Abstract
3D printing technology has grown significantly over the last four decades. Given the complex anatomical variations in congenital heart defects, this technology has been embraced in the field of pediatric cardiology and used for clinical understanding, procedural planning, and education. This chapter will cover 3D printing technology and the applications for patients with congenital heart disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- 3D printing
- Congenital heart disease
- Tetralogy of Fallot
- Transposition of the great arteries
- Atrial septal defect
- Fontan
- Cardiac catheterization
24.1 Introduction
Stereolithography, or as it is more commonly known, 3D printing, was first performed by Charles Hull in 1983 when he used the technique to produce a small plastic cup [1], and his first patent was issued in 1986 [2]. Since that time, there has been a rapid expansion of the use of 3D printing, initially in the fields of engineering and manufacturing, but more recently, in medicine. One field in which 3D printing has been embraced is congenital heart disease (CHD). Given the complex and variable nature of CHD, the ability to visualize and hold these hearts in your hand has been a game changer. In this chapter, we will explore some of the technical aspects of 3D printing, image acquisition in CHD, applications and limitations of 3D printing, and future uses of the technology.
24.2 The Process of 3D Printing
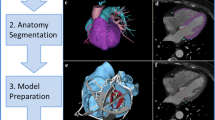
3D printing, or additive manufacturing, is the process of successive layering of a material to generate a structure with length, width, and height. The steps of digital model development and preparation are illustrated in Fig. 24.1. The first step is to acquire a digital 3D dataset, and for CHD, this is typically from computed tomography (CT) or magnetic resonance imaging (MRI). Once the dataset is acquired, the areas of interest for printing must then be identified and highlighted, a process called segmentation (Fig. 24.1), and usually converted to stereolithographic (STL) file formatting, the same that Charles Hull developed. The segmented dataset is then sliced, or converted into a stack of cross sections, which will become the printed layers (Fig. 24.1). The sliced file also has instructions for the 3D printer to physically print the object. The process of segmentation and slicing can take minutes to hours, and the print time can be as long as 24 h, depending on the size and complexity of the structure to be printed.
Process of 3D model generation for a neonate with pulmonary atresia, intact ventricular septum, and a tortuous patent ductus arteriosus. (a) Once a 3D dataset is obtained (typically from CT or MR imaging), the structures of interest are highlighted, a process known as segmentation. This shows axial, coronal, and sagittal projections with the segmented structures highlighted in orange and the 3D reconstruction in the upper right box. (b) Once the model has been cleaned and checked for errors, it is imported into slicing software which will generate support structures (gray) which are needed during the printing process and provide instructions for the movements for the specific 3D printed to be used
The most common 3D printing technologies in use today are fused deposition modeling (FDM), selective laser sintering (SLS), stereolithography (SLA), PolyJet, and digital light processing (DLP). Each has its own advantages and disadvantages, and a full analysis is beyond the scope of this chapter, but some examples of the printing process and final products from FDM and DLP printers are shown in Fig. 24.2.
The process of 3D model printing for a neonate with pulmonary atresia, intact ventricular septum, and a tortuous patent ductus arteriosus. (a–c) An example of fused deposition modelling (FDM) printing which heats and extrudes filament and draws the layers to create a 3D model. Once the model is complete, there are support structures which are needed during the print but are removed afterwards to produce the final model. (d–f) An example of digital light processing (DLP) printing which uses a light source to photocure resin material to generate the layers and create a 3D model. Once the model is complete, there are support structures which are needed during the print but are removed afterwards to produce the final model. Photos for (d–f) provided by Jason Pedersen
Some of the earliest descriptions of 3D printing in CHD came in 2006 when several groups reported printing models of various defects, including direct comparisons between the printed model, standard angiography, and direct visual inspection at the time of surgery [3, 4]. 3D datasets that have typically been used for CHD are CT or MRI, but they need to be high quality for accurate model generation. There is also a need for expertise in software and hardware manipulation [5, 6]. Several groups have quantitatively compared 3D-printed models to the original CT datasets and found high fidelity in the reproductions [7, 8].
While it is ideal to minimize exposure to ionizing radiation in patients with CHD, many have metallic implants (stents, occluder devices, and coils) which, even if safe for MR imaging, could cause enough image distortion to render MRI useless. In addition, image acquisition time is still longer for MRI such that infants need to be anesthetized to obtain diagnostic images. In response, cardiac CT scans with dose-reduction techniques have been used for infants with a variety of CHD and were found to generate excellent-quality images [9]. There have also been improvements in MR post-processing techniques so 3D modeling from MRI may become more commonplace [10]. While CT typically provides higher resolution imaging for 3D model generation, our group has been able to successfully use MRI to generate 3D models on newborn infants weighing less than 3 kg (Fig. 24.3).
