Abstract
The normal sleep cycle is defined by variations in blood pressure, heart rate, and cardiac events. While these variations may be cardioprotective in a normal pattern of sleep, sleep apnea disrupts the normal sleep-cardiovascular interaction. Sleep-disordered breathing is common in people with heart failure, increasing the cardiovascular morbidity and mortality of these patients. In addition, obstructive and central sleep apnea can happen in the same patient, increasing the difficulty of diagnosis and treatment. Due to the increased risk of cardiovascular events, the correct diagnosis and approach are crucial in these patients. The authors intend to present the most recent data about the treatment of these conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Heart failure
- Sleep-disordered breathing
- Obstructive sleep apnea
- Central sleep apnea
- Noninvasive ventilation
- Treatment
Introduction
Sleep-disordered breathing (SDB) is common in patients with cardiovascular diseases, including in heart failure (HF) [1]. Patients with HF often have sleep problems due to periods of pulmonary fluid overload, which leads to classical symptoms of orthopnea, paroxysmal nocturnal dyspnea, and nocturia. A recent position paper of the European Society of Cardiology showed that almost 75% [2] of patients with HF report some degree of sleep disruption. Despite that, many patients remain undiagnosed in our current clinical practice.
SDB is associated with several major pathophysiological abnormalities [3], which include the following:
-
1.
Intrathoracic pressures swings (which contribute to changes in ventricular repolarization and increased risk of sudden cardiac death)
-
2.
Sleep reduction and fragmentation (decreased sleep efficiency and increased inflammation)
-
3.
Cyclical hypoxemia and reoxygenation
-
4.
Sympathetic system activation (worsened by coexisting HF)
-
5.
Endothelial dysfunction and increased thrombosis
Due to the increased risk of cardiovascular events as a consequence of these abnormalities, an adequate approach is crucial. In reality, several studies [3,4,5] showed that coexisting HF and sleep apnea increase the risk of developing malignant ventricular arrythmias leading to increased risk of sudden cardiac death.
SDB can be divided in two main groups: (1) obstructive sleep apnea (OSA) and (2) central sleep apnea (CSA). Both OSA and CSA disrupt the normal sleep-heart interaction and impose similar autonomic, chemical, mechanical, and inflammatory burden in the cardiovascular system. In addition, OSA and CSA can co-occur in the same patient, increasing the difficulty in establishing a diagnosis and treatment scheme.
Despite OSA and CSA having a different etiology, they share a common pattern of recurring cycles of apnea, hypoxia, hypercapnia, and arousal from sleep, and both appear to be associated with increased sympathetic activity. As a consequence, a myocardial oxygen mismatch of demand and supply can occur, and the already debilitated heart just isn’t able to deal with this increased workload, which paves the way to the establishment of ischemia or arrhythmias [1, 3, 4]. As HF becomes more severe, prevalence of OSA and CSA becomes more and more common leading to an increased risk of complications.
Obstructive Sleep Apnea
Definition
Over 50% of patients with HF have SDB [1], which includes obstructive, central, or mixed sleep apnea. OSA prevalence varies between 20 and 60% in this population and is typically more associated with heart failure with preserved ejection fraction (HFpEF). However, it may contribute to the progression of both heart failure with reduced (HFrEF) and preserved ejection fraction, potentially reflecting an important modifiable risk factor [6].
Sleep apnea is characterized by repeated breathing pauses (apneas or hypopneas) that may result in sleep fragmentation, excessive daytime sleepiness, and blood oxygen desaturation. According to the American Academy of Sleep Medicine, apneas are defined as a drop of at least 90% in respiratory flow from baseline during at least 10 s and hypopneas as a drop of at least 30% during at least 10 s accompanied by an oxygen desaturation of at least 3% or by an arousal [7]. In OSA, contrarily to what happens in CSA, there are thoracoabdominal excursions, generating a negative intrathoracic pressure that will be central to its pathophysiological consequences.
Pathophysiology
OSA is a heterogenous disorder with both anatomical and functional mechanisms occurring separately or in combination and contributing to upper airway occlusion. They can be roughly divided into four phenotypic traits [8]: high loop gain, reduced pharyngeal dilator muscle activity, an anatomically narrow pharyngeal airway, and a low respiratory arousal threshold leading to recurrent awakenings.
