Abstract
The current study evaluated the effects of ultrafine granulated blast furnace slag (GBFS) on the compressive strength development of Portland cement mortar. The previous studies have investigated the effects of ultrafine slag (UFS) with sizes of around 3–5 μm on the concrete performance. Despite the great potential of smaller size GBFS particles to enhance the concrete performance, such effects are not clearly known. In this study, UFS particles with sizes of around 1 and 0.6 μm were prepared using a planetary ball mill. UFS was then used to replace Portland cement by 5, 10, and 15 wt.% in preparing mortar specimens. The compressive strength of the specimens was measured at different ages. Isothermal calorimetry was also used to provide insight into the strength development mechanisms of specimens. The results showed that UFS powders significantly increased the 1-d and 3-d compressive strength of mortar specimens by up to 46 and 52%, respectively. The compressive strength increase was proportional to the replacement level of Portland cement with UFS powders. Compared to the 1 μm UFS, only a minor enhancement in 1-day compressive strength of the specimens containing 0.6 μm UFS was observed. The 28-day compressive strength of all specimens was similar regardless of their UFS content. The isothermal calorimetry results showed that the UFS powders increased the early hydration rate of Portland cement. A preliminary analysis of energy consumption of UFS preparation showed that partial replacement of Portland cement with UFS could result in cementitious binders with less GHG emission.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Portland cement concrete is the most widely used man-made product on the earth. About 30 billion tons of Portland cement concrete are produced globally per year [9]. This has made the concrete industry one of the largest consumers of natural resources and a major source of anthropogenic greenhouse gas (GHG) emissions. The production of Portland cement is the primary source of GHG emissions in the concrete production process. Considering the increasing demand for concrete due to population growth in urban areas and the need for concrete structures and infrastructure, the cement and concrete industry should pursue applicable strategies to satisfy both the increasing demand and the need for reducing the environmental impacts of the cement and concrete production.
One of the low-cost and practical methods of reducing the GHG emissions of the cement and concrete industry is the utilization of supplementary cementitious materials (SCMs) as Portland cement replacements in concrete manufacturing. The use of SCMs as partial substitutions for Portland cement can have economic and environmental advantages and benefit the concrete performance. However, due to the slower reactivity of most SCMs compared to the Portland cement at early ages, the early-age compressive strength of the cementitious materials with SCMs has mainly resulted from the hydration of Portland cement [15]. Hence, the appropriate replacement level of Portland cement with SCMs should be carefully determined. When a small or moderate amount of SCMs is used in the cementitious binder, SCMs can increase the hydration rate of the Portland cement because of the filler and nucleation effect at early ages. In addition, the later-age compressive strength of concrete will be further enhanced due to the pozzolanic and/or hydraulic reaction of SCMs. However, for the high replacement levels of Portland cement with SCMs, due to the lower reactivity of SCMs and lower Portland cement content, the obtained cementitious binder contains less hydration products at early ages. Therefore, the early-age compressive strength of the resulted concrete is typically low. That would be a limiting factor toward using large volumes of SCMs in many applications.
One of the methods for increasing the reactivity of SCMs is reducing their particle size through fine or ultrafine grinding of particles. Grinding of the particles produces powders with an increased surface area and reactivity. Nowadays, different types of milling with a high-energy efficiency have been invented to reduce the particle size of different materials into different ranges. The previous studies investigated the fine or ultrafine grinding of fly ash, granulated blast furnace slag (GBFS), and kaolinite clay [8, 10, 11, 16].
For the case of GBFS, Kumar et al. [8] used attrition milling to obtain ultrafine GBFS with a mean diameter of around 4 μm. The 28-d compressive strength of mortar samples containing 60 and 70 wt.% of ultrafine GBFS was approximately 15 and 36% higher than that of the sample with only Portland cement. Bouaziz et al. [5] used a high-energy planetary ball mill and obtained ultrafine GBFS with a mean diameter of 5 μm. The obtained particles were then used to replace 45 wt.% of Portland cement in preparing paste samples. Both short and long-term compressive strengths of the samples containing ultrafine GBFS obtained from high-energy milling were improved by up to 10% compared to the reference Portland cement paste.
Sharmila and Dhinakaran [14] utilized ultrafine GBFS with a particle size of 5 μm to replace 5, 10, and 15 wt.% of Portland cement in preparing high-strength concrete. They observed that the ultrafine GBFS accelerated the compressive strength gain of concrete at the early ages. The 7-d compressive strength of concretes with 5, 10, and 15 wt.% of ultrafine GBFS was equal to 63%, 74%, and 60% of their 28-d compressive, respectively. Another study obtained GBFS particles with a mean diameter of 3 μm through a wet-grinding process using water as a wetting agent in a stirred media mill [16]. The obtained slurry was utilized to replace 50% of Portland cement in mortar specimens to measure the activity index of the obtained ultrafine GBFS. The results showed that the activity index of GBFS prior to the wet grinding was 90.8%, which increased to 126.5% for GBFS after 50 min of milling [16].
