Abstract
Cotula cinerea of the Asteraceae family is a traditional plant which grows in desert area. The plant is endowed with various biological activities due to the presence of several secondary metabolites. Owing to its use in traditional folk medicine and its interesting biological effects, the plant has been subjected to many scientific explorations resulting in the publication of a multitude of papers in a wide range of scientific fields including phytochemistry, biological activities, and toxicology. The objective of this chapter is to report and gather previous studies on Cotula cinerea regarding its botanical description, geographic distribution, bioactive compounds, toxicology, and in vivo and in vitro biological properties. The phytochemical analysis was carried out by several spectroscopic methods, and the obtained results showed the richness of this plant in several phytochemicals including phenolic compounds, volatile compounds, sesquiterpene lactones, and others. Studies on Cotula cinerea showed the harmlessness of this plant since its tested extracts were not toxic even at higher doses. The evaluation of the pharmacological activities of the essential oil and the extracts of Cotula cinerea have shown that the plant has significant antibacterial, antifungal, antioxidant, anticancer, analgesic, anti-inflammatory, and antipyretic effects. This review showed that even if a number of publications have been reported on the plant, research on Cotula cinerea remains an open research area of good interest. It is hoped that the information presented here will be beneficial and useful for further studies that will eventually lead to the development of therapeutic agents from this plant.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
For several years, plants have played a major role in the art of healing throughout the world. The use of medicinal plants or herbal preparations is increasingly popular. Thus, according to estimates, 80% of the world’s population depends mainly on traditional medicine (WHO 2012). The use of traditional practices based on medicinal plants is explained by several reasons such as the high cost of pharmaceutical products, the sociocultural habits of the populations, the need to have therapeutic options for resistant pathogens, and the existence of diseases for which there is no effective treatment (Duke et al. 1993; Cox and Balik 1994).
Medicinal plants are extremely numerous. Indeed, estimates indicate that more than 13,000 species of medicinal plants are used as traditional remedies by various cultures around the world (Tyler 1994). Plants used in traditional medicine contain a wide range of chemical substances and compounds, such as phenolic compounds (phenolic acids, flavonoids, quinones, coumarins, lignans, stilbenes, tannins, etc.), nitrogen compounds (alkaloids, amines, etc.), vitamins, terpenoids, and certain other endogenous metabolites. Polyphenols are known for their significant antioxidant activities, as they can act by direct scavenging of ROS (reactive oxygen species) (Halliwell and Cross 1994). They are also present as ingredients in several cosmetic preparations used in the treatment of cellular aging and skin protection (Menaa et al. 2014). Thus, the chemical composition of plants can be used to treat chronic and infectious diseases.
According to the World Health Organization (WHO), approximately 80% of the world’s population still uses herbal medicines to treat several diseases (World Health Organization 2008).
In order to contribute to the valorization of medicinal plants, we have chosen to establish this review on the Cotula cinerea plant which is distributed in the desert regions especially in North Africa.
Cotula cinerea belongs to the Asteraceae family and is widely distributed in sandy and desert soils (Ahmed et al. 1987). It is known as “Gartoufa” and is used in traditional Moroccan medicine used to treat colic, cough, diarrhea, migraine, headaches, and digestive disorders (Bellakhdar 1997).
Moreover, several studies on Cotula cinerea have been based not only on the species itself but also on several variations which make the difference of the biological effects namely the geographical origin, the climate, the parts of the plant used, the extraction solvent and the harvest period.
Previous work done on this plant has scientifically proven several biological properties and effects (Fig. 1), such as antibacterial, antifungal, antioxidant, anticancer, anti-inflammatory, analgesic, and antipyretic activities.
The study of essential oil and extracts from different parts of Cotula cinerea showed the presence of several chemical classes, including phenolic acids, flavonoids, and terpenoids (Mekhadmi et al. 2023; Chlif et al. 2022; Guaouguaou et al. 2020b).
On the other hand, studies on the chemical composition of Cotula cinerea have also been analyzed to suggest this species as a new source of medicines, thus justifying its traditional uses.
In this review, we tried to find the link between the ethnomedicinal use, the pharmacological properties tested, and the chemical composition which could be the origin of its biological properties.
2 Research Methodology
The literary synthesis on Cotula cinerea botanical description, traditional medicinal application, chemical composition, biological activities, and toxicity of Cotula cinerea extracts have been collected, analyzed, and summarized in this review.
Scientific search engines such as PubMed, Science Direct, Springer Link, Web of Science, Scopus, Wiley Online, and Google Scholar were used to collect all published papers on this species.
In this work, many key words and scientific terms have been used such as Cotula cinerea, essential oil, extracts, chemical composition of Cotula cinerea, acute toxicity of Cotula cinerea, analgesic effect of Cotula cinerea, antioxidant effect of Cotula cinerea, cytotoxic activity of Cotula cinerea, antimicrobial activity of Cotula cinerea, antipyretic activity of Cotula cinerea, and anti-inflammatory activity of Cotula cinerea. All published papers containing the name Cotula cinerea have been cited in this review. To identify other relevant articles, reference lists of retrieved articles were also searched. All data has been discussed in the text and organized in tables to summarize.
3 Results and Discussion
3.1 Botanical Description
The Cotula cinerea is a Saharo-Arabic species common throughout the Sahara, in somewhat sandy soils, being very aromatic is used to flavor tea. It is a woolly-looking annual plant with prostrate stems and golden-yellow flowers (Fig. 2). Its stems are 10 to 40 cm in diameter, laid down, and then straightened (Ozenda 1993; Benhouhou 2005). The leaves are woolly, whitish, and thick, and the upper parts are divided into three to five obtuse. Concerning the flowers, they are flower heads 6 to 10 mm in diameter, woolly involucre, tubular, brown in button then golden yellow when they open (Ozenda 1993; Benhouhou 2005). Brocchia cinerea is the synonym of Cotula cinerea and has several vernacular names such as Chihia, Chouihia, Robita, and Al gartoufa (Quezel and Santa 1962; Dupont and Guignard 2004).
General morphology of Cotula cinerea (Ozenda 1967)
3.2 Geographic Distribution
The Cotula cinerea is a xerophytic plant that grows in desert conditions and requires an average annual rainfall of 100 mm. The Cotula cinerea species is widely encountered throughout the Sahara (Djellouli et al. 2013). It grows in ergs and little sandy soils. Geographically, it is widely distributed in North Africa, particularly in the Saharan regions of Morocco, Algeria and Egypt (Ahmed et al. 1987; Boulos 1983; Ozenda 1993; Markouk et al. 1999a, b).