Loop gain reflects the magnitude of change in respiratory drive-in response to a given disturbance. When it is excessive, like in OSA, a minor increase in PaCO2 provokes an excessive increase in ventilation (may cause the inward suction of the upper airway muscles). The consequent fall of PaCO2 results in an also exaggerated reduction of the respiratory drive that may well be an apnea if it drops below the apneic threshold. On the other hand, these patients also exhibit a low CO2 reserve, which is defined as the difference between eupneic PaCO2 and the apnea threshold, and that is generally associated with a ventilatory overshoot that will precipitate a central apnea [1, 8].
Predisposing factors for upper airway collapse like old age, male sex, obesity, high neck circumference, and retrognathism are also relevant in HF-related OSA. However, the tendency for obstruction is even more pronounced in HF owing to pharyngeal edema caused by rostral fluid shift during sleep. The pharyngeal dilator muscle activity exerted by the genioglossus is also reduced during sleep, increasing even more the risk of airway collapse.
Pathophysiological Consequences
Obstructive sleep apnea may accelerate HF progression through various mechanisms. The negative intrathoracic pressure generated by the respiratory muscles trying to inspirate against a closed airway increases venous return to the right heart and, consequently, the preload, causing the septum to move leftward. The same negative pressure is also responsible for increasing transmural pressure and left ventricular afterload. As a result, left ventricular function can be seriously compromised in OSA [1, 3].
Apnea and hypopnea are also responsible for other mechanisms that affect the cardiovascular functioning, like sympathetic nervous system activation (acutely related to arrhythmias and ischemia, chronically to left ventricle hypertrophy), hypoxemia (related to oxidative stress and inflammation), and pulmonary vasoconstriction (may cause cor pulmonale) [9].
Diagnosis
The gold standard test [1, 3] for sleep disorders is the in-hospital polysomnography (PSG). However, this is a costly exam that requires specialized personnel and facilities and, as such, is not widely available. Multichannel sleep polygraphy (PG) or home sleep apnea test (HSAT) with oxygen saturation are other options that have the advantage of being performed out of the hospital and without the need of attending personnel. They have some important limitations that should be known in order to avoid underdiagnosis. Firstly, the sleep data is less precise with a HSAT and doesn’t allow the calculation of the apnea-hypopnea index (AHI). In its place, we calculate the respiratory event index (REI). Secondly, hypopnea with arousal but without significant desaturation cannot be picked up without an electroencephalogram monitoring. Thirdly and finally, PSG allows an easier identification of periods of wakefulness, and therefore AHI can be recorded during sleep only, whereas in PG AHI is calculated throughout the recording period regardless of sleep pattern, and this may lead to an underestimation of the severity of the sleeping disorder [1, 3].
A diagnosis of OSA requires at least an AHI/REI of 5/h. Then the disease severity is categorized in three groups according to the AHI/REI cutoffs: mild between 5 and 15, moderate between 15 and 30, and severe with more than 30 events per hour [1, 3].
However, it’s difficult to identify which patients should be tested for OSA, as most of them don’t present with typical symptoms like daytime sleepiness thanks to the overactivated sympathetic nervous symptoms, rendering screening questionnaires like the Epworth Sleepiness Scale useless. Patient’s characteristics and risk factors and input from a bedpartner can be a good starting point. On a next step, a recording of nocturnal oxygen saturation via a finger probe can indicate those who have to undergo a more thorough sleep study (those who present with at least 12.5 desaturations/hour are at bigger risk of developing sleep-disordered breathing) [1, 3].
Treatment
Ideally, each patient should be treated according to his/her phenotype.
High Loop Gain
Two medications have been used to treat OSA without HF and were previously used to treat CSA associated with HF. Acetazolamide reduces loop gain by increasing plant gain but also increases chemosensitivity to CO2 [10]. Some studies indicated increased carbonic anhydrase activity is reportedly associated with severity of OSA and related hypoxemia [11]. As such, a combination of three effects (decreasing plant gain, being a mild diuretic, and moving the alkalotic pH toward normal values, further improving periodic breathing) can make this agent a useful weapon against OSA, but more trials are needed.
