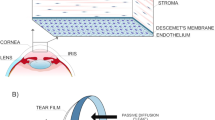
Abstract
The human cornea is a five-layered tissue that provides two-thirds of the total refractive power of the eye, and it is the first barrier protecting the intraocular content. The corneal endothelium, the inner layer, is in charge of maintaining the cornea in a relatively dehydrated state and therefore transparent. The endothelial cell layer failure leads to corneal swelling, loss of transparency and blindness.
Currently, the only effective and probed way to restore endothelial function universally is to perform an allogenic graft. Since Melles revolutionized the field by describing a method to dissect only Descemet Membrane (DM) from the recipient eye, leaving the posterior lamella intact, and after Price and Gorovoy pioneered Descemet stripping endothelial keratoplasty (DSEK), a variety of endothelial keratoplasty techniques have taken over. However, there is a scarcity of donors to adequate to high and increasing demand.
Cell culture techniques make it possible to expand ex vivo the corneal endothelial cells (CEC) to subsequently inject a cell solution into the anterior chamber, or else to manufacture constructs made up of acellular corneal stroma, acellular Descemet membrane or carriers manufactured by tissue bioengineering, and colonized by CEC expanded ex vivo, which could then be grafted onto the recipient. Nowadays, we are in an outstanding position to develop corneal endothelial cell sheets for endothelial keratoplasty: reproducible and well-defined culturing methods and conditions have been achieved in the last decades. Regardless of advances in promoting human CEC proliferation, the achieved capacity for expanding human CECs is still highly limited; new sources of CECs are therefore sought. The use of extraocular cells capable of differentiating into corneal endothelial cells is highly desirable. Recent advances have been achieved in differentiation protocols from embryonic stem cells and adipose-derived mesenchymal stem cells.
New advances in biomimetic materials and manufacturing protocols such as electrospinning, nanolithography, vitrification, and advances in novel 3D printing techniques such as LIFT, laser-assisted bioprinting, and others will aid in the search for a donor-independent biocompatible carrier.
Further development of these and previous approaches, by defining the growth factors, the signaling pathways implicated in directed differentiation, the use of more practical cells to derive hCECs, and the in vivo demonstration of functionality are urgently needed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Corneal endothelial therapeutics has been transformed by lamellar endothelial transplants.
-
Recent developments in endothelial cell culture techniques make it possible to expand ex vivo the corneal endothelial cells.
-
Expanded cells can be delivered subsequently by direct injection into the anterior chamber or in sheet constructs made up of different materials.
-
Recent advances have been achieved in differentiation protocols from extraocular cells capable of differentiating into corneal endothelial cells such as embryonic stem cells and adipose-derived mesenchymal stem cells.
Introduction to Corneal Endothelial Transplant
The cornea is a five-layered tissue that provides two-thirds of the total refractive power of the eye, and it is the first barrier protecting the intraocular content. The corneal endothelium, the inner layer, is in charge of maintaining the cornea in a relatively dehydrated state and therefore transparent. The endothelial cell layer failure leads to corneal swelling, loss of transparency, and blindness. In the past, penetrating keratoplasty (PK) had been the gold standard surgical treatment of corneal diseases for any layer, including primary endothelial diseases [1]. Central endothelial cell density (ECD, expressed in cells per mm2) decreases at an average rate of about 0.6% per year in normal corneas throughout adult life [2]. In a normal individual, this decline in endothelial cells (EC) does not impair corneal transparency, even in centenarians, and only if the density falls below the threshold of 300–500 cells per mm2, irreversible corneal edema can lead to blindness [3]. This event can occur following intraocular surgeries, traumas, or dystrophies. In fact, blindness due to corneal edema is the indication of corneal grafting of one in every three recipients.
Human corneal endothelium is held in a nonreplicative state within the eye [4]. It has been a common belief that in vivo, corneal endothelium has limited wound-healing capacity, mainly by using residual EC which, by enlargement and migration, covers the space left by the lost cells without division [5]. Joyce [6] demonstrated that hCECs are arrested in the G1-phase of the cell cycle in vivo. Mitotic inhibition has been suggested to be due to contact-dependent inhibition and the transforming growth factor beta (TGF-ß) found within the aqueous humor [4]. However, a series of clinical observations suggest the ability of endothelial regeneration in vivo from the human corneal periphery after implanting free floating Descemet membrane in the anterior chamber or in the newly described technique known as Descemet stripping only in selected cases [3, 7, 8].
Currently, the only effective and proven way to restore endothelial function universally is to perform an allogenic graft. Since Melles [9] revolutionized the field in 2004 describing descemetorrhexis, a method to dissect only Descemet Membrane (DM) from the recipient eye, leaving the posterior lamella intact, and after Price [10] and Gorovoy [11] pioneered the procedure known as Descemet stripping endothelial keratoplasty (DSEK) [10], a variety of endothelial keratoplasty techniques have taken over PK as the elective procedure in endothelial keratoplasty. Nowadays, all the different approaches include “descemetorrhexis,” and the difference lies in the tissue grafted:
-
1.
In DSAEK, or Descemet stripping automated endothelial keratoplasty, the graft is prepared using a microkeratome and includes not only DM and endothelium but also part of the posterior stroma [11]. It has been widely adopted, and the eye bank produces precut tissue which is used directly by the surgeon [12]. Although the correlation between preoperative graft thickness and clinical outcomes has been disputed [13, 14], there is a tendency to believe that thinner grafts are associated with better visual acuity. Ultra-thin DSAEK is a variant of the technique where grafts are around 100 microns to improve the visual acuity of standard DSAEK [15].
-
2.
In DMEK, or Descemet membrane endothelial keratoplasty, a step forward in the endothelial keratoplasty developed by Melles [16, 17], the graft consists of endothelium and DM without any stroma, around 10–15 microns thick. Compared to DSAEK, DMEK has better visual outcomes, faster recovery time, and a lower immune rejection rate. It is the gold standard in the treatment of endothelial diseases, although it has not been adopted everywhere yet, due to the higher surgical skills needed. In settings with the scarcity of donor tissue, this technique has evolved to hemi-DMEK [18] or quarter-DMEK [19], allowing one donor to provide tissue for several recipients by dividing the graft into two or four pieces, respectively.
-
3.
In DSO, or Descemet stripping only, there is no grafting, only a descemetorrhexis, and relies on primary healing of the peripheral endothelium [8]. There is a need for longer term comparison studies, but it has several advantages over the other two procedures, it requires only basic skills, it does not need donor tissue, there is no risk of rejection, and there are no early postoperative complications such as DM detachment. On the other hand, a good peripheral endothelial cell count is needed, the disease must be limited to the 5 mm-central part, and although it may provide similar visual outcomes to DMEK, it requires longer periods to achieve transparency with lower endothelial cell counts as a baseline point. The instillation of ROCK inhibitors has been used to speed up recovery and to salvage failing cases [20].