3.3 Ethnobotanical Use
The ethnopharmacological studies have shown that Cotula cinerea is widely used to treat colic, cough, diarrhea, migraine, headaches, and digestive disorders (Bellakhdar 1997). In traditional medical practice, it is used as an antiseptic, antipyretic, analgesic, anti-inflammatory, and antibacterial agent, as well as for the treatment of rheumatism (Beloued 2005; Hammiche and Maiza 2006).
The use of Cotula cinerea in traditional medicine is generally administered in the form of decoction, maceration, infusion and inhalation (Bellakhdar 1997; Djellouli et al. 2013).
3.4 Phytochemistry
In view of its use in traditional folk medicine and its interesting biological activities, Cotula cinerea has undergone several phytochemical investigations. These resulted in the identification and/or the isolation of a huge number of natural products and a wide variety of bioactive secondary metabolites pertaining to different families such as essential oil terpenoids, phenolic compounds, saponins, germacranolides, and other phytochemical compounds (Lakhdar 2018). Among these phytochemical families, the main terpenoids, phenolics, and other phytochemicals previously identified and reported in Cotula cinerea are gathered and described below.
3.4.1 Essential Oil Terpenoids
Cotula cinerea essential oils have been subjected to several research studies. The species is widely known as odoriferous and aromatic plant used in the south of Morocco to flavor hot beverages such as tea. The odoriferous and aromatic properties of the plant are due to the presence of essential oil with various volatiles and aromatic compounds. Essential oils of the aerial parts of the plant from different geographical regions in Algeria, Egypt and Morocco have been extracted through hydrodistillation and subjected to qualitative and quantitative analysis through GC-MS techniques. The main detected compounds have been gathered in Table 1 and the structures of some of these compounds are presented in Fig. 3.
Examination of the reported data showed that the obtained essential oils varied both qualitatively and quantitatively according to the corresponding country and even from region to region within the same country. Such variations were thus observed for samples from Algeria (Mekhadmi et al. 2023; Bouziane et al. 2013; Atef et al. 2015; Djellouli et al. 2015), Egypt (Fournier et al. 1989; Fathy et al. 2017), and Morocco (Boussoula et al. 2016; El Bouzidi et al. 2011; Guaouguaou et al. 2020a, b; Chlif et al. 2021; Hamdouch et al. 2022). It should be indicated that this variation could be due to several factors, such as the harvest site and the vegetation stage of the plant of the plant in addition to other exogenous conditions including climate, soil composition, harvesting time and extraction method. It should also be noted that the differences in the phytochemical composition of plants extracts are not specific only to essential oil but usually observed both qualitatively and quantitatively for the major if not any phytochemical metabolite. However, and even if the investigated samples are from different geographical regions, some commonalities could be observed in addition to some disparities in the phytochemical composition of the investigated Cotula cinerea essential oils. Thus, on the 14 investigated samples, the compound thujone was indicated among the major detected and quantified compounds in 11 samples with percentages up to 50% of the total quantified compounds. Thus, thujone was among the major compounds for Cotula cinerea samples from Algeria (Mekhadmi et al. 2023; Ghouti et al. 2018a, b; Bouziane et al. 2013; Atef et al. 2015), from Egypt (Fournier et al. 1989; Fathy et al. 2017; Larbi et al. 2018), and from Morocco (El Bouzidi et al. 2011; Kasrati et al. 2015; Chlif et al. 2021; Hamdouch et al. 2022; Agour et al. 2022). This could also be noted for santolinatriene which was detected among the most abundant compounds in Cotula cinerea essential oil from Algeria with percentages ranging from 4% to 18.58% (Mekhadmi et al. 2023; Atef et al. 2015; Bouziane et al. 2013). This was also observed for essential oil samples from Morocco with relative abundance ranging from 7.2% to 16.45% (Boussoula et al. 2016; El Bouzidi et al. 2011; Guaouguaou et al. 2020a, b; Hamdouch et al. 2022; Chlif et al. 2021). This compound was reported among the major compounds in all the Moroccan explored Cotula cinerea essential oils. Finally, santolinatriene was reported with a weak percentage or not detected in samples from Algeria (Mekhadmi et al. 2023; Djellouli et al. 2015) and Egypt (Fournier et al. 1989; Fathy et al. 2017). In addition to thujone and santolinatriene, camphor was also detected with a relatively high abundance in the major investigated samples with percentages varying from 4.90% to 65.5%. These higher percentages (50% and 65.5%) were observed for two samples from Egypt (Fournier et al. 1989; Fathy et al. 2017).
Besides these samples which phytochemical compositions were relatively homogenous on the qualitative level with thujone, santolinatriene, camphor as major compounds and for which differences were observed on the quantitative aspect of these compounds, analysis of other Cotula cinerea samples showed a peculiar phytochemical composition. This was the case for the essential oil sample from Algeria for which kassane (34.3%) was indicated as major compounds (Mekhadmi et al. 2023). This was also the case for 3-carene which was present as major phytochemicals (30.99%) for the Cotula cinerea essential oil from Eastern Algeria (Atef et al. 2015). This especial fact appeared also for trans citral (24.01%) and iso-3-thujanol (47.38%) detected as major compounds in samples from Algeria (Djellouli et al. 2015) and Morocco (Boussoula et al. 2016) respectively.
In addition to the volatile compounds constituting the essential oils of Cotula cinerea, its nonvolatile secondary metabolites have also been the subject of several scientific investigations. The different compounds have been separated through various chromatographic techniques including high performance liquid chromatography or column chromatography and have been characterized through hyphenated coupled compounds such as UHPLC-MS and MSn techniques with the use of powerful high-resolution MS detectors with time of flight analyzers. Some compounds have also been characterized after isolation through one-dimensional (1D) and two-dimensional (2D) homonuclear and heteronuclear NMR spectroscopy techniques. Several identified compounds pertaining to various families such as phenolic acids, flavonoids, and germacranolides are discussed below.
3.4.2 Phenolic Acids
Cotula cinerea was reported to contain some phenolic acids. The phytochemical analysis of the plant summarized in Table 2 showed the presence of various phenolic compounds pertaining to phenolic acids. The structures of some of these derivatives previously reported in the plant are presented in Fig. 4. Among these, six mono- (chlorogenic acid and its isomers) and di- (3,4; 3,5; and 4,5) caffeoylquinic acid derivatives with different substitution site have been reported in Cotula cinerea from Morocco (Khallouki et al. 2015). Similar derivatives have also reported in a sample from Algeria with one mono and two dicaffeoylquinic acid adducts (Ghouti et al. 2018a, b). Another sample of the plant from Dakhla (Morocco) was also found to contain phenolic acids derivatives including caffeic acid, coumaric acids, and some other derivatives (Guaouguaou et al. 2020b).
