitro techniques of micropropagation is an effective means for quick and extensive propagation of aromatic and medicinal plants in order to achieve high homogeneity of progeny (Ardelean et al. 2018).
One of the first studies devoted to secondary metabolites in basil carried out by Kintzios et al. (2003) reported rosmarinic acid accumulation in various liquid culture and cell suspensions immobilized in calcium alginate. Rosmarinic acid generally represented around 90% of the total phenolic content in every type of culture (callus, cell suspension, and immobilized cells). Rosmarinic acid accumulation in cell suspensions varied considerably with time and maximum values were observed during the second and fourth weeks of culture. In addition, cell immobilization in 1.5% calcium alginate completely inhibited rosmarinic acid production. Additionally, the accumulation of monoterpenes, sesquiterpenes, phenolic acids, anthocyanins, phenylpropanoids, and other PC with antioxidant potential in callus culture of sweet basil was shown in the study of Makri and Kintzios (2008).
The antioxidant activities and phenolic content of many Ocimum basilicum subspecies are still unknown, despite the fact that basil is widely used in a range of foods and medicines. Consequently, Bajomo et al. (2022) examined 22 commercial basil cultivars and divided them into distinct chemotypes using similarities in characteristics of phenolic acids. Furthermore, total phenolic content, oxygen radical absorbance capacity (ORAC), and cupric ion-reducing antioxidant capacity (CUPRAC) were all significantly influenced by the cultivar type. The findings demonstrated that a chemotype of caffeic acid-rich basil cultivars was defined as having the highest total phenolic content and strongest antioxidant capabilities. Also, significant variations in the content of phenolics among basil morphotypes were observed, e.g., high content of phenolics and better antioxidant activity was demonstrated for green Genovese-type basil in contrast to lettuce-leaf basil (Bajomo et al. 2022). When compounds with biological activities in in vitro cultures of O. basilicum were detailly examined, it can be seen that researchers mostly focused on an effective methods of biologically active metabolites production. For this purpose, they mainly used hairy roots, callus, or suspension cultures and applicate as elicitors 6-benzylaminopurine (BAP), 2,4-dichlorophenoxyacetic acid (2,4-D), indole-3-acetic acid (IAA), 1-naphthylacetic acid (NAA), thidiazuron (TDZ), kinetin (KIN), methyl jasmonate (MeJ), melatonin, salicylic acid, chitosan, cadmium chloride, silver nitrate, yeast, and different light intensities (reviewed by Jakovljević et al. 2022).
According to some authors, optimization of callus culture conditions could be used as a low-cost and suitable basis of important phenolic acids: rosmarinic and cichoric acid, with substantial antioxidant activity (Rady and Nazif 2005; Abdel Rahman et al. 2015; Nazir et al. 2019). The supplementation of MS medium (Murashige and Skoog 1962) with 1 mg L−1 BA and 0.25 mg L−1 IAA led to the accumulation of high concentrations of rosmarinic acid in callus culture of O. americanum (Rady and Nazif 2005) and O. basilicum (Abdel Rahman et al. 2015). Nazir et al. (2020c) described the procedure for callus culture of sweet basil cv. ‘Thai basil’, according to which, MS medium with 1 mg L−1 NAA and 5 mg L−1 BAP was the best choice for the accumulation of fresh and dry biomass, and for the increased production of phenolics. Similarly, Nazir et al. (2019) cultured callus through the leaf explants on MS medium supplemented with various plant growth regulators (PGRs), such as α-naphthalene acetic acid (NAA), thidiazuron (TDZ), and 6-benzylaminopurine (BAP). According to this study, the highest mass growth (23.2 g L−1 DW) was achieved with 2.5 mg L−1 NAA, together with high total phenolic content (210.7 mg L−1) and flavonoid (196.4 mg L−1) production. What is more, the varying phenolic acid accumulation was proved through the HPLC (high-performance liquid chromatography) analysis: caffeic (44.67 mg g−1 DW), rosmarinic (52.22 mg g−1 DW), and chicoric acid (43.89 mg g−1 DW) as well as anthocyanins: peonidin (10.77 mg g−1 DW) and cyanidin (16.39 mg g−1 DW) as an effect of the PGRs treatment. Wongsen et al. (2015) demonstrated that O. basilicum callus had the highest fresh weight and the highest levels of flavonoids, phenolics, ascorbic acid, and β-carotene (7.38 mg g−1 FW, 6.54 mg g−1 FW, 0.64 mg g−1 FW, and 0.08 mg g−1 FW, respectively) after 7 days on the medium with 0.5 mg L−1 2,4-D. Comparable results were obtained by Hakkim et al. (2011a), where the highest callus biomass production of O. sanctum grown on media with 0.1 mg L−1 KIN and 1 mg L−1 2,4-D correlated with a high rosmarinic acid concentration. Bhuvaneshwari et al. (2016) grew O. basilicum and O. tenuiflorum from nodal explants in vitro on MS media with various concentrations of BAP, either alone or with KIN (0.25–2.0 mg L−1) and IAA (0.25–2.0 mg L−1). The maximum shoot induction was achieved for both species when 1 mg mL−1 BAP and 0.5 mg mL−1 KIN were combined (99% and 98%, respectively), and the leaves thus obtained had several times higher content of eugenol and total phenolic compared to leaves from field-grown plants. The highest concentration of total PC (185 mg g−1 DW) was obtained for O. tenuiflorum, whereas both O. basilicum and O. tenuiflorum contained comparable amounts of eugenol (approximately 85 μg g−1). Additionally, the association and importance between total phenolics concentration and eugenol in all in vitro grown plant parts of O. basilicum and O. tenuiflorum were established. The holy basil (O. sanctum) chemical composition and antioxidant properties were examined in the study by Hakkim et al. (2007). Parts of plants (inflorescence, stems, and leaves) grown under natural conditions in field were compared with corresponding callus cultures produced from each explant in vitro. For the first time, the distribution of PC, i.e., rosmarinic acid, ursolic acid, sinapic acid, carnosic acid, isothymusin, and eugenol, in each organ was examined using HPLC. Rosmarinic acid was found to be the most abundant phenolic acid in all callus extracts when compared to plants grown in the field. In comparison to the plants cultivated in the field, holy basil callus accumulated rosmarinic acid 10.8-fold more. The prior study by Kintzios et al. (2003), which discovered a significant increase in the concentration of rosmarinic acid in callus of O. basilicum, gives support to these findings. Additionally, 2,4-D and KIN used as PGRs for callus culture can generate the highest mass production with great secondary metabolite content, according to Kintzios et al. (2003). What is more, in all callus culture testing systems, the antioxidant activity increased, and the extracts obtained from callus had greater antioxidant activity than the extracts derived from field-grown plants at the same concentration of growth regulators. The information from the Kintzios et al. (2003) study revealed that in vitro callus cultures rather than field-grown plant parts of holy basil might be used to isolate rosmarinic acid with a high level.
