Abstract
Ocimum basilicum L. var purpurascens (purple basil) contains medicinally valuable metabolites. Light greatly influences the physiological processes, including biomass accumulation and secondary metabolites production in medicinal plants. Herein, we investigated the influence of different spectral lights on the biosynthesis of phenylpropanoid metabolites in purple basil callus cultures. Growth kinetics was østudied for a total of 49 days, with 7 days of sampling time. Among the various treatments, blue light resulted in maximum biomass accumulation, total phenolic content (TPC), total flavonoid content (TFC) and antioxidant DPPH, FRAP and ABTS activities, as compared to controls. Moreover, blue light also encouraged higher superoxide dismutase activity while the red light was found effective for enhanced peroxidase activity. HPLC analysis revealed enhanced rosmarinic acid (87.62 mg/g DW) and anthocyanins (cyanidin: 0.15 mg/g DW and peonidin: 0.13 mg/g DW) contents under dark grown callus cultures which were almost 1.55, 1.25 and 1.18–fold greater than controls, respectively. Conversely, red light caused maximum production of cichoric acid (14.65 mg/g DW). Moreover, a positive correlation occurred among the accumulation of phenolic and flavonoids and antioxidant activities. These results suggest that light quality strongly influences medicinally valuable phenylpropanoid metabolites biosynthesis along with antioxidant potential in in vitro cultures of purple basil.
Key message
Light-enhanced precious metabolites in callus of Basil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ocimum basilicum L., commonly called “Basil”, is a widely recognized plant species of Lamiaceae family with diverse medicinal properties. O. basilicum is usually found in Africa, India and South Asia. As a herbal medicines, Ocimum genus have been cultivated around the globe since ancient times (Hakkim et al. 2013). Food and cosmetic industries provide huge market space for use of essential oils, obtained from basil (Vani et al. 2009).
Most commercial basil cultivars available in the market belong to the species O. basilicum (Wang et al. 2004; Iwai et al. 2010). Purple basil (Ocimum basilicum L. var purpurascens) is one of the cultivars of Ocimum with a traditional sweet basil flavour (Lee et al. 2014). Purple basil is known for its ornamental value and is also a good source of secondary metabolites (Phippen and Simon 1998), mainly phenylpropanoid metabolites which include anthocyanins, caffeic acid, chicoric acid and rosmarinic acid (Gang et al. 2001; Kiferle et al. 2011; Allan et al. 2008; Flanigan and Niemeyer 2014; Bertoli et al. 2013). These secondary metabolites play a key role against oxidative damage and variety of environmental stresses (La Camera et al. 2004). Rosmarinic acid is considered to be a one of the most significant caffeic acid ester present in Ocimum spp., having various pharmacological and therapeutics properties (Lee and Scagel 2009; Zheng and Wang 2001; Javanmardi et al. 2002; Shan et al. 2005) such as antioxidant, astringent, antibacterial, antiviral and anti-inflammatory (Pereira et al. 2009; Mastaneh et al. 2014), which make it a chief mediator for health endorsing assets, as well as, useful for cosmetic and food industries (Le Claire et al. 2005; Park et al. 2008; Petersen et al. 2009). Plant phytochemicals tend to act as strong antioxidant agents by cession of electron(s) (Aruoma 1998). Hydrogen atom transfer (HAT)-based mechanism and electron transfer (ET)-based mechanism are two main mechanisms through which antioxidants usually neutralize free radicals (Prior et al. 2005). Distinct in vitro-based protocols have been designed to study the mode of action and antioxidant activity of plant phytochemicals. Among these assays, ABTS (2,2-azinobis (3-ethylbenzthiazoline-6-sulphonic acid) assay is considered as a HAT-based reaction, whereas, FRAP (ferric reducing antioxidant power) assay operates via ET mechanism. On the other hand, the most commonly used is DPPH (2,2-diphenyl-1-picrylhydrazyl) assay which is mixed type that operates through both HAT and ET mechanisms (Prior et al. 2005). These assays provide not only antioxidant activity but also information regarding the complex molecular mechanisms involved in free radical scavenging activity. Plants are exposed to harsh environmental conditions on daily basis such as drought, temperature fluctuations, oxidative damage and light intensity. These environmental factors trigger molecular, physiological and morphological changes in plants via activation of cascade of reactions (Ahmad et al. 2012). Influence of stress on plant metabolites production has previously been studied (Trejo-Espino et al. 2011; Nagella and Murthy 2011; Lee and Shuler 2000). Physical factors and availability of suitable nutrients play a key role in optimum productivity of these metabolites (Matsubara et al. 1989; Karwasara and Dixit 2013). Light is an important physical factor, playing fundamental role in plant development, morphogenesis and regulation of phytochemicals biosynthesis (Kim et al. 2004; Shohael et al. 2006; Khan et al. 2013; Abbasi et al. 2007). Various studies have proposed the regulatory effect of light intensity and quality on enhanced metabolites biosynthesis in various plant species cultures (Ellis and Roberts 1978; Senger 1987; Exonomou 1987). High pressure sodium lamps (HPS-light), incandescent light lamps, metal halide lamps and tubular fluorescent lamps are commonly employed as a source of light for in vitro culturing of plants. However, these light sources contain low quality wavelengths that are ineffective for growth stimulation (Kim et al. 2004). Alternatively, LEDs have low thermal emissions, narrow bandwidth, long life, wavelength specificity and low degradation, and are thus considered suitable for in vitro culturing of plant species (Gupta and Jatothu 2013; Yeh and Chung 2009; Yam and Hassan 2005). LEDs have been tested in many plants such as wheat, pepper, lettuce and spinach etc. for improved morphogenesis (Okamoto et al. 1996; Jao and Fang 2004; Jao et al. 2005; Nhut et al. 2000; Yanagi and Okamoto 1994). Studies indicated blue and red lights to be more effective, nevertheless, mode of action and response to light is variable among different plant species. Herein, for the first time, we report light quality effect on antioxidant potential as well as production of valuable metabolites in O. basilicum L. var purpurascens callus cultures.
