Abstract
Recently proposed dynamic functional connectivity (dFC) approach, which focuses on the degree of spatial-temporal variability in regional-pair-wise functional connectivity (FC), is able to detect neural network alternations as the core neural basis of schizophrenia (SZ). Moreover, from the perspective of “emergence” in complex network science, the importance of the establishment of a method to evaluate the neural interactions in the whole brain network, not separating each pair-wise interaction, is emphasized. We proposed the micro-state approach based on the whole-brain instantaneous frequency distribution as one of these methods; this approach opens a new avenue as an evaluation method to detect cognitive function impairment and pathology. Thus, we hypothesized that the application of this micro-state approach to neural activity could detect the other aspects of brain network alterations for SZ previously elucidated in conventional FC and dFC. We applied the micro-state approach to electroencephalography (EEG) signals of SZ patients and healthy controls. The results revealed the alternation of dynamical leading phase transitions between the frontal and occipital regions and right and left hemispheric regions at the beta and gamma bands. This alternation suggested the corpus callosum impairments and abnormal enhancement of functional hub structure at the fast bands as topology of whole brain functional network. Thus, our proposed micro-state approach succeeded in detecting the state transition alternations concerning SZ pathology. This approach might contribute in elucidating new aspects of dFC, thereby resulting in the discovery of a biomarker for SZ.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Schizophrenia (SZ) is a severe, chronic, and intractable mental disorder characterized by distinct positive symptoms, such as delusions, hallucinations, and thought disorder, and negative symptoms, such as decreased or lost motivation, emotional flatness, which impair cognitive function [1, 2]. Early therapeutic intervention has a significant impact on the prognosis [1]. Therefore, in addition to the conventional diagnosis based on the medical interview, devising objective and quantitative biomarkers is desirable to facilitate an early diagnosis. Disconnection of neural networks in SZ is an effective candidate for a biomarker of SZ, since the disconnection of neural networks in brain results in SZ symptoms according to the widely known disconnection hypothesis [3].
Studies based on electroencephalograms (EEGs) and functional magnetic resonance imaging (fMRI) have revealed that abnormalities in the interactions of neural activity between brain regions, which is measured by functional connectivity (FC) defined as synchronization/information flow of neural activity, reflect the pathology of SZ [4] (reviewed in [5]). Particularly, the combination of EEG signals with a high temporal resolution and phase synchronization approach using the high spatial-temporal resolution by reducing the volume conduction demonstrated the frequency-band and region-specific abnormality of FC in SZ, i.e., SZ patients have reduced beta band FCs centered in the frontal region and gamma band FCs throughout a wide-range of brain regions [6].
While, the temporal complexity of local neural activity in individual brain regions strongly correlates with the global network structure of FC, such as node degree and centrality [7, 8]; therefore the complexity reflects the global interaction of neural activity among a wide range of brain regions [6, 9]. In SZ, significant increases in complexity at slow temporal scales were identified in the front-center-temporal regions [10]. This abnormal high complexity may be attributed to the temporal disorganization of neural activity [10] (reviewed in [11]). This complexity is also affected by age, symptoms, and medication [11].
In addition to FC and local regional complexity in neural activity, recent findings of dynamic functional connectivity (dFC), defined by the degree of variation in the FC strength, demonstrated that dFC more accurately reflects the cognitive abilities than does static FC in SZ [12]. Additionally, by combining clustering and graph analysis methods with FC transitions in a time window, spatial and temporal patterns of network dynamics in the whole brain have been revealed [5, 13]. In dFC evaluation by fMRI, temporal changes in the coherence of blood oxygen level dependent (BOLD) signals in brain regions using sliding time window analysis have been used [14]. Moreover, studies on dFC of EEG and magnetoencephalogram (MEG) with high temporal resolution have been conducted[15]. Considering the application of dFC to EEG and MEG, although EEG and MEG have a higher temporal resolution than fMRI, the detection of instantaneous dynamics in the order of several milliseconds is challenging owing to the sliding window process used in the dFC estimation methods [15]. To tackle this issue, recent studies using a method called dynamical phase synchronization (DPS), which is defined by the temporal complexity of instantaneous phase difference of neural activity between regions, successfully detected moment-to-moment network dynamics in dFC during aging by decomposing the EEG signal [9].