Among the most successful method for enhancing a plant’s production of beneficial secondary metabolites is elicitation. Elicitors, both biotic and abiotic, are widely used to improve plant metabolite production in cell cultures by speeding up the process and resulting in higher culture volumes and product concentrations (Mulabagal and Tsay 2004; Yue et al. 2016). One of the most significant benefits of cell cultures for the synthesis of secondary metabolites is the rapid multiplication of cells biomass through organogenesis or somatic embryogenesis, which typically results in a rise in the metabolic rates of developed cells (Jakovljević et al. 2022). Melatonin’s effect on O. basilicum (sweet basil) callus initiation and PC production were examined by Duran et al. in 2019. According to the phytochemical evaluation, calluses grew in media with 100 and 200 μM of melatonin had the highest total phenolic acid concentrations (784.6 μg g−1 and 335.2 μg g−1, respectively), as opposed to calluses produced with MS alone (192.0 μg g−1). The findings showed that PC such as rosmarinic acid, caffeic acid, vanillin, and p-coumaric acid accumulated differently in response to melatonin. The amount of rosmarinic acid (754.2 μg g−1) significantly increased in the callus grown on 100 μM melatonin medium by about fivefold, compared to the callus in the control group. In addition, the presence of the following aromatic compounds was found: 1,8-cineole, DL-limonene, methyl eugenol, 3-methylbutanal, 2-methylbutanal, hexanal, furan-2-carboxaldehyde, benzaldehyde, and bergamotene. Other results reported by Bahcesular et al. (2020) showed that melatonin and salt stress applications decreased total phenolics and total flavonoids; rosmarinic acid was not detected under salinity, while the concentration of caffeic acid and cichoric acid was decreased. Açıkgöz (2020) investigated biotic and abiotic elicitors that activate the defense mechanisms of plants in order to obtain an increasing concentration of biologically active metabolites in O. basilicum cell suspension cultures. The silver nitrate (AgNO3), cadmium chloride (CdCl2), and yeast extract were applied at various concentrations, and their effects were studied on the cell growth, cell viability, total phenolic and flavonoid contents, and pharmaceutical active ingredients. Among the investigated elicitors, the treatment with 200 mg L−1 of yeast extract gave the greatest total phenolic and flavonoid concentrations. The HPLC analysis revealed that the yeast extract treatment caused the highest accumulation of rosmarinic acid (21.28 mg g−1 DW of rosmarinic acid in 200 mg L−1 of yeast extract treatment) and chicoric acid (6.45 mg g−1 DW of chicoric acid in 50 mg L−1 of yeast extract treatment) which were 0.92 and 1.25 times higher than in the control, respectively. Compared to the control culture, the application of 50 mg L−1 of yeast extract resulted in an increase of rutin for 1.91 times (6.54 mg g−1 DW) and isoquercetin for 1.86 times (3.72 mg g−1 DW), respectively. The AgNO3 treatment (25 μM) resulted in the highest levels of linalool and estragole compared to the control culture (4.37 g g−1 DW and 3.30 g g−1 DW, respectively). Açıkgöz (2021) used sorbitol as an elicitor and showed that 50 mg L−1 of it was the most effective in terms of total phenolics, while 200 mg L−1 treatment was most effective for total flavonoid content. Based on HPLC analysis, the highest concentrations of rosmarinic acid and chicoric acid (12.32 mg g−1 DW and 4.52 mg g−1 DW, respectively) were obtained in 25 mg L−1 of sorbitol and 100 mg L−1 treatment, whereas the 50 mg L−1 of sorbitol affected the optimum biosynthesis of rutin (6.78 mg g−1 DW), isoquercetin (4.12 mg g−1 DW), linalool (4.58 μg g−1 DW), and methyl chavicol (4.40 μg g−1 DW) compared to the control culture. According to these findings, adding AgNO3, CdCl2, yeast extract, and sorbitol to cell suspension cultures of O. basilicum may be a suitable way to increase medicinal active components, particularly terpenoids and phenolics.
In the sweet basil hairy root cultures, Marzouk (2009) identified six triterpene acids including ursolic, oleanolic, betulinic, alphitolic, euscaphic, and 3-epimaslinic. Using A. rhizogenes strain LBA 9402 (with a kanamycin-resistant gene as a selectable marker), hairy roots were induced on stem and leaf pieces of O. basilicum. The extracts obtained from hairy roots showed a significant hepatoprotective potential against CCl4-induced oxidative stress in female albino rats and demonstrated inhibition two- to threefold that of silymarin used as the positive control. Received results showed that hairy root culture had the same metabolic profile as the non-transformed roots but showed a tendency to accumulate secondary metabolites at higher amounts. The study of Srivastava et al. (2016) reported A. rhizogenes-mediated transformation of basil cultivars (‘holy green’, ‘red rubin’, and ‘Subja’) for hairy root establishing and selection for the production of PC. Hairy root growth was explant and virulence dependent. Differences between cultivars in the content of total phenolics, rosmarinic acid, and caffeic acid were observed. They varied among cultivars, with the highest rosmarinic acid content recorded in 60 days of culture (76.41 mg g−1 DW). It can be concluded that rosmarinic acid synthesis in hairy roots of ‘holy green’, ‘red rubin’, and ‘Subja’ basil cultivars are age dependent. The caffeic acid (in concentration from 0.11 mg g−1 DW to 1.74 mg g−1 DW) was detected in all tested samples, and the trend of this compound’s content differed between the tested lines of hairy roots within the age. On this basis, three superior lines of hairy roots were chosen because they had higher biomass production, rosmarinic acid, and antioxidant capacity compared to untransformed roots. Prior studies by Tada et al. (1996) also corroborated the same trend involving the five clones of hairy roots grown well in MS, B5 (Gamborg et al. 1968), and woody plant (McCown and Lloyd 1981) liquid media. A high content of rosmarinic acid (14.1% DW) was recorded in the MS medium together with low content of other phenolics, including lithospermic acid (1.70% DW) and lithospermic acid B (0.17% DW). In addition to the commonly occurring rosmarinic acid, Pandey et al. (2019) recorded the presence of pentacyclic triterpenes in the suspension culture of O. basilicum for the first time: betulinic, oleanolic, and ursolic acids. The highest amount of rosmarinic acid (15.73 mg g−1 DW) was produced, followed by betulinic acid (14.63 mg g−1 DW), ursolic acid (4.71 mg g−1 DW), and oleanolic acid (0.91 mg g−1 DW). The cell suspension culture produced several times higher content of these pentacyclic triterpenes than the in vivo control leaves. Moreover, the promising influence of MeJ was ascertained on the overall productivities of sweet basil suspension culture relating to three investigated metabolites: rosmarinic, betulinic, and ursolic acids, compared to control culture. Though MeJ in the concentrations of 200 and 300 μM were successful in increasing the production of ursolic acid, only the lower MeJ concentration (200 μM) was successful in increasing the amount of betulinic acid, supporting the prior discoveries by Pandey et al. (2015). This conclusion is consistent with several past observations where MeJ induced the production of rosmarinic acid in several Ocimum species (Mathew and Sankar 2012). Misra et al. (2014) examined the transcriptional responses in sweet basil following MeJ treatment, which is thought to be an elicitor of secondary metabolites, and they found 388 potential MeJ-responsive individual transcripts. Transcript research reveals that MeJ upregulates transcripts of the numerous secondary metabolic pathways, including terpenoids and flavonoids, in addition to directing its production and stress responses.