Cultured Corneal Endothelial Cells
Human corneal endothelial cells (hCECs) are arrested at G1 phase of the cell cycle, and do not proliferate in vivo, in part due to contact inhibition but also presumably because of lack of growth factor stimulation even when damage to the endothelial layer occurs [21]. Therefore, the supply of human corneal tissue is limited; therefore, in vitro CEC culture is an option to increase the number of cells for potential therapeutic purposes. However, this is challenging by the very biology of CECs, and it is important to consider several factors:
Donor Factors
Age of Donors
For cell culture of corneal endothelial cells, it is essential to start from a source of viable and proliferating cells, i.e., young human corneal tissue. Most human corneas are used for transplantation, leaving those of old donors with less endothelial cell count for research. It has been shown that in the corneas of young donors (<30 years old), the mean cell density is 3000 cells/mm2, while in old donors (> 50 years old), it is 2700 cells/mm2 [22].
There are also differences in cellular morphology within these two groups, young donor endothelial cells show homogeneous hexagonal morphology, while older donor cells were polymorphic. Proliferative capability was maintained from young donor CECs, maintaining their morphology and characteristics until the third passage, while old donor CECs were senescent earlier during the culture [23] hence culturing cells from old donors is more challenging.
Tissue Preservation
The quality of donor corneas also depends on tissue preservation conditions. There are two fundamental methods, maintenance in Optisol-GS and organ culture. Optisol-GS corneal storage medium (Bausch & Lomb, Irvine, California), a hybrid of K-sol and DexSol media containing chondroitin sulfate and dextran, is stored at 2 °C to 8 °C for 14 days [24]. Meanwhile, organ culture maintains the corneas between 31 °C and 37 °C for up to 28 days, using different culture media. Most of these media are supplemented with serum such as CorneaMax, but serum-free media such as Human Endothelial-SFM is also used [25]. Viability comparison studies showed a dead cell percentage of 9.34% ± 4% and 0.46% ± 0.3% in Optisol-GS and organ culture, respectively [26]. Nevertheless, successful cell culture was obtained from tissue preserved in both conditions. Although the viability is higher with organ culture, in both cases proliferation, hexagonal morphology and expression of typical CECs markers are achieved.
Cell Isolation Protocols
Isolation of hCECs is one of the most critical steps for a successful culture. The most commonly used method is the peel-and-digest protocol by Peh’s Laboratory [23]. The endothelium along with the Descemet membrane is separated from the rest of the cornea, and this is enzymatically digested by collagenase. This enzyme gently digests the junctions of the endothelial cells to the Descemet membrane (DM), consisting mainly of ECM proteins like collagen IV. The intercellular junctions mediated by ZO-1 are maintained as well as cell-to-basement membrane interactions [23]. Other enzymatic methods have also been tried, such as trypsin, causing complete degradation of the CECs when too aggressive, or separation by EDTA and pipetting, a technique by which the CECs did not maintain viability either [27, 28].
Cell viability is checked routinely in eye banks using Trypan blue positive cell count, using Trypan Blue staining (0.25%), and counting blue stained cells as dead cells. Using this method and a hemocytometer for counting, viability and plating density can also be checked after cell isolation [26].
Coatings
In vivo, endothelial cells adhere to the Descemet membrane via extracellular matrix proteins. The extracellular matrix is composed of different collagens, laminin, and fibronectin among others. With the idea of creating a biomimetic environment, these and other cell adhesion coatings have been evaluated for culturing endothelial cells. Comparison of wells precoated with Fibronectin, Poly-D-Lysine, Collagen I, Fibronectin/Collagen I, or FNC Coating Mix [29] showed that the coating with the higher adhesion with almost 100% of cells attached after rinsing while maintaining cell morphology was FNC Coating Mix, followed by Collagen I and Fibronectin/Collagen I (with 90% of cells attached). On the downside, FNC Coating Mix is a commercially formulated reagent containing bovine fibronectin and bovine collagen I among other components so it is useful for cell culture but not suitable for clinical studies [29, 30]. Other studies have shown collagen IV as an optimal coating for the culture of CECs for tissue engineering as it is part of the endothelial basement membrane [31].
Media
Different culture media have been used for the expansion of CECs, usually with a dual approach, with a proliferation medium followed by a maintenance medium.
For proliferation, combinations of one or two media with external growth factors have been used. The media include DMEM, DMEM/F12, Opti-MEM-I, and Ham’s F12/M199, compared by Peh [23]. CECs cultured with DMEM or DMEM/F12 do not go beyond the first or second passage, while using Opti-MEM-I or Ham’s F12/M199, the cells start to show typical endothelial markers such as Na+/K+ ATPase or ZO-1 from passage 3 [23]. As human CECs do not proliferate, external factors and supplements have been used to overcome the cell cycle arrest such as serum, ascorbic acid, FGF, or insulin [32]. However, hexagonal morphology was not achieved by culturing in proliferation media alone. For the maintenance of CECs five media were compared, including HCEC growth medium (F99), MEM with FCS, and humanized endothelial SFM, the latter being the one with the best results in terms of lower endothelial cell apoptosis [33, 34].
Parekh [35] cultured CECs using only a proliferation medium based on Ham’s F12/M199. Other groups used Opti-MEM-I with 8% FBS and supplemented with ROCK inhibitor (Y-27632) [36,37,38]. One of the most effective protocols is Peh’s Laboratory [23], which uses a proliferation media with Ham’s F12/M199 with 5% FBS, 20 μg/mL ascorbic acid, 1% ITS, 10 ng/mL FGF2 and 1% antibiotic/antimycotic combined a maintenance media Human endothelial-SFM 4% FBS, 50 μg/mL gentamicin, and 1% antibiotic/antimycotic (Fig. 36.1).
Carriers for DSAEK and DMEK
Following the isolation of hCECs, the next step is to engineer a scaffold mimicking DM and use it as a graft. This scaffold needs to provide a favorable environment for endothelial cell expansion and maintenance as well as a robust tissue that can be handled easily for transplantation. In recent decades, studies have been carried out using both natural and synthetic materials that can serve as grafts with CECs. Today, in addition to using biomaterials as scaffolds, their use is being studied to increase cell viability and long-term transplantation success [39].
Natural Scaffolds
Natural scaffolds can be obtained from different animal sources, which mimic components of DM, improving biocompatibility, proliferation, and maintaining the phenotype of CECs. However, since they are derived from animals, their composition is not well defined, and the resulting scaffolds show little optical transparency and weak mechanical properties.