Most of the previous studies reduced the particle size of GBFS into the ranges of 3–5 μm. However, the effects of smaller size GBFS particles on the performance of cementitious systems are not clearly understood. The current study aims to fill this research gap by investigating the influence of ultrafine GBFS with a mean particle size of about 1 μm and smaller on the hydration and mechanical properties of cementitious mortar samples. Compressive strength measurements and isothermal microcalorimetry were conducted to investigate the strength development and hydration heat of samples. Finally, the energy efficiency, limitations, and advantages of grinding the GBFS particles were also discussed.
2 Materials and Method
2.1 Materials
Ordinary Portland cement type general use cement (GU/GUL cement, QUIKRETE) complying with the Canadian standard (CSA A3001) was used in this study. A commercial GBFS (Lafarge) was used for preparing the ultrafine slag (UFS) powders. In addition, a high-early strength Portland cement (HE cement) from Lafarge was utilized to compare the early-age strength development of the proposed mixtures with the HE cement. The oxide composition of the cementitious materials is presented in Table 1. A polycarboxylate-based superplasticizer (Glenium 3030, Master Builders Solutions) was also used in preparing the mortar samples incorporating UFS particles to keep the slump flow of the samples constant.
2.2 Sample Preparation and Testing
The GBFS was ground to ultrafine powders with two different particles sizes (UFS1 and UFS2) using a laboratory-scale planetary ball mill (Restch PM 100 CM). The UFS particles were characterized by dynamic light scattering (DLS) and scanning electron microscopy coupled with energy dispersive X-ray spectroscopy (SEM/EDX).
Reference mortar samples were prepared according to the ASTM C109 (2021) for the compressive strength test. The obtained UFS powders were used to replace the GU cement at 5, 10, and 15 wt.%, as indicated in Table 2. The UFS powders were first shear-mixed in water containing the superplasticizer for preparing the samples. Then, the mixtures were mechanically mixed according to the ASTM C305 [3]. The mortars were cast in cubic molds with the dimension of 50 mm × 50 mm × 50 mm. The samples were de-molded after 24 h and were kept in a fog room until the specified testing age. The compressive strength tests were performed at ages of 1, 3, 7, and 28 d, according to the ASTM C109 [1].
Cement paste samples with a water to cementitious materials ratio of 0.42 were also prepared for the isothermal calorimetry test. The test was conducted using an isothermal microcalorimeter (TAM Air, TA instruments) according to the [2]. Quartz sand with the same thermal mass as the tested specimens was put in reference cells to measure the baseline of the experiments 24 h before the main experiments. Approximately 40 g of the cementitious pastes, according to the binder composition of Table 2, were prepared in a plastic container. Around 9–10 g of each paste were poured into a glass ampoule and then were placed in testing cells of the calorimeter approximately 5 min after mixing the binders with water. The heat evolution of samples was recorded for 96 h.
3 Results and Discussion
3.1 Characteristics of the UFS Particles
The particle size of the UFS powders as determined by the DLS method is presented in Fig. 1. The results are the average of 3 measurements. The average particle size of the UFS powder was around 1.0 and 0.6 µm for UFS1 and UFS2, respectively.
Figure 2 shows the SEM images of UFS1 and UFS2 particles compared to the as-received GBFS. EDX analysis was also used to define the elemental composition of the powders. Before and after milling, the main elements detected for the obtained powders were Ca, Si, Al, and Mg. Possible iron and chromium contamination of the final powder by elements from balls and jars was not detected.
3.2 Compressive Strength of the Mortar Samples
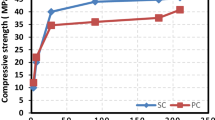
Figure 3 shows the compressive strength of the mortar samples at the ages of 1, 3, 7, and 28 d. The compressive strength of the samples containing UFS particles was higher than that of the samples made with only GU cement at the early ages. The compressive strength increase was more pronounced at the higher replacement levels of the GU cement with the UFS particles. The average 1-d compressive strength of R-GU was 11.2 MPa, while the average 1-day compressive strength of UFS1-15 and UFS2-15 was 15.2 and 16.4 MPa, respectively. The average 3-d compressive strength of R-GU, UFS1-15, and UFS2-15 was 20.8, 31.9, and 32 MPa, which indicated an approximately 52% increase due to the use of the UFS particles. Mortar samples containing 10 and 15 wt.% of UFS gained more than 70% of their 28-d compressive strength at the age of 3 d. The 1-d and 3-d compressive strength of R-GU samples were around 65% of the compressive strength of R-HE samples at a similar age. Replacing GU cement with 5, 10, and 15 wt.% of UFS increased the 1-d and 3-d compressive strength of samples to the level of around 75, 83, and 90% of the compressive strength of R-HE samples at similar ages.