Materials and methods
Seeds germination and callus induction
Seeds of purple basil were taken from National Agriculture Research Center, Pakistan. Superficial sterilization of seeds was done by adopting modified protocol of Abbasi et al. (2010). In brief, seeds were treated with mercuric chloride (0.1%) and ethanol (70%) for 30 s and 1 min, respectively. After that, seeds were washed three times with dH2O (autoclaved) and placed on MS media (Murashige and Skoog 1962), supplemented with carbon source (sucrose: 3%) and gelling agent (agar: 0.8%) at a pH of 5.6–5.7 prior to autoclave. Seeds were allowed to grow under controlled environment i.e. light/dark cycle (16/8 h) and temperature (25 ± 2 °C). Callus culture was established using leaf explants (0.5 cm2) from 28 days old plantlets (in vitro derived) cultured on MS media additionally supplied with 2.5 mg/L NAA, as previously optimized by Nazir et al. (2019). Experiment was carried out under controlled conditions i.e. light/dark cycle (16/8 h) and temperature (25 ± 2 °C) in growth room and callus was further sub-cultured after 28 days on same optimized hormonal media.
Spectral lights treatment
On explant-derived callus, different monochromatic lights were used as elicitors which included photoperiod cycle (light/dark i.e. 16/8 h), White light (24 h, wavelength 400–700 nm), Darkness (24 h), Red light (24 h, wavelength 660 nm), Green light (24 h, wavelength 510 nm), Blue light (24 h, wavelength 460 nm) and Yellow light (24 h, wavelength 570 nm). Culture grown under photoperiod condition was considered as control. Optimized hormonal concentration (2.5 mg/L NAA) was used for callus growth under different sources of monochromatic lights (40–50 µMol m− 2 s− 1 intensity). Experimentation was done thrice. For estimation of biomass (FW, DW) and analysis of phytochemicals, harvesting was carried out at 7 days interval for each light treatment.
Phytochemical analysis
Total phenolic and flavonoid contents
For the estimation of total phenolic and flavonoid contents (TPC & TFC), the previous procedure was employed with slight modifications (Zahir et al. 2014). Briefly, dried powder (100 mg) of each tested sample was thoroughly mixed with 99.9% methanol (500 µL) by vortexing (5 min) and sonication (half an hour) at room temperature, followed by 10 min centrifugation at 15000 rpm. Supernatant was finally assembled and saved for future use at 4 °C.
TPC was evaluated following a previously modified protocol (Velioglu et al. 1998). In brief, the extracted sample (20 µL), Na2CO3 (90 µL) and FC-reagent (90 µL) were mixed. Microplate reader was used to measure absorbance at 630 nm. Phenolic content was expressed as gallic acid equivalents (GAE)/g of DW and gallic acid was also used as a control standard. Total Phenolic Production (TPP) was determined by multiplying the TPC value with a dry weight of the respective sample. For the evaluation of TFC, aluminum chloride assay, as previously described by Ahmad et al. (2010), was used. Potassium acetate (10 µL), distilled water (160 µL) and aluminum chloride (10 µL) were mixed with test sample (20 µL). Absorbance was then taken with microplate reader at 630 nm, after half an hour of incubation. Quercetin was employed as positive control and flavonoid content was expressed as quercetin equivalents per gram (QE)/g DW of sample. Total flavonoid (TFP) was then determined via multiplying TFC value with respective dry weight of the tested sample.
Free radical scavenging activity (FRSA)
FRSA of tested samples was examined via DPPH reagent, as designated earlier (Abbasi et al. 2010). In short, the solution DPPH (180 µL) was added in the test sample (20 µL). Prior to taking absorbance at 517 nm with microplate reader, solution was placed at 37 °C in dark for one hour. Final concentrations of ascorbic acid (40, 20, 10 and 05 µg/mL) and DMSO was used as positive and negative control, respectively. FRSA was then calculated with formula:
where AE is the mixture absorbance at 517 nm with sample addition, while AD is only DPPH solution absorbance.
Ferric reducing antioxidant power (FRAP) assay
FRAP assay was also done according to the previously reported procedure (Benzie and Strain 1996). In brief, 190 µL FRAP solution and 10 µL of calli extract were mixed and then placed for 15 min at 25 ± 1 °C. Afterwards, OD was measured with microplate reader at 630 nm. FRAP solution constitute acetate buffer (pH 3.6, 300 mM), FeCl3·6H2O (20 mM) and 10 mM TPTZ in a ratio of 10:1:1 (v/v/v), respectively. Experiment was performed three times. Antioxidant activity was expressed as TEAC (Trolox C equivalent antioxidant capacity).
Antioxidant ABTS assay
ABTS antioxidant assay (Velioglu et al. 1998) was also done for further screening of antioxidant potential of extracts. Briefly, to make ABTS solution, equal quantity of 7 mM ABTS salt was added to 2.45 mM potassium per sulphate and kept in darkness for 16 h. After that, absorbance was taken, and mixture was then placed again for 15 min in the dark (25 ± 1 °C) and absorbance was again measured at 734 nm with microplate reader. Experiment was repeated three times and the values were expressed as TEAC equivalent.
Enzymatic antioxidant activities
Enzymatic antioxidant activities (peroxidase (POD) and superoxide dismutase (SOD)) were estimated using fresh samples of callus cultures. Procedure designed by Nayyar and Gupta (2006) was employed with minor adjustments for extraction of fresh samples. Buffer (1 mL) of potassium phosphate (50 mM, pH 7.0) having 1% PVP was added to 100 mg of fresh sample. Mixing and grinding of samples was done using Mortar and pestle, followed by half an hour centrifugation at 15000 rpm. Supernatant collected was kept at 4 °C for enzymatic activities. POD activity was performed using previous protocol (Lagrimini 1980). Firstly, fresh sample extract (20 µL), 0.1 mL distilled H2O, 20 µL hydrogen peroxide (27.5 mM; 10 ×), 20 µL guaiacol (100 mM, 10 ×) and potassium phosphate buffer (40 µL; 50 mM; pH 7) were mixed and then, with the aid of a microplate reader, solution absorbance was measured at 470 nm. To determine SOD activity, Giannopolitis and Ries (1977) method was employed. Briefly, 20 µL EDTA (1 mM), 20 µL methionine (130 mM), 60 µL calli extract (fresh), 2 µL riboflavin (0.02 mM), 78 µL phosphate buffer (50 mM, pH 7) and 20 µL NBT (0.75 mM) were mixed to prepare the reaction mixture. Further, prior to taking absorbance, the fluorescent white light was used for 7 min incubation of reaction mixture. Absorbance was taken by microplate reader at 660 nm. The following formula was then used to calculate POD and SOD values:
where A = Absorbance; E = Extinction coefficient (6.39 mM− 1 cm− 1; C = enzyme concentration (value calculated in nM/min/mg FW) & L = Length of wall (0.25 cm).