Oxygen is another drug therapy that downregulates the loop gain. However, the few studies that do exist did not include OSA patients with HF [10].
Reduced Pharyngeal Dilator Muscle Tone
Hypoglossal nerve stimulation is currently somewhat popular as a therapeutic target, but it has been used mainly to treat OSA in the general population or in patients who reject or don’t tolerate positive airway pressure (PAP) devices.
Treatment with noradrenergic agonists (e.g., tricyclic antidepressants) may represent another possible therapeutic target, as noradrenergic withdrawal may be responsible for the pharyngeal hypotonia that occurs during the non-rapid eye movement sleep. However, associated cardiotoxicity may limit their use in the population with HF.
Tongue and pharyngeal training may also be considered [10].
Anatomically Narrow Airway
Oral appliances have been used to treat OSA, including in patients with HF. These include the mandibular advancement splints (MAS) that work by lifting the mandible forward and stabilizing the upper airway and that can be particularly useful with patients with retrognathism [3, 5].
Upper airway surgery may also be considered, but the current evidence is mixed. Recently, patients with moderate or severe OSA who didn’t tolerate or refused PAP or MAS that underwent surgery reported an improvement regarding the daytime sleepiness, but evidence is still scarce [3].
Low Respiratory Arousal Threshold
This might represent the more controversial therapeutic target. The idea behind the use of hypnotics like trazodone is that if the arousal could be delayed, the dilator muscles could be recruited and reestablish normal breathing. However, concerns regarding possible deleterious effects of prolonging the obstructive events are valid and can’t be, at the moment, excluded.
Multimodal Approaches
Exercise and weight loss probably have a pleotropic effect, reducing the rostral movement of fluid and promoting the stabilization of the ventilation. Optimization of the medical therapy should also not be forgotten, as it will also reduce the rostral movement of fluid and improve cardiac output [10].
PAP devices, which include continuous (CPAP) and bilevel (BiPAP) devices, constitute the most effective treatment option for patients with OSA and HF, even if their long-term adherence is still very problematic.
CPAP is probably the more used modality, and its efficacy can be explained by various mechanisms, which include prevention of the pharynx collapse, generation of a positive end-expiratory pressure that prevents alveoli collapse secondary to pulmonary edema, reduced work of breathing, higher alveolar recruitment, improved gas exchanges, and reduced right to left intrapulmonary shunting of the blood. Besides that, the reduction of both left ventricle preload and afterload may improve cardiac function in some patients.
However, it should be noted that the vast majority of the evidence that nowadays supports the use of CPAP in patients with HF concerns HFrEF and not HFpEF that may actually be more prevalent in patients with OSA. As such, more trials in this particular subpopulation are needed [1, 9, 10].
Central Sleep Apnea
Definition
In a simple way, we can define CSA as the cessation of breathing without thoracoabdominal effort. It is characterized by a breathing instability with a periodic crescendo-decrescendo pattern alternating with central apneas [12] (cycle length superior to 40 s), often with some upper airway narrowing.
Prevalence
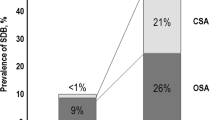
According to the literature [3,4,5, 9], the incidence of SDB is 53% and 48% in HFrEF and HFpEF, respectively. As the severity of HF increases, the episodes of CSA become increasingly more common, and its severity rises. However, despite the prevalence of CSA in HF being strikingly high, this condition continues to be seriously underdiagnosed.
Some studies showed a prevalence of 29–40% in HFrEF. In contrast, CSA in HFpEF remains less acknowledged, with some studies reporting an incidence of only 17%.
Regarding the risk factors [12] to SDB and mainly CSA, the ones that have been implied so far are age (older people), gender (male), genetics (gene-dependent development of respiratory center has been implied to play a role in SDB), obesity (a classic risk factor for OSA), atrial fibrillation, hypocapnia, leptin (low levels), and smoking.