The FC approach, including dynamic FC, focuses on pair-wise neural interactions among complex brain interactions [6, 9]. In the SZ, the degree of FC at the resting state alternates; especially, in a default mode network (DMN) exhibiting intrinsic activities, this alternation becomes significant [16]. However, brain functions are produced in global and multiple neural activities, and not only in local neural activity [17]. Several recent studies highlighted the importance of evaluating the brain from the perspective of “emergence” of complex systems, where new functions are created through the interaction of multiple elements [18]. “Emergence” research in complex network science have revealed that merely capturing pair-wise neural interaction is insufficient for evaluating complex neural network structures characterized as the brain [19]. Therefore, the need to incorporate an integrated approach for capturing neural interactions of the entire brain has been noted [18]. Considering one of these approaches, in our recent research, from the viewpoint of “emergence”, a new micro-state method based on the instantaneous frequency (called as IF micro-state) dynamics was proposed, which enables the evaluation of the neural activity of the entire brain rather than the pair-wise neural interaction between brain regions [20]. Subsequently, in a study on AD patients, this approach revealed that maintaining the occipital leading phase was more difficult in the AD group than in the healthy control group and its degree exhibits the correlation with cognitive function in AD [20].
Thus, considering the fact that cognitive dysfunction is also a core symptom in SZ, we hypothesized that our proposed IF micro-state approach, which can evaluate the integration of the instantaneous frequency dynamic of the whole brain without disassembling pair-wise neural interactions, would provide a new understanding of the relationship between the network dynamics of SZ. To validate our hypothesis, we define EEG signals as micro-states based on instantaneous frequencies in various frequency bands, and evaluated the dynamics of state transitions.
2 Materials and Methods
2.1 Participants
This study employed the same participants as those in our previous study concerning the abnormality of FC in SZ [6]. The SZ group consisted of 21 right-handed participants.The age-and sex-matched healthy control group (HC) consisted of 31 right-handed healthy participants. The demographic characteristics of the two groups are summarized in Table 1. Participants with major neurological diseases, previous electroshock or significant head trauma, or a history of drug or alcohol dependence were excluded. The HC participants were recruited from among staff members at the Kanazawa University Hospital and their family members. They had no personal or family history of psychiatric or neurological diseases, as confirmed by both a self-reported past history and a psychiatric examination of the present mental state using the axis I criteria of the Diagnostic and Statistical Manual-Fourth Edition (DSM-IV). Patients in the SZ group were recruited from the outpatients of Kanazawa University Hospital and met the criteria for DSM-IV SZ at the time of the study, and were subsequently diagnosed with SZ by a specialized clinical psychiatrist. The patients were diagnosed with SZ by an expert clinical psychiatrist. No patient had been treated with neuroleptics before the EEG recording. The Brief Psychiatric Rating Scale (BPRS) was used to assess the patient’s symptoms on the day of the EEG recording. The data were approved by the Ethics Committee of Kanazawa University and were conducted in accordance with the aims of the Declaration of Helsinki. Moreover, all the participants agreed to participate in the study upon understanding the study. Informed consent was obtained from the all the participants.
2.2 EEG Recordings
EEG data were recorded from 16 electrodes in accordance with the International 10-20 System: Fp1, Fp2, F3, F4, Fz, F7, F8, C3, C4, P3, P4, Pz, T5, T6, O1, and O2. The reference electrode was placed in the linked earlobes, and eye movements during the EEG recording were monitored using an additional electrooculogram. The impedance at each electrode was maintained below \(5k\Omega \). EEG was obtained at a sampling frequency of 200 Hz with a band pass filter of 1.5-60 Hz, and stored for offline analysis using an 18-channel system (EEG-44189; Nihon Kohden; Tokyo, Japan). The participants were instructed to lie down with their eyes closed in a soundproof, light-controlled, and electrically shielded recording room; EEG was recorded for 10–15 min in a resting-state condition. Artifacts (e.g., muscle activities, eye movements, or blinks) were visually identified and carefully excluded. One continuous 60-s artifact-free epoch was extracted from every participant. A PLI study in SZ demonstrated network abnormalities in beta and gamma bands [6]. Therefore, we focused on the whole brain IF micro-state of the beta and gamma bands. For each epoch, band-pass filtering was performed to isolate the conventional frequency bands as follows: beta (13-30 Hz), and gamma (30-60 Hz) bands. The first and last 5-s epochs were eliminated from the analysis to avoid interference related to the band pass filtering procedure.