Initially, as with coatings, the use of natural polymer from the extracellular matrix such as collagen was considered because of its biocompatibility properties, low immunogenicity, and degradability. However, the laminas were not consistent, difficult to handle, and easily degraded by proteases. To solve this, different hardening techniques have been used, such as chemical crosslinking or physical crosslinking by ultraviolet light, rendering suboptimal results [40, 41]. Over time, technologies have appeared that allow for the creation of plastic compressed collagen films, based on Real Architecture for 3D Tissues (RAFT) that allow rapid production of grafts with improved mechanical properties without compromising biosafety; however, transparency is not adequate, and there are no in vivo studies yet [42].
Other natural polymers have been tried such as gelatin or chitosan. Gelatin has great porosity, permeability to water, helps cell adhesion, and is widely available [43]. However, gelatin hydrogels do not provide stability as a graft, and there is a risk of carrying bovine spongiform encephalopathy due to the source of gelatin [44]. Chitosan is a biomimetic polysaccharide derived from chitin and has great biocompatibility but low strength. To create a hard construct, it was combined with other natural materials, and a graft consisting of hydroxyethyl chitosan, gelatin, and chondroitin sulfate was created and tested on CECs, showing promising results but causing in vivo inflammation in animal models [45].
An approach using silk fibroin precoated with collagen type IV, has also been evaluated for human CEC culture [46]. Silk fibroin a natural fibrin derived from silk has low immunogenicity and good transparency but on its own cannot maintain a CEC culture, lacks elasticity and mechanical strength, and can cause hypersensitivity. Using non-mulberry silk combined with other materials shows better biocompatibility, but further studies need to be done [47].
Other biologically derived scaffolds are membranes such as amniotic membrane, decellularized cornea, and human anterior lens capsule. In both, the high dependency on the human donor is a limitation.
The human amniotic membrane is a collagen-based scaffold that can be used intact, decellularized or lyophilized and possesses anti-inflammatory, anti-fibrosis, and anti-angiogenic properties that reduce potential graft rejection and have been used in other ocular applications [27]. The main problems are availability and lack of mass manufacturing, sub-optimal transparency with a low biodegradation rate in long-term transplantation, and risk of contamination and transmission of infectious diseases [48].
Decellularized corneas provide the perfect substrate for CECs to grow while maintaining optimal transparency and ultrastructure. Decellularization removes native cells and other immunogenic compounds while preserving the structural and functional proteins of the stroma [49]. Different corneal scaffolds have been used, from porcine corneas to human. Due to a low number of donated corneas and a lengthy decellularization process, obtaining various lamellae per cornea with the femtosecond laser method is vital for the usage of this material as a scaffold [30]. There are various studies with clinical applications leading to corneal edema relief [50, 51].
Human crystalline lens capsule is composed of collagen IV and sulfated glycosaminoglycans. The anterior lens capsule is a byproduct of cataract surgery and presents biomechanical properties similar to DMEK grafts, can be used decellularized with good biocompatibility and inherent transparency; however, there are limitations due to their small diameter and high dependency on the supply of cadaveric eye donors [52].
In addition, a natural material xenograft using decellularized fish scales is being assessed. It presents a collagen I pattern similar to the human cornea and provides a cost-effective available substrate for corneal grafts. CECs adhesion is adequate but can be improved with FNC coating, and proliferation is irregular, but post-modification fish scale scaffolds show some promise due to their inherent transparency being similar to DSAEK grafts [53].
Synthetic Scaffolds
There are interesting materials because their properties such as the structure, shape, chemical composition, mechanical strength, and durability can be customized. Therefore, many authors try to find the best synthetic scaffold-based to regenerate the corneal endothelium.
Kruse [54] compared scaffolds of poly (methyl-methacrylate) (PMMA), poly (lactic-co-glycolic acid) (PLGA) and polycaprolactone (PCL) for the culture of hCECs. PLGA fibers were spun from a solution with a mass concentration of 5 w/v% in 75% chloroform and 25% methanol. PCL fibers were spun from a 14 w/v% solution in 75% CHCl3 and 25% MeOH. PMMA fibers were produced from a 16 w/v% solution of 75% CHCl3 and 25% MeOH. Even using identical production parameters, the three scaffolds differed significantly in terms of viscosity, pore size, thickness, and light transmittance. Then, 40,000 cells/cm2 of human corneal endothelial cell line (HCEC-12) were seeded onto the scaffolds and cultured for a week. The results revealed that HCEC-12 mainly grew on the surface and retained physiological morphology, but the formation of a uniform monolayer was not evident in PLGA. The PCL scaffold maintained high cell viability, while PMMA showed cytotoxicity. In conclusion, PLGA and PCL electro-spun scaffolds showed similar biocompatibility, but only PLGA maintained the characteristic polygonal shape of hCECs.
Poly (ethylene glycol) (PEG)-based hydrogel films containing sebacoyl chloride (SebCl) and 5 w/v% of α, ω-dihydroxy-poly (ε-caprolactone) (PCL) dissolved in dichloromethane showed similar tensile strengths to human corneal tissue and more than 98% optical transparency activity [55]. In vitro analysis performed with sheep CECs on hydrogel films resulted in 100% confluence with natural morphology after 7 days. In vivo studies revealed that the cell-free hydrogel implanted on the inner surface of ovine corneas for 28 days showed no toxicity or inflammatory response and did not compromise the native CEC function, as the corneas maintained their optical transparency.
Synthetic hydrogels of poly-ε-lysine crosslinked 60% with octanedioic-acid to a polymer density of 0.066 g/mL using N-hydroxysulfosuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), produced a thin, transparent, porous, and robust substrate for corneal endothelial cells culture [56]. Their results demonstrated that functionalization of the poly-ε-lysine hydrogel with arginine-glycine-aspartic acid (RGD) provides a suitable surface for 5-week culture of primary porcine hCECs and facilitates the generation of the confluent monolayer with of ZO-1 and Na+/K+ ATPase expression.
Combination of Natural and Synthetic Materials
Other authors combine natural and synthetic polymers to create a biomaterial with the advantages of both. The mechanical properties of the synthetic scaffolds and the extracellular matrix (ECM) proteins of natural ones.
Kim [57] created the Col-I-PLGA scaffold by combining the appropriate mechanical strength of the 5 w/v% PLGA films as a substrate with 5 μg/cm2 collagen I coating to enhance its biocompatibility. This polymer adequately resembled the required surface properties to facilitate adhesion, migration, and proliferation of primary rabbit corneal endothelial cells, as well as roughness, appropriate hydrophilicity, stability, and water uptake, compared to bare PLGA films. Also, the cultured cells on Col I-PLGA scaffolds showed significant enhancement in the expression of corneal endothelial cell-associated marker genes such as aquaporin and Na+/K+ ATPase, along with well-maintained cell morphology.
