Abstract
Supercritical fluid (SCF) extraction has emerged as an effective and efficient method for separating important phytoconstituents. The extraction process is simple and environmentally friendly, generating minimal to no waste. This procedure offers various advantages over traditional extraction techniques. This chapter discusses the procedure, advantages, and different types of phytoconstituents isolated using supercritical fluids, with a preference for natural products.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Supercritical fluid (SCF) extraction is an analytical method to separate the analyte from the sample matrix using supercritical fluids as solvents (Hedrick et al. 1992). This technique is rapid, inexpensive, sustainable, and simple to execute, compared to the traditional Soxhlet extraction, where solvent costs are usually high, requiring several hours accompanied by an additional concentration step that aids pollution (Sapkale et al. 2010).
SCF extraction was initiated along with supercritical fluid chromatography in the late twentieth century for isolating forensically relevant compounds (Khaw et al. 2017). It later gained popularity when supercritical toluene was used mainly in the petroleum industry with many commercial interests.
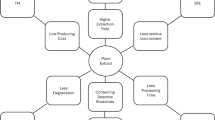
Over the last few years, SCF extraction has gained recognition for its many established advantages, particularly supercritical carbon dioxide, because of its easy-to-use properties (2017). CO2 has a near ambient critical temperature of 31 °C, allowing many biological materials and natural products to be processed around 35 °C without denaturation. It is being extensively used in decaffeination and power generation processes and is widely used to extract natural products, leaving no toxic residues behind (Khaw et al. 2017).
The advantage of supercritical CO2 (ScCO2) is that its extraction properties can be precisely varied with just minute changes in temperature and pressure. The properties can also be modified using solvents like ethanol (Camel 2001). Other than CO2, various solvents are used to extract bioactive components from plants, namely, propane, DME, SF2, and ethanol (Bizaj et al. 2021).
2 Methodology/Mechanism
A supercritical fluid is a substance whose thermodynamic properties are higher than the critical temperature and pressure of the source compound. The maximum temperature, beyond which the gaseous state of a substance cannot be liquified, irrespective of the amount of pressure applied, is called the critical temperature of the substance. Critical pressure is the minimum pressure required to condense a gaseous substance to a liquid at its critical temperature (Alekseev et al. 2020). For carbon dioxide, the critical temperature is 304.2 K and 73.0 atm.
In the supercritical region, a homogenous fluid materializes, which has unique physiochemical properties. In this region, the surface tension of the supercritical fluid is equal to zero, the dissolving and swelling capacity increases, and the viscosity decreases (Alekseev et al. 2020). The physiochemical properties can be modulated by changing the parameters of the supercritical state. The density of supercritical fluids changes with variations in pressure and temperature; a slight increase in pressure can cause a drastic increase in the density of the supercritical fluid, which in turn causes an increase in the solubility of the supercritical solvent. Once extraction is complete, solvent recovery is relatively simple due to the volatility of the supercritical fluid leaving behind the extracted analyte (Pourmortazavi et al. 2014). Such manipulations of the physiochemical properties make SCFs an excellent solvent for extraction due to their high selectivity, solubility, and extraction efficiency (Yousefi et al. 2019).
The setup (Fig. 1) for supercritical fluid extraction involves a pump, a pressurized compartment, and a collecting vessel. The solvent is commonly stored in a tank connected to a pressurized pump. The commonly used solvent is CO2, pumped into the system as a liquid below 5 °C and at around 50 bars of pressure. The fluid is cooled to remain a liquid but heated to critical condition after pressurization. The pressure must be maintained in the extraction cell, and heating should be provided to counteract the cooling caused by the adiabatic expansion of the CO2. Raw material from which the natural product is extracted is placed in the extraction cell, where pressure and temperature are controlled. The raw material is also pre-treated to modulate the moisture content and particle size for optimal extraction. The supercritical fluid is allowed to enter the pressurized extraction cell, where the natural products to be extracted dissolve in the supercritical fluid based on its solubility, which in turn is dictated by its density and pressure. Once the extraction is completed, the fluid with the dissolved natural product is passed through a chamber with lesser pressure, reducing the fluid’s dissolving power, and the natural product gets precipitated out. The depressurization of the supercritical CO2 causes the fluid to become a gas and can be collected separately for further use (Sapkale et al. 2010).
Table 1 lists the chemicals/phytochemicals extracted from the species. Some of the chemicals listed in this table are generic in name, as the literature does not specify the individual compound. The table is arranged in alphabetical order of the species name.
References
2017 Supercritical Fluid Extraction. Natural Food Flavors and Colorants
Abd Aziz NA, Hasham R, Sarmidi MR, Suhaimi SH, Idris MKH (2021) A review on extraction techniques and therapeutic value of polar bioactives from Asian medicinal herbs: case study on Orthosiphon aristatus, Eurycoma longifolia and Andrographis paniculata. Saudi Pharm J 29:143–165
Abd Hamid IA, Ismail N, Abd Rahman N (2018) Supercritical carbon dioxide extraction of selected herbal leaves: an overview. In: 3rd International Conference on Global Sustainability and Chemical Engineering, ICGSCE 2017
Abdul Aziz AH, Putra NR, Kong H, che yunus, M. A. (2020) Supercritical carbon dioxide extraction of sinensetin, isosinensetin, and rosmarinic acid from Orthosiphon stamineus leaves: optimization and modeling. Arab J Sci Eng 45:7467–7476
Abdul Khalil HPS, Lai TK, Tye YY, Rizal S, Chong EWN, Yap SW, Hamzah AA, Nurul Fazita MR, Paridah MT (2018) A review of extractions of seaweed hydrocolloids: properties and applications. Express Polym Lett 12:296–317
Abdullah A, Gani SSA, Mokhtar NFM, Hin TYY, Haiyee ZA, Mustafa S (2018) Supercritical carbon dioxide extraction of red pitaya (Hylocereus polyrhizus) seeds: response surface optimization, fatty acid composition and physicochemical properties. Malays Appl Biol 47:39–46
Adekenov SM, Baysarov GM, Khabarov IA, Polyakov VV (2020) Flavonoids of populus balsamifera L. Buds and methods for their isolation. Khimiya Rastitel’nogo Syr’ya:181–188
Ahmed Baloch H, Nizamuddin S, Siddiqui MTH, Mubarak NM, Dumbre DK, Srinivasan MP, Griffin GJ (2018) Sub-supercritical liquefaction of sugarcane bagasse for production of bio-oil and char: effect of two solvents. J Environ Chem Eng 6:6589–6601
AIP Conference Proceedings (2018) 4th International Conference on Engineering, Technology, and Industrial Application: Human-Dedicated Sustainable Product and Process Design: Materials, Resources, and Energy, ICETIA 2017