3.4.3 Flavonoids
A phytochemical investigation on the flavonoids of Cotula cinerea from Egypt was carried out since the seventies of the last century where free quercetin and kaempferol in addition to quercetrin and kaempferitrin have been identified (Mahran et al. 1976) as indicated in Table 2.
The phytochemical investigations of various Cotula cinerea extracts revealed the presence of several flavonoids. The content of flavonoids reported in the plant was higher either qualitatively or quantitatively than that of phenolic acids. The major detected flavonoids are presented in Table 2 and the structures of some of them are presented in Fig. 5.
In 1987, flavones and flavonols derivatives have been reported in an hydroethanolic extract of Cotula cinerea from Egypt (Ahmed et al. 1987). Free and glycosylated adducts with both C- and O-glycosylated adducts have been reported in this study. Among the flavones, free luteolin and apigenin in addition to their glycosides have been reported while glycosides’ adducts of quercetin have been identified within the flavonols subgroup. In another sample from Algeria seventeen flavonoids have been isolated and structurally elucidated (Dendougui et al. 2012). Among the identified compounds in this study, free apigenin and luteolin have been evidenced. Additionally, several methoxylated, mono-, and diglycosylated derivatives of luteolin, apigenin, and quercetin have also been reported. Glycosides of luteolin and quercetin with hexoses and pentoses moieties have also been found in another sample from Algeria (Ghouti et al. 2018a, b). Some phytochemicals of this sample were acylated with malonyl derivatives of luteolin and quercetin hexosides.
In addition to Cotula cinerea from Algeria and Egypt which were found to contain flavonoids, such phytochemicals have also been reported to occur in populations from Morocco. The flavone derivative luteolin 4’-O-glucoside was thus reported in a sample from Errachidia region (Khallouki et al. 2015). Another more thorough investigation on a sample from Dakhla region (Morocco) has been recently reported (Guaouguaou et al. 2020b). In this study, free apigenin, luteolin and kaempferol have been found in the hydro ethanolic extract in addition to several methoxylated and glycosylated derivatives of flavones (apigenin, luteolin) and flavonols (kaempferol, quercetin).
3.4.4 Sulfated Flavonoids
In addition to the flavonoids discussed above, Cotula cinerea from Morocco was also found to contain the sulfated flavonoids indicated in Table 2 and Fig. 5. Thus, kaempferol 3-sulfate 7-O-glucoside, kaempferol 3-O-sulfate, luteolin 7-O-sulfate in addition to apigenin 7-O-sulfate have been evidenced in the hydroethanolic extracts of the plant. The presence of such sulfated flavonoids has been previously identified in species other than Cotula cinerea (Teles et al. 2018). Their occurrence in this plant agree with the fact that sulfated flavonoids are reported to occur in some specific plant families such as Asteraceae to which Cotula cinerea belong (Teles et al. 2018). This is also in agreement with the fact that such compounds were also reported to occur in species occurring in arid habitats such as Moroccan Sahara region from which the studied Cotula cinerea sample has been harvested.
3.4.5 Other Phytochemical Compounds
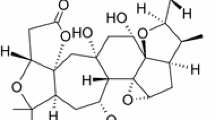
In addition to essential oil terpenoids and phenolic compounds, several other metabolites have been identified in Cotula cinerea plant from different geographical regions as indicated in Table 2 and Figs. 6 and 7. The phytochemical study of Cotula cinerea extracts from Egypt has been conducted in 1985 and afforded to the characterization of compounds pertaining to spiroketal enol-ether, sesquiterpene lactones, germacranolides, and guaianolide (Metwally et al. 1985) in addition to Isofraxidin and scopoletin derivatives (Greger and Hofer 1985). Among the latter are farnochrol, drimartol A, acetyldrimartol A, acetyldrimartol B, pectachol, pectachol B, acetylpectachol B in addition to scopofarnol and farnesylscopoletin (Fig. 6 and Table 2). Another sample from the Egyptian eastern desert was also found to contain several spiroketal enolethers, lactones, germacranolides, eudesmanolides, guaianolides and glaucolides (Jakupovic et al. 1988). A germacranolide compound (1α,6α-dihydroxygermacra-4E,9Z,11(13)-trien-12,8 α-olide) which structure is indicated in Fig. 7 was isolated and identified from Cotula cinerea hydroethanolic extract from Algeria (Dendougui et al. 2012). In a relatively recent investigation on Cotula cinerea extract from Morocco, tatridin and dehydrotatridin derivatives (Fig. 7) have been detected (Guaouguaou et al. 2020b).
In addition to these phytochemicals other derivatives have been isolated from the dichloromethane extract through bioguided isolation affording five main sesquiterpene lactones (Cimmino et al. 2021). These were shown to be three guaiantrianolides (6-acetoxy-1β-hydroxyguaiantrienolide, 6-acetoxy-1α-hydroxyguaiantrienolide, 6-acetoxy-10-β-hydroxyguaiantrienolide) and two germacranolides (haagenolide and 1,10-epoxyhaagenolide) (Fig. 7).
3.5 Pharmacological Investigation
3.5.1 Antimicrobial Activity
The evaluation of the antimicrobial activity (bacterial and fungal) of Cotula cinerea extracts and essential oil harvested in different regions of the world has been reported in numerous studies (Table 3). Several researchers have shown that Cotula cinerea has broad-spectrum antimicrobial activity when tested against several pathogenic bacteria and fungi. The results of the various tests of the antimicrobial activity of Cotula cinerea extracts and essential oil are grouped in Table 3.
The antibacterial test of Cotula cinerea essential oil on Escherichia coli and Staphylococcus aureus showed strong inhibition with a diameter ranging from (16.70 to 14.64 mm) (Mekhadmi et al. 2023).
Hamdouch et al. (2022) showed that the evaluation of the bacterial activity by the essential oil of Cotula cinerea against three bacterial strains (Staphylococcus aureus, Listeria innocua, Pseudomonas aeruginosa) showed that the minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) are important (Table 3).
