Abstract
Ultrasound elastography has developed many applications during the last decade, as it is getting more available in newer US scanners, and radiologists and other medical providers explore their capabilities. In this chapter, we discuss the features of muscle and fasciae when studied with elastography and share some technical clues to perform the study, both with strain and with shear wave types of elastography. Further, we revise applications of elastography that have been developed in many fields, including the study of traumatic injuries, primary muscle disorders, compartment syndrome, painful syndromes, and sarcopenia. Finally, we reveal some limitations and future perspectives for elastography in the field of muscle and fascial pathologies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
Nowadays, ultrasound (US) has begun to be one of the most important radiological tools in the assessment of many entities affecting the musculoskeletal system [1]. There is no doubt about the great benefit of using the US for evaluating traumatic muscle injuries and primary muscle pathologies, and following them up to their healing or further degeneration, with the possibility of real-time evaluation, and the advantage of being a radiation-free and inexpensive diagnostic tool [2]. Nevertheless, biomechanical properties of tissues are difficult to assess with B-mode US, even having symptomatic patients [3, 4], and that is why ultrasound elastography may find a role in the path to the diagnosis of, not just traumatic injuries, but also muscle pathologies. In the following chapter, we will point out how elastography may be an added tool for diagnosing muscular entities with ultrasound. To date, there is just one [5] indexed clinical guideline on the Pubmed database regarding the use of ultrasound elastography in the musculoskeletal system, dated 2013, with just one indication, cerebral spasticity. Further advances since that time have come that we think are worth revising.
Elastography is an ideal added function to the US as a diagnostic test for muscle diseases, as far as it is a noninvasive technique with the ability to quantify the mechanical properties of muscle and can assist clinicians in the diagnosis and evaluation of the progression of diseases and serve as a mean to monitor the effects of treatments [6].
Current literature is trending toward shear wave elastography (SWE) instead of strain elastography (SE) in many fields, and it is expected to be that way in research conditions owing to its capacity to quantify stiffness, which allows comparisons and statistical analysis, as opposed to SE, which offers qualitative information. Nevertheless, since elastography isn’t in many clinical guidelines, not all the scanners in daily practice offer that functionality, particularly older models; in the field of musculoskeletal imaging some devices offer SE, some SWE, or even, they may not be equipped with elastography at all. We think it is important to stress that it is not always mandatory to measure the stiffness of tissues, sometimes the color-coded elastogram may give us the added information needed to diagnose or follow a particular case, and for that purpose, we can use the contralateral side of the patient as a control, an intrinsic advantage in the assessment of the musculoskeletal system.
5.1.1 Peculiarities of Muscle and Fascia Affecting Elastography and Practical Recommendations
Many features of muscle and fasciae significantly influence the elastographic evaluation of such structures; they can be related to mechanical, histologic, anatomic, pathologic, variations between individuals, and the sampling technique [7].
Muscle fibers, collagen, and myofibrils are arranged in longitudinal units that confer significant anisotropic properties to the muscles. Shear waves propagate easier when examined in the parallel than in the perpendicular plane, with significantly higher stiffness values in the short axis than in the longitudinal axis (Fig. 5.1). Also, measuring muscular modulus in the transverse plane, relative to the muscle’s longitudinal axis, can comprise inter and intra-observer reproducibility [8]. Furthermore, muscle inner arrangement of fibers is varied, from parallel, fan-shaped, circular, pennate (feather-like), multipenneate to fusiform [9], a factor that introduces a technical difficulty at the time of standardizing the examination technique. Because of that fact, if elastography is used to compare muscle modulus to a previous value in the same patient, or a published standard, measurements need to be done by scanning the muscle in the same axis used in the control exam [10].
Muscles are dynamic units that change their stiffness depending on their state at the time of sampling, increasing during contraction and maximal extension, and decreasing when in a relaxed state [11, 12] (Fig. 5.2). According to the results of Wang et al. [13], the shear modulus of the vastus intermedius does not change significantly in the relaxed state between elderly and young healthy individuals, nevertheless, in younger individuals, the stiffness is greater than in the elderly during contraction. Although it makes sense biologically, such results can’t apply to all muscles [14]. Interestingly, SWE can be used as a technique for measuring slow muscle contractions, as it can generate elastograms at a speed of 1 Hz [6].
Elastographic variation with muscle contraction. Elastograms of the biceps femoris muscle in a plane paralleling muscle fibers. Note the increased stiffness detection during muscle contraction (right), compared to the resting state (left). The elastogram colors move upward in the stiffness scales, indicating increased stiffness
Good reproducibility of results can be obtained with SWE [8, 12, 15], but it is mandatory to systematically evaluate each muscle following the same patient and probe positioning if their elastographic modulus values or elastograms are intended to be compared to an index value or used in the follow-up of a given individual.
Finally, It is important to note that in SWE, the stiffness can be expressed by different manufacturers as Young’s modulus (kPa), shear modulus (kPa), or shear wave velocity (m·s−1), all of them correlated positively [7]. Contrary to the classic recommendation of applying a generous amount of gel to perform US elastography [7], according to Abdulrahman et al. results [16], in the evaluation of muscles, the best reliability is yielded by using the shear wave velocity as the unit of measurement, applying minimal probe load instead of using a standoff gel, and in depths less than 4 cm.
5.1.2 Elastography of the Normal Muscle and Fascia
In normal conditions, muscle has a heterogenous, although organized US B mode appearance, produced by the many tissue interfaces between the muscle fibers and fascicles, and the interstitial structures like perimysium and fasciae. Those interstitial structures are essential for muscle function because they transmit the force generated in muscle fibers to tendons and bones. Also, nerves and vessels traverse muscles and fascial and interfascial plains, providing more image heterogeneity. Such tissue heterogeneity of the normal muscle is also seen in the US strain elastograms, with muscle fibers accounting for the majority of the image, corresponding to low stiffness, and interspersed fibrofatty interstitium and fasciae between the muscle fibers and bellies, of higher stiffness [17, 18]. Nevertheless, the shear wave elastogram of the normal muscle is more homogenous (Fig. 5.3).