HPLC analysis
Extraction and HPLC separation were performed as described by Nazir et al. (2019). To quantify phenylpropanoid metabolites in callus cultures of purple basil, HPLC with standard grade chemicals (Sigma Aldrich) were used (Takemiya et al. 2005). Hypersil PEP 300 C18 column (250 × 4.6 mm, 5 µm), equipped with a guard column Alltech (10 × 4.1 mm) was utilized to perform separation at 35 °C and compounds separation was observed at 210 and 250 nm wavelength via a Varian HPLC system (equipped with a degasser (Metachem Degassit), Prostar 335 Photodiode Array Detector and autosampler (Varian Prostar 410)). Quantification of the compounds was accomplished on the basis of reliable reference standards and retention times. Two HPLC-grade solvents were used in mobile phase: Solvent A = HCOOH/H2O, pH 2.1 and solvent B = CH3OH. Throughout 1-hour run, composition of mobile phase varied with a nonlinear gradient 8% B (0 min), 12% B (11 min), 30% B (17 min), 33% B (28 min), 100% B (30–35 min), 8% B (36 min) at a flow rate of 1 mL/min. A 10 min re-equilibration time was used among individual runs. All the tested samples were examined thrice, and the values were expressed as µg/mg DW of the sample.
Statistical analysis
Whole experiments were carried out thrice. Microsoft Excel was used to calculate means ± standard deviation of replicates and Origin software (Origin Lab Corporation, Wellesley Hills, MA, USA) was used to draw all the graphs. Tukey’s multiple comparison test was employed for calculating significant differences. One-way analysis of variance (ANOVA) with significant difference p < 0.05 was used to compare the means of different treatments.
Results and discussion
Effect of light quality on biomass accumulation & growth kinetics
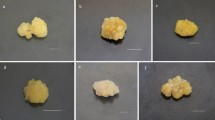
Light quality as well as light quantity are necessary factors which affect the development and composition of plant (Bian et al. 2015). Red light is usually considered to be most efficient in the process of photosynthesis while other spectra are essential for plant growth and physiology. Blue light, for instance, controls the opening of stomata, thus improve access to transpiration and carbon dioxide (CO2) and is also necessary to inhibit “red light syndrome” (Davis and Burns 2016). Light acts as an abiotic elicitor to increase the accumulation of valuable metabolites (Fazal et al. 2016b). Various plant species callus cultures have been previously exposed to light for enhanced metabolites biosynthesis (Fazal et al. 2016a; Nadeem et al. 2018; Shohael et al. 2006). Herein, maximum callus biomass accumulation in purple basil was observed for 35 days period with 7 days sampling time under different LEDs treatments. Morphological variations were observed with visual eye and are presented in Fig. 1. All the spectral lights resulted in shorter lag phases. However, elongated log phases were observed for most of the LEDs treatments as well as control, starting from day 7 up to day 35. Biomass accumulation suddenly increased from day 28 to day 35 during the log phases. Moreover, from day 35 to 42, a shorter stationary phase occurred. From day 42 to 49, a decline phase was observed for all the LEDs treatments and control. Among the various LEDs treatments, maximum biomass (18.83 g/L DW) accumulation was shown by blue light, followed by white (18.34 g/L DW) and yellow (18.2 g/L DW) lights at day 35 of growth kinetics (Fig. 2a, b, e). However, biomass accumulation for green light (15.8 g/L DW), darkness (15.5 g/L DW) and red light (14.8 g/L DW) were lower than control (17.7 g/L DW) (Fig. 2g). Overall, blue spectrum was found efficient for enhanced biomass accumulation and callus induction. Blue light has the ability to increase the chlorophyll content which results in increased photosynthesis (Lobiuc et al. 2017; Nadeem et al. 2018) and subsequent increase in carbon and carbohydrate accumulation. Effect of light wavelength is highly dependent on plant species, type of explant and in vitro cell culture (Andi et al. 2018; Liu et al. 2006).
Growth kinetics and biomass (Fresh Weight and Dry Weight) accumulation at optimized hormonal conditions under different spectral lights. a Blue light treatment, b Yellow light treatment, c Green light treatment, d Red light treatment, e White light treatment, f Dark treatment, g Control. Values are means ± SE from triplicates. Columns with similar alphabets are not significantly different (p < 0.05) according to Tukey’s test
Effect of light quality on phenolic and flavonoids accumulation
Plant phenolic and flavonoids have a powerful biological activity, which makes it necessary to assess them (Sulaiman and Balachandran 2012). Here in, we explored the influence of spectral lights to enhance phenolic and flavonoids in purple basil calli grown under different spectral lights for a duration of 7 weeks with 7 days of sampling time (Fig. 3). Calli grown under blue spectrum increased the TPC (20.1 mg/g DW), followed by red (19.7 mg/g DW), green (18.7 mg/g DW) and dark (18.2 mg/g DW) spectra, respectively (Fig. 3a). Similarly, maximum TPP was also observed for blue light (378.3 mg/L), followed by green (295.63 mg/L) and red (283.9 mg/l) lights, respectively (Fig. 3c). There exists a considerable evidence for light induction and synthesis of phenolic by blue light (Engelsma 1969; Kim et al. 2014; Fazal et al. 2016b). Studies indicate that PAL expression is stimulated by blue light, which is a key enzyme involved in phenylpropanoid biosynthetic pathway (Heo et al. 2012; Son et al. 2012).