Pathophysiology
Complex pathways of medullary and aortic receptor chemosensitivity are the key mechanisms of CSA. Simply put, its pathogenesis reflects an amplified chemoreflex and a prolonged circulation delay [8, 12] between the chemoreceptors localized in the carotid artery and the pulmonary capillaries. Cyclic periods of hyperventilation lead to decreased levels of CO2, which will fall below apnea threshold, causing apnea/hypopnea.
Another mechanism implied in CSA is related with the fluid shift that occurs at nighttime. During the day, fluid tends to accumulate in the legs due to gravity, but when the patient is sleeping, a redistribution of fluids in the body happens. With the increased rostral fluid shift, some of it may additionally accumulate in the lungs, leading to hyperventilation and hypocapnia. As a result, this mechanism will drive PaCO2 below the apnea threshold and cause CSA-Cheyne-Stokes breathing [3, 12].
In short, patients with HF are more vulnerable to the development of CSA because the increased chemosensitivity leads to an excessive ventilation during arousals, lowering PaCO2 excessively. When sleep occurs again, the levels of PaCO2 are below the apnea threshold, causing central apnea—high loop gain mechanism [3, 4].
Diagnostic Criteria
The diagnosis of CSA should fulfill all the following criteria [12]:
-
(a)
Presence of symptoms (one or more of the following, sleepiness, difficulty initiating or maintaining sleep, awakenings, snoring, witnessed apneas, and awakening short of breath) or atrial fibrillation/flutter, congestive HF, or a neurologic disorder.
-
(b)
Polysomnography findings (should present all the following, >5 central apneas and/or central hypopneas per hour of sleep, central events >50% of total events, and pattern of ventilation with criteria for Cheyne-Stokes breathing).
-
(c)
The disorder cannot be explained by another cause.
One interesting evolution in the last years is the increasing use of cardiac device algorithms to detect and quantify SDB. As the number of HF patients with cardiac implantable devices increases, they are being used more frequently [1] to detect this kind of sleep abnormalities with reasonable sensitivity for moderate/severe SDB.
Consequences of CSA
CSA induces a cascade of cyclic variations in heart rate, blood pressure, respiratory volume, partial pressure of oxygen, and carbon dioxide that leads to long-lasting structural changes (like left and right ventricular hypertrophy). Hypoxia itself induces overactivation of the sympathetic nervous system (SNS), which leads to peripheral vasoconstriction (PV). The presence of persistent overactivation of the SNS and PV leads to microcirculatory changes [8, 12] causing cardiac electrical instability (such as atrial and ventricular arrythmias). Furthermore, oxidative stress precipitates a state of inflammation and endothelial dysfunction. All these changes potentiate progressive deterioration and increased mortality in HF patients.
Treatment
Current guidelines do not provide a unique way of approaching treatment for CSA. In addition, some clinical trials using positive airway pressure suffered from significant deficiencies, casting doubt on their results. All of these factors raised questions about how to best treat these patients.
Nowadays, we can divide the treatment of CSA in four major pillars: lifestyle modifications, pharmacological treatment, devices inducing positive airway pressure, and neurostimulation. The authors will explore each one of these points, focusing our attention on the use of positive airway pressure in CSA.
Lifestyle Modifications
Lifestyle modifications [2, 8, 12, 13] in OSA are clearly beneficial, but the advantages in CSA remain uncertain. Weight loss benefits on CSA have not been demonstrated, but it should be included in the treatment strategy of CSA patients. In addition, exercise training and physical activity should be implemented and incremented in HF patients with CSA to decrease symptomatic burden.
Pharmacological Treatment
Optimizing guideline-directed medical therapy for HF is the first step toward CSA treatment. Pharmacological therapy aims to minimize volume overload and improve functional capacity.
Each drug contributes to reduce the burden of CSA by its own distinct mechanism [13]:
-
Diuretics are used to reduce nocturnal fluid shift and decrease the cardiac filling pressure, which is helpful in OSA and CSA.
-
Beta-blockers are extremely useful because they decrease the nocturnal cardiac sympathetic activation. Carvedilol should be the preferred beta-blocker due to the lack of effect in melatonin inhibition, improving sleep quality and diminishing the CSA severity to some extent.