Koca and Karaman (2015) examined the effects of MeJ, epibrassinolide, spermine, and l-phenylalanine on basil to quantify the production of PC and the activity of PAL. The concentration of total PC, including flavonoids (6.72 mg g−1 and 0.92 mg g−1, respectively), was highest after the 1.0 mM spermine + MeJ application. In this way, the content of rosmarinic and caffeic acids can be significantly enhanced. Still, no alterations were seen in the contents of chicoric acid or PAL activity. The predominant phenolic acid in all samples was rosmarinic, with content ranged from 1.04 to 2.70 mg g−1 FW. Because of this, spermin + MeJ, as well as epibrassinolide + MeJ, can be regarded as efficient elicitors of secondary metabolites production, and these interactions can be crucial for the formation of phytochemicals in plants. O. basilicum var. thyrsiflorum cv. ‘Thai basil’ was the subject of research by Nazir et al. (2021a) which investigated the effect of elicitation with salicylic acid and varied light regimes on the formation of secondary metabolites. The researchers demonstrated that the combination of salicylic acid (10 μM) and constant light significantly improved the antioxidant capacity and content of secondary metabolites including the content of total phenolics (18.7 mg g−1 DW), total flavonoid (7.2 mg g−1 DW), rosmarinic acid (54.35 mg g−1 DW), chicoric acid (64.46 mg g−1 DW), eugenol (0.56 mg g−1 DW), peonidin (0.32 mg g−1 DW), and cyanidin (0.42 mg g−1 DW). The highest concentration of caffeic acid (0.54 mg g−1 DW) was obtained after the photoperiod and 25 μM of salicylic acid.
The study of Hammock (2018) and Sipos et al. (2021) demonstrates that supplemental narrow-wavelength light treatments from light-emitting diodes (LED) sources can be used to manipulate plant development and significantly influence the yield and accumulation of biologically active compounds. According to Shoji et al. (2009) and Lobiuc et al. (2017), especially red and blue LEDs can tailor the induction of improved growth and content of PC in green and red basil microgreens. In the experiment done by Ardelean et al. (2018), the effect of LED and fluorescent lamps was evaluated on in vitro growth and content of PC of basil cultivar ‘Aromat de Buzau’. Basil seedlings were cultivated under red, yellow, green, and blue LEDs with peak wavelengths of 660, 525, 500, and 470 nm, respectively. This study showed that the growth of basil plants was not affected by the LEDs since the greatest fresh biomass and shoot height were recorded after 60 days under control light treatment. However, compared to the other treatments, blue LED light considerably increased the total phenolic and flavonoid content of basil plants. Nazir et al. (2020b) described metabolic variations in the purple basil O. basilicum var. purpurascens callus culture successfully induced by exposure to UV and different monochromatic lights. In comparison to the control, blue light caused the greatest accumulation of callus biomass, total phenolic, and flavonoid contents and the highest antioxidant activity. Nazir et al. (2020b) also demonstrated that dark conditions resulted in higher content of peonidin (0.13 mg g−1 DW), cyanidin (0.15 mg g−1 DW), and rosmarinic acid (87.62 mg g−1), whereas the production of chicoric acid (14.65 mg g−1 DW) was enchased by red light. In the subsequent study, Nazir et al. (2020a) described how melatonin, UV-C, and their combinations can improve phenylpropanoid metabolite production in callus cultures of basil. The highest total phenolic and total flavonoid content (18.4 and 13.4 mg g−1 DW, respectively) was measured in the callus exposed for 50 min to UV-C. HPLC quantification of phenylpropanoid metabolites showed that UV-C (10 min) led to an increased concentration of rosmarinic acid (134.5 mg g−1 DW), whereas UV-C (50 min) led to an increased concentration of cichoric acid, cyanidin, and peonidin (51.52, 0.50, and 0.30 mg g−1 DW, respectively). Therefore, according to the most recent research, the use of elicitors like UV-C and narrow-wavelength LED light can appropriately affect secondary metabolites in purple basil, which suggests that this method may be an alternative to modifications in metabolism in other species to obtain bioactive secondary metabolites. Moreover, according to Hashim et al. (2021), among the several abiotic elicitors, light has drawn interest in boosting secondary metabolite production due to its versatility in terms of wavelengths, affordability, and durability.
5 Biological Activity
When it comes to the human organism, the balance between the pros and cons of free radicals is of vital importance for the normal functioning of the organism, given that oxidative stress triggered by an increased concentration of free radicals in the human organism can lead to various stress-related diseases, including cardiovascular disease, cancer, and diabetes (Shoham et al. 2008; Benhammou et al. 2009). Although the human body has an antioxidant defense system that acts against free radicals, this system that prevents oxidative damage is very often not a sufficient component of cellular antioxidant protection (Rechner et al. 2002). The antioxidants introduced exogenously into the human organism can neutralize or mitigate the negative consequence of free radicals. For this reason, synthetic antioxidants (such as BHT and BHA) have been widely used in different aspects (Stanković et al. 2019). However, despite the confirmed antioxidant abilities and the increased interest in this group of synthetic compounds, some data indicate the toxic and carcinogenic effects of synthetic antioxidants (Jennings and Akoh 2009; Sindhi et al. 2013). Because of this, it is essential to replace synthetic antioxidants with antioxidants derived from natural sources. Secondary metabolites from plant samples are the most significant natural antioxidants (Stanković et al. 2019). They are mainly PC that can remove reactive oxygen species and free radicals and consequently prevent the occurrence of oxidative stress or reduce its harmful consequences. The high reactivity of PC as hydrogen or electron donors, the ability of the aromatic phenolic radical to localize the unpaired electron, the ability to react with transition metal ions, and the removal of superoxide anion radicals, hydroxyl radicals, and hydrogen peroxide are all factors that contribute to their antioxidant capabilities (Falleh et al. 2011; Stanković et al. 2019). Therefore, it is considered that plant polyphenolics have bioactive qualities that may be significant in the treatment of a variety of damages (Sousa et al. 2015; Zengin et al. 2015). As a result, recently, the use of plant antioxidants as additives, functional food, and many types of medical and pharmaceutical materials has increased (Stanković et al. 2019).
Plants of the genus Ocimum are known in many countries around the world based on their pharmaceutical active components, including alkaloids, phenols and flavonoids, terpenoids, and others. That is why basil extracts are of great practical importance in the pharmaceutical industries, food, and cosmetics. When it comes to the pharmaceutical industry, the most valued are essential oil components like linalool and methyl-chavicol (Piras et al. 2018; Talebi et al. 2018; Alkuwayti et al. 2019) and PC like rutin, isoquercetin, rosmarinic acid, and caffeic acid (Kwee and Niemeyer 2011; Jakovljević et al. 2019). The content of these metabolites varies, and it is determined by both genetic and environmental agents such as season, climate, and sampling period. Also, the growth and development processes, the plant parts, and the extraction procedures and further processing of the material additionally affect the quantity and quality of the active components of basil (Alkuwayti et al. 2019; Jakovljević et al. 2019, 2021). The production of biologically active components of basil, due to all the mentioned factors, may be constrained or expensive. The use of conventional planting can be expensive for in-depth basil research or screening of bioactive compounds in numerous genotypes that exist within the genus Ocimum. That is why it is necessary, with smaller financial investments, to establish an easily-managed system for smaller areas (Srivastava 2014). Also, the use of biologically active substances in medicine and pharmacy requires homogeneity and a high degree of purity of the substances (Rao and Ravishankar 2002).