Newer imaging modalities have allowed for acquisition of 3D datasets at the same time as other clinically indicated procedures. 3D rotational angiography (3DRA), which is increasingly performed as part of routine cardiac catheterizations, can produce printable 3D datasets [11, 12]. Due to the concern for increased radiation doses with 3DRA, Fetterly performed simulated radiation dose reductions for 3DRA imaging and found that even after reducing the radiation dose by 72%, there were no significant differences in the quality of the imaging or measurements of the structures of interest [13].
An exciting new development is the use of echocardiographic datasets to generate 3D-printed models without the use of any ionizing radiation. As detailed as CT and MRI are, thin structures, such as the atrioventricular valves, can be difficult to visualize, particularly in smaller patients. Several groups have utilized 3D datasets from either transthoracic or transesophageal echocardiograms to successfully print 3D models [14,15,16,17]. Additionally, others have been able to 3D print models of complex CHD with data obtained from fetal echocardiograms which have been particularly useful for prenatal counseling [18, 19].
Finally, combining all these modalities into hybrid model generation has allowed teams to use the best aspects of each technique to generate accurate 3D-printed models and optimize the clinical data obtained from the models. These models can be used for surgical planning, hemodynamic simulation, or education [14,15,16].
24.3 Current Applications of 3D Printing for Congenital Heart Disease
The use of 3D-printed models in CHD is relatively new [3, 4], but there has been rapid adoption within the field for multiple uses, including procedural planning (surgical and transcatheter), hemodynamic simulations, and education [20]. To support these uses, several studies have demonstrated the accuracy of 3D-printed models within 1 mm of standard clinical imaging [21, 22].
24.3.1 Surgical Planning for Complex CHD Repair
Two of the most challenging forms of CHD to repair surgically are double outlet right ventricle (DORV) and complete atrioventricular septal defects (AVSD) as the complex intracardiac relationships can be quite challenging to visualize by standard transthoracic echo alone and the interventional decisions made can greatly alter the long term outcomes for these patients.
For DORV, the location of the aorta relative to the ventricular septal defect (VSD) determines whether a surgeon can create an intracardiac baffle from the left ventricle, through the VSD and out the aorta to establish biventricular circulation (Fig. 24.4). 3D-printed models have been used to guide the critical decision of either initiating single ventricle palliation and potentially later converting to biventricular circulation [23] or performing biventricular repair as the initial intervention in patients where standard imaging supported single ventricle palliation [24,25,26,27,28,29]. To further understand the potential clinical benefits of a pre-operative 3D-printed model, one team compared outcomes for patients with DORV between those with and those without 3D-printed models. Encouragingly, post-operative ventilator time and ICU stays were significantly shorter in the 3D model group [30].
Utility of 3D models for surgical repair of double outlet right ventricle (DORV). (a, b) Two views of a virtual 3D reconstruction of an infant with DORV (right ventricular free wall removed) with the aorta highlighted in red, the pulmonary artery highlighted in blue, and the ventricular septal defect (VSD) outlined with the dashed line. (c, d) Two views of a 3D-printed model of the same patient with the red line representing the direction of flow from the left ventricle, through the VSD and out the aorta and the blue line representing flow from the right ventricle through the right ventricular outflow tract and out the pulmonary artery
For patients with an AVSD, particularly in the setting of heterotaxy syndrome, it can be difficult to determine patient suitability for single versus biventricular intracardiac repair. A review of the management of AVSD highlighted the beneficial addition of 3D echocardiography and 3D-printed models in helping to determine adequacy of biventricular repairs in borderline cases [31]. A series of patients with complex AVSD achieved conversion to biventricular circulation with the aid of 3D-printed models as part of the standard pre-operative evaluation [32].
In addition to DORV and AVSD, researchers have utilized 3D-printed models for a variety of complex CHD cases, including variants of tetralogy of Fallot [33, 34], transposition of the great arteries [35], anomalous pulmonary venous connection [36], pulmonary artery pseudoaneurysm resection [37] and rerouting of a Fontan pathway [38]. It is felt that 3D-printed models used for surgical planning can reduce the exploration and surgical times, which in turn can improve clinical outcomes. The 3D model can also help in the understanding of complex patient anatomy and can help improve pre-operative communication between the surgeon and the patient and family [37] (Fig. 24.5).
3D-printed model of a neonate with double aortic arch from anterior (a) and posterior (b) views. The double aortic arch (red) with a hypoplastic left segment (*) enwraps the trachea and esophagus (white). These models can help families to understand the anatomy of their child’s congenital heart defect and need for surgical intervention
Overall, 3D printing can be very useful in planning surgical techniques and in choosing between single ventricle, one and a half ventricle, and biventricular repair [39]. It can also improve multidisciplinary team decision-making, patient and family understanding, and education of medical professionals [39, 40].