Albarelli JQ, Santos DT, Meireles MAA (2018) Thermo-economic evaluation of a new approach to extract sugarcane wax integrated to a first and second generation biorefinery. Biomass Bioenergy 119:69–74
Alberti Á, Riethmüller E, Béni S (2018) Characterization of diarylheptanoids: an emerging class of bioactive natural products. J Pharm Biomed Anal 147:13–34
Alekseev ES, Alentiev AY, Belova AS, Bogdan VI, Bogdan TV, Bystrova AV, Gafarova ER, Golubeva EN, Grebenik EA, Gromov OI, Davankov VA (2020) Supercritical fluids in chemistry. Russ Chem Rev 89:1337–1427
Ali A, Chua BL, Chow YH (2019) An insight into the extraction and fractionation technologies of the essential oils and bioactive compounds in Rosmarinus officinalis L.: past, present and future. TrAC 118:338–351
Allawzi M, Allaboun H, Almasri A (2019) CO2 supercritical extraction of essential oil of Jordanian rosemary. J AOAC Int 102:662–665
Almeida LD Jr, Quaglio AEV, de Almeida Costa CAR, di Stasi LC (2017) Intestinal anti-inflammatory activity of ground cherry (Physalis angulata L.) standardized CO2 phytopharmaceutical preparation. World J Gastroenterol 23:4369–4380
Al-Suod H, Ratiu IA, Krakowska-Sieprawska A, Lahuta L, Górecki R, Buszewski B (2019) Supercritical fluid extraction in isolation of cyclitols and sugars from chamomile flowers. J Sep Sci 42:3243–3252
Andrade KS, Poncelet D, Ferreira SRS (2017) Sustainable extraction and encapsulation of pink pepper oil. J Food Eng 204:38–45
Antonova DV, Medarska YN, Stoyanova AS, Nenov NS, Slavov AM, Antonov LM (2021) Chemical profile and sensory evaluation of Bulgarian rose (Rosa damascena Mill.) aroma products, isolated by different techniques. J Essent Oil Res 33:171–181
Antunes-Ricardo M, García-Cayuela T, Mendiola JA, Ibañez E, Gutiérrez-Uribe JA, Cano MP, Guajardo-Flores D (2018) Supercritical CO2 enzyme hydrolysis as a pretreatment for the release of isorhamnetin conjugates from Opuntia ficus-indica (L.) Mill. J Supercrit Fluids 141:21–28
Anusha Siddiqui S, Redha AA, Esmaeili Y, Mehdizadeh M (2022) Novel insights on extraction and encapsulation techniques of elderberry bioactive compounds. Crit Rev Food Sci Nutr:1
Aponte-Buitrago R, Mayorga-Wandurraga H, Moreno-Murillo B (2017) Flavonols and sesquiterpenoids from outer bark and leaves of Croton Polycarpus Benth. (Euphorbiaceae). Boletin Latinoamericano y del Caribe de Plantas Medicinales y Aromaticas 16:471–485
Arias J, Mejía J, Córdoba Y, Martínez JR, Stashenko E, del Valle JM (2020) Optimization of flavonoids extraction from Lippia graveolens and Lippia origanoides chemotypes with ethanol-modified supercritical CO2 after steam distillation. Ind Crop Prod 146:112170
Arumugam S, Thiruganasambantham R (2018) Standardized supercritical Co2 extract of acanthus ilicifolius (Linn.) leaves inhibits the pro-inflammatory cytokine tumor necrosis factor-Α in lipopolysaccharide-activated murine raw 264.7 macrophage cells. Asian J Pharm Clin Res 11:193–198
Attia IB, Zucca P, Marincola FC, Piras A, Rosa A, Chaieb M, Rescigno A (2018) Chemical composition and antioxidant potential differences between Cynomorium coccineum L. growing in Italy and in Tunisia: effect of environmental stress. Diversity 10
Averyanova EV, Shkolnikova MN, Tsyganok SN, Shakura VA (2018) Intensification of the process of ultrasonic extraction of dehydroquercetin from wood waste. In: 19th International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices, EDM 2018. 312–317
Axiotis E, Petrakis EA, Halabalaki M, Mitakou S (2020) Phytochemical profile and biological activity of endemic Sideritis sipylea Boiss. In North Aegean Greek islands. Molecules 25
Aydi A, Zibetti AW, Al-Khazaal AZ, Eladeb A, Adberraba M, Barth D (2020) Supercritical CO2 extraction of extracted oil from Pistacia lentiscus L.: mathematical modeling, economic evaluation and scale-up. Molecules 25
Ayub MA, Hanif MA, Sarfraz RA, Shahid M (2018) Biological activity of boswellia serrata roxb. Oleo gum resin essential oil: effects of extraction by supercritical carbon dioxide and traditional methods. Int J Food Prop 21:808–820
Azahar NI, Mokhtar NM, Arifin MA (2020) Piper betle: a review on its bioactive compounds, pharmacological properties, and extraction process. In: 5th International Conference of Chemical Engineering and Industrial Biotechnology, ICCEIB 2020
Aziz AHA, Yunus MAC, Yian LN, Idham Z, Rithwan F, Hadzri HM, Mustapha AN (2018) Enhancement and optimization of sinensetin extract from orthosiphon stamineus using supercrtitical carbon dioxide extraction. Malaysian J Anal Sci 22:867–876
Bai XN, Cheng J, Liang W, Ma LQ, Liu YB, Shi GL, Wang YN (2012) Antifungal activity of extracts by supercritical carbon dioxide extraction from roots of Stellera chamaejasme L. and analysis of their constituents using GC-MS. In: 2011 International Conference on Information Technology and Agricultural Engineering, ICITAE 2011. Sanya
Bajer T, Surmová S, Eisner A, Ventura K, Bajerová P (2018) Use of simultaneous distillation-extraction, supercritical fluid extraction and solid-phase microextraction for characterisation of the volatile profile of Dipteryx odorata (Aubl.) Willd. Ind Crop Prod 119:313–321
Bakshi RA, Sodhi NS, Wani IA, Khan ZS, Dhillon B, Gani A (2022) Bioactive constituents of saffron plant: extraction, encapsulation and their food and pharmaceutical applications. Appl Food Res 2:100076
Baldino L, Reverchon E (2018) Artemisia annua organic solvent extract, processed by supercritical CO2. J Chem Technol Biotechnol 93:3171–3175
Baldino L, Della Porta G, Osseo LS, Reverchon E, Adami R (2018a) Concentrated oleuropein powder from olive leaves using alcoholic extraction and supercritical CO2 assisted extraction. J Supercrit Fluids 133:65–69
Baldino L, Scognamiglio M, Reverchon E (2018b) Extraction of rotenoids from Derris elliptica using supercritical CO2. J Chem Technol Biotechnol 93:3656–3660
Balkrishna A, Nain P, Joshi M, Khandrika L, Varshney A (2021) Supercritical fluid extract of putranjiva roxburghii wall. Seeds mitigates fertility impairment in a zebrash model. Molecules 26
Balkrishna A, Nain P, Joshi M, Kumar B, Varshney A (2022) Super-critical fluid extract of Bryonopsis laciniosa (Shivlingi) seeds restores fertility in zebrafish models through revival of cytological and anatomical features. J Ovarian Res 15
Banožić M, Gagić T, Čolnik M, Knez Ž, Škerget M, Jerković I, Jokić S (2021) Sequence of supercritical CO2 extraction and subcritical H2O extraction for the separation of tobacco waste into lipophilic and hydrophilic fractions. Chem Eng Res Des 169:103–115
Baranauskienė R, Venskutonis PR (2022) upercritical CO2 Extraction of Narcissus poeticus L. Flowers for the Isolation of Volatile Fragrance Compounds. Molecules 27
Barbosa JR, S. Freitas MM, Oliveira LC, S. Martins LH, Almada-Vilhena AO, Oliveira RM, Pieczarka JC, B. Brasil DDS, Carvalho Junior RN (2020) Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem 330:127173
Barrales FM, Silveira P, Barbosa PDPM, Ruviaro AR, Paulino BN, Pastore GM, Macedo GA, Martinez J (2018) Recovery of phenolic compounds from citrus by-products using pressurized liquids — an application to orange peel. Food Bioprod Process 112:9–21
Bartolomé Ortega A, Cano Calvo A, Szekely E, Škerget M, Knez Ž (2017) Supercritical fluid extraction from Saw Palmetto berries at a pressure range between 300 bar and 450 bar. J Supercrit Fluids 120:132–139
Barzotto ILM, Santos KA, da Silva EA, Sene AC, da Silva NS, Vieira L (2019) Supercritical extraction of Eugenia involucrata leaves: influence of operating conditions on yield and Α-tocopherol content. J Supercrit Fluids 143:55–63