Only minor enhancement in the 1-d compressive strength of the mixtures was observed for samples containing UFS2 compared to those containing UFS1. At 5, 10, and 15 wt.% replacement, the average 1-d compressive strength of samples with UFS2 was only 6, 5, and 8% higher than those of the samples with UFS1, respectively. At later ages (3, 7 and 28 d), no significant difference was observed between samples containing UFS1 and UFS2.
The enhancing effect of UFS on the compressive strength of the samples was reduced at later ages. The average 7-d compressive strength of R-GU was 27.9 MPa, while for the mixtures containing UFS, the average 7-d compressive strength varied between 33.3 and 38.3 MPa. At the age of 28 days, all the samples indicated approximately a similar compressive strength value regardless of their UFS content and size, and the average compressive strength values varied between 41 and 45 MPa for different samples.
3.3 Heat Flow Measurements
Figure 4 shows the normalized heat flow and normalized cumulative heat based on the cement mass for different pastes for a period of 96 h. R-GU hydration heat curve included two peaks. The first peak indicated the formation of calcium-silicate-hydrate (C–S–H) due to the reaction of tri-calcium-silicate (C3S). The second peak was related to ettringite formation due to the hydration of tri-calcium aluminate (C3A) in the presence of gypsum [12].
When UFS1 and UFS2 were added to the mixtures, no significant change was observed in the initiation of the acceleration period. However, a considerable increase in the intensity of the first peak occurred. These changes were more pronounced by increasing the proportion of the UFS particles in the binders. The increased heat flow was likely due to the presence of extra nucleation sites provided by the UFS particles for the formation of C–S–H. In addition, the shearing rate possibly increased due to reduced inter-particle distance in systems containing UFS. The increase in shearing rates speeds up the ion dissolution from the cement grains and results in the enhanced formation of C–S–H [4]. The presence of UFS particles also altered the position of the first peak. In the pastes with UFS, the first peak occurred faster (shifting to the left), and the change was more evident by increasing the UFS content in the binder.
The incorporation of UFS into the cementitious systems also influenced the position and intensity of the second peak. The changes in the second peak were more pronounced by increasing the content of UFS powders in the binders. In UFS1-15 and UFS2-15 curves, the position of the first and second peaks is pretty close to each other. Zunino and Scrivener [18] stated that the sulfate ions are adsorbed on the surface of C–S–H formed from the hydration of C3S. An increase in the rate of C–S–H formation increases the sulfate adsorption on the C–S–H surface; therefore, the depletion of gypsum occurs faster. After depletion of sulfate ions, sulfate is desorbed from the C–S–H and becomes available to react with C3A to form ettringite [18].
Figure 5 presents the heat flow per gram of cement for UFS1-15 and UFS2-15 and R-GU samples for a better comparison of the effect of the particles size of UFS powders on the heat flow curves. The heat flow and hydration peaks of UFS1-15 and UFS2-15 are generally similar. Finer particles of UFS2 had only a minor enhancing effect on the amount of first and second peaks compared to the UFS1 particles. However, the cumulative heat of these samples after 96 h of hydration is approximately the same and is about 30% higher than that of R-GU. The same trend was also observed for mixtures with 5 and 10% replacement of GU with UFS2 and UFS1. The cumulative heat of samples with 5 and 10% GU replacement with the UFS powders was about 7 and 15% higher than that of R-GU at a similar age.
4 Potential Environmental Advantages and Disadvantages of Using Ultrafine Slag
Reducing the particle size of GBFS to UFS needs extra energy consumption for grinding that produces additional GHG emissions. In most cases, electricity is used as energy for grinding. The extra electrical energy and GHG emissions should be considered to evaluate the environmental advantages and disadvantages of UFS utilization in concrete. The GHG emission of electricity generation and consumption varies between different regions. For example, in different regions of Canada, the GHG emission for 1 kWh electricity consumption varied between 0.0013 and 0.89 kg of CO2-eq in 2019 [7]. The GHG emissions associated with electricity consumption are higher in regions where sources such as coal and natural gas are used for electricity generation. On the other hand, electricity generation by nuclear, hydro, wind, and solar sources in some areas results in less GHG emissions [7].