The Staphylococcus aureus is the bacterial strain that was inhibited with a low concentration (MIC = 0.5 μL/mL and MBC = 4 μL/mL), followed by Pseudomonas aeruginosa (MIC = 0.6 μL/mL and MBC = 1.5 μL/mL) and in third position is Listeria innocua aeruginosa (MIC = 0.8 μL/mL and MCB = 3.5 μL/mL) (Hamdouch et al. 2022).
On the other hand, the antibacterial activity of Cotula cinerea essential oil was evaluated on two bacterial strains (Escherichia coli and Pseudomonas aeruginosa) (Table 3).
The results obtained show that the two bacteria tested have a low sensitivity vis-à-vis the different prepared concentrations of this essential oil compared to the negative control (the inhibitory zones of the two strains vary between 6 and 9 mm and those of Amoxyclav between 13 and 15 mm) (Mahboub et al. 2021).
The study of the antimicrobial activity of the methanolic extract of Cotula cinerea revealed the effectiveness of this extract on the strains: Escherichia coli, Escherichia coli ATCC 25922 and Pseudomonas aeruginosa and their inhibition diameter varies between 7.1 ± 0.6 mm and 23.2 ± 0.3 mm (Table 3).
Also, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus aureus ATCC 25923, and Proteus mirabilis showed high sensitivity toward the Cotula cinerea methanolic extract with a large inhibition diameter (28.7 ± 0.5 mm, 23.7 ± 0.6 mm, 21.7 ± 0.2 mm, and 36.7 ± 0.1 mm, respectively). Thus, the zones of inhibition measured exceed that of the antibiotic tested as reference (Amoxicillin) (Djahra et al. 2020).
The study of the antimicrobial activity of Cotula cinerea essential oil on bacterial strains showed that Enterobacter cloacae and Escherichia coli are moderately sensitive (ɸ = 14 mm and ɸ = 13 mm, respectively) (Table 3). In addition, Pseudomonas aeruginosa is the most sensitive strain to this essential oil with a zone of inhibition of 15.23 mm.
On the other hand, the Staphylococcus aureus strain is more resistant with an inhibition zone of 6 mm. However, Fusarium sporotrichioides showed strong resistance to different concentrations of Cotula cinerea essential oil, and no mycelial growth was observed (Mehani et al. 2019).
The evaluation of the antibacterial activity of Cotula cinerea essential oil by the disk diffusion method on four strains of bacteria (Bacillus cereus, Bacillus subtilis, Micrococcus luteus, Pseudomonas aeruginosa) and on a yeast (Candida albicans) has revealed the effectiveness of this essential oil with moderate minimum inhibitory concentration (MIC). The Bacillus subtilis strain gave the best inhibition with MIC = 0.303 mg/mL (Table 3) (Ghouti et al. 2018a).
The study of the antimicrobial activity of Cotula cinerea hydroethanolic extract and the infusion extract tested on ten microbial strains (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Proteus mirabilis, Morganella morganii, Enterococcus faecalis, Listeria monocytogenes, MRSA, MSSA, and Candida albicans) showed inhibitory effects that ranged from moderate to weak and which are expressed as minimum inhibitory concentrations (MIC), minimum bactericidal concentrations (MBC) and minimum fungicidal concentrations (MFC) (Table 3). The MIC values varied between 5 and 20 mg/mL and the inhibitory effect of these extracts tested against all bacterial strains was more bacteriostatic than bactericidal (Ghouti et al. 2018b).
The results of the study of Cotula cinerea aqueous extract on Fusarium graminearum and on Fusarium sporotrichioides (Table 3) revealed the effectiveness of this extract in inhibiting the growth of mycelia with the two concentrations of 10% and 20%. The growth inhibition zones of these two fungi are ɸ = 39 ± 0.57 mm and ɸ = 50 ± 0.57 mm, respectively (Salhi et al. 2017).
The antibacterial and antifungal activity of Cotula cinerea essential oil showed significant inhibitory effects (Table 3). For the four bacterial strains (Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Micrococcus luteus), they were all inhibited at a concentration of 1/500 v/v. However, molds (Asper gillusniger, Penicillium digitatum, and Penicillium expansum) were less inhibited than bacteria, and their growth was stopped at a concentration of 1/250 v/v (Boussoula et al. 2016). Concerning the four strains of fungi (Gloeophyllum trabeum, Coniophora puteana, Poria placenta, and Coriolus versicolor), only Poria placenta which presented the greatest vulnerability compared with Cotula cinerea essential oil with a low concentration of 1/2000 v /v. For Coniophora puteana, it was inhibited with a concentration of 1/1000 v/v. However, Coriolus versicolor and Gloeophyllum trabeum showed resistance to the Cotula cinerea essential oil, and they were inhibited only with the concentration 1/500 v/v (Boussoula et al. 2016).
The antibacterial activity of Cotula cinerea essential oil tested on Enterococcus faecium showed a very high inhibitory effect (50 mm) by comparing with the diameter of antibiotic inhibition (Lincomycin: 32 mm) (Atef et al. 2015). Also, Escherichia coli, Morganella morganii, Proteus vulgaris, Staphylococcus aureus, and Acinetobacter baumannii showed great sensitivity to this essential oil with the concentrations (1/1, 1/2, 1/4, 1/8) where the diameter of inhibition varied between 50 mm and 21 mm (Table 3). However, Citrobacter freundii and Klebsiella pneumoniae showed great sensitivity just at the three concentrations (1/1, 1/2, 1/4) with an inhibition diameter which reached 50 mm. Pseudomonas aeruginosa showed strong resistance with all tested concentrations of Cotula cinerea essential oil (Atef et al. 2015).
The antimicrobial activity of Cotula cinerea n-butanol and petroleum ether extracts tested on Klebsiella pneumoniae showed a major inhibitory effect (16.67 ± 5.77 mm and 17 ± 1.73 mm respectively) (Table 3). Also, a strong activity against Staphylococcus aureus was revealed with the n-butanol and ethyl acetate extracts where the zones of inhibition were 12 ± 5.20 mm and 11.67 ± 3.79 mm, respectively. However, weak antimicrobial activity was observed against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Candida albicans with the concentration 0.25 mg/mL (Bensizerara et al. 2013).
Analysis of the antimicrobial activity of Cotula cinerea essential oil showed strong activity against all Candida genus yeasts studied (Candida albicans CCMM L4, Candida albicans CCMM L5, Candida krusei, Candida glabrata, and Candida parapsilosis) (Table 3), with inhibition zones ranging from 19.3 to 25.3 mm (Bouzidi et al. 2011).