Bayrak S, Sökmen M, Aytaç E, Sökmen A (2019) Conventional and supercritical fluid extraction (SFE) of colchicine from Colchicum speciosum. Ind Crop Prod 128:80–84
Bendif H, Adouni K, Miara MD, Baranauskienė R, Kraujalis P, Venskutonis PR, Nabavi SM, Maggi F (2018a) Essential oils (EOs), pressurized liquid extracts (PLE) and carbon dioxide supercritical fluid extracts (SFE-CO2) from Algerian thymus munbyanus as valuable sources of antioxidants to be used on an industrial level. Food Chem 260:289–298
Bendif H, Lazali M, Souilah N, Miara MD, Kazernavičiūtė R, Baranauskienė R, Venskutonis PR, Maggi F (2018b) Supercritical CO2 extracts and essential oils from Teucrium polium L. growing in Algeria: chemical composition and antioxidant activity. J Essent Oil Res 30:488–497
Bendif H, Miara MD, Kalboussi Z, Grauzdytė D, Povilaitis D, Venskutonis PR, Maggi F (2018c) Supercritical CO2 extraction of Rosmarinus eriocalyx growing in Algeria: chemical composition and antioxidant activity of extracts and their solid plant materials. Ind Crop Prod 111:768–774
Benito-Román O, Rodríguez-Perrino M, Sanz MT, Melgosa R, Beltrán S (2018) Supercritical carbon dioxide extraction of quinoa oil: study of the influence of process parameters on the extraction yield and oil quality. J Supercrit Fluids 139:62–71
Bettaieb Rebey I, Bourgou S, Detry P, Wannes WA, Kenny T, Ksouri R, Sellami IH, Fauconnier ML (eds) (2019) Green extraction of fennel and Anise edible oils using bio-based solvent and supercritical fluid: assessment of chemical composition, antioxidant property, and oxidative stability. Food Bioprocess Technol 12:1798–1807
Bezerra FWF, Costa WAD, Oliveira MSD, Aguiar Andrade EHD, Carvalho RNDJ (2018) Transesterification of palm pressed-fibers (Elaeis guineensis Jacq.) oil by supercritical fluid carbon dioxide with entrainer ethanol. J Supercrit Fluids 136:136–143
Bhatt V, Mahesh Kumar M, Periyar Selvam S (2018) Antimicrobial effect of ajwain seed ethanolic extract against food borne pathogenic bacteria. Int Food Res J 25:908–912
Bizaj K, Škerget M, Košir IJ, Knez Ž (2021) Sub- and supercritical extraction of slovenian hops (Humulus lupulus l.) aurora variety using different solvents. Plan Theory 10
Bobinaitė R, Kraujalis P, Tamkutė L, Urbonavičienė D, Viškelis P, Venskutonis PR (2020) Recovery of bioactive substances from rowanberry pomace by consecutive extraction with supercritical carbon dioxide and pressurized solvents. J Ind Eng Chem 85:152–160
Bogolitsyn K, Krasikova A, Gusakova M, Ivakhnov A, Gravitis J (2019) Selective extraction of terpenoid compounds of Juniperus communis L. wood in the medium of a binary solvent (supercritical CO2 with modifier). Phytochem Anal 30:609–616
Bouazzaoui N, Bouajila J, Camy S, Mulengi JK, Condoret JS (2018) Fatty acid composition, cytotoxicity and anti-inflammatory evaluation of melon (Cucumis melo L. Inodorus) seed oil extracted by supercritical carbon dioxide. Sep Sci Technol (Philadelphia) 53:2622–2627
Buranachokpaisan K, Muangrat R, Chalermchat Y (2021) Supercritical CO2 extraction of residual oil from pressed sesame seed cake: optimization and its physicochemical properties. J Food Process Preserv 45
Burčová Z, Kreps F, Greifová M, Jablonský M, Ház A, Schmidt Š, Šurina I (2018) Antibacterial and antifungal activity of phytosterols and methyl dehydroabietate of Norway spruce bark extracts. J Biotechnol 282:18–24
Bursać Kovačević D, Maras M, Barba FJ, Granato D, Roohinejad S, Mallikarjunan K, Montesano D, Lorenzo JM, Putnik P (2018) Innovative technologies for the recovery of phytochemicals from Stevia rebaudiana Bertoni leaves: A review. Food Chem 268:513–521
Busatta C, Barbosa J, Cardoso RI, Paroul N, Rodrigues M, Oliveira D, Oliveira JV, Cansian RL (2017) Chemical profiles of essential oils of marjoram (Origanum majorana) and oregano (Origanum vulgare) obtained by hydrodistillation and supercritical CO2. J Essent Oil Res 29:367–374
Buszewski B, Rafińska K, Cvetanović A, Walczak J, Krakowska A, Rudnicka J, Zeković Z (2019) Phytochemical analysis and biological activity of Lupinus luteus seeds extracts obtained by supercritical fluid extraction. Phytochem Lett 30:338–348
Cadena-Carrera S, Tramontin DP, Bella Cruz A, Bella Cruz RC, Müller JM, Hense H (2019) Biological activity of extracts from guayusa leaves (Ilex guayusa Loes.) obtained by supercritical CO2 and ethanol as cosolvent. J Supercrit Fluids 152:104543
Camel V (2001) Recent extraction techniques for solid matrices—supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: their potential and pitfalls. Analyst 126:1182–1193
Cante RC, Gallo M, Varriale L, Garella I, Nigro R (2022) Recovery of carotenoids from tomato pomace using a hydrofluorocarbon solvent in sub-critical conditions. Appl Sci (Switzerland) 12
Carmona PAO, Garcia LC, Ribeiro JAA, Valadares LF, Marçal AF, de França LF, Mendonça S (2018) Effect of solids content and spray-drying operating conditions on the carotenoids microencapsulation from pressed palm fiber oil extracted with supercritical CO2. Food Bioprocess Technol 11:1703–1718
Carrara VS, Filho LC, Garcia VAS, Faiões VS, Cunha-Júnior EF, Torres-Santos EC, Cortez DAG (2017) Supercritical fluid extraction of pyrrolidine alkaloid from leaves of Piper amalago L. Evid-based Complement Alternat Med 2017
Casas MP, López-Hortas L, Díaz-Reinoso B, Moure A, Domínguez H (2021) Supercritical CO2 extracts from Acacia dealbata flowers. J Supercrit Fluids 173:105223
Cejudo Bastante C, Casas Cardoso L, Fernández Ponce MT, Mantell Serrano C, Martínez de la Ossa-Fernández EJ (2018) Characterization of olive leaf extract polyphenols loaded by supercritical solvent impregnation into PET/PP food packaging films. J Supercrit Fluids 140:196–206
Chan YH, Yusup S, Quitain AT, Chai YH, Uemura Y, Loh SK (2018) Extraction of palm kernel shell derived pyrolysis oil by supercritical carbon dioxide: evaluation and modeling of phenol solubility. Biomass Bioenergy 116:106–112
Chañi-Paucar LO, Johner JCF, Zabot GL, Meireles MAA (2022) Technical and economic evaluation of supercritical CO2 extraction of oil from Sucupira branca seeds. J Supercrit Fluids 181:105494
Chaturvedi M, Rani R, Sharma D, Yadav JP (2020) Effect of temperature and pressure on antimycobacterial activity of Curcuma caesia extract by supercritical fluid extraction method. Int J Mycobacteriol 9:296–302
Chen F, Sun H, Ni H, Hong P, Li L, Yang Y, Jiang Z (2018a) Pummelo essential oil: extraction, volatiles, storage, and application. J Food Sci Technol (China) 36:1–10
Chen HJ, Chen JW, Li X (2018b) Chemical constituents from the rhizome of Saururus chinensis. Chin Pharm J 53:340–345
Cheng X, Qi Z, Burdyny T, Kong T, Sinton D (2018) Low pressure supercritical CO2 extraction of astaxanthin from Haematococcus pluvialis demonstrated on a microfluidic chip. Bioresour Technol 250:481–485
Chhouk K, Kanda H, Goto M (2018) Efficacy of supercritical carbon dioxide integrated hydrothermal extraction of Khmer medicinal plants with potential pharmaceutical activity. J Environ Chem Eng 6:2944–2956
Chi XF, Yue HL, Zhao XH, Hu FZ (2016) Obtaining alantolactone and isoalantolactone from Inula racemose Hook.f. by optimized supercritical fluid extraction. Ind Crop Prod 79:63–69
Choi SA, Lee HS (2018) Insecticidal activities of Russia coriander oils and these constituents against Sitophilus oryzae and Sitophilus zeamais. J Appl Biol Chem 61:239–243