-
Angiotensin-converting enzyme inhibitors contribute to CSA improvement thanks to the ability to reduce ventricular afterload and improve cardiac output.
-
Angiotensin receptor-neprilysin inhibitors have also a clear benefit in the reduction of CSA symptoms due to their ability to reduce pulmonary congestion.
-
Two other potential drugs, with limited evidence, are theophylline and acetazolamide [2, 3, 8, 12]. Theophylline has been able to decrease the periodic breathing and the AHI, while acetazolamide acts by reducing pulmonary congestion and lowering the apneic threshold through neural plasticity. These drugs should only be considered after optimization with standard medical therapy and imply a cautious follow-up due to their potential side effects (e.g., risk of arrythmia with the use of theophylline due to its narrow therapeutic window).
Although it is not a pharmacological strategy, resynchronizing therapy may also have beneficial effects on CSA, as the improvement of cardiac pumping function causes a considerable decrease in AHI in CSA patients.
Nonpositive Airway Pressure Modalities
We can divide these interventions in two main modalities [12]: nocturnal oxygen supplementation and nocturnal supplemental CO2.
-
(a)
Nocturnal oxygen supplementation: by improving oxygen delivery to cardiomyocytes and influencing controller gain and by decreasing hypoxemia and increasing the cerebral CO2, this therapy could decrease AHI by 50%. Current evidence is scarce, and the recent LOFT-HF trial was not able to further clarify the outcomes of this strategy on hospital admissions and mortality (the study was terminated because of lack of funding).
-
(b)
Nocturnal supplemental CO2 [12]: in theory, this approach leads to suppression of CSA by shifting pCO2 above the apneic threshold. Despite that, CO2 therapy is not recommended for clinical use.
Positive Airway Pressure Modalities
We can divide these interventions in three main modalities [3, 4, 8, 12, 14]: continuous positive airway pressure (CPAP), bilevel positive airway pressure (BiPAP), and adaptive servoventilation (ASV).
-
(a)
CPAP: it is considered the gold standard for the initial treatment of CSA [8, 12, 13] related to HF, as it reduces AHI and improves the saturation profile. Patients classified as responders showed decreased AHI, virtually nonexistent oxyhemoglobin desaturation, and decreased nocturnal ventricular arrhythmias when compared with nonresponders. This allows patient division in two major groups with important repercussions on treatment strategy. Indeed, it seems likely that the hemodynamics of the cardiovascular system predict patient response to CPAP.
However, the benefits of this strategy in CSA are less well-established when comparing with OSA. Some of the main trials and their results are exposed in the fourth point of this chapter.
-
(b)
BiPAP: ST mode should only be considered if there isn’t any clinical response with CPAP, ASV, and oxygen supplementation [12]. BiPAP facilitates expiration and increases tidal volume. However, the latter mechanism may cause hypocapnia and provoke periodic or worsened CSA, effects that may be even more pronounced when there is a large difference between inspiratory and expiratory pressures (>7 cm H2O). Despite the scarce data regarding BiPAP in CSA, the contradictory results of the studies raise questions about the utility of BiPAP in this setting.
-
(c)
ASV: it is a modality aimed to deliver optimal positive pressure to maintain ventilation. Early studies showed a potential benefit with this modality (lower AHI and left ventricular ejection fraction recovery). However nowadays this modality, thanks to the results of the SERVE-HF trial [15], is considered unacceptable for patients with HFrEF. This study revealed an increased risk of cardiovascular events, but further reviews raised questions about its methodology (lack of information regarding right ventricular function, poor compliance of the patients, obsolete algorithm in the ASV device). In conclusion, ASV is only indicated for the treatment of CSA in patients with ejection fraction >45% [8, 12].
Neurostimulation
A new modality of therapy, unilateral phrenic nerve stimulation [2, 3, 12, 13], has emerged as a potential strategy for this kind of patients. HF European Society of Cardiology Guidelines stated that “implantable phrenic nerve stimulation can be considered for symptomatic relief,” with some trials revealing an improvement in CSA that was associated with reduction in sleep arousals, better sleep quality, and improvement in quality of life.