Strain elastogram (image in the right square in a and both images in b) of healthy muscle and corresponding B mode ultrasound imaging (image in the left square in a). Quadratus lumborum muscle in the longitudinal plane with the patient in the prone and relaxed position. With this equipment, the elastographic pattern appears heterogeneous
The term “fascia” is broad and differentiates fascia itself from the fascial system [19]. For this chapter, we will call fascia only the deep fascia, specially epimysium, the interstitial membrane encasing individual muscles, and aponeurosis, a fascial expansion to attach muscles that require wide attachment to bones or other muscles. Other components in the fascial system will be referred to as interstitium, encasing endomysium, epimysium, perimysium, and interfascial planes. Although joint capsules, tendons, and ligaments, belong to the fascial system according to the current Fascia Research Society glossary [20], they will not be discussed in this chapter.
Finally, it is fair to acknowledge the effort made by many investigations to establish normality values of shear wave elastography corresponding to different muscles along the human anatomy, with varied values corresponding to different muscles, and patient and probe positioning, which soon could provide a reference for comparison in clinical practice or new research projects [7] (Table 5.1).
5.2 Clinical Applications
A summary of the findings in different clinical conditions can be found in Table 5.2.
5.2.1 Primary and Secondary Muscle Disorders
In this section, we will speak about a heterogeneous group of diseases that share their affinity for muscle involvement, giving rise to a varied spectrum of clinical conditions in which knowing the biomechanics of the muscle can help the clinician to establish the current state of the disease and guide the therapeutic strategy.
5.2.1.1 Inflammatory Myositis
Idiopathic inflammatory myopathies (IIM) are a rare group of acquired skeletal muscle diseases, unified by immune-mediated muscle damage, that results in muscle fiber loss and weakness, including a broad spectrum of diseases such as inclusion body myositis, polymyositis, dermatomyositis, and even thyroid disorders. However, all of them share clinical presentations in the form of subacute or chronic muscle weakness courses and a biopsy with the presence of inflammatory infiltrates or disorders that suggest an immune-mediated cause. Most of them are pathologies that should be diagnosed early because they could receive curative treatment. These diseases are diagnosed from a combination of clinical presentation, laboratory tests, and biopsy [27, 28]. The main diagnostic target would be to be able to monitor the inflammatory load. A good correlation was found between elastography and specific muscle biomarkers like the levels of creatine kinase (CK) and lactate dehydrogenase (LDH) [29]. Despite the subtypes of IIM, with heterogeneous clinical and muscular involvement, they share the same pattern of findings, consisting of inflammation/edema, followed by fatty infiltration and eventually muscle atrophy. Some studies focus on the deltoid and vastus lateralis muscles, because of their common involvement in IIM, showing lower values of shear wave speed in these patients, compared to healthy controls [25]. Song et al. [30] found a significant correlation between semi-quantitative US strain elastography rates and pathological scores in biopsy samples in patients with IIM. In that study they could not find a significant correlation with qualitative US and magnetic resonance imaging (MRI) analysis, stressing the added value of elastography in the evaluation of these patients, especially in the early stages of the disease. Finally, a significant advantage of elastography is that it allows monitoring the evolution of the disease in a simple and noninvasive way.
5.2.1.2 Duchenne Dystrophy
Inherited myopathies and muscular dystrophies encase a varied group of diseases with a genetic origin, that present with weakness, motor delay, and respiratory and bulbar dysfunction. Duchenne dystrophy is the most common among them, with a prevalence of 1/3300 males [31]. In Duchenne dystrophy, there is an involvement of the dystrophin gene that makes muscle contraction impossible and clinically manifests itself with proximal weakness, already evident at a very early age, about 5 years, leading to the impossibility of independent ambulation over 13 years without treatment, with early cardiorespiratory involvement that implies high morbidity and mortality in adolescent age. The affected musculature presents an excessive deposit of fibroadipose tissue, with loss of muscle mass and its function, showing atrophy and diffuse increase in echogenicity in B mode ultrasound, as well as an increase in stiffness in the elastographic evaluation. There is a characteristic pattern of involvement: predominant in gluteus maximus and quadriceps muscles with relative sparing of the gracilis, sartorius, and semimembranosus muscles and, in the lower leg, predominant in the superficial posterolateral muscles more than the deep posterior and anterior leg muscles. SWE measurements are generally increased compared to age-matched controls, especially the gluteus maximus (mean 27 kPa versus 21.9 kPa), and tibialis anterior muscles (mean 96.8 kPa versus 23.1 kPa) [32]. Furthermore, a study analyzed changes in SWE values in patients with DMD over 12 months, and elastography proved to be a good candidate for a monitoring tool [33].
5.2.1.3 Cerebral Palsy
Cerebral palsy (CP) is a nonprogressive (though not unchanging) disorder of movement and posture pathology associated with an impairment of the developing fetal or infant brain (up to 2 years old). It represents the first cause of disability in childhood. It has a prevalence of 1.5–2.5 per 1000 live births and is estimated to affect around 17 million people worldwide. Although the symptoms can be very diverse depending on the brain involvement (motor deficit, epilepsy, behavioral changes, etc.), some of the most frequent complications are musculoskeletal deformations and hypertonia (spasticity, dystonia, and stiffness) [34, 35]. CP is one of the most common disorders associated with secondary muscle changes. It has been shown that patients with CP have greater shear modulus (rigidity) and also that there is more variability in stiffness than in patients without CP [36]. It has also been demonstrated significantly different shear wave speeds between the less and more affected sides [37], and that muscle stiffness assessed using elastography is related to clinical presentations according to the MAS score (modified Ashworth scale) [38], which measures clinically the resistance during passive soft tissue stretching and it is used as a simple measure of spasticity. Given that the most frequent subtype of cerebral palsy is that associated with spasticity, the target in these cases is to be able to guide the rehabilitation techniques on which the treatment is based, and also to monitor the botulin toxin A (BoNT-A) treatment. Clear associations between post-BoNT-A changes in elastography measurements and clinical scale scores for spasticity have been proved [39]. Also, elastography can be useful to plan the best interval between doses as it has been used to quantify the duration of treatment effect, resulting in <3 months post injection [40] (Fig. 5.4).