Chou HY, Lee C, Pan JL, Wen ZH, Huang SH, Lan CWJ, Liu WT, Hour TC, Hseu YC, Hwang BH, Cheng KC, Wang HMD (2016) Enriched astaxanthin extract from haematococcus pluvialis augments growth factor secretions to increase cell proliferation and induces MMP1 degradation to enhance collagen production in human dermal fibroblasts. Int J Mol Sci 17
Colorado D, Fernandez M, Orozco J, Lopera Y, Muñoz DL, Acín S, Balcazar N (2020) Metabolic activity of anthocyanin extracts loaded into non-ionic niosomes in diet-induced obese mice. Pharm Res 37
Confortin TC, Todero I, Luft L, Teixeira AL, Mazutti MA, Zabot GL, Tres MV (2019) Valorization of solanum viarum dunal by extracting bioactive compounds from roots and fruits using ultrasound and supercritical CO2. Braz J Chem Eng 36:1689–1702
Cordeiro RM, de S. e Silva AP, Pinto RH, da Costa WA, da Silva SH, de Souza Pinheiro WB, Arruda MS, Carvalho Junior RN (2019) Supercritical CO 2 extraction of ucuúba (Virola surinamensis) seed oil: global yield, kinetic data, fatty acid profile, and antimicrobial activities. Chem Eng Commun 206:86–97
Correa M, Mesomo MC, Pianoski KE, Torres YR, Corazza ML (2016) Extraction of inflorescences of Musa paradisiaca L. using supercritical CO2 and compressed propane. J Supercrit Fluids 113:128–135
Cunha VM, da Silva MP, de Sousa SH, do Nascimento Bezerra P, Menezes EG, da Silva NJ, da Silva Banna DA, Araújo ME, de Carvalho Junior RN (2019) Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J Supercrit Fluids 144:81–90
Cvetković T, Ranilović J, Gajari D, Tomić-Obrdalj H, Šubarić D, Moslavac T, Cikoš AM, Jokić S (2020) Podravka and slavonka varieties of pepper seeds (Capsicum annuum L.) as a new source of highly nutritional edible oil. Foods 9:1262
da Silva IC, Oliveira PF, Barbosa GM, Wessjohann LA, Cardozo-Filho L, Holandino C, Muzitano MF, Leal IC (2020) Passiflora mucronata leaves extracts obtained from different methodologies: A phytochemical study based on cytotoxic and apoptosis activities of triterpenes and phytosterols constituents. Brazilian J Pharm Sci 56
da Silva M, Trancoso J, Tormen L, Bombardelli MM, Corazza ML, Bainy EM (2022) Extraction of compounds from Moringa oleifera leaves using supercritical CO2 plus ethanol as a cosolvent. J Food Process Eng 45
Dąbrowski G, Konopka I, Czaplicki S (2018) Supercritical CO2 extraction in chia oils production: impact of process duration and CO-solvent addition. Food Sci Biotechnol 27:677–686
Damjanović-Vratnica B, Perović S, LU T, Santos R (2016) Effect of matrix pretreatment on the supercritical CO2 extraction of satureja montana essential oil. Chem Ind Chem Eng Q 22:201–209
Damrongwattanakool N, Raviyan P (2018) Enrichment of vitamin e in palm fatty acid distillate using sequential-cooling urea–Fatty acid complexation. Songklanakarin J Sci Technol 40:1175–1180
Daraee A, Ghoreishi SM, Hedayati A (2019) Supercritical CO2 extraction of chlorogenic acid from sunflower (Helianthus annuus) seed kernels: modeling and optimization by response surface methodology. J Supercrit Fluids 144:19–27
de Aguiar AC, Osorio-Tobón JF, Silva LP, Barbero GF, Martinez J (2018) Economic analysis of oleoresin production from malagueta peppers (Capsicum frutescens) by supercritical fluid extraction. J Supercrit Fluids 133:86–93
de Aguiar AC, da Fonseca Machado AP, Angolini CF, de Morais DR, Baseggio AM, Eberlin MN, Junior MR, Martinez J (2019) Sequential high-pressure extraction to obtain capsinoids and phenolic compounds from biquinho pepper (Capsicum chinense). J Supercrit Fluids 150:112–121
de Cerqueira MD, de Souza-Neta LC, Guedes ML, Rivelino R, Cruz FG (2013) Myrciaine, a new nicotinic ester from Myrcia blanchetiana (Myrtaceae). Tetrahedron Lett 54:1421–1423
de Melo MM, Carius B, Simões MM, Portugal I, Saraiva J, Silva CM (2020) Supercritical CO2 extraction of V. vinifera leaves: influence of cosolvents and particle size on removal kinetics and selectivity to target compounds. J Supercrit Fluids 165:104959
de Oliveira NA, Mazzali MR, Fukumasu H, Goncalves CB, de Oliveira AL (2019) Composition and physical properties of babassu seed (Orbignya phalerata) oil obtained by supercritical CO2 extraction. J Supercrit Fluids 150:21–29
de Souza Junior ET, Siqueira LM, Almeida RN, Lucas AM, Silva CG, Cassel E, Vargas RM (2020) Comparison of different extraction techniques of zingiber officinale essential oil. Braz Arch Biol Technol 63
Dehghanizadeh M, Brewer CE (2020) Guayule resin: Chemistry, extraction, and applications. 2020 ASABE Annual International Meeting
Demirkoz AB, Karakaş M, Bayramoğlu P, Üner M (2018) Analysis of volatile flavour components by dynamic headspace analysis/gas chromatography-mass spectrometry in roasted pistachio extracts using supercritical carbon dioxide extraction and sensory analysis. Int J Food Prop 21:972–981
Deng SB, Chen JP, Chen YZ, Yu CQ, Yang Y, Wu SH, Chen CZ (2018) Chemical composition analysis of extracts from Ficus Hirta using supercritical fluid. In: 2018 International Conference on Computer Information and Automation Engineering, ICCIAE 2018
Derrien M, Aghabararnejad M, Gosselin A, Desjardins Y, Angers P, Boumghar Y (2018) Optimization of supercritical carbon dioxide extraction of lutein and chlorophyll from spinach by-products using response surface methodology. LWT 93:79–87
Devi V, Khanam S (2019a) Development of generalized and simplified models for supercritical fluid extraction: case study of papaya (Carica papaya) seed oil. Chem Eng Res Des 150:341–358
Devi V, Khanam S (2019b) Study of ω-6 linoleic and ω-3 α-linolenic acids of hemp (Cannabis sativa) seed oil extracted by supercritical CO 2 extraction: CCD optimization. J Environ Chem Eng 7:1–10
Di Sanzo GD, Mehariya S, Martino M, Larocca V, Casella P, Chianese S, Musmarra D, Balducchi R, Molino A (2018) Supercritical carbon dioxide extraction of astaxanthin, lutein, and fatty acids from haematococcus pluvialis microalgae. Mar Drugs 16
Dienaite, L., Pukalskiene, M., Pereira, C. V., Matias, A. A. & Venskutonis, P. R. 2020. Valorization of european cranberry bush (viburnum opulus l.) berry pomace extracts isolated with pressurized ethanol and water by assessing their phytochemical composition, antioxidant, and antiproliferative activities. Foods, 9
Dienaitė L, Baranauskienė R, Rimantas Venskutonis P (2021) Lipophilic extracts isolated from European cranberry bush (Viburnum opulus) and sea buckthorn (Hippophae rhamnoides) berry pomace by supercritical CO2 – promising bioactive ingredients for foods and nutraceuticals. Food Chem 348:129047
Djapic N (2018) Corallina officinalis chemical compounds obtained by supercritical fluid extraction. AACL Bioflux 11:422–428
Dong A, Li W, Wang H, Zou Y (2020) Extraction of Dihydrofisetin from Rhus punjabensis var. sinica Rehd. Fruit by supercritical CO2 and its antioxidant activity in vitro. Chem Ind Forest Prod 40:108–114
Dorado Achicanoy D, Hurtado Benavides A, Martínez-Correa HA (2018) Study of supercritical CO2 extraction of tamarillo (Cyphomandra Betacea) seed oil containing high added value compounds. Electrophoresis 39:1917–1925
Eissa M, Hashim YZHY, Zainurin NAA (eds) (2018) Aquilaria malaccensis leaf as an alternative source of antiinflammatory compounds. Int J Adv Sci Eng Inform Technol 8:1625–1632
El-Shamy S, Farag MA (2021) Novel trends in extraction and optimization methods of bioactives recovery from pomegranate fruit biowastes: valorization purposes for industrial applications. Food Chem 365:130465