Growth kinetics and total phenolic content (TPC), total flavonoid content (TFC), along with their productions (TPP & TFP) at optimized hormonal conditions under different spectral lights. a TPC (mg/g DW), b TFC (mg/g DW), c TPP (mg/L), d TFP (mg/L). Values are means ± SE from triplicates. Columns with similar alphabets are not significantly different (p < 0.05) according to Tukey’s test
Moreover, blue light is also involved in cis form hydroxycinnamic acids biosynthesis which has minimal inhibitory potential as compared to trans-form (Billett et al. 1981). Thus, blue light has a role in stimulating the biosynthetic pathways of metabolites, resulting in high amount of phenolic content and their production (Nam et al. 2018; Nadeem et al. 2018). Similar observations were previously reported for the accumulation of phenolic compounds under the influence of blue and red lights in buck wheat (Lee and Chen 2014). Maximum total flavonoid content (TFC) was also detected in log phase (day 35) for blue light (13.9 mg/g DW), and subsequently by red (13.4 mg/g DW), dark (12.9 mg/g DW) and green (11.8 mg/g DW) spectra, respectively (Fig. 3b). Similarly, higher total phenolic production (TFP) was observed for blue spectrum (263.3 mg/L), followed by white (207.2 mg/L) and red (199.9 mg/L) spectra, respectively (Fig. 3d). Likewise, it was stated that blue spectrum encourages maximum flavonoid accumulation in O. basilicum callus cultures (Nadeem et al. 2018).
The results of our study indicated that blue spectrum is more efficient in phenolic and flavonoids accumulation, as compared to other spectra. Possible link exists between blue light and phenylpropanoids production by the process of photosynthesis. Phototrophins and cryptochromes are specific photoreceptors of blue light. These receptors perceive specific wavelengths of light and thus regulate functional adaptations and photo-morphogenic responses (Takemiya et al. 2005). Cryptochromes have been shown to induce the expression of genes associated with flavonoids biosynthesis (Wade et al. 2001; Liu et al. 2018). Lights of shorter wavelength, including the blue light, have a vital role in flavonoids biosynthesis in buck wheat seedling (Nam et al. 2018). Likewise, blue light has been shown to enhance the contents of flavonoids and phenolic acids in numerous other plant species (Wang et al. 2010; Liu et al. 2016).
Effect of light quality on antioxidant capacity
The antioxidant capacity of callus culture was estimated using DPPH, ABTS and FRAP assays, due to their ease of use, sensitivity, inexpensiveness and simplicity. The results of these antioxidant activities are reported in Fig. 4. As the accumulation of biomass was found optimal at log phase, therefore, harvesting was done for all the treated cultures at the end of log phase (day 35) to assess the antioxidant potential. DPPH antioxidant activity provided the overall antioxidant ability of purple basil callus. Here, higher free radical activity (DPPH) was demonstrated in culture treated with blue light (94%), followed by white (90%) and dark (87%) treatments, respectively (Fig. 4a). The antioxidant power of callus cultured under different spectral lights was also measured using FRAP assay. Extracts total antioxidant and single antioxidant power was analyzed by using this assay (Siddhuraju et al. 2002). The FRAP assay showed a large difference in antioxidant reduction profile of purple basil in different LEDs treatments. As shown in Fig. 4b, the reducing ability of blue light has highest activity than other lights. The FRAP values (TEAC µM) were in the order: blue (630.6) > white (592.0) > dark (504.9) > green (507.9) > red (501.5). The FRAP assay for blue spectrum was 1.4-folds higher than control, while, yellow light displayed the lowest activity. Wu et al. (2007) noticed that blue light has positive significant effect to improve the antioxidant capacity of the sprouted pea seed. Similarly, higher DPPH and FRAP activities in soybean, treated with blue light, was also reported (Azad et al. 2018). Likewise, blue light also resulted in highest antioxidant ABTS assay (Fig. 4b). Callus cultures of purple basil treated with different LEDs displayed the ABTS activities in the following order: blue (937.3 TEAC µM) > white (879.0 TEAC µM) > dark (807.1 TEAC µM) > green (800.1 TEAC µM) > red (790.7 TEAC µM) > yellow (781.1 TEAC µM). To assess the antioxidant potential of plant extracts, ABTS and DPPH assays have previously been employed, which depends on the capability of electron transfer antioxidant to decrease oxidants (Li et al. 2008). It has been shown that blue light results in highest ABTS assay in buckwheat sprouts, which might be linked to maximum TPC and TFC (Nam et al. 2018), which is in harmony with the results of our study. Furthermore, a correlation occurred between the accumulation of phenolic and flavonoids and antioxidant assays in our study. Therefore, the enhanced antioxidant activities (DPPH, FRAP, ABTS) in cultures treated with blue light might be due to increased contents of phenolic and flavonoid (Table 1).
Effect of light quality on enzymatic antioxidant activities
Herein, we also determined the influence of spectral lights on POD (peroxidase) and SOD (superoxide dismutase) activities in purple basil callus cultures to measure their role in stress situations. Highest POD activity was shown by red light which was 1.5-fold higher than control, while dark grown cultures displayed minimum POD activity (Fig. 5b). The highest POD activity for cultures grown under red spectrum might be because of harmful effects of increased production of H2O2 or over production of its poisonous active oxygen derivatives, as due to oxidative stress (Younas et al. 2018). It was also concluded that red light enhanced POD besides other antioxidant enzymes in strawberry plantlets (Yonghua et al. 2005). Similarly, red light resulted in increased protease and peroxidase activities in Artemisia (Tariq et al. 2014). On the other hand, blue light resulted in highest SOD activity (0.246 nM/min/mg FW), followed by red spectrum (0.194 nM/min/mg FW), while minimal SOD activity was found for yellow light (Fig. 5a). Previously, Khan et al. (2018) also concluded that blue LED causes optimum POD activity in Fagonia indica callus cultures, whereas, Younas et al. (2018) reported that yellow light results in lowest SOD in S. marianum L. cultures. Lee et al. (2009) concluded that peroxidase is a reference enzyme which is mainly used for the blanching treatment due to its high thermal resistance and is mainly involved in the decomposition of polyphenols (Bhat and Sharma 2016). During this oxidation, highly reactive oxidant species such as H2O2 are produced. It is considered that blue light treatment leads to less degradation of polyphenols that might be the reason that blue light showed a high phenolic content but less POD activity.