Palchesko [58] demonstrated that bovine CECs cultured in vitro on a polydimethylsiloxane surface with an elastic modulus of 50 kPa previously coated with collagen IV grew in monolayer with a polygonal morphology and positive staining for the characteristic endothelial marker ZO-1.
Rizwan [59] produced an improved gelatin methacrylate hydrogel named GelMA+ and UV crosslinking. GelMA+ showed an eight-fold increase in mechanical strength and slower degradation compared to regular GelMA. In addition, primary human CECs at passage 3 from donor corneas reached confluence in a monolayer with rise ZO-1 expression, higher cell density and cell size homogeneity on GelMA+ carrier compared to GelMA.
Wang [60] hybridized chitosan and polycaprolactone (PCL) and cultured bovine corneal endothelial cells on this scaffold and reported that the cells reached confluence on day 11, displayed a normal polygonal morphology and showed ZO-1, Na+/K+ ATPase expression after 14 days of incubation on the 25% PCL and 75% chitosan blend membrane.
An alternative method to is cell sheet engineering. Cells were cultured on the surface of a stimuli-sensitive polymer that allows controlled cell adhesion and detachment without using proteolytic enzymes.
Several studies have shown that Poly-N-isopropylacrylamide (PIPAAm) is a good temperature-responsive polymer for generating hCEC sheets. Their chains display hydrophobic properties at 37 °C so the cultured cells could adhere and proliferate on the polymer. In contrast, by lowering the culture temperature to 20 °C, the polymer turns into a hydrophilic state with fully extended chains, so the formed cell sheets spontaneously detach from the surface with intact ECM proteins. The harvested hCECs, which exhibit hexagonal morphology with the presence of microvilli and cellular interconnections, were transferred to gelatin disc supports for transplantation into the anterior chamber of rabbit models. After 2 weeks, the hCEC film was attached to the denuded surface of Descemet’s membrane with tight junction formation (ZO-1) between cells [61,62,63,64]. This approach has not gone clinically forward because cultured corneal endothelial sheets, as cell monolayers, are highly fragile and technically difficult to transplant into the anterior chamber. To overcome this problem, some researchers have transplanted cultured corneal endothelial sheets with a carrier, but they have adhered only temporarily before eventually detaching, with the exception of corneal stromal laminas, which is a limited source and whose necessity hinders the advantages of transplantation of cultured CEC [65]. However, this thermoresponsive polymer has been used for patient therapy to enable corneal epithelial reconstruction [66].
Stem Cells Induced Differentiation to Human Corneal Endothelial Cells
Since the corneal endothelium was shown to be derived from neural crest [67, 68], most approaches to induce corneal endothelial cell differentiation from stem cells in vitro started mimicking the developmental process. The strategy consisted of a first phase in which stem cells were differentiated into neural crest cells and a second stage in which corneal endothelial cells were further differentiated from these neural crest cells.
Three labs, McCabe [69], Ali [70], and Wagoner [71], independently derived corneal endothelium from pluripotent stem cells under chemically defined conditions with a first step called “dual inhibition” to promote neural crest cell induction, either embryonic stem cells (ESC) or induced pluripotent stem cells (iPSC). McCabe [69] and Ali [70] used 10 μM TGF beta signaling inhibitor SB431542 and 500 ng/mL BMP signaling inhibitor Noggin in a basal medium of DMEM-F12, knock out serum replacement, non-essential AA, and 8 ng/mL fibroblast growth factor 2 (FGF2). However, Wagoner [71] used 3 μM GSK-3 inhibitor CHIR99021 instead of a BMP signaling blocker in a basal medium of DMEM/F12, bovine serum albumin (BSA), 50 μg/ml (+)-sodium L-ascorbate, 10 μg/mL transferrin, 10 ng/mL Heregulin β-1, 200 ng/mL IGF-I, and 8 ng/mL FGF2. After a minimum of 3 days, the dual inhibitors were replaced by 10 ng/mL platelet-derived growth factor B (PDGF-BB), 10 ng/mL Dickkopf-related protein 2 (DKK-2), and 0.1 × B27 supplement for at least 7 days to generate hexagonal corneal endothelial-like cells. Their analyses revealed increased expression of corneal endothelial cell-associated markers such as ZO-1 and Na+/K+ ATPase α1 (ATP1A1) as well as the key Descemet’s membrane protein, Collagen type VIII (COL8A1 and COL8A2).
At the same time, Zhao and Afshari [72] used a three-step chemical method. A first dual inhibition step like previous researchers with 5 μM SB431542 and 50 nM BMP signaling inhibitor LDN193189, adding a Wnt inhibitor 1 μM IWP2 to raise eye field stem cell development in a priming medium of DMEM/F12, N2, B27, BSA, nonessential AA for 6 days. Next, they derived neural crest cells from these stem cells using an induction medium of DMEM/F12 50:50, N2, B27, 0.3 mM 2-phospho-l-ascorbic acid supplemented with 3 μM CHIR99021. Lastly, they were able to differentiate neural crest cells into corneal endothelial-like cells, which expressed Na+/K+ ATPase, ZO-1, and N-cadherin, with human endothelial-SFM, 5% FBS, 0.3 mM 2-phosphate ascorbic acid, 1 μM SB431542, and a 2.5 μM ROCK inhibitor H-1125.
In our opinion, both Ali [70] and Wagoner [71] are better protocols than the others mentioned above because both have been able to achieve the generation of CECs using cells from adult patients. This would be advantageous because the risk of rejection may be reduced when patient-specific autologous cells are used for the treatment of corneal endothelial disorders. Among them, Ali et al. [70] show the highest advantage because with only 20 days of procedure, they generated CECs with 90.82% proteome similarity to a human corneal endothelium (Figs. 36.2 and 36.3).
On the other hand, three other labs derived corneal endothelial-like cells from stem cells using different cell-conditioned media. Obviously, these approaches with conditioned media are less applicable to clinical practice, as undefined factors and concentrations of the molecules present in the conditioned media prevent their safe and reproducible use:
Zhang [73] derived corneal endothelial-like cells from human ESCs by co-culture for 5 days with human corneal stroma cells in a basal medium contained DMEM/F12 supplemented with 10% FBS, B27, 20 ng/ml EGF and 40 ng/ml bFGF to generate an outgrowth of precursors of neural crest cells which expressed CD73 and FoxC1. Next, the medium was changed to SV-40 transformed human lens epithelial cell-conditioned medium for 14 additional days to obtain a monolayer of corneal endothelial-like cells with positive signals for Na+/K+ ATPase, ZO-1, vimentin, and N-cadherin.