Fang X, Du M, Luo F, Jin Y (2015) Physicochemical properties and lipid composition of camellia seed oil (camellia oleifera abel.) extracted using different methods. Food Sci Technol Res 21:779–785
Fang L, Wang X, Guo L, Liu Q (2018) Antioxidant, anti-microbial properties and chemical composition of cumin essential oils extracted by three methods. Open Chem 16:291–297
Favareto R, Teixeira MB, Soares FAL, Belisário CM, Cabral JF, da Silva EA, Moia TA, Cardozo-Filho L (eds) (2019) Extraction of bioactive compounds of leaves of duguetia furfuracea (annonaceae) using green and organic solvents. Braz J Chem Eng 36:549–556
Felföldi-Gáva A, Szarka S, Simándi B, Blazics B, Simon B, Kéry A (2012) Supercritical fluid extraction of Alnus glutinosa (L.) Gaertn. J Supercrit Fluids 61:55–61
Ferrentino G, Haman N, Morozova K, Tonon G, Scampicchio M (2021) Phenolic compounds extracted from spruce (Picea abies) by supercritical carbon dioxide as antimicrobial agents against gram-positive bacteria assessed by isothermal calorimetry. J Therm Anal Calorim 145:3093–3103
Ferro DM, Mazzutti S, Vitali L, Müller CM, Ferreira SR (2019) Integrated extraction approach to increase the recovery of antioxidant compounds from Sida rhombifolia leaves. J Supercrit Fluids 149:10–19
Fornari T, de Molina AR, Reglero G (2014) Phenolic diterpenes from rosemary as enhancement agents of usual chemotherapeutic drugs for colorectal cancer therapy. New Developments in Terpenes Research
Frohlich PC, Santos KA, Palú F, Cardozo-Filho L, da Silva C, da Silva EA (2019) Evaluation of the effects of temperature and pressure on the extraction of eugenol from clove (Syzygium aromaticum) leaves using supercritical CO2. J Supercrit Fluids 143:313–320
Gallo-Molina AC, Castro-Vargas HI, Garzón-Méndez WF, Ramírez JA, Monroy ZJ, King JW (2019) Extraction, isolation and purification of tetrahydrocannabinol from the Cannabis sativa L. plant using supercritical fluid extraction and solid phase extraction. J Supercrit Fluids 146:208–216
Gan Z, Liang Z, Chen X, Wen X, Wang Y, Li M, Ni Y (2016) Separation and preparation of 6-gingerol from molecular distillation residue of Yunnan ginger rhizomes by high-speed counter-current chromatography and the antioxidant activity of ginger oils in vitro. J Chromatogr B Anal Technol Biomed Life Sci 1011:99–107
García-Pérez JS, Cuéllar-Bermúdez SP, de la Cruz-Quiroz R, Arévalo-Gallegos A, Esquivel-Hernandez DA, Rodríguez-Rodríguez J, García-García R, Iqbal HM, Parra-Saldivar R (2019) Supercritical CO 2 -based tailor made valorization of Origanum vulgare L extracts: A green approach to extract high-value compounds with applied perspectives. J Environ Manag 232:796–802
García-Pérez JS, Cuéllar-Bermúdez SP, Arévalo-Gallegos A, Salinas-Salazar C, Rodríguez-Rodríguez J, de la Cruz-Quiroz R, Iqbal HM, Parra-Saldívar R (2020) Influence of supercritical CO2 extraction on fatty acids profile, volatile compounds and bioactivities from Rosmarinus officinalis. Waste Biomass Valorization 11:1527–1537
Gavarić A, Vidović S, Aladić K, Jokić S, Vladić J (2021) Supercritical CO2extraction ofMarrubium vulgare: intensification of marrubiin. RSC Adv 11:9067–9075
Gawron G, Krzyczkowski W, Lemke K, Ołdak A, Kadziński L, Banecki B (2019) Nigella sativa seed extract applicability in preparations against methicillin-resistant Staphylococcus aureus and effects on human dermal fibroblasts viability. J Ethnopharmacol 244:112135
Ghosh S, Chatterjee D, Das S, Bhattacharjee P (2013) Supercritical carbon dioxide extraction of eugenol-rich fraction from Ocimum sanctum Linn and a comparative evaluation with other extraction techniques: process optimization and phytochemical characterization. Ind Crop Prod 47:78–85
Ghosh S, Bhattacharjee P, Das S (2015) 1,8-cineol-rich cardamom seed (Elettaria cardamomum) extracts using green technologies and conventional extractions: process analysis, phytochemical characterization, and food application. Sep Sci Technol (Philadelphia) 50:1974–1985
Górnaś P, Siger A, Rudzińska M, Grygier A, Marszałkiewicz S, Ying Q, Sobieszczańska N, Segliņa D (2019) Impact of the extraction technique and genotype on the oil yield and composition of lipophilic compounds in the oil recovered from Japanese Quince (Chaenomeles japonica) seeds. Eur J Lipid Sci Technol 121:1800262
Gustinelli G, Eliasson L, Svelander C, Alminger M, Ahrné L (2018) Supercritical CO2 extraction of bilberry (Vaccinium myrtillus L.) seed oil: fatty acid composition and antioxidant activity. J Supercrit Fluids 135:91–97
Gwatidzo L, Botha BM, Mccrindle RI, Combrinck S (eds) (2014) Extraction and identification of phytosterols in manketti (Schinziophyton rautanenii) nut oil. JAOCS 91:783–794
Gyawali R, Jeon DH, Moon J, Kim H, Song YW, Hyun HB, Jeong D, Cho SK (2012a) Chemical composition and antiproliferative activity of supercritical extract of Citrus grandis (L.) Osbeck fruits from Korea. J Essent Oil-Bear Plants 15:915–925
Gyawali R, Moon JY, Jeon DH, Kim HJ, Song YW, Hyun HB, Kang TH, Moon KS, Jeong S, Kim JC, Ahn KS, Cho SK (2012b) Chemical composition and antiproliferative activity of supercritical CO2 extracts from citrus fruits. Food Sci Technol Res 18:813–823
Győri E, Varga A, Fábián I, Lázár I (eds) (2019) Supercritical CO2 extraction and selective adsorption of aroma materials of selected spice plants in functionalized silica aerogels. J Supercrit Fluids 148:16–23
Haddadin MSY, Haddadin JS (2015) Lycopene extraction from tomato pomace with supercritical carbon dioxide: effect of pressures, temperatures and CO2 flow rates and evaluation of antioxidant activity and stability of lycopene. Pak J Nutr 14:942–956
Hadinezhad M, Rowland O, Hosseinian F (2015) The fatty acid profile and phenolic composition of Descurainia sophia seeds extracted by supercritical CO2. JAOCS 92:1379–1390
Haiyee ZA, Mohd Shah SH, Ismail K, Hashim N, Wan Ismail WI (2016) Quality parameters of Curcuma longa L. extracts by supercritical fluid extraction (SFE) and ultrasonic assisted extraction (UAE). Malaysian J Anal Sci 20:626–632
Hamburger M (2002) Isatis tinctoria - from the rediscovery of an ancient medicinal plant towards a novel anti-inflammatory phytopharmaceutical. Phytochem Rev 1:333–344
Hamdan S, Daood HG (2011) Changes in the chlorophyll and carotenoid content and composition of ground thyme leaves as a function of supercritical carbon dioxide and subcritical propane extraction. Acta Aliment 40:8–18
Hameed A, Arun AB, Ho HP, Chang CMJ, Rekha PD, Lee MR, Singh S, Young CC (2011) Supercritical carbon dioxide micronization of zeaxanthin from moderately thermophilic bacteria muricauda lutaonensis CC-HSB-11T. J Agric Food Chem 59:4119–4124
Hamid IAA, Mustapa AN, Ismail N, Abdullah Z (2013) Solubility prediction of mangosteen peel oil in Supercritical Carbon Dioxide using Neural Network. In: 2013 IEEE Business Engineering and Industrial Applications Colloquium, BEIAC 2013, Langkawi. pp. 91–96
Hamid MA, Bakar NA, Park CS, Ramli F, Wan WR (2018) Optimisation of alpha mangostin extraction using supercritical CO2 from garcinia mangostana. Chem Eng Trans 63:577–582
Han CN, Kang CH (2015) Extraction of genistein from sophora flavescens with supercritical carbon dioxide. Korean Chem Eng Res 53:445–449
Han JH, Wu QF, Xu B, Zhou SL, Ding F (2016) Quality characteristics of soybean germ oil obtained by innovative subcritical butane experimental equipment. Qual Assur Safety Crops Foods 8:369–377