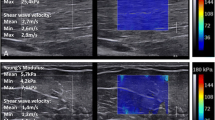
Shear wave elastography in an adult patient affected by cerebral palsy. In B mode the medial and lateral gastrocnemius muscles appear equally hyperechogenic, as seen on the background of the elastograms. The medial gastrocnemius (a) showed a shear modulus between 142 and 197 kPa, whereas the lateral gastrocnemius (b) showed a shear modulus between 24 and 36 kPa. This reflects an asymmetric involvement of these muscles, probably with a larger amount of fibrosis in the medial gastrocnemius. Note that in healthy individuals, the stiffness is greater in the lateral gastrocnemius
5.2.2 Traumatic Muscle Injury
Muscle injuries have three pathophysiologic phases: destructive, reparative, and remodeling. The destructive phase takes place at the time of the injury, depending on the severity of the lesion it will produce or not a hematoma in situ and a separation gap between muscle fibers/interstitium stumps. The reparative phase occurs around the second day after an injury and produces a scar, revascularization, and regeneration of myofibrils at the site of the lesion. The remodeling phase occurs later when the scar tissue reorganizes and the regenerated myofibrils maturate, leading to the recovery of muscle function. In US elastography, the destructive phase will show the injured tissue as a focal decrease in muscle stiffness, corresponding to the hematoma, and overdistended or torn myofibrils. Small tears may be difficult to detect with B mode US, however, the elastogram can show the softened area, confirming the diagnosis. In the reparative phase, the elastography will depict the scar tissue as an area of increased stiffness that can extend beyond the injury seen in B mode US [41,42,43]. Furthermore, it has been suggested as a good prognostic sign, the presence of decreased stiffness muscle adjacent to the scar tissue seen on B mode US (“elastic ribbed appearance”), as it is less prone to re-injury and behaves biomechanically better during contraction [41, 42] (Figs. 5.5 and 5.6).
This is a patient with insidious onset pain after prolonged exercise. The ultrasound (on the left) is practically normal, however, the elastography (below) shows focal softening. During contraction (on the right) a hypoechoic zone (dashed line) is observed that corresponds to a tear and coincides with the softening depicted in the shear wave elastogram
Evolution of a sports injury of medial gastrocnemius, “tennis leg.” US B-mode and corresponding strain elastogram images in the longitudinal plane of the distal medial gastrocnemius aponeurosis. The first US demonstrated a distal myoaponeurotic tear with focal softening of the injured tissues. Note the green area corresponding to the hematoma and muscle stumps (arrowheads), and the “red” softened fascia (arrows). In the 2 months follow-up study, a massive hematoma extending proximally through the myofascial junction and deep venous thrombosis (not shown) were detected. The elastogram showed a softened myoaponeurotic junction, indicating immature scaring. At 6 months follow-up the patient still has mild symptoms, the hematoma significantly diminished but the scarring process is incomplete and heterogeneous. At the distal end, the scar has areas of softening and the underlying muscle is hard. At the proximal end, the fascial scar stiffness is comparable to the normal fascia, and the underlying muscle has a normal pattern
Fascial tissues, including the rest of the interstitium, have viscoelastic and inhomogeneous properties that affect their biomechanics even more than myofibrils. The loose component in the interstitium works as a sliding system and accounts for its viscous properties. Also, in normal conditions, the fascial tissues change their elastographic behavior depending on the position of the joints related to their insertions. For instance, the gastrocnemius’s fasciae appear soft in a relaxed state and progressively and significantly increase their stiffness according to different degrees of ankle dorsiflexion [44], a factor that needs to be taken into account when performing the study (Fig. 5.7).
Elastographic changes in gastrocnemius fascia according to the position of the joints. (a) Relaxed state. (b) Active contraction, (c) ankle dorsiflexion. B-mode on the left and strain elastogram on the right. The dashed box encases the deep fascia of the medial gastrocnemius. In the relaxed position, the fascia appears with an intermediate softness (green), and in active contraction and ankle dorsiflexion, the stiffness increases both in the muscle and in the fascia (blue)
After a fascia injury, the elastographic changes occur following the same phases described above in the case of muscle injury. Well, in the chronic phase the fibrotic tissue and the altered loose interstitial component can become stiffer than normal tissue, losing sliding properties and sometimes affecting the muscle function, a phenomenon that patients may refer to as “tightness”[45] (Fig. 5.8).
This is a patient with a previous tennis leg injury who undergoes a follow-up ultrasound. B-mode US continues to show a nonspecific hypoechoic area. Shear wave elastography demonstrates stiff scar tissue in the myoaponeurotic junction, indicating a mature scar. Nevertheless, there is also increased stiffness in the soleus muscle, as opposed to the good prognostic “elastic ribbed appearance” sign, indicating in this case, incomplete restoration of the aponeurosis properties
Nerve endings run through the interstitium. The neuromuscular system is based on sensory information that allows measuring the length and speed of muscle fibers constantly, thus maintaining tension and being sensitive to stretching in all phases of contraction and relaxation. Hyperexcitability of the motor neuron can lead to spastic hypertonia when muscle responsiveness is impaired [6]. Also, the modification in the sliding properties of the interstitium after a muscle injury can lead to abnormal movement of the nociceptors within it during muscle function, and misinterpret such mechanical dysfunction as pain [45].