2.3 Estimation of the Dynamic State Based on the Instantaneous Frequency Distribution
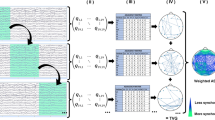
We defined the state of brain activity by studying the dynamics of the instantaneous frequencies of EEG signals. Using the time series of the instantaneous frequencies of the EEG signals; the following process [20] was used to estimate the state of brain activity (Fig.1).
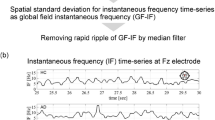
The Hilbert transform was used to estimate the instantaneous phase \(\theta (t)\) (\(-\pi \le \theta \le \pi \)) for each frequency band. This instantaneous phase has a phase noise called phase slip, which is a large deviation from the set frequency range. Therefore, by performing median filtering, we obtained continuous instantaneous frequencies \(I\!F (t)\) (\(-\infty \le I\!F(t) \le +\infty \)) without phase slip. This method was employed in our previous study [9]. Against this instantaneous frequencies \(I\!F_i (t)\) (i: electrode position), the frequency was Z-scored among all the electrodes. The z-scored \(dI\!F_i(t)\) of the HC and SZ groups were classified into k clusters by the k-means method. Here, the center of the cluster was determined by the z-scored \(dI\!F_i(t)\) of HC. In this study, we set the cluster size to \(k=2,3,4\). We set these cluster sizes to approximate the number of clusters generally used in micro-states (reviewed in [21, 22]).
2.4 Statistical Analysis
To evaluate the dynamic characteristics of state transitions classified by k-means, we used the emergence probabilities of each state and its state transition probability. A t-test was used to evaluate the difference in the emergence probabilities and the state transition probabilities between the HC and SZ groups. A Benjamini-Hochberg false discovery (FDR) correction was applied to the t-values for multiple comparisons of transition probabilities (size of p-values: \(k\times k\) state transitions \(\times \) frequency bands), and for the emergence probabilities of IF micro-state (size of p-values: k state \(\times \) 2 frequency bands) with \(q<0.05\).
(a) Topographs of the mean values of the Z-scored frequency \(dI\!F_i(t)\) in the healthy controls (HC) and schizophrenia (SZ) groups based on the duration for each state (#1 and #2) (cluster size \(k=2\), left and right parts exhibit beta and gamma bands, respectively). In both groups, the region-specific leading phase, such as the frontal and occipital leading, was confirmed. (b) Emergence probability of state #1 and #2. The significant large difference between the HC and SZ groups for the emergence probabilities were confirmed (\(q<0.05\)), which is squared by a solid blue circle. (Color figure online)
(a) Topographs of the mean values of Z-scored frequency \(dI\!F_i(t)\) in the HC and SZ groups based on the duration for each state (#1, #2, and #3) (cluster size \(k=3\), left and right parts exhibit beta and gamma bands, respectively). In both the groups, region-specific leading phase, such as the right and left frontal and occipital leading, was confirmed. (b) Emergence probability of states #1, #2, and #3. The significant large difference between the HC and SZ groups for the emergence probabilities was confirmed (\(q<0.05\)), which is squared by a solid blue circle. (Color figure online)
(a) Topographs of the mean values of the Z-scored frequency \(dI\!F_i(t)\) in the HC and SZ groups based on the duration for each state (#1, #2, #3, and #4) (cluster size \(k=4\), left and right parts exhibit the beta and gamma bands, respectively). In both the groups, region-specific leading phase, such as the right/left frontal and the right/left occipital leading phase, was confirmed. (b) Emergence probability of state #1, #2, #3, and #4. The significant large difference between the HC and SZ groups for the emergence probabilities was confirmed (\(q<0.05\)), which is squared by a solid blue circle. (Color figure online)
3 Results