Haro-González JN, Castillo-Herrera GA, Martínez-Velázquez M, Espinosa-Andrews H (2021) Clove essential oil (Syzygium aromaticum l. myrtaceae): extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 26
Hartati, Salleh LM, Pagarra H, Rachmawaty (2018) Response Surfaces of Linoleic Acid of Swietenia Mahagoni in Supercritical Carbon Dioxide. In: 2nd International Conference on Statistics, Mathematics, Teaching, and Research 2017, ICSMTR 2017, 2018
Hedrick JL, Mulcahey LJ, Taylor LT (1992) Supercritical fluid extraction. Mikrochim Acta 108:115–132
Hernández SMP, Estévez JJ, Giraldo LJL, Méndez CJM (2019) Supercritical extraction of bioactive compounds from cocoa husk: study of the main parameters. Revista Facultad de Ingenieria:95–105
Hrabovski N, Sinadinović-Fišer S, Nikolovski B, Sovilj M, Borota O (2012) Phytosterols in pumpkin seed oil extracted by organic solvents and supercritical CO 2. Eur J Lipid Sci Technol 114:1204–1211
Hu Q, Xu J, Chen S, Yang F (2004) Antioxidant activity of extracts of black sesame seed (Sesamum indicum L.) by supercritical carbon dioxide extraction. J Agric Food Chem 52:943–947
Idham Z, Putra NR, Aziz AHA, Zaini AS, Rasidek NAM, Mili N, Yunus MAC (eds) (2021) Improvement of extraction and stability of anthocyanins, the natural red pigment from roselle calyces using supercritical carbon dioxide extraction. J CO2 Util 56:101839
Idowu S, Adekoya AE, Igiehon OO, Idowu AT (2021) Clove (Syzygium aromaticum) spices: a review on their bioactivities, current use, and potential application in dairy products. J Food Measure Charact 15:3419–3435
Inakuma T (2015) Study of carotenoid activity in vegetables : application to food development. Nippon Shokuhin Kagaku Kogaku Kaishi 62:263–273
Ixtaina VY, Nolasco SM, Tomás MC (2014) Characterization of Argentinean chia seed oil obtained by different processes: a multivariate study. In: Seed oil: biological properties, health benefits and commercial applications
Jaafar SNBS, Haimer E, Liebner F, Böhmdorfer S, Potthast A, Rosenau T (2011) Empty palm fruit bunches-A CO 2-based biorefinery concept. J Biobaased Mater Bioenergy 5:225–233
Jafarian Asl P, Niazmand R, Jahani M (2020) Theoretical and experimental assessment of supercritical CO2 in the extraction of phytosterols from rapeseed oil deodorizer distillates. J Food Eng 269:109748
Jahongir H, Miansong Z, Amankeldi I, Yu Z, Changheng L (2019) The influence of particle size on supercritical extraction of dog rose (Rosa canina) seed oil. J King Saud Univ 31:140–143
Jaski JM, Barão CE, Morais Lião L, da Silva Pinto V, Zanoelo EF, Cardozo-Filho L (2019) β-Cyclodextrin complexation of extracts of olive leaves obtained by pressurized liquid extraction. Ind Crop Prod 129:662–672
Jiang Q, Liu W, Li X, Zhang T, Wang Y, Liu X (2016) Detection of related substances in polyene phosphatidyl choline extracted from soybean and in its commercial capsule by comprehensive supercritical fluid chromatography with mass spectrometry compared with HPLC with evaporative light scattering detection. J Sep Sci 39:350–357
Jokić S, Molnar M, Jakovljević M, Aladić K, Jerković I (2018) Optimization of supercritical CO2 extraction of Salvia officinalis L. leaves targeted on oxygenated monoterpenes, Α-humulene, viridiflorol and manool. J Supercrit Fluids 133:253–262
Jokić S, Molnar M, Cikoš AM, Jakovljević M, Šafranko S, Jerković I (2020) Separation of selected bioactive compounds from orange peel using the sequence of supercritical CO2 extraction and ultrasound solvent extraction: optimization of limonene and hesperidin content. Sep Sci Technol (Philadelphia) 55:2799–2811
Karimi M, Raofie F (2019) Micronization of vincristine extracted from Catharanthus roseus by expansion of supercritical fluid solution. J Supercrit Fluids 146:172–179
Kaushik S, Dar L, Kaushik S, Yadav JP (2021) Anti-dengue activity of super critical extract and isolated oleanolic acid of Leucas cephalotes using in vitro and in silico approach. BMC Complement Med Ther 21
Kessler JC, Vieira VA, Martins IM, Manrique YA, Afonso A, Ferreira P, Mandim F, Ferreira ICFR, Barros L, Rodrigues AE, Dias MM (2022) Obtaining aromatic extracts from Portuguese thymus mastichina L. by hydrodistillation and supercritical fluid extraction with CO2 as potential Flavouring additives for food applications. Molecules 27
Kha TC, Phan-Tai H, Nguyen MH (2014) Effects of pre-treatments on the yield and carotenoid content of Gac oil using supercritical carbon dioxide extraction. J Food Eng 120:44–49
Khalil AA, Rahman UU, Khan MR, Sahar A, Mehmood T, Khan M (2017) Essential oil eugenol: Sources, extraction techniques and nutraceutical perspectives. RSC Adv 7:32669–32681
Khaw KY, Parat MO, Shaw PN, Falconer JR (2017) Solvent supercritical fluid technologies to extract bioactive compounds from natural sources: a review. Molecules 22
Khoddami A, Man YBC, Roberts TH (2014) Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur J Lipid Sci Technol 116:553–562
Klejdus B, Lojková L, Lapčík O, Koblovská R, Moravcová J, Kubáň V (2005) Supercritical fluid extraction of isoflavones from biological samples with ultra-fast high-performance liquid chromatography/mass spectrometry. J Sep Sci 28:1334–1346
Konar N, Dalabasmaz S, Poyrazoglu ES, Artik N, Colak A (2014) The determination of the caffeic acid derivatives of Echinacea purpurea aerial parts under various extraction conditions by supercritical fluid extraction (SFE). J Supercrit Fluids 89:128–136
Kueh BWB, Yusup S, Osman N (2018) Supercritical carbon dioxide extraction of Melaleuca cajuputi leaves for herbicides allelopathy: optimization and kinetics modelling. J CO2 Util 24:220–227
Kumar S, Dhanani T, Shah S (2014) Extraction of three bioactive diterpenoids from andrographis paniculata: effect of the extraction techniques on extract composition and quantification of three andrographolides using high-performance liquid chromatography. J Chromatogr Sci 52:1043–1050
Kumoro AC, Hasan M, Singh H (2019) Extraction of Andrographolide from Andrographis paniculata dried leaves using supercritical CO2 and ethanol mixture. Ind Eng Chem Res 58:742–751
Kuo CF, Su JD, Chiu CH, Peng CC, Chang CH, Sung TY, Huang SH, Lee WC, Chyau CC (2011) Anti-inflammatory effects of supercritical carbon dioxide extract and its isolated carnosic acid from rosmarinus officinalis leaves. J Agric Food Chem 59:3674–3685
Kyriakoudi A, Z. Tsimidou M (2018) Latest advances in the extraction and determination of saffron apocarotenoids. Electrophoresis 39:1846–1859
Lee YS, Park KY, Ji SH, Jo GS, Lee SK (2018) Effect of harvest seasons and extraction methods on the nutritional and functional components of Seomcho (Spinacia oleraecea L.). Korean J Food Preserv 25:682–688
Lefebvre T, Destandau E, Lesellier E (2021) Sequential extraction of carnosic acid, rosmarinic acid and pigments (carotenoids and chlorophylls) from Rosemary by online supercritical fluid extraction-supercritical fluid chromatography. J Chromatogr A 1639:461709
Leyva-Jiménez FJ, Lozano-Sánchez J, Fernández-Ochoa Á, Cádiz-Gurrea MDLL, Arraéz-Román D, Segura-Carretero A (2020) Optimized extraction of phenylpropanoids and flavonoids from lemon verbena leaves by supercritical fluid system using response surface methodology. Foods 9
Li J, Zhang J, Wang M (2016a) Extraction of flavonoids from the flowers of abelmoschus manihot (l.) medic by modified supercritical co2 extraction and determination of antioxidant and anti-adipogenic activity. Molecules 21
Li J, Zhang X, Liu Y (2016b) Supercritical carbon dioxide extraction of Ganoderma lucidum spore lipids. LWT 70:16–23