Effect of light quality on accumulation of phenylpropanoid metabolites
As an abiotic elicitor, light directly or indirectly influences the growth as well as development of plant species (OuYang et al. 2015; Adams et al. 1996; Stefano and Rosario 2003). Not only the presence or absence of light but also the changes in light quality influence plant responses (Felippe 1979). Previously, light has been shown to stimulate metabolites production in numerous callus culture systems (Shohael et al. 2006; Exonomou 1987; Fazal et al. 2016b). Rosmarinic acid (RA) is a key phenolic compound of basil. Therefore, we also examined the light quality effect on callus cultures of purple basil for RA biosynthesis. All spectral lights exhibited maximum biosynthesis of RA in day 35 (log phase) of growth kinetics, followed by a shorter stationary phase (from day 35 to 42) and then decline phase (from day 42 onwards), as shown in Table 2. Maximum biosynthesis of RA in log phase was observed for darkness (87.62 mg/g DW), followed by red (85.33 mg/g DW), white (82.16 mg/g DW) and blue (77.57 mg/g DW) spectra, respectively. RA content under control was 56.30 mg/g DW. Light and dark periods play a significant role in provoking responses in plants, particularly the synthesis of secondary metabolites (Mir et al. 2017). As a defense strategy, plants accumulate secondary metabolites (Ferrandino and Lovisolo 2014), synthesized via phenylpropanoid pathway (Iriti and Faoro 2009). RA is the key metabolite of basil plant, synthesised through phenylpropanoid pathway (Shiga et al. 2009). In current study, high RA accumulation in dark-grown callus cultures may be due to the fact that continuous dark creates stress conditions and hence, stimulates signal transduction cascade of phenylpropanoid pathway for increment in production of RA. The secondary metabolites undergo photo-block in continuous light conditions, resulting in photoconversion (Mir et al. 2017). The increase in RA might be because of the accumulation of higher levels of reactive oxygen species (ROS) by cytochrome P450. Non-enzymatic scavengers of ROS, like RA, are produced as a defense mechanism (Iwai et al. 2010).
Anthocyanins are pigments, produced in plants, which are affected by numerous environmental factors, mainly light (Gould 2006). Cyanidin and peonidin are major anthocyanins that have previously been identified in these purple basil cultures (Phippen and Simon 1998). In this study, anthocyanins and RA were accumulated in correlated manner. Maximum biosynthesis of cyanidin (0.152 mg/g DW) and peonidin (0.135 mg/g DW) were noted in the dark treatment, followed by red (cyanidin: 0.147 mg/g DW & peonidin: 0.1217 mg/g DW) and white (cyanidin: 0.133 mg/g DW & peonidin: 0.127 mg/g DW) lights, respectively (Table 2). Conversely, yellow and green spectra showed lower anthocyanins, as compared to control. From the point of process scale-up, necessity of light irradiation is a problem. However, several types of photo-bioreactors have been designed but it is expensive to operate a photo-bioreactor with light of high intensity. Therefore, production of anthocyanins in the dark is preferable for commercial applications. In literature, reports are available for few plant cell cultures to produce anthocyanins in the dark such as Fragaria ananassa (Nakamura et al. 1999), Aralia cordata (Sakamoto et al. 1994), Bupleurum falcatum (Hiraoka et al. 1986), Vitis hybrid (Yamakawa et al. 1983) and Daucus carota (Dougall et al. 1980). Reasons for this increase is yet not clear. Nakamura et al. (1999) proposed that high anthocyanin in strawberry callus grown in the dark might be due to genetic mutation that changed the way of expression in an anthocyanin synthetic pathway. In plant cells, anthocyanins are biosynthesized through the phenylpropanoid metabolic pathway and flavonoid metabolic pathway. Another reason may be that, in callus cultured on solid medium, cells on the surface of a clump were always irradiated with strong light intensity. Continuous light irradiation might damage the cells on the surface of clumps, resulting in low anthocyanin productivity (Nakamura et al. 1999). Same will be true for the current findings in our study.
Current study indicated that the total anthocyanin and RA contents increased in response to different LEDs treatments and this might be due to the fact that their initial biosynthetic pathways are common (Petersen, 2003). Nadeem et al. (2018) reported higher anthocyanin contents in basil cultures grown under red spectrum. Another major metabolite found in basil species is cichoric acid which carries diverse medicinal benefits (Lee and Scagel 2009). Here, higher production of cichoric acid was noted during the log phase (day 35) in callus treated with red light (14.65 mg/g DW), followed by white (12.76 mg/g DW) and dark (12.52 mg/g DW) treatments, respectively. Studies indicate that the expression of genes including CYP98A6, PAL and C4H are induced by white light. These genes are thought to be involved in cichoric acid biosynthesis (Shoji et al. 2009; Shiga et al. 2009).
Caffeic acid is another valuable metabolite produced in basil. Here, we also evaluated the influence of spectral lights on caffeic acid accumulation in purple basil callus cultures. No significant effect was observed in caffeic acid production, as presented in Table 2. Since caffeic acid is considered as a precursor of RA, therefore, lower caffeic acid accumulation in our study might be because of its turnover to RA (Petersen and Simmonds 2003; Nazir et al. 2019; Petersen et al. 2009).
Conclusions
Light quality strongly influences the biosynthesis of valuable metabolites in purple basil callus cultures. Among the different LEDs used in this study, blue spectrum is the best for biomass, as well as, phenolic and flavonoids accumulation. Additionally, blue light also encourages superoxide dismutase as well as other antioxidant activities (DPPH, FRAP and ABTS). Conversely, red light is effective in peroxidase activity. Moreover, there is a strong correlation between the accumulation of phenolic and flavonoids and antioxidant activities in purple basil calli grown under different spectral lights. HPLC analysis indicated that red light is effective for cichoric acid accumulation while darkness favors enhanced RA and anthocyanins (peonidin and cyanidin) contents in callus cultures of purple basil. These results demonstrate that LEDs provide a promising elicitation approach for increased accumulation of valuable phenylpropanoid metabolites in in vitro cultures of Ocimum basilicum L. var purpurascens.