Because of its abundant phenolic acid and flavonoid content, O. basilicum is exploited in cosmetic and pharmaceutical treatments. It can act in the prevention of heart illnesses, inflammation reduction, and the occurrence of malignancies and diabetes because of its powerful antioxidant activity (Mastaneh et al. 2014). Antioxidants originating from plants can be extracted using a variety of techniques and conditions (process time, lightning) and with different solvents. A significant number of phenolic acids and flavonoids, which are holders of antioxidant activity, are produced during the maceration with organic solvents (Vidović et al. 2012). Teofilović et al. (2017) reported that the total phenolic content of ground parts of sweet basil ranged from 5.17 mg g−1 DW to 65.25 mg g−1 DW of extract and the content of the total flavonoids from 0.11 mg g−1 DW to 40.63 mg g−1 DW of extract. With longer extraction times, more polar solvents, and more plant fragmentation, the overall extraction yield increased. The extract produced by extraction with 96% ethanol for 30 min had the highest total phenolic content, while the extract produced by chloroform for 30 min had the highest flavonoid level. Properties of leaves from ‘Genovese’, ‘dark opal’, and ‘sweet Thai’ basil to phenolics accumulation were determined in the presence of potassium ions. The 5.0-mM potassium rate increased the concentration of total PC, with higher rosmarinic and chicoric acid concentrations compared to a lower (1.0 mM) potassium treatment level. The phenolic composition and antioxidant properties of basil were also affected by the type of cultivar. The cultivar ‘sweet Thai’ had lower total phenolics content and ferric-reducing antioxidant power (FRAP) compared to ‘Genovese’ and ‘dark opal’. The concentrations of anthocyanin, unaffected by the amount of potassium ions, differed remarkably among the cultivars. Purple ‘dark opal’ basil showed higher anthocyanin levels than ‘Genovese’ and ‘sweet Thai’.
Shafique et al. (2011) investigated the antimicrobial activities of various extracts obtained from in vitro and in vivo grown plants of O. basilicum and showed that extracts from basil grown in vitro demonstrated better antimicrobial activity against Gram-positive bacteria compared to extracts obtained from in vivo grown plants. Significant antioxidant activity was demonstrated for seedlings of various basil genotypes grown in vitro under conditions of nutrient deprivation (Jakovljević et al. 2019) with values similar to standardized extracts of ginkgo or green tea. Considering the purity of substances in standardized extracts and the mixture of substances in basil samples, it can be concluded that basil grown in vitro can be an important source of natural antioxidants. Guirgis et al. (2007) and Homhuan et al. (2008) showed that the application of gamma rays to basil callus culture can increase its antioxidant capacity. Wongsen et al. (2015) examined callus culture as a source of antioxidative compounds. It has been shown that the callus, originating from the leaf explants of O. basilicum, is a significant source of β-carotene, ascorbic acid, phenolics, and flavonoids but also of total antioxidant activity and that these biologically active substances can be obtained in high concentrations for 7 days after callus induction. Extracts originating from the callus tissue of purple basil show a protective effect in stress conditions caused by radiation, indicating the great practical potential that the in vitro callus culture of this basil variety possess (Nazir et al. 2019). The influence of different abiotic and biotic stimuli on the secondary metabolites production in the cell suspension of O. basilicum was studied by Açıkgöz (2020). It was shown that the antioxidant activity, the content of total phenolics, and the concentration of total flavonoids are the highest with the addition of yeast extract (200 mg L−1). Srivastava et al. (2016) determined that by the genetic transformation of three different varieties of basil (‘holy green’ ‘red rubin’, and ‘Genovese’) with A. rhizogenes significantly higher antioxidant activity can be achieved compared to non-transformed roots. The culture of genetically transformed hairy roots of O. basilicum shows a tendency to accumulate rosmarinic acid in a concentration three times higher compared to control (non-transformed) roots, whereby the synthesis of this phenolic acid can be further improved by the application of biotic stimulants. Rosmarinic acid synthesized in genetically transformed roots shows significant antimicrobial activity and has a significant effect on Pseudomonas aeruginosa (Bais et al. 2002). According to the study of Darrag et al. (2022), the volatile secondary metabolites obtained from the cell suspension of O. basilicum may be useful as a bio-insecticide against Rhynchophorus ferrugineus.
6 Concluding Remarks
In recent years, there can be seen an improvement in the implementation of in vitro tissue culture with the goal of producing compounds of interest originating from basil plants. This is mainly due to the fact that in vitro production of bioactive substances is stable, predictable and reliable, independent of geographic position, seasonal fluctuations, and environmental factors, and it allows content modification to achieve the production of bioactive compounds with the proper quantitative and qualitative composition. Callus culture, cell suspension, and genetically transformed roots provide the opportunities of obtaining biologically active compounds at higher concentrations compared to the standard cultivation methods. Through the hairy root culture, callus, or cell suspension culture, it is possible to obtain a significant amount of phenolic acids, particularly rosmarinic acid, caffeic acid, chicoric acid, betulinic acid, and flavonoids cyanidin and peonidin. These secondary metabolites possess various biological activities which can contribute to human health, and it is proved that extracts originating from basil grown in vitro act as antioxidant and antimicrobial agents.
References
Abdel Rahman RAI, El-Wakil H, Abdelsalam NR, Elsaadany RMA (2015) In vitro production of rosmarinic acid from basil (Ocimum basilicum L.) and lemon balm (Melissa officinalis L.). Middle East J Appl Sci 5:47–51
Açıkgöz MA (2020) Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind Crop Prod 148:112278. https://doi.org/10.1016/j.indcrop.2020.112278
Açıkgöz MA (2021) Effects of sorbitol on the production of phenolic compounds and terpenoids in the cell suspension cultures of Ocimum basilicum L. Biologia 76:395–409. https://doi.org/10.2478/s11756-020-00581-0
Alkuwayti MA, El-Sherif F, Yap YK, Khattab S (2019) Foliar application of Moringa oleifera leaves extract altered stress-responsive gene expression and enhanced bioactive compounds composition in Ocimum basilicum. S Afr J Bot. https://doi.org/10.1016/j.sajb.2019.08.001