Li L, Wu Y, Yuan SW, Yang J, Yang YF, Ding G, Xiao W (2016c) Extraction and purification process of total fatty acid in Brassica campestris pollen. Zhongguo Zhong yao za zhi 41:226–232
Lima RN, Ribeiro AS, Cardozo-Filho L, Vedoy D, Alves PB (2019) Extraction from leaves of piper klotzschianum using supercritical carbon dioxide and co-solvents. J Supercrit Fluids 147:205–212
Lima RN, Santos ADC, Ribeiro AS, Cardozo-Filho L, Freitas LS, Barison A, Costa EV, Alves PB (2020) Selective amides extraction and biological activity from Piper hispidum leaves using the supercritical extraction. J Supercrit Fluids 157:104712
Liu X (2018) Optimization of Supercritical CO2 Fluid Extraction Conditions of Saponins from Spina Gleditsiae. In: 2018 International Conference on Advanced Electronic Materials, Computers and Materials Engineering, AEMCME 2018
Long T, Lv X, Xu Y, Yang G, Xu LY, Li S (2019) Supercritical fluid CO2 extraction of three polymethoxyflavones from Citri reticulatae pericarpium and subsequent preparative separation by continuous high-speed counter-current chromatography. J Chromatogr B Anal Technol Biomed Life Sci 1124:284–289
Luan ZJ, Li PP, Li D, Meng XP, Sun J (2020) Optimization of supercritical-CO2 extraction of Iris lactea seed oil: component analysis and antioxidant activity of the oil. Ind Crop Prod 152:112553
Machado BAS, Silva RPD, Barreto GDA, Costa SS, da Silva DF, Brandão HN, da Rocha JLC, Dellagostin OA, Henriques JAP, Umsza-Guez MA, Padilha FF (2016) Chemical composition and biological activity of extracts obtained by supercritical extraction and ethanolic extraction of brown, green and red propolis derived from different geographic regions in Brazil. PLoS ONE 11
Maia JD, Ávila CRD, Mezzomo N, Lanza M (2018) Evaluation of bioactive extracts of mangaba (Hancornia speciosa) using low and high pressure processes. J Supercrit Fluids 135:198–210
Marić B, Abramović B, Ilić N, Krulj J, Kojić J, Perović J, Bodroža-Solarov M, Teslić N (2020) Valorization of red raspberry (Rubus idaeus L.) seeds as a source of health beneficial compounds: extraction by different methods. J Food Process Preserv 44
Martins PF, de Melo MMR, Sarmento P, Silva CM (2016) Supercritical fluid extraction of sterols from Eichhornia crassipes biomass using pure and modified carbon dioxide. Enhancement of stigmasterol yield and extract concentration. J Supercrit Fluids 107:441–449
Marzorati S, Friscione D, Picchi E, Verotta L (2020) Cannabidiol from inflorescences of Cannabis sativa L.: green extraction and purification processes. Ind Crop Prod 155:112816
Masghati S, Ghoreishi SM (2018) Supercritical CO2 extraction of cinnamaldehyde and eugenol from cinnamon bark: optimization of operating conditions via response surface methodology. J Supercrit Fluids 140:62–71
Mehariya S, Iovine A, Di Sanzo G, Larocca V, Martino M, Leone G, Casella P, Karatza D, Marino T, Musmarra D, Molino A (2019) Supercritical fluid extraction of lutein from scenedesmus almeriensis. Molecules 24
Memon AH, Hamil MSR, Laghari M, Rithwan F, Zhari S, Saeed MAA, Ismail Z, Majid AMSA (2016) A comparative study of conventional and supercritical fluid extraction methods for the recovery of secondary metabolites from Syzygium campanulatum Korth. J Zhejiang Univ Sci B 17:683–691
Meneses MA, Caputo G, Scognamiglio M, Reverchon E, Adami R (2015) Antioxidant phenolic compounds recovery from Mangifera indica L. by-products by supercritical antisolvent extraction. J Food Eng 163:45–53
Mesquita PC, Rodrigues LGG, Mazzutti S, da Silva M, Vitali L, Lanza M (2021) Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.). Food Chem X:12
Miȩkus N, Iqbal A, Marszałek K, Puchalski C, Świergiel A (2019) Green chemistry extractions of carotenoids from daucus carota L.-Supercritical carbon dioxide and enzyme-assisted methods. Molecules 24
Mihalcea L, Turturică M, Cucolea EI, Dănilă GM, Dumitrașcu L, Coman G, Constantin OE, Grigore-Gurgu L, Stănciuc N (2021) CO2 supercritical fluid extraction of oleoresins from sea buckthorn pomace: evidence of advanced bioactive profile and selected functionality. Antioxidants 10
Minteguiaga M, Catalán CAN, Cassel E, Dellacassa E (2021) The “other Vassoura oil” and volatile fractions from Baccharis uncinella DC. (Asteraceae) as potential sources for flavor and fragrance industry. In: Volatile oils: production, composition and uses
Mohamad N, Ramli N, Abd-Aziz S, Ibrahim MF (2019) Comparison of hydro-distillation, hydro-distillation with enzyme-assisted and supercritical fluid for the extraction of essential oil from pineapple peels. 3 Biotech 9
Mu J, Wu G, Chen Z, Brennan CS, Tran K, Dilrukshi HNN, Shi C, Zhen H, Hui X (2021) Identification of the fatty acids profiles in supercritical CO2 fluid and Soxhlet extraction of Samara oil from different cultivars of Elaeagnus mollis Diels seeds. J Food Compos Anal 101:103982
Nastić N, Borrás-Linares I, Lozano-Sánchez J, Švarc-Gajić J, Segura-Carretero A (2018) Optimization of the extraction of phytochemicals from black mulberry (Morus nigra L.) leaves. J Ind Eng Chem 68:282–292
Náthia-Neves G, Vardanega R, Martinez Urango AC, Meireles MAA (2020) Supercritical CO2 extraction of α-bisabolol from different parts of candeia wood (Eremanthus erythropappus). J Supercrit Fluids 166:105026
Occhipinti A, Capuzzo A, Bossi S, Milanesi C, Maffei ME (2013) Comparative analysis of supercritical CO2 extracts and essential oils from an Ocimum basilicum chemotype particularly rich in T-cadinol. J Essent Oil Res 25:272–277
Ouédraogo JCW, Dicko C, Kini FB, Bonzi-Coulibaly YL, Dey ES (2018) Enhanced extraction of flavonoids from Odontonema strictum leaves with antioxidant activity using supercritical carbon dioxide fluid combined with ethanol. J Supercrit Fluids 131:66–71
Özcan MM, Ören D (2019) Comparative of physico-chemical properties of wheat germ oil extracted with cold press and supercritical co2 extraction. Iran J Chem Chem Eng 38:167–174
Pal S, Bhattacharjee P (2018) Spray dried powder of lutein-rich supercritical carbon dioxide extract of gamma-irradiated marigold flowers: process optimization, characterization and food application. Powder Technol 327:512–523
Pavić V, Jakovljević M, Molnar M, Jokić S (2019) Extraction of carnosic acid and carnosol from sage (Salvia officinalis l.) leaves by supercritical fluid extraction and their antioxidant and antibacterial activity. Plan Theory 8
Pavlović N, Vidović S, Vladić J, Popović L, Moslavac T, Jakobović S, Jokić S (2018) Recovery of tocopherols, amygdalin, and fatty acids from apricot kernel oil: cold pressing versus supercritical carbon dioxide. Eur J Lipid Sci Technol 120
Pires FB, Dolwitsch CB, Ugalde GA, Menezes BB, Fontana MEZ, Rieffeld RC, Sagrillo MR, Essi L, Mazutti MA, da Rosa MB, Pizzutti IR (2021) Chemical study, antioxidant activity, and genotoxicity and cytotoxicity evaluation of Ruellia angustiflora. Nat Prod Res 35:5317–5322
Pise VH, Shirkole SS, Thorat BN (2022) Visualization of oil cells and preservation during drying of betel leaf (piper betel) using hot-stage microscopy. Dry Technol 40:2494
Pourmortazavi SM, Rahimi-Nasrabadi M, Hajimirsadeghi SS (2014) Supercritical fluid technology in analytical chemistry - review. Curr Anal Chem 10:3–28
Priyanka, Khanam S (2018) Influence of operating parameters on supercritical fluid extraction of essential oil from turmeric root. J Clean Prod 188:816–824