Markouk et al. (1999a, b), tested the antibacterial activity of two extracts (n-butanol and ethyl acetate) of Cotula cinerea on eight bacterial strains (Pseudomonas fluorescens 456-2, Pseudomonas savastanoui T12-10, Pseudomonas savastanoui 73-29/88, Bacillus sp. VP5, Bacillus brevis VP7, Bacillus sp. 326, Bacillus sphaericus 324, and Bacillus sp. 459-1) (Table 3). They found the n-butanol extract to be highly effective against the bacterial strains tested with minimum inhibitory concentrations ranging from 12 to 200 μg/mL. Furthermore, Pseudomonas fluorescens 456-2 and Bacillus sp. 459-1 were inhibited at a low concentration of 12 μg/mL. However, the ethyl acetate extract inhibited the growth of all the bacteria studied at a concentration of 200 μg/mL (Markouk et al. 1999a, b).
3.5.2 Antioxidant Activity
The antioxidant effect of Cotula cinerea extracts and essential oil obtained by extracting different parts of the plant has been proven by several studies. The antioxidant activity of this plant was carried out by the DPPH, ABTS, reducing power, β-carotene bleaching inhibition, TBARS inhibition, FRAP, and ORAC tests. Table 4 brings together all the work on the antioxidant activity of Cotula cinerea.
Hamdouch et al. (2022) showed that Cotula cinerea essential oil has a low antioxidant activity compared to the selected positive controls (butylhydroxytoluene (BHT) and Cov-iox T50) with an IC50 of 0.080 ± 0.014 mg/mL (Table 4).
On the other hand, Mahboub et al. (2021) showed that the Cotula cinerea essential oil evaluated for its antioxidant power using the DPPH test showed a moderate antioxidant effect compared to ascorbic acid (IC50 = 79.28 mg/mL) (Table 4).
The study of the antioxidant activity of Cotula cinerea essential oil and extracts was carried out by two methods: DPPH and ABTS and several concentrations were tested (Guaouguaou et al. 2020a).
The DPPH test showed a higher antioxidant effect than that of ABTS and this for three extracts of Cotula cinerea (hexane, ethyl acetate, and n-butanol) with IC50 values of 0.0602, 0.0644 and 0.0641 mg/mL respectively (Table 4). For the essential oil of the same plant, it showed a moderate effect (IC50 = 0.1832 mg/mL). However, the ABTS test showed that Cotula cinerea essential oil has powerful antioxidant activity compared to the other extracts tested (IC50 = 0.0093 mg/mL). Also, the n-butanol extract shows strong antioxidant activity with the ABTS test (0.0698 mg/mL) (Guaouguaou et al. 2020a).
The evaluation of the antioxidant activity of Cotula cinerea essential oil using the DPPH method (Table 4) showed moderate inhibition (IC50 = 28 mg/mL) (Ghouti et al. 2018a).
On the other hand, the evaluation of the antioxidant activity of Cotula cinerea hydroethanolic and infusion extracts was carried out by four tests (DPPH, Reducing power, β-carotene bleaching inhibition and TBARS inhibition) in order to be able to compare their antioxidant effects (Ghouti et al. 2018b) (Table 4). For the TBARS inhibition test, the two hydroethanolic and infusion extracts of Cotula cinerea showed that they are three times more effective than the positive control (TROLOX) with high values: EC50 = 7.4 ± 0. 3 μg/mL and EC50 = 7.5 ± 0.2 μg/mL respectively. Also, for the DPPH test, Ghouti et al. (2018b) showed that Cotula cinerea hydroethanolic and infusion extracts have significant antioxidant activity with EC50 = 26.0 ± 0.1 μg/mL and EC50 = 24.8 ± 0.2 μg/mL respectively (Ghouti et al. 2018b).
The phytochemical identification and fractionation of Cotula cinerea methanol extract, revealed the presence of six compounds (Chlorogenic acid, Neochlorogenic acid, 3,4-Dicaffeoylquinic acid, 3,5-Dicaffeoylquinic acid, 4,5-Dicaffeoylquinic acid, Luteolin-4´-O-glucoside) (Table 4) (Khallouki et al. 2015). These compounds were evaluated for their antioxidant effects using three tests namely DPPH, ABTS and ORAC. Furthermore, Chlorogenic acid showed strong antioxidant activity by the DPPH test with IC50 = 10.5 μM. For the FRAP test, 3,4-Dicaffeoylquinic acid gave an antioxidant effect with the lowest concentration (EC1 = 329 μM). However, neochlorogenic acid was able to inhibit fluorescence with 2.42 units (Khallouki et al. 2015).
3.5.3 Anticancer Activity
As part of promoting medicinal plants, the Cotula cinerea essential oil and extracts have also been targeted to assess their anticancer activity on several cancer cell lines. The methods and results of the antiproliferative activity of this plant have been compiled in Table 5.
The evaluation of the cytotoxic activity of Cotula cinerea extracts harvested in Algeria showed that the hydroethanolic extract has significant and important cytotoxic properties against the four cancer cell lines tested (Ghouti et al. 2018b). The HepG2 line (hepatocellular carcinoma) was inhibited by the lowest concentration and this by the two extracts (hydroethanolic GI50 = 31 ± 2 μg/mL and the infusion extract GI50 = 42 ± 4 μg/mL) (Ghouti et al. 2018b). Furthermore, the Cotula cinerea extracts also exhibited a moderate cytotoxic effect against the MCF-7, NCI-H460, HeLa, and PLP2 lines (Table 5).
The study of the antiproliferative activity of essential oil and three extracts (hexane, ethyl acetate, and n-butanol) of Cotula cinerea carried out by the MTT test against two cell lines RD and VERO (Table 5) showed that for the RD cell line, the hexane extract presents the highest cytotoxic effect with IC50 = 57.21 ± 3.43 μg/mL, followed by the ethyl acetate extract (IC50 = 187.52 ± 6.27 μg/mL), essential oil (IC50 = 173.05 ± 4.46 μg/mL), and lastly, we find the n-butanol extract (IC50 > 500 μg/mL). However, the Vero cell line was better inhibited by the essential oil with IC50 = 72.72 ± 2.18 μg/mL (Guaouguaou et al. 2018).
3.5.4 Other Pharmacological Activities of Cotula cinerea
The evaluation of other pharmacological activities of Cotula cinerea essential oil and extracts has been carried out by several research teams. The results of this work are grouped in Table 6.
Bettayeb et al. (2022) showed that the essential oil of the leaves and flowers of Cotula cinerea administered orally to mice exhibited low toxicity (LD50 = 1131.37 mg/kg, LD50 = 1264.91 mg/kg respectively).