Anandjiwala S, Kalola J, Rajani M (2006) Quantification of eugenol, luteolin, ursolic acid, and oleanolic acid in black (Krishna Tulasi) and green (Sri Tulasi) varieties of Ocimum sanctum Linn. Using high-performance thin-layer chromatography. JOAC Int 89:1467–1474. https://doi.org/10.1093/JAOAC/89.6.1467
Ardelean M, Ardelean A, Don I, Lobiuc A, Burducea M (2018) Effect of LED lighting on growth and phenolic content on in vitro seedlings of Ocimum basilicum L. cultivar Aromat de Buzeau. Food Environ Safety J 17(1):66–73
Avetisyan A, Markosian A, Petrosyan M, Sahakyan N, Babayan A, Aloyan S, Trchounian A (2017) Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement Altern Med 17:60. https://doi.org/10.1186/s12906-017-1587-5
Bączek K, Kosakowska O, Gniewosz M, Gientka I, Węglarz Z (2019) Sweet basil (Ocimum basilicum L.) productivity and raw material quality from organic cultivation. Agronomy 9:279. https://doi.org/10.3390/agronomy9060279
Bahcesular B, Yildirim ED, Karaçocuk M, Kulak M, Karaman S (2020) Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind Crop Prod 146:112165. https://doi.org/10.1016/j.indcrop.2020.112165
Bais HP, Walker TS, Schweizer HP, Vivanco JM (2002) Root specific elicitation and antimicrobial activity of rosmarinic acid in hairy root cultures of Ocimum basilicum. Plant Physiol Biochem 40:983–995. https://doi.org/10.1016/S0981-9428(02)01460-2
Bajomo EM, Aing MS, Ford LS, Niemeyer ED (2022) Chemotyping of commercially available basil (Ocimum basilicum L.) varieties: cultivar and morphotype influence phenolic acid composition and antioxidant properties. NFS J 26:1–9. https://doi.org/10.1016/j.nfs.2022.01.001
Baque MA, Lee EJ, Paek KY (2010) Medium salt strength induced changes in growth, physiology and secondary metabolite content in adventitious roots of Morinda citrifolia: the role of antioxidant enzymes and phenylalanine ammonia lyase. Plant Cell Rep 29:685–694
Benhammou N, Bekkara FA, Kadifkova-Panovska T (2009) Antioxidant activity of methanolic extracts and some bioactive compounds of Atriplex halimus. C R Chim 12:1259–1266
Bhuvaneshwari K, Gokulanathan A, Jayanthi M, Govindasamy V, Milella L, Lee S, Yang DC, Girija S (2016) Can Ocimum basilicum L. and Ocimum tenuiflorum L. in vitro culture be a potential source of secondary metabolites. Food Chem 194:55–60. https://doi.org/10.1016/j.foodchem.2015.07.136
Carović-Stanko K, Liber Z, Besendorfer V, Javornik B, Bohanec B, Kolak I, Satovic Z (2010) Genetic relations among basil taxa (Ocimum L.) based on molecular markers, nuclear DNA content, and chromosome number. Plant Syst Evol 285:13–22
Carović-Stanko K, Šalinović A, Grdiša M, Liber Z, Kolak I, Satovic Z (2011) Efficiency of morphological trait descriptors in discrimination of Ocimum basilicum L. accessions. Plant Biosyst 145(2):298–305
Castronuovo D, Russo D, Libonati R, Faraone I, Candido V, Picuno P, Andrade P, Valentao P, Milella L (2019) Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Ocimum basilicum L.). Sci Hortic 254:91–98. https://doi.org/10.1016/j.scienta.2019.04.077
Chowdhury T, Mandal A, Roy SC, De Sarker D (2017) Diversity of the genus Ocimum (Lamiaceae) through morpho-molecular (RAPD) and chemical (GC-MS) analysis. J Genet Eng Biotechnol 15(1):275–286
Cooper CS, Grover PL (eds) (2012) Chemical carcinogenesis and mutagenesis II. Springer, Berlin/Heidelberg, pp 164–170
D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R (2007) Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43:348–361
da Silva DM, de Souza KRD, Boas LVV, Alves YS, Alves JD (2017) The effect of magnesium nutrition on the antioxidant response of coffee seedlings under heat stress. Sci Hortic 224:115–125
da Silva WMF, Kringel DH, de Souza EJD, da Rosa Zavareze E, Guerra Dias AR (2022) Basil essential oil: methods of extraction, chemical composition, biological activities, and food applications. Food Bioprocess Technol 15:1–27. https://doi.org/10.1007/s11947-021-02690-3
Daneshian A, Gurbuz B, Cosge B, Ipek A (2009) Chemical components of essential oils from basil (Ocimum basilicum L.) grown at different nitrogen levels. Int J Nat Eng Sci 3(3):9–13
Darrag HM, Almuhanna HT, Hakami EH, Alhojaily SM (2022) Analysis of volatile secondary metabolites in Ocimum basilicum cell suspensions: inhibition, in silico molecular docking, and an ADMET analysis against proteolytic enzymes of Rhynchophorus ferrugineus. Plants 11(21):2949. https://doi.org/10.3390/plants11212949
de Miguel M, Guevara MÁ, Sánchez-Gómez D, de María N, Díaz LM, Mancha JA, Fernandez de Simon B, Cadahía E, Desai N, Aranda I, Cervera MT (2016) Organ-specific metabolic responses to drought in Pinus pinaster Ait. Plant Physiol Biochem 102:17–26
Dudai N, Poljakoff-Mayber A, Mayer AM, Putievsky E, Lerner HR (1999) Essential oils as allelochemicals and their potential use as bioherbicides. J Chem Ecol 25:1079–1089
Duran RE, Kilic S, Coskun Y (2019) Melatonin influence on in vitro callus induction and phenolic compound production in sweet basil (Ocimum basilicum L.). In Vitro Cell Dev Biol-Plant 55:468–475. https://doi.org/10.1007/s11627-019-10006-6
Elansary HO, Mahmoud EA (2015) In vitro antioxidant and antiproliferative activities of six international basil cultivars. Nat Prod Res 29:2149–2154
Falleh H, Ksouri R, Medini F, Guyot S, Abdelly C, Magné C (2011) Antioxidant activity and phenolic composition of the medicinal and edible halophyte Mesembryanthemum edule L. Ind Crop Prod 34:1066–1071
Filip S, Pavlić B, Vidović S, Vladić J, Zeković Z (2017) Optimization of microwave-assisted extraction of polyphenolic compounds from Ocimum basilicum by response surface methodology. Food Anal Methods 10:2270–2280. https://doi.org/10.1007/s12161-017-0792-7
Flanigan PM, Niemeyer ED (2014) Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem 164:518–526
Fraser CM, Chapple C (2011) The phenylpropanoid pathway in Arabidopsis. The Arabidopsis Book 9:e0152
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky R (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125(2):539–555. https://doi.org/10.1104/pp.125.2.539
Ghasemzadeh A, Ashkani S, Baghdadi A, Pazoki A, Jaafar HZE, Rahmat A (2016) Improvement in flavonoids and phenolic acids production and pharmaceutical quality of sweet basil (Ocimum basilicum L.) by ultraviolet-B irradiation. Molecules 21:1203. https://doi.org/10.3390/molecules21091203
Giri A, Narasu ML (2000) Transgenic hairy roots: recent trends and applications. Biotechnol Adv 18(1):1–22. https://doi.org/10.1016/S0734-9750(99)00016-6
Grayer RG, Kite GC, Goldstone FJ, Bryan SE, Paton A, Putievsky E (1996) Infraspecific taxonomy and essential oil chemotypes in basil. Ocimum basilicum. Phytochemistry 43:1033–1039. https://doi.org/10.1016/S0031-9422(96)00429-3
Grayer R, Kite GC, Veitch NC, Eckert MR, Marin PD, Senanayake P, Paton AJ (2002) Leaf flavonoids glycosides as chemosystematic characters in Ocimum. Biochem Syst Ecol 30:327–342. https://doi.org/10.1016/S0305-1978(01)00103-X