In terms of return-to-play time, the goal is to achieve muscle function restoration before restarting the sports practice. Based on MRI and medical records, previous studies showed return-to-play times between a few days and 7 weeks for calf injuries [46, 47], probably reflecting heterogeneity in the inclusion of pure muscular and myotendinous junction injuries, the latter known to have a longer repairing time [48]. Yoshida et al. [49] found that musculotendinous junction injuries in the calf tend to restore their normal stiffness between 8 and 12 weeks, longer than previous reports. A possible application for this finding is to follow up with elastography on the healing process of the myotendinous junction’s injury to guide the optimal return-to-play time, avoiding potential re-injury.
Blunt trauma has been studied in animals with SWE demonstrating a transient increase in muscle stiffness after the traumatic injury, that correlated with increased collagen fibers (fibrosis). Fibrosis was found to be higher in the group without treatment than in the treatment group. Thus, elastography could be used to demonstrate the tissue alterations after blunt trauma, follow the recovery phase, and monitor the response to therapies [50].
Literature is limited in using SWE in patients with delayed onset muscle soreness (DOMS), a type of transient muscle injury induced by extenuating exercise. So far, US elastography does not appear to have a practical application in this field. It has been found a transient increase in muscle stiffness after vigorous exercise under controlled conditions [51,52,53], nevertheless, Agten et al. did not find a correlation between the observed increase in stiffness suffered by the affected muscles and clinical symptoms nor quantitative MRI parameters, which by counterpart, had significant correlation with pain [53].
5.2.3 Painful Muscle Disorders
Regional myofascial pain is a clinical syndrome characterized by the development of pain related to trigger points [54]. Although its exact etiology is not fully known, it appears to be related to a protracted spontaneous contraction of a selected muscle band (taut band), that leads to ischemia in the affected muscle, which in turn activates nociceptors and produces pain, in a vicious cycle. The main goal of the management is to stop that cycle. Previous publications showed how the US can demonstrate these trigger points, as hypoechoic rounded areas with altered echotexture [55], and nowadays with the added tool of SWE, we can also measure their stiffness. As shown by Ertekin et al. [56] SWE is a suitable test to detect latent trigger points, clinically silent points that may convert into active and symptomatic [57], and to monitor the effects of physical therapy, referred to by them as the most effective treatment option, demonstrating a significant decline in muscle stiffness, accompanying a clinical improvement, after 4 weeks of treatment. Nevertheless, literature is limited in this field and the study by Valera-Calero et al. [58] did not find a significant correlation between muscle stiffness measured by SWE and clinical severity markers, although they demonstrated increased stiffness in the trigger points and also in the rest of the affected muscle. That said, more research is needed to clarify the actual utility of demonstrating the increased stiffness in trigger points and muscles of patients suffering from this syndrome.
Additionally, elastography can be used to demonstrate muscle tension, a painful syndrome that is readily common in daily practice. Some studies have investigated this topic, but so far, the results have not been homogeneous demonstrating increased or decreased stiffness in the painful areas, although there were differences in the methodological approach to measurements. The relationship seems more constant in the case of neck and back pain, in which increased stiffness can be readily observed with SE [59] (Fig. 5.9). This finding could be used in the future to guide therapies and follow-up their effects.
Examples of painful muscle tension. (a and b) belongs to a patient with acute lumbar back pain. (a) is longitudinal imaging of the left quadratus lumborum muscle, with B-mode and corresponding strain elastogram. The elastographic pattern of the muscle is normal. (b) shows the symptomatic right quadratus lumborum, with diffusely increased stiffness. (c) Another patient with left cervical pain, in which the shear wave elastogram in the transverse plane, shows diffusely increased stiffness in the left multifidus muscle
5.2.4 Acute Compartment Syndrome
Acute compartment syndrome (ACS) is an orthopedic emergency caused by increased interstitial pressure, the intracompartmental pressure (ICP), in a closed osteofascial compartment, usually affecting the limbs. It can be derived from many causes, like fractures or compression injuries, that lead to hypoxia, with potentially serious consequences if untreated, like muscular necrosis, limb dysfunction, amputation, and death [60]. The diagnosis is difficult, usually clinical and invasive methods, like Whitesides needle intracompartmental manometry [61]. SWE is a promising tool to assess the ICP as found by Zhang et al. [62], they manage to demonstrate a statistically significant increase in muscular stiffness in patients with suspected ACS from many causes. In their study, the group treated with fasciotomy showed a several times increase in Young’s modulus of the affected compartment, compared to the unaffected limb, ranging from 2 to 9.74. At the time of writing this chapter, there are ongoing studies in this field with unpublished results, that we hope to support the described findings, and enhance the potential role of SWE in such cases, providing a clinical guide to diagnosis and treatment.
5.2.5 Plantar Fasciitis
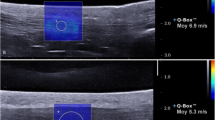
This is a self-limiting but life-altering condition in which a painful sensation is felt when pressing on the heel, exacerbated by periods of non-loading. It is the most common cause of nontraumatic heel pain and develops after degeneration of the plantar fascia at its calcaneal enthesis due to multifactorial causes. The diagnosis can usually be sufficed by clinical history and physical examination, nevertheless, the US can confirm the diagnosis and guide minimally invasive treatments [63,64,65]. As described in a recent meta-analysis by Wu et al. [66], the plantar fascia is significantly less stiff in patients with plantar fasciitis than in asymptomatic individuals (Fig. 5.10). They found this feature can be demonstrated both with SE and SWE, although further investigations are needed to evaluate the impact of this finding in the diagnosis and treatment of patients with plantar fasciitis.