Main Clinical Trials: Heart Failure and Noninvasive Ventilation
The use of noninvasive positive pressure ventilation can improve hemodynamics and ultimately be considered an adjuvant treatment in HF. Several clinical trials were published in the last years, some of them generating doubts about their findings among the scientific community. Tables 1 and 2 present the main findings of the major clinical [5, 13, 15] trials performed in HF patients with SDB.
The reasons why these trials failed to demonstrate cardiovascular benefits were intensely discussed, with the consensus being that the need for better phenotyping of SDB and poor CPAP adherence were important biases found in all of them.
Despite these findings, post hoc analysis of CERCAS, SAVE, and RICCADSA trials showed reduction of cardiovascular events in patients who had CPAP adherence >4 h per night.
Promising data was revealed during the 2022 European Society of Cardiology Congress regarding ADVENT-HF trial, as the usage of ASV (focused in peak flow targeted adaptative servoventilation) in CSA didn’t result in increased cardiovascular events. However, a cautious approach to these findings is warranted, as the data has not been published and peer reviewed until the moment this chapter was redacted.
Nasal High-Flow Therapy in CSA
Nasal high-flow (NHF) therapy is a promising treatment strategy to reduce CSA. Thanks to the absence of the negative effects associated to intrathoracic pressure, this modality combines the positive effects of a mild level of continuous positive pressure with the use of oxygen therapy. Moreover, the pressure reached in the oropharyngeal cavity is actually very low [16] (maximum of 5 cm H2O), and this fact will translate into even lower intrathoracic pressures. On the other hand, arterial carbon dioxide pressures decrease with this treatment (washout of dead space area), leading to potential central apnea induced by hypocapnia and its subsequent cardiovascular events. In line with this finding, one small study [16] showed an increased risk of cardiac arrythmias in patients treated with NHF. Although only five patients were included, it did raise questions about the safety of this therapeutic strategy. It is therefore necessary to conduct clinical trials for further clarification on possible benefits of this strategy.
Heart Failure with Preserved Ejection Fraction and Sleep Breathing Disorders
Some recent clinical trials [3, 4, 12] revealed that ASV can improve diastolic function and symptoms and decrease the level of natriuretic peptides concentration in patients with HFpEF and CSA. Current treatment recommendations with positive airway pressure devices are similar to the ones used in patients with HFrEF, the main difference being that ASV is indicated for treatment of CSA related with HF in patients with ejection fraction >45% [3, 12, 13]. Despite that, no robust clinical trials have yet been conducted to evaluate the true impact of this type of noninvasive ventilation on the cardiovascular outcomes.
Conclusion
SDB is found in at least 50% (some estimates point to 75%) of patients with HF and is associated with a worse prognosis. Pathophysiological mechanisms found in SDB include cyclic hypoxemia and sympathetic nervous system activation, although OSA is associated with thoracoabdominal excursions while CSA is not. The gold standard for its diagnosis is the PSG, but simpler or more accessible methods like PG or recording of nocturnal oxygen saturation via a finger probe can also be used and are still very helpful. CPAP and optimized medical therapy remained as the therapeutic cornerstones for both OSA and CSA, but newer strategies are being pursued and studied.
Key Points
-
SDB is still highly prevalent in patients with HF, despite all the various clinical breakthroughs in the last decades.
-
Untreated severe sleep apnea increases the risk for cardiovascular events (ischemia and arrythmias).
-
Positive airway pressure treatment is useful both in OSA and CSA.
-
The ADVENT-HF trial should bring us new data regarding the use of ASV in patients with HFrEF and CSA.
-
There is scarce data about the use and benefits of nasal high-flow therapy in HF patients with CSA.
References
Pearse SG, Cowie MR, Sharma R, Vazir A. Sleep-disordered breathing in heart failure. Eur Cardiol Rev. 2015;10(2):89–94. https://doi.org/10.15420/ecr.2015.10.2.89.