The parts of (a) for Figs. 2, 3, and 4 demonstrate the group average for the temporal mean \(dI\!F_i(t)\) among the evaluation duration at the beta and gamma bands, in the cases with cluster size \(k=2,3,4\), respectively, in the HC and SZ groups. In the parts of (b) for Figs. 2, 3, and 4, the emergence probabilities and the t-values for these probabilities between the HC and SZ groups are shown. Although common region-specific topological features between the HC and SZ groups were observed, significant differences between the HC and SZ groups in the emergence probability were identified. Particularly, in the case of cluster size \(k=2\) (see Fig. 2), at both the beta and gamma bands, the states for the frontal and occipital leading phases were confirmed. The emergence probability for the frontal (occipital) leading phase of SZ significantly decreased (increased). Considering cluster size \(k=3\) (see Fig. 3), at both the beta and gamma bands, the states for the left frontal leading phase, the right frontal leading phase, and the occipital (especially, left occipital in gamma band) leading phase were confirmed. The emergence probabilities of the gamma band for the left occipital (right frontal) leading phase of SZ significantly decreased (increased). Considering cluster size \(k=4\) (see Fig. 4), at both the beta and gamma bands, the states for the left frontal leading phase, the right frontal leading phase, the left occipital leading phase and the right occipital leading phase were confirmed. In the beta band, the emergence probabilities for the right occipital leading phase of SZ significantly decreased. In the gamma band, the emergence probabilities for the left occipital leading phase of SZ significantly decreased; however, the emergence probabilities for the left frontal and right frontal leading phase of SZ significantly decreased.
(a) Mean values of the state transition probabilities for the beta band in the healthy control (HC) and schizophrenia (SZ) groups. (b) t-value of the state transition probabilities between the SZ and HC groups. A larger (smaller) t-value corresponds to a higher (lower) probability of SZ compared to that for HC. The parts surrounded by squares indicate the t-value satisfying the criteria of Benjamini-Hochberg false discovery correction. The probabilities of the SZ group were significantly smaller \(q<0.05\) than that of the HC group.
(a) Mean values of the state transition probabilities for the gamma band in the healthy control (HC) and schizophrenia (SZ) groups. (b) t-value of the state transition probabilities between the SZ and HC groups. A larger (smaller) t-value corresponds to a higher (lower) probability of SZ compared to that for HC. The parts surrounded by squares indicate the t-value satisfying the criteria of the Benjamini-Hochberg false discovery correction. The probabilities of the SZ group were significantly smaller \(q<0.05\) than that of the HC group.
The parts of (a) for Figs. 5 and 6 demonstrate the mean of the state transition probabilities of the HC and SZ groups in the beta and gamma bands, respectively. The parts of (b) for Figs. 5 and 6 demonstrate the t-values of the state transition probabilities between the HC and SZ groups. Considering the beta band (see Fig. 5), the state transition probability of SZ becomes significantly larger from the occipital to the frontal leading phase state and smaller from the occipital to the occipital in \(k=2\) case, larger from the occipital to the left frontal leading phase state, and smaller from the occipital to the occipital in \(k=3\) case, larger from the right occipital to the left occipital leading phase state, and smaller from the right occipital to the right occipital in \(k=4\) case. Considering the gamma band (see Fig. 6), the state transition probability of SZ shows significantly larger transitions to the occipital leading phase state in \(k=2,3,4\) case; becomes smaller from the frontal to the frontal and occipital leading phase state in \(k=2\), smaller from the occipital and right frontal to the right frontal leading phase state in \(k=3\), and smaller from the left occipital to the left frontal leading phase state in \(k=4\).
4 Discussion and Conclusions
To reveal the neural network dynamics of the whole brain in SZ, we introduced a method for estimating IF micro-states distribution of the whole brain using EEG. Concerning the emergence probability of IF micro-state, the emergence probability for the state of the occipital (frontal) leading phase increased (decreased) in the SZ groups and all the cluster sizes (\(k=2,3,4\)). Furthermore, the alternation of the state transition probability in SZ between the frontal and occipital leading phase state in the beta and gamma bands was observed in the case of cluster size \(k=2\). Similarly, in the cluster size \(k=3,4\), the alternation of the state transition probability of SZ between the right and left hemispheric leading phase state in the beta and gamma bands was observed.