Abbreviations
- ABTS:
-
2,2-Azinobis 3-ethylbenzthiazoline-6-sulphonic acid
- FRAP:
-
Ferric reducing antioxidant power
- DPPH:
-
2, 2-Diphenyl-1-picrylhydrazyl
- LEDs:
-
Light-emitting diodes
- MS:
-
Murashige and Skoog
- DMSO:
-
Dimethyl sulfoxide
- NAA:
-
α-Naphthalene acetic acid
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
- TFP:
-
Total flavonoid production
- TPP:
-
Total phenolic production
- FW:
-
Fresh weight
- DW:
-
Dry weight
- FRSA:
-
Free radical scavenging activity
- POD:
-
Peroxidase
- SOD:
-
Superoxide dismutase
- TEAC:
-
Trolox C equivalent antioxidant capacity
- RA:
-
Rosmarinic acid
References
Abbasi BH, Khan MA, Mahmood T, Ahmad M, Chaudhary MF, Khan MA (2010) Shoot regeneration and free-radical scavenging activity in Silybum marianum L. Plant Cell Tissue Organ Cult 101:371–376
Abbasi BH, Tian C-L, Murch SJ, Saxena PK, Liu C-Z (2007) Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep 26:1367–1372
Adams S, Kunz B, Weidenborner M (1996) Mycelial deformations of Cladosporium herbarum due to the application of eugenol or carvacrol. J Essent Oil Res 8:535–540
Ahmad N, Fazal H, Abbasi BH, Rashid M, Mahmood T, Fatima N (2010) Efficient regeneration and antioxidant potential in regenerated tissues of Piper nigrum L. Plant Cell Tissue Organ Cult 102:129–134
Ahmad P, Kumar A, Gupta A, Hu X, Azooz MM, Sharma S (2012) Polyamines: role in plants under abiotic stress. Crop production for agricultural improvement. Springer, New York, pp 491–512
Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13:99–102
Andi SA, Gholami M, Ford CM (2018) The effect of methyl jasmonate and light irradiation treatments on the stilbenoid biosynthetic pathway in Vitis vinifera cell suspension cultures. Nat Prod Res 32:909–917
Aruoma OI (1998) Free radicals, oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc 75:199–212
Azad M, Kim W, Park C, Cho D (2018) Effect of artificial LED light and far infrared irradiation on phenolic compound, isoflavones and antioxidant capacity in soybean (Glycine max L.) sprout. Foods 7:174
Benzie IF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem 239:70–76
Bertoli A, Lucchesini M, Mensuali-Sodi A, Leonardi M, Doveri S, Magnabosco A, Pistelli L (2013) Aroma characterisation and UV elicitation of purple basil from different plant tissue cultures. Food Chem 141:776–787
Bhat S, Sharma HK (2016) Combined effect of blanching and sonication on quality parameters of bottle gourd (Lagenaria siceraria) juice. Ultra Sonochem 33:182–189
Bian ZH, Yang QC, Liu WK (2015) Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: a review. J Sci Food Agri 95:869–877
Billett EE, Grayer-Barkmeijer RJ, Johnson C, Harborne J (1981) The effect of blue light on free and esterified phenolic acids in etiolated gherkin tissues. Phytochemistry 20:1259–1263
La Camera S, Gouzerh G, Dhondt S, Hoffmann L, Fritig B, Legrand M, Heitz T (2004) Metabolic reprogramming in plant innate immunity: the contributions of phenylpropanoid and oxylipin pathways. Immunol Rev 198:267–284
Le Claire E, Schwaiger S, Banaigs B, Stuppner H, Gafner F (2005) Distribution of a new rosmarinic acid derivative in Eryngium alpinum L. and other Apiaceae. J Agric Food Chem 53:4367–4372
Davis PA, Burns C (2016) Photobiology in protected horticulture. Food Energy Secur 5:223–238
Dougall DK, Johnson JM, Whitten GH (1980) A clonal analysis of anthocyanin accumulation by cell cultures of wild carrot. Planta 149:292–297
Ellis R, Roberts E (1978) Towards a rational basis for testing seed quality. In: Proceedings-Easter School in Agricultural Science, University of Nottingham
Engelsma G (1969) The influence of light of different spectral regions on the synthesis of phenolic compounds in gherkin seedlings, in relation to photomorphogenesis: VI. phenol synthesis and photoperiodism. Acta Bot Neerl 18:347–352
Exonomou A (1987) Light treatments to improve efficiency of in vitro propagation systems. Hort Sci 22:751–754
Fazal H, Abbasi BH, Ahmad N, Ali M (2016a) Elicitation of medicinally important antioxidant secondary metabolites with silver and gold nanoparticles in callus cultures of Prunella vulgaris L. Appl Biochem Biotech 180:1076–1092
Fazal H, Abbasi BH, Ahmad N, Ali SS, Akbar F, Kanwal F (2016) Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J Photochem Photobiol B 159:1–7
Felippe G (1979) Fotomorfogenese. FERRI, MG Fisiologia Vegetal. EPU, Sao Paulo
Ferrandino A, Lovisolo C (2014) Abiotic stress effects on grapevine (Vitis vinifera L.). Focus on abscisic acid-mediated consequences on secondary metabolism and berry quality. Environ Exp Bot 103:138–147
Flanigan PM, Niemeyer ED (2014) Effect of cultivar on phenolic levels, anthocyanin composition and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem 164:518–526
Gang DR, Wang J, Dudareva N, Nam KH, Simon JE, Lewinsohn E, Pichersky E (2001) An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant Physiol 125:539–555
Giannopolitis CN, Ries SK (1977) Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol 59:309–314
Gould K (2006) Flavonoid functions in plants. In: Andersen ØM, Markham KR (eds) Flavonoids: chemistry biochemistry and applications. CRC Press, Boca Raton, pp 397–441
Gupta SD, Jatothu B (2013) Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol Rep 7:211–220
Hakkim FL, Arivazhagan G, Boopathy R (2013) Antioxidant property of selected Ocimum species and their secondary metabolite content. J Med Plants Res 2:250–257
Heo J-W, Kang D-H, Bang H-S, Hong S-G, Chun C-H, Kang K-K (2012) Early growth, pigmentation, protein content, and phenylalanine ammonia-lyase activity of red curled lettuces grown under different lighting conditions. Korean J Hortic Sci Technol 30:6–12
Hiraoka N, Kodama T, Tomita Y (1986) Selection of Bupleurum falcatum callus line producing anthocyanins in darkness. J Nat Prod 49:470–474
Iriti M, Faoro F (2009) Bioactivity of grape chemicals for human health. Nat Prod Commun 4:1934578 × 0900400502