Jaarsma T, Hill L, Bayes-Genis A, et al. Self-care of heart failure patients: practical management recommendations from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2021;23(1):157–74. https://doi.org/10.1002/Ejhf.2008.
Cowie MR, Linz D, Redline S, Somers VK, Simonds AK. Sleep disordered breathing and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78(6):608–24. https://doi.org/10.1016/J.Jacc.2021.05.048.
Mehra R. Sleep apnea and the heart. Cleve Clin J Med. 2019;86:10–8. https://doi.org/10.3949/CCJM.86.S1.03.
Haruki N, Floras JS. Sleep-disordered breathing in heart failure: a therapeutic dilemma. Circ J. 2017;81(7):903–12. https://doi.org/10.1253/Circj.CJ-17-0440.
Javaheri S, Javaheri S. Obstructive sleep apnea in heart failure: current knowledge and future directions. J Clin Med. 2022;11(12):4–9. https://doi.org/10.3390/jcm11123458.
Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. J Clin Sleep Med. 2012;8(5):597–619. https://doi.org/10.5664/Jcsm.2172.
Javaheri S, Brown LK, Khayat RN. Update on apneas of heart failure with reduced ejection fraction: emphasis on the physiology of treatment: Part 2: central sleep apnea. Chest. 2020;157(6):1637–46. https://doi.org/10.1016/j.chest.2019.12.020.
Khattak HK, Hayat F, Pamboukian SV, Hahn HS, Schwartz BP, Stein PK. Obstructive sleep apnea in heart failure: review of prevalence, treatment with continuous positive airway pressure, and prognosis. Texas Hear Inst J. 2018;45(3):151–61. https://doi.org/10.14503/THIJ-15-5678.
Javaheri S, Brown LK, Abraham WT, Khayat R. Apneas of heart failure and phenotype-guided treatments: Part one: OSA. Chest. 2020;157(2):394–402. https://doi.org/10.1016/J.Chest.2019.02.407.
Wang T, Eskandari D, Zou D, Grote L, Hedner J. Increased carbonic anhydrase activity is associated with sleep apnea severity and related hypoxemia. Sleep. 2015;38(7):1067–1073B. https://doi.org/10.5665/Sleep.4814.
Terziyski K, Draganova A. Central sleep apnea with cheyne-stokes breathing in heart failure—from research to clinical practice and beyond. Adv Exp Med Biol. 2018;(1067):327–51. https://doi.org/10.1007/5584_2018_146.
Fudim M, Shahid I, Emani S, et al. Evaluation and treatment of central sleep apnea in patients with heart failure. Curr Probl Cardiol. 2022;47(12):101364. https://doi.org/10.1016/j.cpcardiol.2022.101364.
Orr JE, Safwan Badr M, Ayappa I, et al. Research priorities for patients with heart failure and central sleep apnea an Official American Thoracic Society research statement. Am J Respir Crit Care Med. 2021;203(6):E11–24. https://doi.org/10.1164/Rccm.202101-0190ST.
Cowie MR, Woehrle H, Wegscheider K, et al. Rationale and design of the SERVE-HF Study: treatment of sleep-disordered breathing with predominant central sleep apnoea with adaptive servo-ventilation in patients with chronic heart failure. Eur J Heart Fail. 2013;15(8):937–43. https://doi.org/10.1093/Eurjhf/hft051.
Meijer PM, Oudman KWE, Van der Leest S, et al. Nasal high flow therapy in heart failure patients with central sleep apnea: a report of disproportional occurrence of cardiac arrhythmias. Sleep Med. 2021;79:119–21. https://doi.org/10.1016/J.Sleep.2021.01.002.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Miranda, H., Miranda, S., Cimbron, M., Barros, N. (2023). Sleep Breathing in Heart Failure. In: Esquinas, A.M., De Vito, A., Barbetakis, N. (eds) Upper Airway Disorders and Noninvasive Mechanical Ventilation. Springer, Cham. https://doi.org/10.1007/978-3-031-32487-1_30
Download citation
DOI: https://doi.org/10.1007/978-3-031-32487-1_30
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-32486-4
Online ISBN: 978-3-031-32487-1
eBook Packages: MedicineMedicine (R0)