Under the same conditions, Bettayeb et al. (2022) also showed that the essential oil of the leaves and flowers of Cotula cinerea possesses a significant anti-inflammatory effect at concentrations that do not exceed 300 mg/kg with percentages of 86, 16% for the leaves and 80.87% for the flowers (Table 6).
The experimental study of the acute oral toxicity of the aqueous extract of the dry and fresh aerial parts of Cotula cinerea at several doses (200, 400, 600 and 800 mg/kg) showed that these extracts did not cause any mortality or signs of toxicity in Wistar rats (Chlif et al. 2022).
On the other hand, the oral administration of the aqueous extract of the fresh and dry aerial parts at a dose of 200 mg/kg reduced the edema 3 h after the injection of carrageenan, with a percentage inhibition of 36.84% and 39.47%, respectively (Chlif et al. 2022). Furthermore, the study of the analgesic activity of the aqueous extract of fresh and dry aerial parts at a dose of 200 mg/kg and 400 mg/kg on rats by injecting 0.6% acetic acid showed a significant analgesic effect (Table 6). The aqueous extract of the dry aerial parts presents a higher percentage of inhibition (43.15% at the dose of 200 mg and 50.71% at the dose of 400 mg/kg) than that of the fresh parts (32.14% at the 200 mg dose and 45.51% at the 400 mg/kg dose) (Chlif et al. 2022).
By the same team and under the same conditions, Chlif et al. (2022) evaluated the antipyretic activity of the aqueous extract of the fresh and dry aerial parts of Cotula cinerea by the method Brewer’s yeast-induced pyrexia model in rats using two different concentrations (200 and 400 mg/kg). The results showed that these extracts possess a significant antipyretic activity after 4 h of administration. The aqueous extract of the fresh parts reduced the rectal temperature for the two concentrations tested (36.62 ± 0.24°C at the dose of 200 mg and 37.74 ± 0.25 °C at the dose of 400 mg/kg). However, the aqueous extract of the dry parts was more effective in reducing the rectal temperature at the dose of 400 mg/kg (37.73 ± 0.26°C and 36.86 ± 0.41 °C, respectively) (Chlif et al. 2022).
The evaluation of the anti-inflammatory activity of Cotula cinerea extracts (hydroethanolic and infusion extracts) was evaluated by the Murine macrophage-like RAW method 264.7 cells and quantified through the nitric oxide (NO) production (Table 6).
The results obtained showed a lower inhibition of the production of NO compared to the positive control and this by the two extracts (the hydroethanolic extract (EC50 = 105 ± 9 μg/mL) and the infusion extract (EC50 = 122 ± 6 μg/mL)) (Ghouti et al. 2018a, b).
The experimental study of acute oral toxicity of the Cotula cinerea essential oil and extracts administered orally at a dose of 2000 mg/kg showed that this plant showed no particular signs of toxicity, no lethality or no mortality was observed in treated mice (Guaouguaou et al. 2020a).
On the other hand, Guaouguaou et al. (2020a) used two methods (Tail flick and Hot plate) to evaluate the central analgesic activity of the Cotula cinerea essential oil and three extracts (hexane, ethyl acetate, and n-butanol) at a dose of 500 mg/kg. The results obtained showed that from the 45th minute, the reaction of the animals increased with the two methods used (Tail flick and Hot plate) and this for the four extracts tested and also for the positive control (Table 6). For analgesic effect observed by Tail flick method of ethyl acetate extract was 14.46 s, then n-butanol extract with 14.69 s, followed by essential oil (10.84 s) and of the hexane extract (10.22 s). However, the analgesic effect evaluated by the Hot plate method was higher than the first method (Tail flick). The n-butanol extract prolonged the reaction time to the thermal stimulus with a reaction time of 25.56 s, followed by the ethyl acetate extract (25.16 s). For the essential oil and the hexane extract, they showed moderate analgesic activity with a reaction time of 22.16 s and 22.25 s respectively (Guaouguaou et al. 2020a).
The evaluation of the antipyretic activity of Cotula cinerea of three extracts (ethyl ether, ethyl acetate, and n-butanol) by the method Brewer’s yeast-induced pyrexia model in rats (Table 6) showed that ether ethyl and ethyl acetate extract reduced fever with a percentage of 89.43% and 90.12%, respectively (Larhsini et al. 2002). In the same direction and always on the same extracts cited in the work of Larhsini et al. (2002), Markouk et al. (1999a, b) showed that the oral administration of three extracts (ethyl ether, ethyl acetate, and n-butanol) of Cotula cinerea caused no mortality at doses of 1, 2, 3, 4, 5, and 6 g/kg and also the animals remained without physiological abnormality. On the other hand, the ethyl acetate and n-butanol extracts showed a moderate analgesic effect with inhibition percentages of 50% and 40.21%, respectively (Table 6). However, the ethyl ether extract gave a percentage inhibition of (62.49%) which is close to that of the positive control (acetylsalicylic acid) with a percentage inhibition of 73.9% (Markouk et al. 1999a, b).
4 Conclusion and Future Perspectives
This review was conducted to report all studies containing Cotula cinerea that describe its botanical description, medicinal use, chemical composition, toxicity, and pharmacological properties. Ethnopharmacological studies indicate that Cotula cinerea is widely used in traditional medicine to treat colic, cough, diarrhea, migraine and digestive disorders.
On the other hand, the pharmacological and toxicological activities carried out in vivo and in vitro on the various extracts and the essential oil of Cotula cinerea revealed numerous effects, namely the antibacterial, antifungal, antioxidant, anticancer, anti-inflammatory, analgesic, and antipyretic activities.
The Cotula cinerea extracts and essential oil have shown remarkable antibacterial and antifungal effects against several bacteria and fungi. The antioxidant activity of Cotula cinerea extracts and essential oil was evaluated in vitro by several tests. The results obtained showed significant antioxidant effects. The results of the antiproliferative activity of Cotula cinerea extracts and essential oil show significant and encouraging cytotoxic effects against the cancer cell lines tested, and could then be considered as a source of new antitumor agents. The toxicity study reveals that the Cotula cinerea extracts and essential oil do not cause any signs of mortality or signs of toxicity when administered to animals orally. With regard to the analgesic effect of Cotula cinerea extracts and essential oil, the results obtained showed a significant analgesic effect and this for all the methods used.