Iwai M, Ohta M, Tsuchiya H, Suzuki T (2010) Enhanced accumulation of caffeic acid, rosmarinic acid and luteolin-glucoside in red perilla cultivated under red diode laser and blue LED illumination followed by UV-A irradiation. J Funct Foods 2:66–70
Jao R-C, Fang W (2004) Effects of frequency and duty ratio on the growth of potato plantlets in vitro using light-emitting diodes. HortScience 39:375–379
Jao R-C, Lai C-C, Fang W, Chang S-F (2005) Effects of red light on the growth of Zantedeschia plantlets in vitro and tuber formation using light-emitting diodes. HortScience 40:436–438
Javanmardi J, Khalighi A, Kashi A, Bais H, Vivanco J (2002) Chemical characterization of basil (Ocimum basilicum L.) found in local accessions and used in traditional medicines in Iran. J Agric Food Chem 50:5878–5883
Karwasara VS, Dixit VK (2013) Culture medium optimization for camptothecin production in cell suspension cultures of Nothapodytes nimmoniana (J. Grah.) Mabberley. Plant Biotechnol Rep 7:357–369
Khan M, Khan R, Ahmed M, Muhammad N, Khan M, Khan H, Atlas N, Khan F (2013) Biological screening of methanolic crude extracts of Caralluma tuberculata. Int J Indig Med Plants 46:2051–4263
Khan T, Ullah MA, Garros L, Hano C, Abbasi BH (2018) Synergistic effects of melatonin and distinct spectral lights for enhanced production of anti-cancerous compounds in callus cultures of Fagonia indica. J Photochem Photobiol B 190:163–171
Kiferle C, Lucchesini M, Mensuali-Sodi A, Maggini R, Raffaelli A, Pardossi A (2011) Rosmarinic acid content in basil plants grown in vitro and in hydroponics. Cent Eur J Biol 6:946–957
Kim S-J, Hahn E-J, Heo J-W, Paek K-Y (2004) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Kim E-Y, Park S-A, Park B-J, Lee Y, Oh M-M (2014) Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic Environ Biotechnol 55:506–513
Lagrimini L (1980) Plant peroxidases: under-and over-expression in transgenic plants and physiological consequences. Plant Peroxidases 1990:59–69
Lee P-L, Chen J-T (2014) Plant regeneration via callus culture and subsequent in vitro flowering of Dendrobium huoshanense. Acta Physiol Plant 36:2619–2625
Lee J, Scagel CF (2009) Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem 115:650–656
Lee S-W, Seo JM, Lee M-K, Chun J-H, Antonisamy P, Arasu MV, Suzuki T, Al-Dhabi NA, Kim S-J (2014) Influence of different LED lamps on the production of phenolic compounds in common and Tartary buckwheat sprouts. Ind Crops Prod 54:320–326
Lee CW, Shuler ML (2000) The effect of inoculum density and conditioned medium on the production of ajmalicine and catharanthine from immobilized Catharanthus roseus cells. Biotechnol Bioeng 67:61–71
Lee MRF, Tweed JKS, Minchin FR, Winters (2009) Red clover polyphenol oxidase: activation, activity and efficacy under grazing. Anim Feed Sci Technol 149:250–264
Li H-B, Wong C-C, Cheng K-W, Chen F (2008) Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci Technol 41:385–390
Liu Y, Fang S, Yang W, Shang X, Fu X (2018) Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J Photochem Photobiol B 179:66–73
Liu Z, Qi J-L, Chen L, Zhang M-S, Wang X-Q, Pang Y-J, Yang Y-H (2006) Effect of light on gene expression and shikonin formation in cultured Onosma paniculatum cells. Plant Cell Tissue Org Cult 84:38
Liu B, Zhang Y, Zhang K, Fang H, Zhang X, Fu R, Qiu X, Xu R (2016) The efficient tissue culture system of Orostachys fimbriata. Agric Sci 7:175
Lobiuc A, Vasilache V, Oroian M, Stoleru T, Burducea M, Pintilie O, Zamfirache M-M (2017) Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. Microgreens. Molecules 22:2111
Mastaneh M, Ahamd M, Taher N, Mehrdad H (2014) Antioxidant effect of Purple basil (Lamiaceae) Phenolics. Orient J Chem 30:1965–1969
Matsubara K, Kitani S, Yoshioka T, Morimoto T, Fujita Y, Yamada Y (1989) High density culture of Coptis japonica cells increases berberine production. J ChemTechnol Biotechnol 46:61–69
Mir MY, Kamili AN, Hassan QP, Tyub S (2017) Effect of Light and Dark Conditions on Biomass Accumulation and Secondary Metabolite Production in Suspension Cultures of Artemisia amygdalina Decne. J Himalaya Ecol Sustain Dev 12:1–6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nadeem M, Abbasi BH, Younas M, Ahmad W, Zahir A, Hano C (2018) LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J Photochem Photobiol B 190:172–178
Nagella P, Murthy HN (2011) Effects of macroelements and nitrogen source on biomass accumulation and withanolide-A production from cell suspension cultures of Withania somnifera (L.) Dunal. Plant Cell Tissue Org Cult 104:119–124
Nakamura M, Takeuchi Y, Miyanaga K, Seki M, Furusaki S (1999) High anthocyanin accumulation in the dark by strawberry (Fragaria ananassa) callus. Biotechnol Lett 21:695–699
Nam TG, Kim D-O, Eom SH (2018) Effects of light sources on major flavonoids and antioxidant activity in common buckwheat sprouts. Food Sci Biotechnol 27:169–176
Nayyar H, Gupta D (2006) Differential sensitivity of C3 and C4 plants to water deficit stress: association with oxidative stress and antioxidants. Environ Exp Bot 58:106–113
Nazir M, Tungmunnithum D, Bose S, Drouet S, Garros L, Giglioli-Guivarc’h N, Abbasi BH, Hano C (2019) Differential production of phenylpropanoid metabolites in callus cultures of Ocimum basilicum L. with distinct in vitro antioxidant activities and in vivo protective effects against UV stress. J Agric Food Chem 67:1847–1859
Nhut DT, Hong L, Watanabe H, Goi M, Tanaka M (2000) Growth of banana plantlets cultured in vitro under red and blue light-emitting diode (LED) irradiation source. In: International Symposium on Tropical and Subtropical Fruits, vol 575, pp 117–124
Okamoto K, Yanagi T, Takita S, Tanaka M, Higuchi T, Ushida Y, Watanabe H (1996) Development of plant growth apparatus using blue and red LED as artificial light source. In: International Symposium on Plant Production in Closed Ecosystems, vol 440, pp 111–116
OuYang F, Mao J-F, Wang J, Zhang S, Li Y (2015) Transcriptome analysis reveals that red and blue light regulate growth and phytohormone metabolism in Norway spruce [Picea abies (L.) Karst.]. PLoS ONE 10:e0127896