Chen [74] promoted neural crest cell differentiation from mouse ESC and mouse iPSC by culturing them in a first stage with embryonic body differentiation medium adding 1 μM all-trans retinoic acid during 4 days. Then, they induced differentiation towards corneal endothelial cells by exposing them for 14–17 days to conditioned medium collected from rabbit lens epithelial cell culture medium. The differentiated cells presented an up-regulation of corneal endothelial cell-associated marker genes as Aquaporin-1, ZO-1, Na+/K+ ATPase, N-cadherin, and Collage type VIII compared with undifferentiated cells.
In search for adult stem cells capable of CEC differentiation, Bosch [75] used dental pulp stem cells. They transdifferentiated these stem cells into neural crest stem cells with an induction medium consisting of DMEM-F12 supplemented with 1× B-27, 1× N-2, 20 ng/mL EGF, 20 ng/ml FGF2, 5 ng/mL heparin, and 2 mM L-alanyl-l-glutamine. On day 4, an adequate number of cells showed up-regulation of neural crest stem cells markers such as AP2, Nestin, and p75; therefore, these cells were cultured in the human corneal endothelial conditioned medium for a further 15 days to derive corneal endothelial cells. At the end of the differentiation process, gene expression of typical CEC markers like ZO-1, Na+/K+ ATPase pump ATP1A1 and extracellular matrix components COL4A2 and COL8A2 were significantly increased compared to undifferentiated dental pulp stem cells.
Clinical Studies on CEC Transplantation
There are some alternative procedures that are currently evaluated under clinical trials and study the use of carriers and endothelial cells in culture:
-
1.
CECs migrate much more efficiently over intact DM rather than bare corneal stroma in DSO, leading to the idea that for the treatment of FECD, DSO could potentially be improved by increasing the size of the descemetorrhexis to incorporate most of the large guttas, but providing a cell-free Descemet’s membrane graft afterwards to complete a descemetorrhexis. This way it acts as a support for endothelial cells favoring their proliferation and centripetal migration. This technique is known as Descemet membrane transfer (DMT) [76]. Unlike endothelial keratoplasty, it has the advantage of using an acellular graft that is widely available and avoiding problems related to postoperative graft rejection due to the absence of allogeneic endothelium. A clinical trial is currently underway to evaluate the efficacy of DMT for the treatment of FECD in a larger cohort of patients and for longer-term monitoring of its safety and efficacy (ClinicalTrials.gov; identifier: NCT03275896).
-
2.
Cell culture techniques make it possible to expand ex vivo the CEC to subsequently inject a cell solution into the anterior chamber [37], or else to manufacture constructs made up of acellular corneal stroma, acellular Descemet membrane or material manufactured by tissue bioengineering [51], and colonized by expanded CEC. These grafts could then be transplanted onto the recipient in the same way as in the previously seen endothelial keratoplasties. In both approaches, a single population of endothelial cells can be amplified many times for distribution to large numbers of patients. Currently, within the framework of a clinical trial that included 11 patients, it has been found that the injection of cells in suspension is capable of effectively treating corneal edema secondary to various conditions, including Fuchs’ Dystrophy and pseudophakic bullous keratopathy, in addition to secondary corneal edema, argon laser peripheral iridotomy (LPI) or pseudoexfoliation syndrome [37]. At 2 years after cell injection, corneal thickness was less than 600 μm in 10 eyes, and the cornea was thinner than the baseline measure in all 11 eyes. The same study [37], however, also found a relatively broad range of endothelial counts among trial participants 2 years after treatment (mean CEC density, 1534 cells per square millimeter [95% CI, 1213 to 1855]). Each of the 11 eyes maintained corneal transparency. Regarding the efficacy of tissue bioengineered constructs, there are no human data yet, although a clinical trial is currently underway (ClinicalTrials.gov; identifier: NCT04319848).
Concluding Remarks and Future Perspectives
Nowadays, we are in an outstanding position to develop corneal endothelial cell sheets for endothelial keratoplasty: With respect to culture conditions, reproducible and well-defined culturing methods, and conditions have been achieved in the last decades [23, 33, 34, 36, 77,78,79].
Regardless of advances in promoting hCEC proliferation, the achieved capacity for expanding human CECs is still highly limited; new sources of CECs are therefore sought. The use of extraocular cells capable of differentiating into corneal endothelial cells is highly desirable. Recent advances have been achieved in differentiation protocols from adipose-derived mesenchymal stem cells (ADSC) from our lab [80]. Our results broaden the type of cells of autologous extraocular origin that could be employed in the clinical setting for corneal endothelial deficiency. In addition, recent in vivo demonstration of the functionality of hESC-derived hCEC together with nicotinamide [81] provides experimental evidence for a potential approach for treating corneal endothelial dysfunction.
Respect to carriers, so far, the most advantageous carrier is a corneal stroma decellularized lamina [30, 51, 82]. However, this carrier still depends on donors; new advances in biomimetic materials and manufacturing protocols such as electrospinning, electrogradient transport, shear flow, nano-lithography, flow-induced crystallization, vitrification, and advances in novel 3D printing techniques such as LIFT, laser-assisted bioprinting, and fused filament fabrication, and other methods of achieving lamellar parallel bundles of collagen, such as molecular crowding and densification to a liquid crystalline state [83,84,85,86] will aid in the search for a donor-independent biocompatible carrier.
Further development of these and previous approaches by defining the growth factors, the signaling pathways implicated in directed differentiation, the use of more practical cells to derive hCECs, and the in vivo demonstration of functionality are urgently needed.
Take Home Notes
-
Recently, a variety of endothelial keratoplasty techniques to restore endothelial function have taken over the classical allogenic graft. However, there is a scarcity of donors to adequate to high and increasing demand.
-
Nowadays, we are in an outstanding position to develop corneal endothelial cell sheets for endothelial keratoplasty: reproducible and well-defined culturing methods and conditions have been achieved in the last decades.
-
The use of extraocular cells capable of differentiating into corneal endothelial cells from embryonic stem cells and adipose-derived mesenchymal stem cells is readily available.
-
New advances in biomimetic materials and manufacturing protocols such as electrospinning, nanolithography, vitrification, and advances in novel 3D printing techniques and others will aid in the search for a donor-independent biocompatible carrier.
-
Further development of these and previous approaches by defining the growth factors, the signaling pathways implicated in directed differentiation, the use of more practical cells to derive hCECs, and the in vivo demonstration of functionality are urgently needed.