Few studies have reported the required electricity consumption for reducing the particle size of SCMs and, in particular, GBFS. Different types of mills with different energy efficiency have been used for the ultrafine grinding of SCMs [6, 8, 16]. The final particle size of the obtained ultrafine powders after grinding was larger than or equal to 2 µm in these studies. In one of the studies, Sebaibi and Boutouil [13] stated that increasing the Blaine specific surface area of each ton of GBFS from 4500 \(\frac{{{\text{cm}}^2 }}{{\text{g}}}\) (standard GBFS) to 9000 \(\frac{{{\text{cm}}^2 }}{{\text{g}}}\) (GBFS with the mean diameter of 2.5 µm) required 300 kWh more electrical energy. In another study, [6] used a supersonic steam jet mill to reduce the GBFS particle size and reported that 17 kWh electrical energy and 0.88 tons of steam were required to produce one ton of GBFS with a mean particle size of 2.25 µm.
In the current study, the UFS powders were obtained by a laboratory-scale planetary ball mill. The electricity consumption associated with the production of the UFS particles of our study can be different from, and likely higher than, that in bulk production of UFS. However, the electricity consumption analysis of this study showed that the GHG emission resulted from GBFS grinding to obtain 1 ton of UFS1 could range from approximately 11–7500 kg of CO2-eq in different regions of Canada. The GHG emission of grinding will be the lowest in provinces such as Quebec and Manitoba. The GHG emission associated with the production of each ton of Portland cement is 700–900 kg of CO2-eq [17]. Thus, partial replacement of Portland cement with UFS1, at least in provinces with a low GHG emission for electricity generation, results in cementitious binders with less GHG emission. The results presented in Sect. 3.2 also indicated that the use of 5–15 wt.% UFS with type GU Portland cement resulted in a higher early-age compressive strength of the Portland cement mortars. Hence, the utilization of UFS may allow a high replacement level of Portland cement with conventional SCMs in cementitious binders without compromising the early-age properties of the concrete mixtures. Moreover, the compressive strength results showed that the combination of UFS and type GU Portland cement could be considered an alternative for HE cement. Production of UFS2 required further grinding which results in higher electricity consumption and higher GHG emissions compared to the UFS1 production. The compressive strength measurements, however, showed that the enhancing effects of the UFS1 and UFS2 on the compressive strength of the mortars were relatively similar. Thus, a more comprehensive study on the effects of UFS2 particles on the engineering properties of concrete and its environmental impacts will be needed to determine the suitability of using UFS2 in concrete production.
It is also worth mentioning that partial replacement of Portland cement with GBFS also provides other benefits in addition to the potential carbon footprint reduction. The GBFS utilization in concrete avoids landfilling of slag. In addition, reducing the share of Portland cement in concrete preserves natural resources required for the production of Portland cement. Analyzing the effects of replacing type GU Portland cement with UFS on different environmental impacts through a comparative life cycle assessment (LCA), that considers the effects of regional availability of slag, energy use and efficiency of grinding facilities, and transportation of UFS, can provide more insights into the potential advantages and disadvantages of using UFS for different projects.
5 Conclusion
The current study assessed the effect of using UFS as partial Portland cement replacement on the compressive strength, heat flow, and environmental impacts of cementitious mixtures. Two different ultrafine slags with average particle sizes of around 1 (UFS1) and 0.6 μm (UFS2) were obtained by ultrafine grinding and used as 5–15 wt.% replacement of the Portland cement in cementitious mixtures. The main conclusions are summarized as follows:
-
Substitution of type GU Portland cement with 5–15 wt.% UFS powders increased the compressive strength of mortar samples at early ages (1, 3, and 7 d). The improving effects of the UFS powders were more pronounced at 1 and 3 d. This resulted in mortar samples with superior compressive strength, comparable to that of mortars prepared with the HE cement at similar ages. The use of finer UFS2 powders slightly enhanced the 1-d compressive strength of the mortar samples compared to the UFS1 utilization. Its effects, however, were similar to those observed for the UFS1 powders at other testing ages.
-
The calorimetry results indicated that the presence of UFS powders increased the heat evolution of the Portland cement systems. The increase in the heat flow was enhanced by increasing the replacement level of the GU cement with the UFS powders. However, there was only a slight difference in the heat flow curves of samples containing UFS1 and UFS2, and both samples resulted in a similar cumulative heat after 96 h of hydration.