Park SU, Uddin R, Xu H, Kim YK, Lee SY (2008) Biotechnological applications for rosmarinic acid production in plant. Afr J Biotechnol 7(25):4959–4965
Pereira DM, Valentão P, Pereira JA, Andrade PB (2009) Phenolics: from chemistry to biology. MDPI 2202–2211
Petersen M, Abdullah Y, Benner J, Eberle D, Gehlen K, Hücherig S, Janiak V, Kim KH, Sander M, Weitzel C (2009) Evolution of rosmarinic acid biosynthesis. Phytochemistry 70:1663–1679
Petersen M, Simmonds MS (2003) Rosmarinic acid. Phytochemistry 62:121–125
Petersen M (2003) Simmonds. Rosmarinic acid 2:121–125
Phippen WB, Simon JE (1998) Anthocyanins in basil (Ocimum basilicum L.). J Agric Food Chem 46:1734–1738
Prior RL, Wu X, Schaich K (2005) Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem 53:4290–4302
Sakamoto K, Iida K, Sawamura K, Hajiro K, Asada Y, Yoshikawa T, Furuya T (1993) Effects of nutrients on anthocyanin production in cultured cells of Aralia cordata. Phytochemistry 33:357–360
Senger H (1987) Blue light responses: phenomena and occurrence in plants and microorganisms
Shan B, Cai YZ, Sun M, Corke H (2005) Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem 53:7749–7759
Shiga T, Shoji K, Shimada H, Hashida S-n, Goto F, Yoshihara T (2009) Effect of light quality on rosmarinic acid content and antioxidant activity of sweet basil, Ocimum basilicum L. Plant Biotechnol 26:255–259
Shohael A, Ali M, Yu K, Hahn E, Islam R, Paek K (2006) Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem 41:1179–1185
Shoji K, Goto E, Hashida S, Goto F, Yoshihara T (2009) Effect of light quality on the polyphenol content and antioxidant activity of sweet basil (Ocimum basilicum L.). In: VI International Symposium on Light in Horticulture, vol 907, pp 95–99
Siddhuraju P, Mohan P, Becker K (2002) Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem 79:61–67
Son K-H, Park J-H, Kim D, Oh M-M (2012) Leaf shape index, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Korean J Hortic Sci 30:664–672
Stefano M, Rosario M (2003) Effects of light quality on micropropagation of woody species Micropropagation of woody trees and fruits. Springer, Berlin, pp 3–35
Sulaiman C, Balachandran I (2012) Total phenolics and total flavonoids in selected Indian medicinal plants. Indian J Pharm Sci 74:258
Takemiya A, Inoue S-i, Doi M, Kinoshita T, Shimazaki K-i (2005) Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17:1120–1127
Tariq U, Ali M, Abbasi BH (2014) Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J Photochem Photobio B 130:264–271
Trejo-Espino JL, Rodriguez-Monroy M, Vernon-Carter E, Cruz-Sosa F (2011) Establishment and characterization of Prosopis laevigata (Humb. & Bonpl. ex Willd) MC Johnst. cell suspension culture: a biotechnology approach for mesquite gum production. Acta Physiol Plant 33:1687–1695
Vani SR, Cheng S, Chuah C (2009) Comparative study of volatile compounds from genus Ocimum. Am J Appl Sci 6:523
Velioglu Y, Mazza G, Gao L, Oomah B (1998) Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J Agric Food Chem 46:4113–4117
Wade HK, Bibikova TN, Valentine WJ, Jenkins GI (2001) Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J 25:675–685
Wang H, Jiang YP, Yu HJ, Xia XJ, Shi K, Zhou YH, Yu JQ (2010) Light quality affects incidence of powdery mildew, expression of defence-related genes and associated metabolism in cucumber plants. Eur J Plant Pathol 127:125–135
Wang H, Provan GJ, Helliwell K (2004) Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem 87:307–311
Wu M-C, Hou C-Y, Jiang C-M, Wang Y-T, Wang C-Y, Chen H-H, Chang H-M (2007) A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem 101:1753–1758
Yam F, Hassan Z (2005) Innovative advances in LED technology. Microelectron J 36:129–137
Yamakawa T, Kato S, Ishida K, Kodama T, >Minoda Y (1983) Production of anthocyanins by Vitis cells in suspension culture. Agric Biol Chem 47:2185–2191
Yanagi T, Okamoto K (1994) Utilization of super-bright light emitting diodes as an artificial light source for plant growth. In: III International Symposium on Artificial Lighting in Horticulture, vol 418, pp 223–228
Yeh N, Chung J-P (2009) High-brightness LEDs—Energy efficient lighting sources and their potential in indoor plant cultivation. Renew Sust Energy Rev 13:2175–2180
Yonghua Q, Shanglong Z, Asghar S, Lingxiao Z, Qiaoping Q, Kunsong C, Changjie X (2005) Regeneration mechanism of Toyonoka strawberry under different color plastic films. Plant Sci 168:1425–1431
Younas M, Drouet S, Nadeem M, Giglioli-Guivarc’h N, Hano C, Abbasi BH (2018) Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J Photochem Photobiol B 184:61–70
Zahir A, Abbasi BH, Adil M, Anjum S, Zia M (2014) Synergistic effects of drought stress and photoperiods on phenology and secondary metabolism of Silybum marianum. Appl Biochem Biotechnol 174:693–707
Zheng W, Wang SY (2001) Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49:5165–5170
Acknowledgements
Dr. Bilal Haider Abbasi acknowledges research fellowship of Le Studium Institute for Advanced Studies, Loire Valley, Orléans, France.
Author information
Authors and Affiliations
Contributions
MN performed the experiments, analysed and compiled data and prepared the manuscript. MA and MY assisted in sub-culturing and write-up. AS and MS helped in phytochemical analysis. CH and NG performed HPLC and antioxidant assays. BHA apprehended the idea, provided platform to complete this research, supervised the research, and reviewed the paper critically.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sergio J. Ochatt
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nazir, M., Ullah, M.A., Younas, M. et al. Light-mediated biosynthesis of phenylpropanoid metabolites and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Plant Cell Tiss Organ Cult 142, 107–120 (2020). https://doi.org/10.1007/s11240-020-01844-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-020-01844-z