Longitudinal view of the central fibers of the plantar fascia at its insertion on the calcaneus in a patient with clinical suspicion of plantar fasciitis. Although the fascia’s thickness is in the upper limit of normality, and consequently of doubtful imaging diagnosis, the shear wave elastography showed significantly reduced stiffness at the insertional portion compared to the distal portion, confirming the diagnosis of plantar fasciitis
5.2.6 Sarcopenia
Sarcopenia is defined by the European Working Group on Sarcopenia in Older People (EWGSOP), as a syndrome characterised by progressive and generalized loss of skeletal muscle mass and strength, with a risk of adverse outcomes as physical disability, falls, fractures, poor quality of life and death [67, 68]. Its prevalence is increasing, reaching up to 50% of the population older than 80 years [69]. Changes in muscle parameters can start as early as 25, but it is beyond 60, when muscle quantity, quality, and strength begin to decline considerably. Its diagnosis relies on the determination of low muscle strength and, low muscle quantity or quality, and is considered severe when low physical performance is detected [69]. From the imaging side, diagnosis of low muscle quantity may be established by dual X-ray absorptiometry (DXA), CT, MRI, and US [70]. DXA is probably the most accepted imaging test for determining sarcopenia, owing to the validated cut-off values that make it useful in clinical practice. Limitations of DXA include poor evaluation of muscle quality in terms of muscle steatosis, and the fact that may be biased in patients with extracellular water accumulation as those with heart, kidney, and liver failure because DXA cannot differentiate water from lean mass, the key parameter that estimates the amount of non-fatty/non-bony tissues the body [71, 72]. CT is becoming a recurrent tool in many studies to measure muscle quantity, in terms of cross-sectional area, and quality, in terms of muscle density, recognizing that abnormal muscle decrease in area and density. It has the disadvantages of using radiation, being unavailable in some clinical settings, and being unable to work by the patient’s bedside. MRI is a promising tool for the determination of sarcopenia because of the possibility of evaluating muscle quantity and quality with semi-quantitative and quantitative sequences, without the need for radiation exposure. Though, its use is so far limited to research conditions and shares the limitations of unavailability and inability of bedside exploration, has long acquisition times, and there is a lack of cut-off values and standardized protocols [70]. Ultrasound assessment can detect reduced quantity, and quality in the form of volume loss, increased echogenicity reflecting fat infiltration, and changes in pennation angle and fascicle length. US SWE has been proposed as an added tool to measure muscle quality, considering that muscle stiffness reduces while different grades of fatty infiltration occur until it gets completely infiltrated and atrophied, when the stiffness increases, as shown in the case of rotator cuff tendon rupture and associated muscle degeneration [26]. Although the relation between muscle stiffness and aging is still not completely clear, studies show a higher stiffness in the older population [14]. Results show great variability probably due to different techniques, different muscular groups studied, differences in activity level, and different ages in the target population. Sarcopenia determination with ultrasound and SWE is promising because it could overpass disadvantages of CT and MRI, like radiation, high cost, and long acquisition time, but so far shares with them a lack of validated cut-off values, which limits its use in clinical practice [70, 73].
5.3 Limitations
As with any other diagnostic test, elastography has some specific limitations for the evaluation of muscles that should be known in advance, as well as the technical recommendations to avoid them as much as possible [74].
-
SWE measurements may vary from one vendor to another. Although the found variation is minimal, it is unclear if it could be clinically relevant or not [24, 75]. In case of following-up patients or research conditions, use the same equipment to avoid such variability.
-
Skin overpressure may increase the measured stiffness in muscle. When scanning, apply as minimal pressure as possible [76].
-
Variability in measurements due to the scanning angle. Most reproducibility is yielded by measuring stiffness in the longitudinal plane [24] and < 30° relative to muscle fibers orientation [77].
-
Vigorous exercise transiently increases muscle stiffness. Then, if the patients to be tested have exercised, wait at least 48 h after strenuous physical activity, when muscle stiffness returns to baseline [78].
-
Small ROI increases variance in SWE results. The use of a medium-sized ROI (defined as one with an area of 75 mm2) was found to produce better intra-observer agreement [24].
-
Soft tissues over convex bony surfaces may have artificially altered stiffness. To avoid such artifact reorientation of the probe is advised, selecting a scanning area where the bony surface isn’t convex or measuring far from the artifact band (“reflective corridor”) [79].
-
As occurs with B-mode US, the deeper the tissues evaluated, the more US/Shear Waves attenuation (5). Scanning tissues obtain the best results at <4 cm deep [16]. In fact, in patients with thick subcutaneous tissue, elastography may not reach the muscle (Fig. 5.11).
Limitation of elastography due to thick subcutaneous tissue. US study of the abdominal wall in an obese patient. (a) Strain elastography of the subcutaneous fat. (b) Strain elastography of the rectus abdominis muscle (void arrow), about 3.5 cm from the skin surface. Note the quality sensor indicates poor sampling (arrowhead) and the elastogram is not presented (empty box)
-
US elastography is limited by its intrinsic operator dependency and the risk of lacking reproducibility [18]. Do your best to be consistent in performing the test, do 3–5 measurements, and use the mean value. Practice makes perfect: better intra-operator agreement has been found in the case of experienced operators [8].
-
US elastography is affected by the “eggshell effect,” in which harder tissues surrounding a ROI limit the penetration of the compression wave [79]. As far as technically possible, avoid scanning around bony surfaces.
-
Movement of the patient or the probe during the test can produce unreliable results. Try scanning in a stable position and do 3–5 measurements to detect possible motion artifacts [79].
-
Make sure there are no gas bubbles between the patient and the probe, as they could cause an apparent increase in stiffness (Fig. 5.12).