-
A preliminary analysis showed that depending on the source of electricity, obtaining the UFS1 powders could have lower GHG emissions than that required for the Portland cement production. Thus, replacing Portland cement with UFS1 can reduce the embodied GHG emissions of concrete mixtures in addition to enhancing their early-age properties. It will also have other environmental benefits such as landfill avoidance of slag and preservation of the natural resources used for the Portland cement production. Further grinding of UFS1 particles to achieve UFS2 might not be justified due to the small enhancing effects on the compressive strength of the mortar samples of this study. A more comprehensive analysis of the engineering and environmental performance of concrete mixtures incorporating UFS particles will be needed to determine the optimum particle size of the UFS for specific concrete applications.
References
ASTM C109/109M-21 (2021) Standard test method for compressive strength of hydraulic cement mortars (Using 2-in. or cube specimens). ASTM International, West Conshohocken, PA, pp 1–10
ASTM C1702 (2017) Standard test method for measurement of heat of hydration of hydraulic cementitious materials using isothermal conduction calorimetry. ASTM International, West Conshohocken, PA
ASTM C305-20 (2020) Standard practice for mechanical mixing of hydraulic cement pastes and mortars of plastic consistency. ASTM International, West Conshohocken, PA
Berodier E, Scrivener K (2014) Understanding the filler effect on the nucleation and growth of C–S–H. J Am Ceram Soc 97(12):3764–3773
Bouaziz A, Hamzaoui R, Guessasma S, Lakhal R, Achoura D, Leklou N (2017) Efficiency of high energy over conventional milling of granulated blast furnace slag powder to improve mechanical performance of slag cement paste. Powder Technol 308:37–46
Duan S, Wu H, Liao H, Cheng F (2021) Design and experimental study of a blended cement containing high-volume solid waste activated ultrafine powder. Constr Build Mater 303:124504
Environment and Climate Change Canada (2019) 2019 National Inventory Report (NIR)—Part 3
Kumar S, Kumar R, Bandopadhyay A, Alex TC, Kumar BR, Das SK, Mehrotra SP (2008) Mechanical activation of granulated blast furnace slag and its effect on the properties and structure of Portland slag cement. Cem Concr Compos 30(8):679–685
Monteiro PJM, Miller SA, Horvath A (2017) Towards sustainable concrete. Nat Mater 16(7):698–699
Norhasri MSM, Hamidah MS, Fadzil AM, Megawati O (2016) Inclusion of nano metakaolin as additive in ultra high performance concrete (UHPC). Constr Build Mater 127:167–175
Paul KT, Manna ÆSKSÆI, Nando KKCÆGB (2007) Preparation and characterization of nano structured materials from fly ash: a waste from thermal power stations, by high energy ball milling. Nanoscale Res Lett 2(8):397–404
Scrivener KL, Juilland P, Monteiro PJM (2015) Advances in understanding hydration of Portland cement. Cem Concr Res 78:38–56
Sebaibi N, Boutouil M (2020) Reducing energy consumption of prefabricated building elements and lowering the environmental impact of concrete. Eng Struct 213:110594
Sharmila P, Dhinakaran G (2016) Compressive strength, porosity and sorptivity of ultra fine slag based high strength concrete. Constr Build Mater 120:48–53
Skibsted J, Snellings R (2019) Reactivity of supplementary cementitious materials (SCMs) in cement blends. Cem Concr Res 124:105799
Wang Y, He X, Su Y, Yang J, Strnadel B, Wang X (2019) Efficiency of wet-grinding on the mechano-chemical activation of granulated blast furnace slag (GBFS). Constr Build Mater 199:185–193
WBCSD I (2009) Cement technology roadmap: carbon emissions reductions up to 2050. World business council for sustainable development and international energy agency
Zunino F, Scrivener K (2019) The influence of the filler effect on the sulfate requirement of blended cements. Cem Concr Res 126:105918
Acknowledgements
The authors would like to thank the financial and technical support of the Canada Masonry Design Centre and Canadian Concrete Masonry Producers Association (CCMPA). The financial support provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) is also greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 Canadian Society for Civil Engineering
About this paper
Cite this paper
Ghasemalizadeh, S., Khoshnazar, R. (2024). Effect of Ultrafine Granulated Blast Furnace Slag on the Strength Development of Portland Cement Mortar. In: Gupta, R., et al. Proceedings of the Canadian Society of Civil Engineering Annual Conference 2022. CSCE 2022. Lecture Notes in Civil Engineering, vol 359. Springer, Cham. https://doi.org/10.1007/978-3-031-34027-7_56
Download citation
DOI: https://doi.org/10.1007/978-3-031-34027-7_56
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-34026-0
Online ISBN: 978-3-031-34027-7
eBook Packages: EngineeringEngineering (R0)