Grayer RJ, Vieira RF, Price AM, Kite GC, Simon JE, Paton AJ (2004) Characterization of cultivars within species of Ocimum by exudate flavonoid profiles. Biochem Syst Ecol 32:901–913
Guirgis AA, Abd El-Kawi MA, Abbas HN, Araffa AM, Maksoud AI (2007) High rosmarinic acid content in induced mutants and in in vitro elicited sweet basil (Ocimum basilicum L.) callus. Asian J Plant Sci 6:1058–1064. https://doi.org/10.3923/ajps.2007.1058.1064
Gurav TP, Dholakia BB, Giri AP (2021) A glance at the chemodiversity of Ocimum species: trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem Rev 21:879–913. https://doi.org/10.1007/s11101-021-09767-z
Hakkim FL, Shankar CG, Girija S (2007) Chemical composition and antioxidant property of holy basil (Ocimum sanctum L.) leaves, stems, and inflorescence and their in vitro callus cultures. J Agric Food Chem 55:9109–9117. https://doi.org/10.1021/jf071509h
Hakkim FL, Kalyani S, Essa M, Girija S, Song H (2011a) Production of rosmarinic acid in Ocimum sanctum (L.) cell suspension cultures by the influence of growth regulators. Int J Biol Med Res 2:1158–1161
Hakkim FL, Kalyani S, Essa M, Girija S, Song H (2011b) Production of rosmarinic in Ocimum sanctum cell cultures by the influence of sucrose, phenylalanine, yeast extract, and methyl jasmonate. Int J Biol Med Res 2:1070–1074
Hammock HA (2018) The impact of blue and red LED lighting on biomass accumulation, flavor volatile production, and nutrient uptake in hydroponically grown genovese basil. Master’s thesis, University of Tennessee, Knoxville
Hashim M, Ahmad B, Drouet S, Hano C, Abbasi BH, Anjum S (2021) Comparative effects of different light sources on the production of key secondary metabolites in plants in vitro cultures. Plants 10(8):1521. https://doi.org/10.3390/plants10081521
Hassanpour S, Maheri-sis N, Eshratkhah B, Mehmandar FB (2011) Plants and secondary metabolites (Tannins): a review. Int J For Soil Eros 1:47–53
Hiltunen R, Holm Y (2003) Basil: the genus Ocimum. CRC Press, Boca Raton
Homhuan S, Kijwijan B, Wangsomnuk P, Bodhipadma K, Leung DWM (2008) Variation of plants derived from indirect somatic embryogenesis in cotyledon explants of papaya. Sci Asia 34:347–352
Hussain S, Khan F, Cao W, Wu L, Geng M (2016) Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front Plant Sci 7:439
Imen T, Cristina S, Riccardo I, Flavia NI, Zeineb O (2012) Phenolic acids and total antioxidant activity in Ocimum basilicum L. grown under Na2SO4 medium. J Med Plant Res 6:5868–5875
Jakovljević D (2018) Intraspecific variability of primary and secondary metabolism in nutrient deprived seedlings from Ocimum basilicum L. (Lamiaceae). Dissertation, University of Kragujevac
Jakovljević D, Stanković M (2020) Application of Teucrium species: current challenges and further perspectives. In: Teucrium species: biology and applications. Springer, Cham, pp 413–432. https://doi.org/10.1007/978-3-030-52159-2_15
Jakovljević D, Stanković M, Bojović B, Topuzović M (2017) Regulation of early growth and antioxidant defense mechanism of sweet basil seedlings in response to nutrition. Acta Physiol Plant 39(11):243
Jakovljević D, Topuzović M, Stanković M (2019) Nutrient limitation as a tool for the induction of secondary metabolites with antioxidant activity in basil cultivars. Ind Crop Prod 138:111462. https://doi.org/10.1016/j.indcrop.2019.06.025
Jakovljević D, Momčilović J, Bojović B, Stanković M (2021) The short-term metabolic modulation of basil (Ocimum basilicum L. cv.‘Genovese’) after exposure to cold or heat. Plants 10(3):590. https://doi.org/10.3390/plants10030590
Jakovljević D, Stanković M, Warchoł M, Skrzypek E (2022) Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: a review. Plant Cell Tissue Organ Cult:1–19. https://doi.org/10.1007/s11240-022-02286-5
Javanmardi J, Khalighi A, Kashi A, Bais HP, Vivanco JM (2002) Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem 50(21):5878–5883
Jennings BH, Akoh CC (2009) Effectiveness of natural versus synthetic antioxidants in a rice bran oil-based structured lipid. Food Chem 114:1456–1461
Kabera JN, Semana E, Mussa AR, He X (2014) Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol 2:377–392
Karaś M, Jakubczyk A, Szymanowska U, Złotek U, Zielińska E (2017) Digestion and bioavailability of bioactive phytochemicals. Int J Food Sci Technol 52(2):291–305. https://doi.org/10.1111/ijfs.13323
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3(13):1222–1239
Kasem MM (2017) Micropropagation and in vitro secondary metabolites production of Ocimum species. J Plant Production 8(4):473–484
Khater ES, Bahnasawy A, Abass W et al (2021) Production of basil (Ocimum basilicum L.) under different soilless cultures. Sci Rep 11:12754. https://doi.org/10.1038/s41598-021-91986-7
Kintzios S, Makri O, Panagiotopoulos E, Scapeti M (2003) In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol Lett 25:405–408. https://doi.org/10.1023/a:1022402515263
Kintzios S, Kollias H, Straitouris E, Makri O (2004) Scale-up micropropagation of sweet basil (Ocimum basilicum L.) in an airlift bioreactor and accumulation of rosmarinic acid. Biotechnol Lett 26(6):521–523. https://doi.org/10.1023/B:BILE.0000019561.89044.30
Kividompolo M, Hyotylainen T (2007) Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: characterization and quantification of antioxidant phenolic acids. J Chromatogr A 1145:155–164. https://doi.org/10.1016/j.chroma.2007.01.090
Koca N, Karaman Ş (2015) The effects of plant growth regulators and L-phenylalanine on phenolic compounds of sweet basil. Food Chem 166:515–521. https://doi.org/10.1016/j.foodchem.2014.06.065
Kruk J, Aboul-Enein BH, Duchnik E, Marchlewicz M (2022) Antioxidative properties of phenolic compounds and their effect on oxidative stress induced by severe physical exercise. J Physiol Sci 72:19. https://doi.org/10.1186/s12576-022-00845-1
Kwee EM, Niemeyer ED (2011) Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem 128(4):1044–1050
Kwon DY, Kim YB, Kim JK, Park SU (2021) Production of rosmarinic acid and correlated gene expression in hairy root cultures of green and purple basil (Ocimum basilicum L.). Prep Biochem Biotechnol 51(1):35–43. https://doi.org/10.1080/10826068.2020.1789990
Labra M, Miele M, Ledda B, Grassi F, Mazzei M, Sala F (2004) Morphological characterization, essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars. Plant Sci 167(4):725–731
Lattanzio V, Kroon PA, Quideau S, Treutter D (2008) Plant phenolics – secondary metabolites with diverse functions. In: Daayf F, Lattanzio V (eds) Recent advances in polyphenol research, vol 1. Wiley-Blackwell Publishing, Oxford, pp 1–35. https://doi.org/10.1002/9781444302400.ch1
Lattanzio V, Cardinali A, Ruta C, Fortunato IM, Lattanzio VM, Linsalata V, Cicco N (2009) Relationship of secondary metabolism to growth in oregano (Origanum vulgare L.) shoot cultures under nutritional stress. Environ Exp Bot 65:54–62