5.4 Conclusion and Future Perspectives
As has been exhibited in this chapter, elastography has great potential to enter clinical practice in diverse scenarios, soon it will be more available in diagnostic facilities and, its results have been demonstrated to be reproducible. Nevertheless, there is still a trip to go. A limitation in the current literature is the lack of large series establishing normality thresholds, which limits the usability of this technique in daily routine. Standardization is a must in the process of achieving this goal, and it will necessarily need to take into account the sonographer’s preparation and experience, patient positioning, US probe positioning, previous physical activity in the evaluated patient, muscles to be evaluated, and the unit of measurement. The next step will be to put elastography into the context of diseases and see how such findings can influence the treatment or predict a given prognosis, and according to such knowledge, introduce elastography in clinical guidelines.
References
Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;28:5338–51.
Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011;33:1016–24.
Ryan M, Bisset L, Newsham-West R. Should we care about tendon structure? The disconnect between structure and symptoms in tendinopathy. J Orthop Sports Phys Ther. 2015;45:823–5.
Docking SI, Ooi CC, Connell D. Tendinopathy: Is imaging telling us the entire story? J Orthop Sports Phys Ther. 2015;45:842–52.
Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 2: Clinical applications. Ultraschall Med. 2013;34:238–53.
Harmon B, Wells M, Park D, Gao J. Ultrasound elastography in neuromuscular and movement disorders. Clin Imaging. 2019;53:35–42.
Creze M, Nordez A, Soubeyrand M, Rocher L, Maître X, Bellin M-F. Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skelet Radiol. 2018;47:457–71.
Cortez CD, Hermitte L, Ramain A, Mesmann C, Lefort T, Pialat JB. Ultrasound shear wave velocity in skeletal muscle: a reproducibility study. Diagn Interv Imaging. 2016;97:71–9.
Chapter 5: Muscle: anatomy, physiology, and biochemistry. Firestein & Kelley’s textbook of rheumatology. Elsevier; 2021. p. 67–79.
Eby SF, Song P, Chen S, Chen Q, Greenleaf JF, An K-N. Validation of shear wave elastography in skeletal muscle. J Biomech. 2013;46:2381–7.
Shinohara M, Sabra K, Gennisson J-L, Fink M, Tanter M. Real-time visualization of muscle stiffness distribution with ultrasound shear wave imaging during muscle contraction. Muscle Nerve. 2010;42:438–41.
Yoshitake Y, Takai Y, Kanehisa H, Shinohara M. Muscle shear modulus measured with ultrasound shear-wave elastography across a wide range of contraction intensity. Muscle Nerve. 2014;50:103–13.
Wang C-Z, Li T-J, Zheng Y-P. Shear modulus estimation on vastus intermedius of elderly and young females over the entire range of isometric contraction. PLoS One. 2014;9:e101769.
Eby SF, Cloud BA, Brandenburg JE, Giambini H, Song P, Chen S, LeBrasseur NK, An K-N. Shear wave elastography of passive skeletal muscle stiffness: influences of sex and age throughout adulthood. Clin Biomech (Bristol Avon). 2015;30:22–7.
Miyamoto N, Hirata K, Kanehisa H, Yoshitake Y. Validity of measurement of shear modulus by ultrasound shear wave elastography in human pennate muscle. PLoS One. 2015;10:e0124311.
Alfuraih AM, O’Connor P, Hensor E, Tan AL, Emery P, Wakefield RJ. The effect of unit, depth, and probe load on the reliability of muscle shear wave elastography: variables affecting reliability of SWE. J Clin Ultrasound. 2018;46:108–15.
Botar Jid C, Vasilescu D, Damian L, Dumitriu D, Ciurea A, Dudea SM. Musculoskeletal sonoelastography. Pictorial essay. Med Ultrason. 2012;14:239–45.
Winn N, Lalam R, Cassar-Pullicino V. Sonoelastography in the musculoskeletal system: current role and future directions. World J Radiol. 2016;8:868–79.
Schleip R, Jäger H, Klingler W. What is “fascia”? A review of different nomenclatures. J Bodyw Mov Ther. 2012;16:496–502.
Fascia glossary of terms - Fascia Research Society. https://www.fasciaresearchsociety.org/fascia_glossary_of_terms.php. Accessed 6 Oct 2022.
Akagi R, Takahashi H. Acute effect of static stretching on hardness of the gastrocnemius muscle. Med Sci Sports Exerc. 2013;45:1348–54.
Akagi R, Yamashita Y, Ueyasu Y. Age-related differences in muscle shear moduli in the lower extremity. Ultrasound Med Biol. 2015;41:2906–12.
Bouillard K, Jubeau M, Nordez A, Hug F. Effect of vastus lateralis fatigue on load sharing between quadriceps femoris muscles during isometric knee extensions. J Neurophysiol. 2014;111:768–76.
Alfuraih AM, O’Connor P, Tan AL, Hensor E, Emery P, Wakefield RJ. An investigation into the variability between different shear wave elastography systems in muscle. Med Ultrason. 2017;19:392–400.
Paramalingam S, Needham M, Raymond W, Mastaglia F, Lightowler D, Morin N, Counsel P, Keen HI. Muscle shear wave elastography, conventional B mode and power Doppler ultrasonography in healthy adults and patients with autoimmune inflammatory myopathies: a pilot cross-sectional study. BMC Musculoskelet Disord. 2021;22:537.
Rosskopf AB, Ehrmann C, Buck FM, Gerber C, Flück M, Pfirrmann CWA. Quantitative shear-wave US elastography of the supraspinatus muscle: reliability of the method and relation to tendon integrity and muscle quality. Radiology. 2016;278:465–74.