References
Moshirfar M, Thomson AC, Ronquillo Y. Corneal endothelial transplantation. Treasure Islad, FL: StatPearls Publishing; 2022.
Bourne W, Nelson L, Hodge D. Central corneal endothelial cell changes over a ten-year period. Invest Ophthalmol Vis Sci. 1997;38(3):779–82.
He Z, Campolmi N, Gain P, Ha Thi BM, Dumollard JM, Duband S, et al. Revisited microanatomy of the corneal endothelial periphery: new evidence for continuous centripetal migration of endothelial cells in humans. Stem Cells. 2012;30(11):2523–34.
Joyce NC, Harris DL, Mello DM. Mechanisms of mitotic inhibition in corneal endothelium: contact inhibition and TGF-β2. Investig Ophthalmol Vis Sci. 2002;43(7):2152–9.
Sherrard ES. The corneal endothelium in vivo: its response to mild trauma. Exp Eye Res. 1976;22(4):347–57.
Joyce NC, Meklir B, Joyce SJ, Zieske JD. Cell cycle protein expression and proliferative status in human corneal cells. Investig Ophthalmol Vis Sci. 1996;37(4):645–55.
Dirisamer M, Yeh RY, Van Dijk K, Ham L, Dapena I, Melles GRJ. Recipient endothelium may relate to corneal clearance in descemet membrane endothelial transfer. Am J Ophthalmol. 2012;154(2):290–296.e1.
Garcerant D, Hirnschall N, Toalster N, Zhu M, Wen L, Moloney G. Descemet’s stripping without endothelial keratoplasty. Curr Opin Ophthalmol. 2019;30(4):275–85.
Melles GRJ, Wijdh RHJ, Nieuwendaal CP. A technique to excise the descemet membrane from a recipient cornea (Descemetorhexis). Cornea. 2004;23(3):286–8.
Price FW, Price MO. Descemet’s stripping with endothelial keratoplasty in 50 eyes: a refractive neutral corneal transplant. J Refract Surg. 2005;21(4):339–45.
Gorovoy MS. Descemet-stripping automated endothelial keratoplasty. Cornea. 2006;25(8):886–9.
Chen ES, Terry MA, Shamie N, Hoar KL, Friend DJ. Precut tissue in descemet’s stripping automated endothelial keratoplasty. Donor characteristics and early postoperative complications. Ophthalmology. 2008;115(3):497–502.
Tourabaly M, Chetrit Y, Provost J, Georgeon C, Kallel S, Temstet C, et al. Influence of graft thickness and regularity on vision recovery after endothelial keratoplasty. Br J Ophthalmol. 2020;104(9):1317–23.
Perone J-M, Goetz C, Zevering Y, Derumigny A, Bloch F, Vermion J-C, et al. Graft thickness at 6 months postoperatively predicts long-term visual acuity outcomes of descemet stripping automated endothelial keratoplasty for fuchs dystrophy and moderate phakic bullous keratopathy. Cornea. 2021;41(11):1362.
Busin M, Madi S, Santorum P, Scorcia V, Beltz J. Ultrathin descemet’s stripping automated endothelial keratoplasty with the microkeratome double-pass technique: two-year outcomes. Ophthalmology. 2013;120(6):1186–94.
Melles GRJ, Ong TS, Ververs B, van der Wees J. Preliminary clinical results of descemet membrane endothelial keratoplasty. Am J Ophthalmol. 2008;145(2):222.
Melles GRJ, Ong TS, Ververs B, van der Wees J. Descemet membrane endothelial keratoplasty (DMEK). Cornea. 2006;25(8):987–90.
Lam FC, Baydoun L, Dirisamer M, Lie J, Dapena I, Melles GRJ. Hemi-descemet membrane endothelial keratoplasty transplantation: a potential method for increasing the pool of endothelial graft tissue. JAMA Ophthalmol. 2014;132(12):1469–73.
Birbal RS, Ni Dhubhghaill S, Baydoun L, Ham L, Bourgonje VJA, Dapena I, et al. Quarter-descemet membrane endothelial keratoplasty: one- to two-year clinical outcomes. Cornea. 2020;39(3):277–82.
Moloney G, Garcerant Congote D, Hirnschall N, Arsiwalla T, Luiza Mylla Boso A, Toalster N, et al. Descemet stripping only supplemented with topical Ripasudil for Fuchs endothelial dystrophy 12-month outcomes of the Sydney eye hospital study. Cornea. 2021;40(3):320–6.
Joyce NC. Proliferative capacity of corneal endothelial cells. Exp Eye Res. 2012;95(1):16–23.
McGlumphy EJ, Margo JA, Haidara M, Brown CH, Hoover CK, Munir WM. Predictive value of corneal donor demographics on endothelial cell density. Cornea. 2018;37(9):1159–62.
Peh GSL, Toh KP, Wu FY, Tan DT, Mehta JS. Cultivation of human corneal endothelial cells isolated from paired donor corneas. PLoS One. 2011;6(12):e28310.
Kitazawa K, Inatomi T, Tanioka H, Kawasaki S, Nakagawa H, Hieda O, et al. The existence of dead cells in donor corneal endothelium preserved with storage media. Br J Ophthalmol. 2017;101(12):1725–30.
Valtink M, Donath P, Engelmann K, Knels L. Effect of different culture media and deswelling agents on survival of human corneal endothelial and epithelial cells in vitro. Graefes Arch Clin Exp Ophthalmol. 2016;254(2):285–95.
Parekh M, Peh G, Mehta JS, Ahmad S, Ponzin D, Ferrari S. Effects of corneal preservation conditions on human corneal endothelial cell culture. Exp Eye Res. 2019;179:93–101.
Ishino Y, Sano Y, Nakamura T, Connon CJ, Rigby H, Fullwood NJ, et al. Amniotic membrane as a carrier for cultivated human corneal endothelial cell transplantation. Investig Ophthalmol Vis Sci. 2004;45(3):800–6.
Engelmann K, Bednarz J, Valtink M. Prospects for endothelial transplantation. Exp Eye Res. 2004;78(3):573–8.
Engler C, Kelliher C, Speck CL, Jun AS. Assessment of attachment factors for primary cultured human corneal endothelial cells. Cornea. 2009;28(9):1050–4.
He Z, Forest F, Bernard A, Gauthier AS, Montard R, Peoc’h M, et al. Cutting and decellularization of multiple corneal stromal lamellae for the bioengineering of endothelial grafts. Investig Ophthalmol Vis Sci. 2016;57(15):6639–51.
Zhu YT, Tighe S, Chen SL, John T, Kao WY, Tseng SCG. Engineering of human corneal endothelial grafts. Curr Ophthalmol Rep. 2015;3(3):207–17.