We further discussed the reason for the region specific alternation of SZ in the emergence probability and state transition probability. In the case of cluster size \(k=2\), differences were found in the frontal-occipital regions of the emergence and state transition probability. A previous study reported the enhancement of hub structure centering at the occipital region in the FC of the fast frequency components of the EEG signals in SZ [23]. The hub structure plays a role in the integration of the neural information process [24]; therefore, the enhancement of this hub structure affects the state occipital leading phase (see the result of \(k=4\) at the beta band in Fig. 4(b), the result of \(k=2,3,4\) at the gamma band in Figs. 2, 3, and 4(b)), especially, might induce the transition of the state occipital leading phase (see the result of \(k=2,3\) at the beta band in Fig. 5 and the result of \(k=2,3,4\) at the gamma band in Fig. 6). Moreover, in the case of cluster size \(k=3,4\), differences of the state transition probability were found in the left and right hemispheric alterations. Many studies have suggested a decrease in the connectivity of the corpus callosum in SZ [25]. The observed alternation of state transition between the left and right hemispheric is considered to be attributed to this abnormality of the corpus callosum in SZ.
Our study had certain limitations. Previous studies of hub location and node centrality reported reduced the average centrality across hubs, shorter path lengths, and significantly weaker centrality within frontal regions [24, 26]. The hub integrates information; however, it is currently only undirected, such as the PLI. The high transition probability to the occipital in the SZ IF micro-state transitions could be attributed to the centrality and hub structures. However, in order to correspond to the leading phase state transition in this study, the directed hub structure in the FC detected by the directed connectivity measures typified as directed PLI and transfer entropy should be considered. Moreover, although MEG is less clinically adaptable than EEG, applying IF-micro-state to MEG with high spatial resolution may lead more detailed characteristics than one for EEG. Additionally, this study used the k-means method as the most convetional clustering method for micro-state clustering; while, other methods such as Ward’s method and furthest neighbor method may be used to capture the other aspects of state transitions. Thus, these points should be dealt with in future studies.
In conclusion, despite the limitations, our proposed IF micro-state based on the instantaneous frequency of the EEG approach succeeded in the detection of state transition alternations concerning SZ pathology. This approach could contribute in the elucidation of new aspects of dFC, which results in the establishment of a biomarker for SZ.
References
Green, M.F., Horan, W.P., Lee, J.: Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry 18(2), 146–161 (2019)
Correll, C.U., Schooler, N.R.: Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiat. Dis. Treat. 16, 519 (2020)
Friston, K.J.: The disconnection hypothesis. Schizoph. Res. 30(2), 115–125 (1998)
Medaglia, J.D., Lynall, M.-E., Bassett, D.S.: Cognitive network neuroscience. J. Cogn. Neurosci.27(8), 1471–1491 (2015)
Cohen, J.R.: The behavioral and cognitive relevance of time-varying, dynamic changes in functional connectivity. NeuroImage 180, 515–525 (2018)
Takahashi, T., Goto, T., Nobukawa, S., Tanaka, Y., Kikuchi, M., Higashima, M., Wada, Y.: Abnormal functional connectivity of high-frequency rhythms in drug-naïve schizophrenia. Clin. Neurophysiol. 129(1), 222–231 (2018)
Sporns, O., Honey, C.J.: RolfKotter. Identification and classification of hubs in brain networks. PLoS ONE 2(10), e1049 (2007)
Misic, B., Vakorin, V.A., Paus, T., McIntosh, A.R.: Functional embedding predicts the variability of neural activity. Front. Syst. Neurosci. 5, 90 (2011)