Amato AA, Barohn RJ. Evaluation and treatment of inflammatory myopathies. J Neurol Neurosurg Psychiatry. 2009;80:1060–8.
Mariampillai K, Granger B, Amelin D, et al. Development of a new classification system for idiopathic inflammatory myopathies based on clinical manifestations and myositis-specific autoantibodies. JAMA Neurol. 2018;75:1528–37.
Botar-Jid C, Damian L, Dudea SM, Vasilescu D, Rednic S, Badea R. The contribution of ultrasonography and sonoelastography in assessment of myositis. Med Ultrason. 2010;12:120–6.
Song Y, Lee S, Yoo DH, Jang K-S, Bae J. Strain sonoelastography of inflammatory myopathies: comparison with clinical examination, magnetic resonance imaging and pathologic findings. Br J Radiol. 2016;89:20160283.
Cardamone M, Darras BT, Ryan MM. Inherited myopathies and muscular dystrophies. Semin Neurol. 2008;28:250–9.
Cornelson SM, Ruff AN, Perillat M, Kettner NW. Sonoelastography of the trunk and lower extremity muscles in a case of Duchenne muscular dystrophy. J Ultrasound. 2021;24:343–7.
Lacourpaille L, Gross R, Hug F, Guével A, Péréon Y, Magot A, Hogrel J-Y, Nordez A. Effects of Duchenne muscular dystrophy on muscle stiffness and response to electrically-induced muscle contraction: a 12-month follow-up. Neuromuscul Disord NMD. 2017;27:214–20.
Bromham N, Dworzynski K, Eunson P, Fairhurst C. Cerebral palsy in adults: summary of NICE guidance. BMJ. 2019;364:l806.
Graham HK, Rosenbaum P, Paneth N, et al. Cerebral palsy. Nat Rev Dis Primer. 2016;2:1–25.
Brandenburg JE, Eby SF, Song P, Kingsley-Berg S, Bamlet W, Sieck GC, An K-N. Quantifying passive muscle stiffness in children with and without cerebral palsy using ultrasound shear wave elastography. Dev Med Child Neurol. 2016;58:1288–94.
Lee SSM, Gaebler-Spira D, Zhang L-Q, Rymer WZ, Steele KM. Use of shear wave ultrasound elastography to quantify muscle properties in cerebral palsy. Clin Biomech (Bristol Avon). 2016;31:20–8.
Vola EA, Albano M, Di Luise C, Servodidio V, Sansone M, Russo S, Corrado B, Servodio Iammarrone C, Caprio MG, Vallone G. Use of ultrasound shear wave to measure muscle stiffness in children with cerebral palsy. J Ultrasound. 2018;21:241–7.
Dağ N, Cerit MN, Şendur HN, Zinnuroğlu M, Muşmal BN, Cindil E, Oktar SÖ. The utility of shear wave elastography in the evaluation of muscle stiffness in patients with cerebral palsy after botulinum toxin A injection. J Med Ultrason. 2020;47:609–15.
Brandenburg JE, Eby SF, Song P, Bamlet WR, Sieck GC, An K-N. Quantifying effect of onabotulinum toxin A on passive muscle stiffness in children with cerebral palsy using ultrasound shear wave elastography. Am J Phys Med Rehabil. 2018;97:500–6.
Paoletta M, Moretti A, Liguori S, Snichelotto F, Menditto I, Toro G, Gimigliano F, Iolascon G. Ultrasound imaging in sport-related muscle injuries: pitfalls and opportunities. Medicina (Mex). 2021;57:1040.
Drakonaki EE, Sudoł-Szopińska I, Sinopidis C, Givissis P. High resolution ultrasound for imaging complications of muscle injury: is there an additional role for elastography? J Ultrason. 2019;19:137–44.
Martínez-Rodríguez R, Galán-Del-Río F, Cantalapiedra JA, Flórez-García MT, Martínez-Martín J, Álvaro-Meca A, Koppenhaver SL, Fernández-de-Las-Peñas C. Reliability and discriminative validity of real-time ultrasound elastography in the assessment of tissue stiffness after calf muscle injury. J Bodyw Mov Ther. 2021;28:463–9.
Zhou J, Lin Y, Zhang J, Si’tu X, Wang J, Pan W, Wang Y. Reliability of shear wave elastography for the assessment of gastrocnemius fascia elasticity in healthy individual. Sci Rep. 2022;12:8698.
Kawai T. Shear wave elastography for chronic musculoskeletal problem. London: IntechOpen; 2022. https://doi.org/10.5772/intechopen.102024.
Hallén A, Ekstrand J. Return to play following muscle injuries in professional footballers. J Sports Sci. 2014;32:1229–36.
Waterworth G, Wein S, Gorelik A, Rotstein AH. MRI assessment of calf injuries in Australian Football League players: findings that influence return to play. Skelet Radiol. 2017;46:343–50.
Comin J, Malliaras P, Baquie P, Barbour T, Connell D. Return to competitive play after hamstring injuries involving disruption of the central tendon. Am J Sports Med. 2013;41:111–5.
Yoshida K, Itoigawa Y, Maruyama Y, Kaneko K. Healing process of gastrocnemius muscle injury on ultrasonography using B-mode imaging, power Doppler imaging, and shear wave elastography. J Ultrasound Med. 2019;38:3239–46.
Zhou X, Wang C, Qiu S, Mao L, Chen F, Chen S. Non-invasive assessment of changes in muscle injury by ultrasound shear wave elastography: an experimental study in contusion model. Ultrasound Med Biol. 2018;44:2759–67.
Niitsu M, Michizaki A, Endo A, Takei H, Yanagisawa O. Muscle hardness measurement by using ultrasound elastography: a feasibility study. Acta Radiol. 2011;52:99–105.