Møller-Pedersen T, Hartmann U, Ehlers N, Engelmann K. Evaluation of potential organ culture media for eye banking using a human corneal endothelial cell growth assay. Graefes Arch Clin Exp Ophthalmol. 2001;239(10):778–82.
Jäckel T, Knels L, Valtink M, Funk RHW, Engelmann K. Serum-free corneal organ culture medium (SFM) but not conventional minimal essential organ culture medium (MEM) protects human corneal endothelial cells from apoptotic and necrotic cell death. Br J Ophthalmol. 2011;95(1):123–30.
Bednarz J, Doubilei V, Wollnik PCM, Engelmann K. Effect of three different media on serum free culture of donor corneas and isolated human corneal endothelial cells. Br J Ophthalmol. 2001;85(12):1416–20.
Parekh M, Romano V, Ruzza A, Kaye SB, Ponzin D, Ahmad S, et al. Culturing discarded peripheral human corneal endothelial cells from the tissues deemed for preloaded DMEK transplants. Cornea. 2019;38(9):1175–81.
Okumura N, Sakamoto Y, Fujii K, Kitano J, Nakano S, Tsujimoto Y, et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci Rep. 2016;6(January):1–11.
Kinoshita S, Koizumi N, Ueno M, Okumura N, Imai K, Tanaka H, et al. Injection of cultured cells with a ROCK inhibitor for bullous keratopathy. N Engl J Med. 2018;378(11):995–1003.
Okumura N, Matsumoto D, Fukui Y, Teramoto M, Imai H, Kurosawa T, et al. Feasibility of cell-based therapy combined with descemetorhexis for treating Fuchs endothelial corneal dystrophy in rabbit model. PLoS One. 2018;13(1):1–14.
Parekh M, Romano V, Hassanin K, Testa V, Wongvisavavit R, Ferrari S, et al. Biomaterials for corneal endothelial cell culture and tissue engineering. J Tissue Eng. 2021;12:2041731421990536.
Koizumi N, Sakamoto Y, Okumura N, Okahara N, Tsuchiya H, Torii R, et al. Cultivated corneal endothelial cell sheet transplantation in a primate model. Investig Ophthalmol Vis Sci. 2007;48(10):4519–26.
Navaratnam J, Utheim T, Rajasekhar V, Shahdadfar A. Substrates for expansion of corneal endothelial cells towards bioengineering of human corneal endothelium. J Funct Biomater. 2015;6(3):917–45.
Levis H, Kureshi A, Massie I, Morgan L, Vernon A, Daniels J. Tissue engineering the cornea: the evolution of RAFT. J Funct Biomater. 2015;6(1):50–65.
Watanabe R, Hayashi R, Kimura Y, Tanaka Y, Kageyama T, Hara S, et al. A novel gelatin hydrogel carrier sheet for corneal endothelial transplantation. Tissue Eng Part A. 2011;17(17–18):2213–9.
Lai JY, Cheng HY, Hui-Kang MD. Investigation of overrun-processed porous hyaluronic acid carriers in corneal endothelial tissue engineering. PLoS One. 2015;10(8):1–20.
Liang Y, Liu W, Han B, Yang C, Ma Q, Zhao W, et al. Fabrication and characters of a corneal endothelial cells scaffold based on chitosan. J Mater Sci Mater Med. 2011;22(1):175–83.
Madden PW, Lai JNX, George KA, Giovenco T, Harkin DG, Chirila TV. Human corneal endothelial cell growth on a silk fibroin membrane. Biomaterials. 2011;32(17):4076–84.
Ramachandran C, Gupta P, Hazra S, Mandal BB. In vitro culture of human corneal endothelium on non-mulberry silk fibroin films for tissue regeneration. Transl Vis Sci Technol. 2020;9(4):1–15.
Mimura T, Yamagami S, Amano S. Corneal endothelial regeneration and tissue engineering. Prog Retin Eye Res. 2013;35:1–17.
Bayyoud T, Thaler S, Hofmann J, Maurus C, Spitzer MS, Bartz-Schmidt KU, et al. Decellularized bovine corneal posterior lamellae as carrier matrix for cultivated human corneal endothelial cells. Curr Eye Res. 2012;37(3):179–86.
Chakraborty J, Roy S, Murab S, Ravani R, Kaur K, Devi S, et al. Modulation of macrophage phenotype, maturation, and graft integration through chondroitin sulfate cross-linking to Decellularized cornea. ACS Biomater Sci Eng. 2019;5(1):165–79.
Arnalich-Montiel F, Moratilla A, Fuentes-Julián S, Aparicio V, Martin MC, Peh G, et al. Treatment of corneal endothelial damage in a rabbit model with a bioengineered graft using human decellularized corneal lamina and cultured human corneal endothelium. PLoS One. 2019;14(11):1–16.
Yoeruek E, Saygili O, Spitzer MS, Tatar O, Bartz-Schmidt KU. Human anterior lens capsule as carrier matrix for cultivated human corneal endothelial cells. Cornea. 2009;28(4):416–20.
Parekh M, Van den Bogerd B, Zakaria N, Ponzin D, Ferrari S. Fish scale-derived scaffolds for culturing human corneal endothelial cells. Stem Cells Int. 2018;2018:8146834.
Kruse M, Walter P, Bauer B, Rütten S, Schaefer K, Plange N, et al. Electro-spun membranes as scaffolds for human corneal endothelial cells. Curr Eye Res. 2018;43(1):1–11.
Ozcelik B, Brown KD, Blencowe A, Ladewig K, Stevens GW, Scheerlinck JPY, et al. Biodegradable and biocompatible poly(ethylene glycol)-based hydrogel films for the regeneration of corneal endothelium. Adv Healthc Mater. 2014;3(9):1496–507.
Kennedy S, Lace R, Carserides C, Gallagher AG, Wellings DA, Williams RL, et al. Poly-ε-lysine based hydrogels as synthetic substrates for the expansion of corneal endothelial cells for transplantation. J Mater Sci Mater Med. 2019;30(9):1–13.
Kim EY, Tripathy N, Cho SA, Lee D, Khang G. Collagen type I–PLGA film as an efficient substratum for corneal endothelial cells regeneration. J Tissue Eng Regen Med. 2017;11(9):2471–8.
Palchesko RN, Lathrop KL, Funderburgh JL, Feinberg AW. In vitro expansion of corneal endothelial cells on biomimetic substrates. Sci Rep. 2015;5:32–4.
Rizwan M, Peh GS, Adnan K, Naso SL, Mendez AR, Mehta JS, et al. In vitro topographical model of Fuchs dystrophy for evaluation of corneal endothelial cell monolayer formation. Adv Healthc Mater. 2016;5(22):2896–910.