This review is an opportunity by which we invite the authors to further pursue their research in order to understand the physiological mechanism behind the biological activities and pharmacological effects of Cotula cinerea extracts. Other pharmacological and toxicological tests seem more than necessary and other therapeutic virtues remain to be revealed in the hope of finding a place for this plant in modern pharmacy.
References
Agour A, Mssillou I, Mechchate H, Es-safi I, Allali A, Barnossi AE, Al Kamaly O, Alshawwa SZ, El Moussaoui A, Bari A et al (2022) Brocchia cinerea (Delile) Vis. Essential oil antimicrobial activity and crop protection against Cowpea weevil Callosobruchus maculatus (Fab.). Plan Theory 11:583
Ahmed AA, El-Sayed NH, el-Negoumy SI, Mabry TJ (1987) Flavonoids of Cotula cinerea. J Nat Prod 50(3):519–520
Atef C, Boualem M, Cherif MM, Youcef H, Azzedine C (2015) Chemical composition and antimicrobial activity of essential oils in Xerophytic plant Cotula cinerea Del (Asteraceae) during two stages of development: flowering and fruiting. J Appl Pharm Sci 5(3):029–034
Bellakhdar J (1997) La pharmacopée marocaine traditionnelle Médecine arabe ancienne et savoirs populaires. Ibis Press - Edition Le Fennec, p 764
Beloued A (2005) Les plantes médicinales d’Algérie. Ed. Office des publications universitaires (OPU), Algiers, p 284
Benhouhou S (2005) Cotula cinerea. In: A guide to medicinal plants in North Africa. Union Internationale pour la conservation de la nature et de ses ressources, pp 99–100
Bensizerara D, Menasria T, Melouka M, Cheriet L, Chenchouni H (2013) Antimicrobial activity of xerophytic plant (Cotula cinerea Delile) extracts against some pathogenic bacteria and fungi. Jordan J Biol Sci 147(916):1–6
Bettayeb Z, Benali FT, Benyamina A, Ouldchikh S, El-Amin SM, Salem H, Keddar YB (2022) Acute toxicity and anti-inflammatory activity of essential oils of Cotula cinerea from southwest Algeria. South Asian J Exp Biol 12(5):609–615
Boulos L (1983) Medicinal Plants of North Africa. Reference Publication Algonac, Michigan, p 286
Boussoula E, Ghanmi M, Satrani B, Alaoui MB, Rhafouri R, Farah A, Amusant N, Chaouch A (2016) Chemical quality, antibacterial and antifungal activities of cotula cinerea essential oil from South Morocco. Environ Sci 12:209–216
Bouziane M, Badjah-Hadj-Ahmed Y, Hadj-Mahammed M (2013) Chemical composition of the essential oil of Brocchia cinerea grown in south eastern of Algeria. Asian J Chem 25(7):3917–3921
Bouzidi LE, Abbad A, Fattarsi K, Hassani L, Leach D, Markouk M et al (2011) Chemical composition and anticandidal properties of the essential oil isolated from aerial parts of Cotula cinerea: a rare and threatened medicinal plant in Morocco. Nat Prod Commun 6(10):1934578X1100601021
Chlif N, Ed-Dra A, Diouri M, El Messaoudi N, Zekkori B, Filali FR, Bentayeb A (2021) Chemical composition, antibacterial and antioxidant activities of essential oils extracted from dry and fresh Brocchia cinerea (Vis.). J Biol Divers 22(4):1741–1749
Chlif N, Bouymajane A, El Majdoub YO, Diouri M, Filali FR, Bentayeb A et al (2022) Phenolic compounds, in vivo anti-inflammatory, analgesic and antipyretic activities of the aqueous extracts from fresh and dry aerial parts of Brocchia cinerea (Vis.). J Pharm Biomed Anal 213:114695
Cimmino A, Roscetto E, Masi M, Tuzi A, Radjai I, Gahdab C, Paolillo R, Guarino A, Catania MR, Evidente A (2021) Sesquiterpene Lactones from Cotula cinerea with Antibiotic Activity against Clinical Isolates of Enterococcus faecalis. Antibiotics 10:819
Cox PA, Balick MJ (1994) The ethnobotanical approach to drug discovery. Sci Am 270(6):82–87
Dendougui H, Seghir S, Jay M, Benayache F, Benayache S (2012) Flavonoids from Cotula Cinerea Del. Int J Med Arom Plants 2(4):589–595
Djahra AB, Benkaddour M, Benkherara S (2020) Evaluation of antimicrobial activity of medicinal plant Cotula cinerea against pathogenic strains. Int J 76(4/1)
Djellouli M, Moussaoui A, Benmehdi H, Ziane L, Belabbes A, Badraoui M et al (2013) Ethnopharmacological studyand phytochemical screening of three plants (Asteraceae family) from the region of South West Algeria. Asian J Nat Appl Sci 2:59–65
Djellouli M, Benmehdi H, Mammeri S, Moussaoui A, Ziane L, Hamidi N (2015) Chemical constituents in the essential oil of the endemic plant Cotula cinerea (Del.) from the South West of Algeria. Asian Pac J Trop Biomed 5(10):870–873
Duke JA, Janick J, Simon JE (1993) Medicinal plants and the pharmaceutical industry. In: New crops. John Wiley and Sons, New York, pp 664–669
Dupont F, Guignard JL (2004) Abrégés botanique systématique moléculaire. 13 édition révisée. Masson, p 283
El Bouzidi L, Abbad A, Fattarsi K, Hassani L, Leach D, Markouk M, Legendre L, Bekkouche K (2011) Chemical composition and Anticandidal properties of the essential oil isolated from aerial parts of Cotula cinerea: a rare and threatened medicinal plant in Morocco. Nat Prod Commun 6(10):1491–1494
Fathy KF et al (2017) Chemical composition and biological activity of essential oil from Cotula cinerea (Del.) growing wildly in the Middle East: a short review. Int J Pharmacogn Chinese Med 1(1):000103
Fournier G, Baghdadi H, Ahmed S, Paris M (1989) Contribution to the study of Cotula cinerea essential oil. Planta Med 55(6):580–580
Ghouti D, Lazouni HA, Moussaoui A, Sari DC (2018a) Chemical profile, in vitro antibacterial and antioxidant activities of Juniperus phoenicea L. and Cotula cinerea (Del.) essential oils from Southwestern Algeria. Phytothérapie 16(S1):S74–S83