Akagi R, Tanaka J, Shikiba T, Takahashi H. Muscle hardness of the triceps brachii before and after a resistance exercise session: a shear wave ultrasound elastography study. Acta Radiol. 2015;56:1487–93.
Agten CA, Buck FM, Dyer L, Flück M, Pfirrmann CWA, Rosskopf AB. Delayed-onset muscle soreness: temporal assessment with quantitative MRI and shear-wave ultrasound elastography. Am J Roentgenol. 2017;208:402–12.
Cummings M, Baldry P. Regional myofascial pain: diagnosis and management. Best Pract Res Clin Rheumatol. 2007;21:367–87.
Sikdar S, Shah JP, Gebreab T, Yen R-H, Gilliams E, Danoff J, Gerber LH. Novel applications of ultrasound technology to visualize and characterize myofascial trigger points and surrounding soft tissue. Arch Phys Med Rehabil. 2009;90:1829–38.
Ertekin E, Kasar ZS, Turkdogan FT. Is early diagnosis of myofascial pain syndrome possible with the detection of latent trigger points by shear wave elastography? Pol J Radiol. 2021;86:e425–31.
Celik D, Mutlu EK. Clinical implication of latent myofascial trigger point. Curr Pain Headache Rep. 2013;17:353.
Valera-Calero JA, Sánchez-Jorge S, Buffet-García J, Varol U, Gallego-Sendarrubias GM, Álvarez-González J. Is shear-wave elastography a clinical severity indicator of myofascial pain syndrome? An observational study. J Clin Med. 2021;10:2895.
Dieterich AV, Haueise A, Gizzi L. Feeling stiff…but what does it mean objectively?: can you measure muscle tension? Schmerz Berl Ger. 2022;36:242–7.
Via AG, Oliva F, Spoliti M, Maffulli N. Acute compartment syndrome. Muscles Ligaments Tendons J. 2015;5:18–22.
Hammerberg EM, Whitesides TE, Seiler JG. The reliability of measurement of tissue pressure in compartment syndrome. J Orthop Trauma. 2012;26:24–31; discussion 32.
Zhang J, Zhang W, Zhou H, Sang L, Liu L, Sun Y, Gong X, Guan H, Yu M. An exploratory study of two-dimensional shear-wave elastography in the diagnosis of acute compartment syndrome. BMC Surg. 2021;21:418.
Irving DB, Cook JL, Young MA, Menz HB. Impact of chronic plantar heel pain on health-related quality of life. J Am Podiatr Med Assoc. 2008;98:283–9.
Rompe JD. Plantar fasciopathy. Sports Med Arthrosc Rev. 2009;17:100–4.
Wearing SC, Smeathers JE, Urry SR, Hennig EM, Hills AP. The pathomechanics of plantar fasciitis. Sports Med Auckl NZ. 2006;36:585–611.
Wu C-H, Chiu Y-H, Chang K-V, Wu W-T, Özçakar L. Ultrasound elastography for the evaluation of plantar fasciitis: a systematic review and meta-analysis. Eur J Radiol. 2022;155:110495.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–23.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31.
Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452.
Chianca V, Albano D, Messina C, Gitto S, Ruffo G, Guarino S, Del Grande F, Sconfienza LM. Sarcopenia: imaging assessment and clinical application. Abdom Radiol N Y. 2022;47:3205–16.
Dn P, Pc O, Ej A, Ks N. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Phys. 1999;277:E489. https://doi.org/10.1152/ajpendo.1999.277.3.E489.
Prado CMM, Heymsfield SB. Lean tissue imaging: a new era for nutritional assessment and intervention. JPEN J Parenter Enteral Nutr. 2014;38:940–53.
Wang J-C, Wu W-T, Chang K-V, Chen L-R, Chi S-Y, Kara M, Özçakar L. Ultrasound imaging for the diagnosis and evaluation of sarcopenia: an umbrella review. Life. 2021;12:9.
Bastijns S, De Cock A-M, Vandewoude M, Perkisas S. Usability and pitfalls of shear-wave elastography for evaluation of muscle quality and its potential in assessing sarcopenia: a review. Ultrasound Med Biol. 2020;46:2891–907.
Dillman JR, Chen S, Davenport MS, Zhao H, Urban MW, Song P, Watcharotone K, Carson PL. Superficial ultrasound shear wave speed measurements in soft and hard elasticity phantoms: repeatability and reproducibility using two ultrasound systems. Pediatr Radiol. 2015;45:376–85.
Kot BCW, Zhang ZJ, Lee AWC, Leung VYF, Fu SN. Elastic modulus of muscle and tendon with shear wave ultrasound elastography: variations with different technical settings. PLoS One. 2012;7:e44348.
Liu J, Qian Z, Wang K, Wu J, Jabran A, Ren L, Ren L. Non-invasive quantitative assessment of muscle force based on ultrasonic shear wave elastography. Ultrasound Med Biol. 2019;45:440–51.
Andonian P, Viallon M, Goff CL, de Bourguignon C, Tourel C, Morel J, Giardini G, Gergelé L, Millet GP, Croisille P. Shear-wave elastography assessments of quadriceps stiffness changes prior to, during and after prolonged exercise: a longitudinal study during an extreme mountain ultra-marathon. PLoS One. 2016;11:e0161855.
Davis LC, Baumer TG, Bey MJ, van Holsbeeck M. Clinical utilization of shear wave elastography in the musculoskeletal system. Ultrasonography. 2019;38:2–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Duitama, I.G., Claramunt, A.A., Gonzalez, P.G. (2023). Muscles and Fasciae. In: Marsico, S., Solano, A. (eds) Elastography of the Musculoskeletal System . Springer, Cham. https://doi.org/10.1007/978-3-031-31054-6_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-31054-6_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-31053-9
Online ISBN: 978-3-031-31054-6
eBook Packages: MedicineMedicine (R0)