Ramli NH, Yusup S, Quitain AT, Johari K, Kueh BWB (2019) Optimization of saponin extracts using microwave-assisted extraction as a sustainable biopesticide to reduce Pomacea canaliculata population in paddy cultivation. Sustain Chem Pharm 11:23–35
Reverchon E, Scognamiglio M, Baldino L (2022) Lycopene extract from tomato concentrate and its co-precipitation with PVP using hybrid supercritical processes. J CO2 Util 64:102157
Rodrigues VH, De Melo MMR, Portugal I, Silva CM (2021) Lupane-type triterpenoids from Acacia dealbata bark extracted by different methods. Ind Crop Prod 170:113734
Rohman A, Irnawati (2020) Pumpkin (Cucurbita maxima) seed oil: chemical composition, antioxidant activities and its authentication analysis. Food Res 4:578–584
Romano R, Aiello A, Pizzolongo F, Rispoli A, De Luca L, Masi P (2020) Characterisation of oleoresins extracted from tomato waste by liquid and supercritical carbon dioxide. Int J Food Sci Technol 55:3334–3342
Salea R, Hiendrawan S, Subroto E, Veriansyah B, Tjandrawinata RR (2018) Supercritical carbon dioxide extraction of citronella oil from cymbopogon winterianus using taguchi orthogonal array design. Int J Appl Pharm 10:147–151
Sánchez-Camargo ADP, Gutiérrez LF, Vargas SM, Martinez-Correa HA, Parada-Alfonso F, Narváez-Cuenca CE (2019) Valorisation of mango peel: proximate composition, supercritical fluid extraction of carotenoids, and application as an antioxidant additive for an edible oil. J Supercrit Fluids 152:104574
Sanjaya RE, Tedjo YY, Kurniawan A, Ju YH, Ayucitra A, Ismadji S (2014) Investigation on supercritical CO2 extraction of phenolic-phytochemicals from an epiphytic plant tuber (Myrmecodia pendans). J CO2 Util 6:26–33
Saotome Y, Imai M (2018) Supercritical carbon dioxide extraction of apigenin from parsley leaves pre-treated to maximize yield. Food Sci Technol Res 24:63–73
Sapkale GN, Patil SM, Surwase US, Bhatbhage PK (2010) Supercritical fluid extraction - a review. Int J Chem Sci 8:729–743
Sharifi-Rad J, Ezzat SM, El Bishbishy MH, Mnayer D, Sharopov F, Kiliç CS, Neagu M, Constantin C, Sharifi-Rad M, Atanassova M, Nicola S, Pignata G, Salehi B, Fokou PVT, Martins N (2020) Rosmarinus plants: key farm concepts towards food applications. Phytother Res 34:1474–1518
Shi LK, Zheng L, Liu RJ, Chang M, Jin QZ, Wang XG (2018) Chemical characterization, oxidative stability, and in vitro antioxidant capacity of sesame oils extracted by supercritical and subcritical techniques and conventional methods: a comparative study using chemometrics. Eur J Lipid Sci Technol 120:1700326
Song L, Liu P, Yan Y, Huang Y, Bai B, Hou X, Zhang L (2019) Supercritical CO2 fluid extraction of flavonoid compounds from Xinjiang jujube (Ziziphus jujuba Mill.) leaves and associated biological activities and flavonoid compositions. Ind Crop Prod 139:111508
Suryawanshi B, Mohanty B (2018) Modeling and optimization: supercritical CO2 extraction of Pongamia pinnata (L.) seed oil. J Environ Chem Eng 6:2660–2673
Swapna Sonale R, Ramalakshmi K, Udaya Sankar K (2018) Characterization of neem (Azadirachta indica A. Juss) seed volatile compounds obtained by supercritical carbon dioxide process. J Food Sci Technol 55:1444–1454
Tang ZX, Ying RF, Lv BF, Yang LH, Xu Z, Yan LQ, Bu JZ, Wei YS (2021) Flaxseed oil: extraction, health benefits and products. Qual Assur Safety Crops Foods 13:1–19
Trucillo P, Campardelli R, Aliakbarian B, Perego P, Reverchon E (2018) Supercritical assisted process for the encapsulation of olive pomace extract into liposomes. J Supercrit Fluids 135:152–159
Tyskiewicz K, Konkol M, Rój E (2019) Supercritical carbon dioxide (scCO2) extraction of phenolic compounds from lavender (Lavandula angustifolia) flowers: A box-Behnken experimental optimization. Molecules 24
Uzel RA (2018) Effect of extraction method and extraction solvent on recovery of phenolic compounds from olive leaves in Kemalpaşa-İzmir (Turkey): Oleuropein recovery as a case example. Sep Sci Technol (Philadelphia) 53:1531–1539
Végh K, Riethmüller E, Hosszú L, Darcsi A, Müller J, Alberti Á, Tóth A, Béni S, Könczöl Á, Balogh GT, Kéry Á (2018) Three newly identified lipophilic flavonoids in Tanacetum parthenium supercritical fluid extract penetrating the Blood-Brain Barrier. J Pharm Biomed Anal 149:488–493
Veiga BA, Hamerski F, Clausen MP, Errico M, De Paula Scheer A, Corazza ML (2021) Compressed fluids extraction methods, yields, antioxidant activities, total phenolics and flavonoids content for Brazilian Mantiqueira hops. J Supercrit Fluids 170:105155
Velikorodov AV, Kovalev VB, Nosachev SB, Tyrkov AG, Morozova LV (2018) Fatty-oxygen composition of seeds oils of some wild-growing and cultivated plants of the Astrakhan Region Obtained by the supercritical fluid extraction method. Khimiya Rastitel’nogo Syr’ya:153–158
Villanueva-Bermejo D, Vázquez E, Villalva M, Santoyo S, Fornari T, Reglero G, García-Risco MR (2019) Simultaneous supercritical fluid extraction of heather (Calluna vulgaris L.) and marigold (Calendula officinalis L.) and anti-inflammatory activity of the extracts. Appl Sci (Switzerland) 9
Villinski JR, Bergeron C, Cannistra JC, Gloer JB, Coleman CM, Ferreira D, Azelmat J, Grenier D, Gafner S (2014) Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J Nat Prod 77:521–526
Wu H, Li J, Jia Y, Xiao Z, Li P, Xie Y, Zhang A, Liu R, Ren Z, Zhao M, Zeng C, Li C (2019) Essential oil extracted from Cymbopogon citronella leaves by supercritical carbon dioxide: antioxidant and antimicrobial activities. J Anal Methods Chem 2019
Yekefallah M, Raofie F (2022) Production of herbal nanocolloids from Rubia tinctorum L. roots by rapid expansion from supercritical solution into suspension system. Ind Crop Prod 176:114286
Yousefi M, Rahimi-Nasrabadi M, Pourmortazavi SM, Wysokowski M, Jesionowski T, Ehrlich H, Mirsadeghi S (2019) Supercritical fluid extraction of essential oils. TrAC 118:182–193
Yu L, Hu X, Xu R, Ba Y, Chen X, Wang X, Cao B, Wu X (2022) Amide alkaloids characterization and neuroprotective properties of Piper nigrum L.: A comparative study with fruits, pericarp, stalks and leaves. Food Chem 368:130832
Zaid R, Mouhouche F, Canela-Garayoa R, Chacón NMO (2020) Supercritical fluid extraction of Algerian Melissa officinalis L. 1753 (Lamiaceae) and its biological activity against two species of the genus Chaitophorus (Homoptera-Aphididae). Arch Phytopathol Plant Protect 53:940–953
Zakharenko AM, Razgonova MP, Pikula KS, Golokhvast KS (2021) Simultaneous determination of 78 compounds of Rhodiola rosea extract by supercritical CO2-extraction and HPLC-ESI-MS/MS spectrometry. Biochem Res Int 2021
Zengin G, Mahomoodally F, Picot-Allain C, Diuzheva A, Jekő J, Cziáky Z, Cvetanović A, Aktumsek A, Zeković Z, Rengasamy KRR (2019) Metabolomic profile of Salvia viridis L. root extracts using HPLC–MS/MS technique and their pharmacological properties: A comparative study. Ind Crop Prod 131:266–280
Zhang ZS, Liu YL, Che LM (2018) Optimization of supercritical carbon dioxide extraction of eucommia ulmoides seed oil and quality evaluation of the oil. J Oleo Sci 67:255–263
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Vidwathpriya, K., Sriranjani, S., Niharika, P.K., Anil Kumar, N.V. (2023). Supercritical Fluid for Extraction and Isolation of Natural Compounds. In: Cruz, J.N. (eds) Drug Discovery and Design Using Natural Products. Springer, Cham. https://doi.org/10.1007/978-3-031-35205-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-031-35205-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35204-1
Online ISBN: 978-3-031-35205-8
eBook Packages: MedicineMedicine (R0)