Ghouti D, Rached W, Abdallah M, Pires TC, Calhelha RC, Alves MJ et al (2018b) Phenolic profile and in vitro bioactive potential of Saharan Juniperus phoenicea L. and Cotula cinerea (Del) growing in Algeria. Food Funct 9(9):4664–4672
Greger H, Hofer O (1985) Sesquiterpene-coumarin ethers and polyacetylenes from Brocchia cinerea. Phytochemistry 24:85–88
Guaouguaou FE, Bebaha MAA, Taghzouti K, Bouyahya A, Bakri Y, Dakka N, Es-Safi NE (2018) Cytotoxicological investigation of the essential oil and the extracts of Cotula cinerea and Salvia verbenaca from Morocco. BioMed Res Int 2018
Guaouguaou FE, Bebaha MA, Taghzouti K, Es-Safi NE (2020a) Phytochemical investigation, acute toxicity, central analgesic and antioxidant activities of extracts and essential oil of Cotula cinerea Del (Asteraceae). Curr Bioact Compd 16(2):164–173
Guaouguaou FE, Ahl Bebaha MA, Yadlapalli S, Taghzouti K, Es-Safi NE (2020b) Structural characterization of bioactive compounds in Cotula cinerea extracts by ultra-high-performance liquid chromatography with photodiode array and high-resolution time-of-flight mass spectrometry detectors. Rapid Commun Mass Spectrom 34(8):e8695
Halliwell B, Cross CE (1994) Oxygen-derived species: their relation to human disease and environmental stress. Environ Health Perspect 102(suppl 10):5–12
Hamdouch A, Bouzid HA, GHARBY S, Asdadi A, Achemchem F, Chebli B, Hassani LMI (2022) Chemical composition, antioxidant and antibacterial activities of Brocchia Cinerea from South-East of Morocco. Arabian J Med Aromat Plants 8(1):1–20
Hammiche V, Maiza K (2006) Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J Ethnopharmacol 105:358–367
Jakupovic J, Abdelaal M, Eid F, Bohlmann F, El-Dahmy S, Sarg T (1988) Further glaucolides and other sesquiterpene lactones from Brocchia cinerea. Phytochemistry 27:2219–2224
Kasrati A, Jamali CA, Bekkouche K, Wohlmuth H, Leach D, Abbad A (2015) Comparative evaluation of antioxidant and insecticidal properties of essential oils from five Moroccan aromatic herbs. J Food Sci Technol 52:2312–2319
Khallouki F, Sellam K, Koyun R, Ricarte I, Alem C, Elrhaffari L, Owen RW (2015) Phytoconstituents and in vitro evaluation of antioxidant capacities of Cotula cinerea (Morocco) methanol extracts. Rec Nat Prod 9(4):572
Lakhdar M (2018) Traditional uses, phytochemistry and biological activities of Cotula cinerea Del: a review. Trop J Pharm Res 17(2):365–373
Larbi BAM, Naima B, Elsharkawy E, Neghmouche NS (2018) Phytochemical characterization, in-vitro cytotoxic and antibacterial activity of Cotula cinerea (Delile) essential oil. J Nat Remedies 18:107–112
Larhsini M, Markouk M, Jaouhari JT, Bekkouche K, Lazrek HB, Jana M (2002) The antipyretic activity of some Moroccan medicinal plants. Phytother Res 16(S1):97–98
Mahboub N, Slimani N, Henni M (2021) Extraction and characterization of the biological activity of essential oils from Cotula cinerea in the region of El Oued (Algeria). Int J Nat Resour Environ 3(2):29–37
Mahran GH, Salah MA, Ansary SM (1976) A study of the flavonoid content of Cotula cinerea Del. Bull Fac Pharm (Cairo Univ.) 14:237
Markouk M, Lazrek HB, Jana M (1999a) Analgesic effect of extracts from Cotula cinerea (L). Phytother Res 13(3):229–230
Markouk M, Redwane A, Lazrek HB, Jana M, Benjama A (1999b) Antibacterial activity of Cotula cinerea extracts. Fitoterapia 70(3):314–316
Mehani M, Mehani I, Segni L, Morcia C, Terzi V (2019) Spontaneous plants of Septentrional Sahara of Algerian: Cotula cinerea and Chamomilla recutita and their biological study. J Appl Biol Sci 13(1):21–24
Mekhadmi NE, Mlik R, Ramdani M, Mouane A, Lakhdari W, Dehliz A et al (2023) Chemical composition and biological properties of Cotula cinerea essential oil from Sahara of Algeria. Biocatal Agric Biotechnol 47:102613
Menaa F, Menaa A, Tréton J (2014) Polyphenols against skin aging. In: Polyphenols in human health and disease. Academic Press, London, pp 819–830
Metwally MA, El-Dahmy S, Jakupovic J, Bohlmann F, Dawidar AM, Metwally SA (1985) Glaucolide-like sesquiterpene lactones from Cotula cinerea. Phytochemistry 25:255–257
Ozenda P (1967) Le rôle des végétaux en radioécologie continentale: le cas du site de Grenoble. Bull, d’inform. Scient, et techn. du CE A, 119:3–8
Ozenda P (1993) Flora of the Sahara. French National Center for Scientific Research, Paris, p French, 270
Quézel P, Santa S (1962) In: Tome II (ed) Nouvelle flore de l’Algérie et des régions désertiques méridionales. CNRS, Paris, p 1170
Salhi N, Mohammed Saghir SA, Terzi V, Brahmi I, Ghedairi N, Bissati S (2017) Antifungal activity of aqueous extracts of some dominant Algerian medicinal plants. BioMed Res Int 2017
Teles YCF, Souza MSR, de Souza MFV (2018) Sulphated flavonoids: biosynthesis, structures, and biological activities. Molecules 23(2):480–490
Tyler VE (1994) Herbs of choice: the therapeutic use of phytomedicinals. Pharmaceutical Products Press (imprint of Haworth Press, Inc.)
World Health Organization (2008) Herbal medicine research and global health: an ethical analysis. Bull World Health Organ 86:577–656
World Health Organization (2012) Traditional medicine: from ancient texts to new drugs. Bull World Health Organ 90(8):557–632
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Guaouguaou, FE., Es-Safi, N.E. (2023). Cotula cinerea as a Source of Natural Products with Potential Biological Activities. In: Cruz, J.N. (eds) Drug Discovery and Design Using Natural Products. Springer, Cham. https://doi.org/10.1007/978-3-031-35205-8_17
Download citation
DOI: https://doi.org/10.1007/978-3-031-35205-8_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-35204-1
Online ISBN: 978-3-031-35205-8
eBook Packages: MedicineMedicine (R0)