Wang YH, Young TH, Wang TJ. Investigating the effect of chitosan/ polycaprolactone blends in differentiation of corneal endothelial cells and extracellular matrix compositions. Exp Eye Res. 2019;185:107679.
Hsiue GH, Lai JY, Chen KH, Hsu WM. A novel strategy for corneal endothelial reconstruction with a bioengineered cell sheet. Transplantation. 2006;81(3):473–6.
Sumide T, Nishida K, Yamato M, Ide T, Hayashida Y, Watanabe K, et al. Functional human corneal endothelial cell sheets harvested from temperature-responsive culture surfaces. FASEB J. 2006;20(2):392–4.
Ide T, Nishida K, Yamato M, Sumide T, Utsumi M, Nozaki T, et al. Structural characterization of bioengineered human corneal endothelial cell sheets fabricated on temperature-responsive culture dishes. Biomaterials. 2006;27(4):607–14.
Lai JY, Chen KH, Hsiue GH. Tissue-engineered human corneal endothelial cell sheet transplantation in a rabbit model using functional biomaterials. Transplantation. 2007;84(10):1222–32.
Okumura N, Koizumi N. Regeneration of the corneal endothelium. Curr Eye Res. 2020;45(3):303–12.
Burillon C, Huot L, Justin V, Nataf S, Chapuis F, Decullier E, et al. Cultured autologous oral mucosal epithelial cell sheet (CAOMECS) transplantation for the treatment of corneal limbal epithelial stem cell deficiency. Investig Ophthalmol Vis Sci. 2012;53(3):1325–31.
Tuft SJ, Coster DJ. The corneal endothelium. Eye. 1990;4(3):389–424.
Zavala J, López Jaime GR, Rodríguez Barrientos CA, Valdez-Garcia J. Corneal endothelium: developmental strategies for regeneration. Eye. 2013;27(5):579–88.
McCabe KL, Kunzevitzky NJ, Chiswell BP, Xia X, Goldberg JL, Lanza R. Efficient generation of human embryonic stem cell-derived corneal endothelial cells by directed differentiation. PLoS One. 2015;10(12):1–24.
Ali M, Kabir F, Raskar S, Renuse S, Na CH, Delannoy M, et al. Generation and proteome profiling of PBMC-originated, iPSC-derived lentoid bodies. Stem Cell Res. 2020;46:101813.
Wagoner MD, Bohrer LR, Aldrich BT, Greiner MA, Mullins RF, Worthington KS, et al. Feeder-free differentiation of cells exhibiting characteristics of corneal endothelium from human induced pluripotent stem cells. Biol Open. 2018;7(5):1–10.
Zhao JJ, Afshari NA. Generation of human corneal endothelial cells via in vitro ocular lineage restriction of pluripotent stem cells. Investig Ophthalmol Vis Sci. 2016;57(15):6878–84.
Zhang K, Pang K, Wu X. Isolation and transplantation of corneal endothelial cell-like cells derived from in-vitro-differentiated human embryonic stem cells. Stem Cells Dev. 2014;23(12):1340–54.
Chen P, Chen JZ, Shao CY, Li CY, Zhang YD, Lu WJ, et al. Treatment with retinoic acid and lens epithelial cell-conditioned medium in vitro directed the differentiation of pluripotent stem cells towards corneal endothelial cell-like cells. Exp Ther Med. 2015;9(2):351–60.
Bosch BM, Salero E, Núñez-Toldrà R, Sabater AL, Gil FJ, Perez RA. Discovering the potential of dental pulp stem cells for corneal endothelial cell production: a proof of concept. Front Bioeng Biotechnol. 2021;96:17724.
Soh YQ, Mehta JS. Regenerative therapy for fuchs endothelial corneal dystrophy. Cornea. 2018;37(4):523–7.
Peh GSL, Chng Z, Ang HP, Cheng TYD, Adnan K, Seah XY, et al. Propagation of human corneal endothelial cells: a novel dual media approach. Cell Transplant. 2015;24(2):287–304.
Peh GSL, Ang HP, Lwin CN, Adnan K, George BL, Seah XY, et al. Regulatory compliant tissue-engineered human corneal endothelial grafts restore corneal function of rabbits with bullous keratopathy. Sci Rep. 2017;7(1):1–17.
Numa K, Imai K, Ueno M, Kitazawa K, Tanaka H, Bush JD, et al. Five-year follow-up of first 11 patients undergoing injection of cultured corneal endothelial cells for corneal endothelial failure. Ophthalmology. 2021;128(4):504–14.
Marta CM, Adrian M, Jorge FD, Francisco AM, De Miguel MP. Improvement of an effective protocol for directed differentiation of human adipose tissue-derived adult mesenchymal stem cells to corneal endothelial cells. Int J Mol Sci. 2021;22(21):11982.
Li Z, Duan H, Jia Y, Zhao C, Li W, Wang X, et al. Long-term corneal recovery by simultaneous delivery of hPSC-derived corneal endothelial precursors and nicotinamide. J Clin Invest. 2022;132(1):1–11.
Wongvisavavit R, Parekh M, Ahmad S, Daniels JT. Challenges in corneal endothelial cell culture. Regen Med. 2021;16(9):871–91.
Brunette I, Roberts CJ, Vidal F, Harissi-Dagher M, Lachaine J, Sheardown H, et al. Alternatives to eye bank native tissue for corneal stromal replacement. Prog Retin Eye Res. 2017;59:97–130.
Matthyssen S, Van den Bogerd B, Dhubhghaill SN, Koppen C, Zakaria N. Corneal regeneration: a review of stromal replacements. Acta Biomater. 2018;69:31–41.
Lagali N. Corneal stromal regeneration: current status and future therapeutic potential. Curr Eye Res. 2020;45(3):278–90.
Alió del Barrio JL, Arnalich-Montiel F, De Miguel MP, El Zarif M, Alió JL. Corneal stroma regeneration: preclinical studies. Exp Eye Res. 2021;202:108314.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
De Miguel, M.P., Cadenas Martín, M., Moratilla, A., Arnalich-Montiel, F. (2023). Cultured Cells for Corneal Endothelial Therapy. In: Alió, J.L., del Barrio, J.L.A. (eds) Modern Keratoplasty. Essentials in Ophthalmology. Springer, Cham. https://doi.org/10.1007/978-3-031-32408-6_36
Download citation
DOI: https://doi.org/10.1007/978-3-031-32408-6_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-32407-9
Online ISBN: 978-3-031-32408-6
eBook Packages: MedicineMedicine (R0)