Lee J, Scagel CF (2009) Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem 115:650–656
Lee SJ, Umano K, Shibamoto T, Lee KG (2005) Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem 91(1):131–137
Lobiuc A, Vasilache V, Pintilie O, Stoleru T, Burducea M, Oroian M, Stoleru T, Burducea M, Pintilie O, Zamfirache MM (2017) Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 22:2111. https://doi.org/10.3390/molecules22122111
Lubbe A, Verpoorte R (2011) Cultivation of medicinal and aromatic plants for specialty industrial materials. Ind Crop Prod 34:785–801. https://doi.org/10.1016/j.indcrop.2011.01.019
Makri O, Kintzios S (2008) Ocimum sp. (Basil): botany, cultivation, pharmaceutical properties and biotechnology. Int J Geogr Inf Syst 13(3):123–150. https://doi.org/10.1300/J044v13n03_10
Marais JPJ, Deavours B, Dixon RA, Ferreira D (2007) The stereochemistry of flavonoids. In: Grotewold E (ed) The science of flavonoids. Springer, New York, pp 1–35. https://doi.org/10.1007/978-0-387-28822-2_1
Marzouk AM (2009) Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. Z Für Nat C 64:201–209. https://doi.org/10.1515/znc-2009-3-409
Mastaneh M, Ahmad M, Taher N, Mehrdad H (2014) Antioxidant effect of Ocimum basilicum cv. Dark Opal (Lamiaceae) phenolics. Orient J Chem 30(4):1965–1969. https://doi.org/10.13005/ojc/300459
Máthé Á, Hassan F, Kader AA (2015) In vitro micropropagation of medicinal and aromatic plants. In: Ákos M (ed) Medicinal and aromatic plants of the world. Springer, Dordrecht, pp 305–336
Mathew R, Sankar PD (2012) Effect of methyl jasmonate and chitosan on growth characteristics of Ocimum basilicum L., Ocimum sanctum L. and Ocimum gratissimum L. cell suspension cultures. Afr J Biotechnol 11:4759–4766. https://doi.org/10.5897/AJB11.3183
McCown BH, Lloyd G (1981) Woody Plant Medium (WPM) – a mineral nutrient formulation for microculture of woody plant species. Hortic Sci 16:453–453
Misra RC, Maiti P, Chanotiya CS, Shanker K, Ghosh S (2014) Methyl jasmonate-elicited transcriptional responses and pentacyclic triterpene biosynthesis in sweet basil. Plant Physiol 164:1028–1044. https://doi.org/10.1104/pp.113.232884
Mulabagal V, Tsay HS (2004) Plant cell cultures-an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nadeem M, Abbasi BH, Younas M, Ahmad W, Zahir A, Hano C (2019) LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J Photochem Photobiol B Biol 190:172–178. https://doi.org/10.1016/j.jphotobiol.2018.09.011
Nazir M, Tungmunnithum D, Bose S, Drouet S, Garros L, Giglioli-Guivarc’h N et al (2019) Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J Agric Food Chem 67:1847–1859. https://doi.org/10.1021/acs.jafc.8b05647
Nazir M, Asad UM, Mumtaz S, Siddiquah A, Shah M, Drouet S, Hano C, Abbasi BH (2020a) Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 25(5):1072. https://doi.org/10.3390/molecules25051072
Nazir M, Ullah MA, Younas M et al (2020b) Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tissue Org Cult 142:107–120. https://doi.org/10.1007/s11240-020-01844-z
Nazir S, Jan H, Tungmunnithum D, Drouet S, Zia M, Hano C, Abbasi BH (2020c) Callus culture of Thai basil is an effective biological system for the production of antioxidants. Molecules 25:4859. https://doi.org/10.3390/molecules25204859
Nazir S, Jan H, Zaman G, Ahmed N, Drouet S, Hano C, Abbasi BH (2021a) Synergistic effects of salicylic acid and light stress on bioactive metabolites in basil callus cultures. Biocatal Agric Biotechnol 37:102176. https://doi.org/10.1016/j.bcab.2021.102176
Nazir S, Jan H, Zaman G, Khan T, Ashraf H, Meer B, Zia M, Drouet S, Hano C, Abbasi BH (2021b) Copper oxide (CuO) and manganese oxide (MnO) nanoparticles induced biomass accumulation, antioxidants biosynthesis and abiotic elicitation of bioactive compounds in callus cultures of Ocimum basilicum (Thai Basil). Artif Cell Nanomed Biotechnol 49:626–634. https://doi.org/10.1080/21691401.2021.1984935
Nguyen PM, Niemeyer ED (2008) Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocimum basilicum L.). J Agric Food Chem 56:8685–8691
Pandey H, Pandey P, Singh S, Gupta R, Banerjee S (2015) Production of anti-cancer triterpene (betulinic acid) from callus cultures of different Ocimum species and its elicitation. Protoplasma 252:647–655. https://doi.org/10.1007/s00709-014-0711-3
Pandey P, Singh S, Banerjee S (2019) Ocimum basilicum suspension culture as resource for bioactive triterpenoids: yield enrichment by elicitation and bioreactor cultivation. Plant Cell Tissue Organ Cult 137:65–75. https://doi.org/10.1007/s11240-018-01552-9
Patel RP, Singh R, Rao BR, Singh RR, Srivastava A, Lal RK (2016) Differential response of genotype × environment on phenology, essential oil yield and quality of natural aroma chemicals of five Ocimum species. Ind Crop Prod 87:210–217
Paton ALAN, Harley RM, Harley MM (1999) Ocimum: an overview of classification and relationships. In: Basil: the genus Ocimum. Harwood Academic, Amsterdam, pp 1–38
Phippen WB, Simon JE (2000) Anthocyanin inheritance and instability in purple basil (Ocimum basilicum L.). J Hered 91(4):289–296
Piras A, Gonçalves MJ, Alves J, Falconieri D, Porcedda S, Maxia A, Salgueiro L (2018) Ocimum tenuiflorum L. and Ocimum basilicum L., two spices of Lamiaceae family with bioactive essential oils. Ind Crop Prod 113:89–97
Politeo O, Jukic M, Milos M (2007) Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem 101(1):379–385
Polyakova MN, Martirosyan YT, Dilovarova TA, Kosobryukhov AA (2015) Photosynthesis and productivity of basil plants (Ocimum basilicum L.) under different irradiation. Agric Biol 50(1):124–130. https://doi.org/10.15389/agrobiology.2015.1.124eng
Pushpangadan P (1974) Studies on reproduction and hybridisation of Ocimum species with view to improving their quality. Ph.D. thesis, Aligarh Muslim University, Aligarh
Pushpangadan P, Bradu BL (1995) Medicinal and aromatic plants. In: Chadha KL, Gupta R (eds) Advances in horticulture. Malhotra Publishing House, New Delhi
Rady MR, Nazif NM (2005) Rosmarinic acid content and RAPD analysis of in vitro regenerated basil (Ocimum americanum) plants. Fitoterapia 76:525–533. https://doi.org/10.1016/j.fitote.2005.04.001
Raimondi G, Orsini F, Maggio A, De Pascale S, Barbieri G (2006) Yield and quality of hydroponically grown sweet basil cultivars. Acta Hortic 723:357–360. https://doi.org/10.17660/ActaHortic.2006.723.48
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rechner AR, Kuhnle P, Bremner GP, Hubbard KP, Moore GCA, Rice-Evans CA (2002) The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med 33:220–235
Saha S, Monroe A, Day MR (2016) Growth, yield, plant quality and nutrition of basil (Ocimum basilicum L.) under soilless agricultural systems. Ann Agric Sci 61:181–186. https://doi.org/10.1016/j.aoas.2016.10.001