Nobukawa, S., Kikuchi, M., Takahashi, T.: Changes in functional connectivity dynamics with aging: a dynamical phase synchronization approach. Neuroimage 188, 357–368 (2019)
Takahash, T., et al.: Antipsychotics reverse abnormal EEG complexity in drug-naive schizophrenia: a multiscale entropy analysis. Neuroimage 51(1), 173–182 (2010)
Fernández, A., Gómez, C., Hornero, R., José López-Ibor, J.: Complexity and schizophrenia. Progr. Neuro-Psychopharm. Biol. Psychiatr. 45, 267–276 (2013)
Dong, D., Duan, M., Wang, Y., Zhang, X., Jia, X., Li, Y., Xin, F., Yao, D., Luo, C.: Reconfiguration of dynamic functional connectivity in sensory and perceptual system in schizophrenia. Cereb. Cortex 29(8), 3577–3589 (2019)
Kang, J., Pae, C., Park, H.-J.: Graph-theoretical analysis for energy landscape reveals the organization of state transitions in the resting-state human cerebral cortex. PLoS ONE 14(9), e0222161 (2019)
Allen, E.A., et al.: Tracking whole-brain connectivity dynamics in the resting state. Cerebral Cortex 24(3), 663–676 (2014))
Tewarie, P., et al.: Tracking dynamic brain networks using high temporal resolution meg measures of functional connectivity. Neuroimage 200, 38–50 (2019)
Hu, M.L., et al.: A review of the functional and anatomical default mode network iMn schizophrenia. Neurosci. Bull. 33(1), 73–84 (2017)
Yin, W., et al.: The emergence of a functionally flexible brain during early infancy. Proc. Natl. Acad. Sci. 117(38), 23904–23913 (2020)
Sporns, O., Betzel, R.F.: Modular brain networks. Ann. Rev. Psychol. 67, 613–640 (2016)
Battiston, F., Cencetti, G., Iacopini, I., Latora, V., Lucas, M., Patania, A., Young, J.-G., Petri, G.: Networks beyond pairwise interactions: structure and dynamics. Phys. Rep. 874, 1–92 (2020)
Nobukawa, S., Ikeda, T., Kikuchi, M., Takahashi, T.: Dynamical characteristics of state transition defined by neural activity of phase in alzheimer’s disease. In: Mantoro, T., Lee, M., Ayu, M.A., Wong, K.W., Hidayanto, A.N. (eds.) ICONIP 2021. CCIS, vol. 1517, pp. 46–54. Springer, Cham (2021). https://doi.org/10.1007/978-3-030-92310-5_6
Khanna, A., Pascual-Leone, A., Michel, C.M., Farzan, F.: Microstates in resting-state EEG: current status and future directions: Neurosci. Biobeh. Rev. 49, 105–113 (2015)
Strelets, V., et al.: Chronic schizophrenics with positive symptomatology have shortened EEG microstate durations. Clin. Neurophysiol. 14, (11), 2043–2051 (2003)
Krukow, P., Jonak, K., Karpiński, R., Karakuła-Juchnowicz, H.: Abnormalities in hubs location and nodes centrality predict cognitive slowing and increased performance variability in first-episode schizophrenia patients. Sci. Rep. 9(1), 1–13 (2019)
van den Heuvel, M.P., Mandl, R.C.W. Stam, C.J., Kahn, R.S., Hulshoff Pol, H.E.: Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J. Neurosci. 30(47), 15915–15926 (2010)
Najjar, S., Pearlman, D.M.: Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophrenia Res. 161(1), 102–112 (2015)
Jalili, M., Knyazeva, M.G.: EEG-based functional networks in schizophrenia. Comput. Biol. Med. 41(12), 1178–1186 (2011)
Acknowledgment
This study was supported by JSPS KAKENHI for a Grant-in-Aid for Scientific Research (C) (Grant No. 22K12183) (SN). This study was partially supported by the JST CREST (Grant No. JPMJCR17A4).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Ebina, D., Nobukawa, S., Ikeda, T., Kikuchi, M., Takahashi, T. (2023). Dynamic Characteristics of Micro-state Transition Defined by Instantaneous Frequency in the Electroencephalography of Schizophrenia Patients. In: Tanveer, M., Agarwal, S., Ozawa, S., Ekbal, A., Jatowt, A. (eds) Neural Information Processing. ICONIP 2022. Lecture Notes in Computer Science, vol 13624. Springer, Cham. https://doi.org/10.1007/978-3-031-30108-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-30108-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-30107-0
Online ISBN: 978-3-031-30108-7
eBook Packages: Computer ScienceComputer Science (R0)