Santana-Gálvez J, Jacobo-Velázquez DA (2017) Classification of phenolic compounds. In: LML N, Gutierrez-Uribe JA (eds) Phenolic compounds in food, 1st edn. CRC Press, New York, pp 3–21
Shafique M, Khan SJ, Khan NH (2011) Comparative study for antibacterial potential of in vitro and in vivo grown Ocimum basilicum L. plant extracts. Pak J Biochem Mol Biol 44:113–117
Shahrajabian MH, Sun W, Cheng Q (2020) Chemical components and pharmacological benefits of basil (Ocimum basilicum): a review. Int J Food Prop 23:1961–1970. https://doi.org/10.1080/10942912.2020.1828456
Sharan S, Sarin NB, Mukhopadhyay K (2019) Elicitor-mediated enhanced accumulation of ursolic acid and eugenol in hairy root cultures of Ocimum tenuiflorum L. is age, dose, and duration dependent. S Afr J Bot 124:199–210. https://doi.org/10.1016/j.sajb.2019.05.009
Shoham A, Hadziahmetovic M, Dunaief JL, Mydlarski MB, Schipper HM (2008) Oxidative stress in diseases of the human cornea. Free Radic Biol Med 45:1047–1055
Shoji K, Goto E, Hashida S, Goto F, Yoshihara T (2009) Effect of light quality on the polyphenol content and antioxidant activity of sweet basil (Ocimum basilicum L.). In: Proceedings of the VI international symposium on light in horticulture, Tsukuba, pp 95–99
Sindhi V, Gupta V, Sharma K, Bhatnagar S, Kumari R, Dhaka N (2013) Potential applications of antioxidants. A review. J Pharm Res 7:828–835
Sipos L, Balázs L, Székely G, Jung A, Sárosi S, Radácsi P, Csambalik L (2021) Optimization of basil (Ocimum basilicum L.) production in LED light environments – a review. Sci Hortic 289:110486. https://doi.org/10.1016/j.scienta.2021.110486
Sousa EO, Miranda CMBA, Nobre CB, Boligon AA, Athayde ML, Costa JGM (2015) Phytochemical analysis and antioxidant activities of Lantana camara and Lantana montevidensis extracts. Ind Crop Prod 70:7–15
Srivastava S, Cahill DM, Conlan XA, Adholeya A (2014) A novel in vitro whole plant system for analysis of polyphenolics and their antioxidant potential in cultivars of Ocimum basilicum. J Agric Food Chem 62(41):10064–10075
Srivastava S, Conlan XA, Adholeya A, Cahill DM (2016) Elite hairy roots of Ocimum basilicum as a new source of rosmarinic acid and antioxidants. Plant Cell Tissue Organ Cult 126:19–32. https://doi.org/10.1007/s11240-016-0973-x
Stalikas CD (2007) Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci 30:3268–3295. https://doi.org/10.1002/jssc.200700261
Stanković M, Jakovljević D, Stojadinov M, Stevanović ZD (2019) Halophyte species as a source of secondary metabolites with antioxidant activity. In: Ecophysiology, abiotic stress responses and utilization of halophytes. Springer, Singapore, pp 289–312
Strazzer P, Guzzo F, Levi M (2011) Correlated accumulation of anthocyanins and rosmarinic acid in mechanically stressed red cell suspensions of basil (Ocimum basilicum). J Plant Physiol 168(3):288–293. https://doi.org/10.1016/j.jplph.2010.07.020
Tada H, Murakami Y, Omoto T, Shimomura K, Ishimaru K (1996) Rosmarinic acid and related phenolics in hairy root cultures of Ocimum basilicum. Phytochemistry 42:431–434. https://doi.org/10.1016/0031-9422(96)00005-2
Talebi M, Moghaddam M, Pirbalouti AG (2018) Methyl jasmonate effects on volatile oil compounds and antioxidant activity of leaf extract of two basil cultivars under salinity stress. Acta Physiol Plant 40(2):34
Tang GB, Song BZ, Zhao LL, Sang XS, Wan HH, Zhang J, Yao YC (2013) Repellent and attractive effects of herbs on insects in pear orchards intercropped with aromatic plants. Agrofor Syst 87:273–285
Tegeder M, Weber APM (2006) Metabolite transporters in the control of plant primary metabolism. In: Plaxton WC, McManus MT (eds) Annual plant reviews volume 22: control of primary metabolism in plants. Blackwell Publishing Ltd, Oxford. https://doi.org/10.1002/9780470988640.ch4
Telci I, Elmastas M, Sahin A (2009) Chemical composition and antioxidant activity of Ocimum minimum essential oils. Chem Nat Compd 45:568
Teofilović B, Grujić-Letić N, Goločorbin-Kon S, Stojanović S, Vastag G, Gadžurić S (2017) Experimental and chemometric study of antioxidant capacity of basil (Ocimum basilicum) extracts. Ind Crop Prod 100:176–182. https://doi.org/10.1016/j.indcrop.2017.02.039
Tzin V, Galili G (2010) New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol Plant 3(6):956–972
Vidović S, Zeković Z, Lepojević Ž, Radojković M, Jokić S, Anačkov G (2012) Optimization of the Ocimum basilicum L. extraction process regarding the antioxidant activity. Acta Periodica Technologica 43:315–323. https://doi.org/10.2298/APT1243315V
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3(1):2–20
Vyas P, Mukhopadhyay K (2014) Development of a rapid and high frequency Agrobacterium rhizogenes mediated transformation protocol for Ocimum tenuiflorum. Biologia 69(6):765–770. https://doi.org/10.2478/s11756-014-0375-7
Wongsen W, Bodhipadma K, Noichinda S, Leung DWM (2015) Influence of different 2, 4-D concentrations on antioxidant contents and activities in sweet basil leaf-derived callus during proliferation. Int Food Res J 22:638
Yue W, Ming QL, Lin B, Rahman K, Zheng CJ, Han T, Qin LP (2016) Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit Rev Biotechnol 36:215–232. https://doi.org/10.3109/07388551.2014.923986
Zabka M, Pavela R, Prokinova E (2014) Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 112:443–448
Zagoto M, Cardia GFE, da Rocha EMT, Mourão KSM, Janeiro V, Cuman RKN, Pinto AA, Contiero RL, Freitas PSL (2021) Biological activities of basil essential oil: a review of the current evidence. Res Soc Dev 10:e363101220409. https://doi.org/10.33448/rsd-v10i12.20409
Zahran E, Abdelmohsen U, Khalil H, Desoukey S, Ahmed M, Kamel M (2020) Diversity, phytochemical and medicinal potential of the genus Ocimum L. (Lamiaceae). Phytochem Rev 19. https://springerlink.bibliotecabuap.elogim.com/article/10.1007/s11101-020-09690-9
Zengin G, Uysal S, Ceylan R, Aktumsek A (2015) Phenolic constituent, antioxidative and tyrosinase inhibitory activity of Ornithogalum narbonense L. from Turkey: a phytochemical study. Ind Crop Prod 70:1–6
Zhang X, Liu CJ (2015) Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol Plant 8(1):17–27
Acknowledgments
This work was supported by the Ministry of Science, Technological Development, and Innovation of the Republic of Serbia (Agreements No. 451-03-47/2023-01/200122).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jakovljević, D., Skrzypek, E., Stanković, M., Warchoł, M. (2023). Phytochemical Diversity and Biological Activity of Basil (Ocimum L.) Secondary Metabolites Produced In Vitro. In: Kumar, N., S. Singh, R. (eds) Biosynthesis of Bioactive Compounds in Medicinal and Aromatic Plants. Food Bioactive Ingredients. Springer, Cham. https://doi.org/10.1007/978-3-031-35221-8_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-35221-8_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35220-1
Online ISBN: 978-3-031-35221-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)