24.3.2 Mechanical Cardiac Support and Heart Transplantation
It is inevitable that some patients with CHD will develop heart failure and require mechanical cardiac support and possible heart transplantation. Many of these patients will have unusual intracardiac and intrathoracic post-operative anatomy, and 3D-printed models can be used to plan for ventricular assist device (VAD) implantation [41]. This can be particularly important for adults with CHD as the multiple prior surgeries can make re-intervention more challenging and higher risk. And while these patients could benefit from mechanical cardiac support, their anatomy and risk factors may preclude it. 3D-printed models can help identify the best approach for placing mechanical support devices, increasing surgeon confidence to undertake the procedure, and may lead to shorter procedure times as well [42].
For those who go on to heart transplantation, 3D-printed models can help surgeons anticipate and plan for problems that may arise during dissection or implantation of the donor heart. Models can be particularly useful in determining any special dimensions and requirements of the donor heart, such as extra donor venous tissue to “undo” Fontan procedures [43,44,45]. Better pre-operative planning can also reduce cross-clamp and total operative time, further reducing patient morbidity [44].
24.3.3 Cardiac Catheterization
The field of interventional congenital cardiology has always pushed the envelope to develop minimally-invasive approaches to minimize morbidities for this fragile population. The complex anatomy can be challenging to understand with angiography alone, so 3D-printed models have been embraced by the interventional community to optimize the planning and safety of interventional catheterizations. There are almost as many uses of 3D-printed models to guide transcatheter interventions as there are CHDs. 3D-printed models have been used for coronary artery fistula closure [46, 47], ductus arteriosus stenting to maintain pulmonary blood flow [48], closure of an atrial baffle leak in a patient with a crisscross heart [49], device closure of a right ventricular outflow tract pseudoaneurysm [50], device closure of a ruptured sinus of Valsalva aneurysm [51], transcatheter Fontan completion [52], closure of an unroofed coronary sinus [53], recanalization of a chronically occluded branch pulmonary artery [54] and percutaneous edge-to-edge repair of systemic atrioventricular valve regurgitation [55]. For each of these reports, the ability to simulate and practice intervention, as well as improved communication with the care team and patients, were noted benefits of the 3D-printed models.
The first reported use of a 3D-printed model to guide a catheterization was in 2014 when Olivieri and colleagues printed a 3D model of the heart of a patient with pulmonary venous baffle obstruction after a Mustard procedure to aid in planning successful transcatheter stent placement [56]. This was followed by reports of 3D-printed models to guide catheter-based interventions on complex aortic coarctation and arch hypoplasia [57, 58].
Device closure of atrial septal defects (ASDs) is one of the earliest transcatheter interventions, but the septal geometry can be complex and hard to assess by standard imaging techniques, such as echocardiography, such that some patients who could benefit from device closure instead undergo surgical closure. In particular, some patients appear to have insufficient tissue rims around the ASD on echocardiogram to allow for safe placement of standard occluder devices. Several groups have found that 3D-printed models can allow for more accurate visualization and assessment of the tissue rims [59] and for in vitro test closure of complex ASDs with standard and off-label closure devices [60, 61].
An exciting extension of 3D printing in planning transcatheter interventions is the treatment of superior sinus venosus ASDs and partial anomalous pulmonary venous return, which have historically required surgical closure. However, using 3D-printed models and bench simulations of covered stent placement has allowed planning to create the “missing” wall between the superior vena cava and the pulmonary veins (Fig. 24.6). The team at Evelina London Children’s Hospital in the United Kingdom has pioneered this technique with good medium-term outcomes [62,63,64]. 3D model planning allows for selection of candidates with anatomy favorable for the transcatheter approach, and the procedure has successfully been replicated at other centers around the world [65].
Planning for transcatheter correction of anomalous right-sided pulmonary venous return and a superior sinus venosus atrial septal defect. (a) 3D virtual model (anterior right atrial wall cut away) demonstrating the anomalous right-sided pulmonary veins entering the superior vena cava (*), the superior sinus venosus atrial septal defect (dotted line), and the normal entry of the azygous vein (†). (b) Virtual implantation of a covered stent (white) to recreate the back wall of the superior vena cava, rerouting the anomalous pulmonary venous drainage and closing the atrial septal defect. (c) 3D-printed model for pre-procedure simulation. Note that the azygous vein is not obstructed by the covered stent
3D-printed models have been used in electrophysiology catheter interventions as well. A number of adults with CHD will develop conduction abnormalities related to the underlying CHD or as a consequence of their prior interventions. Atypical anatomy is the norm for these patients and unusual approaches may be needed to successfully perform ablation procedures or place transvenous pacemaker leads (Fig. 24.7). Many patients with ventricular looping abnormalities can have unusual coronary sinus anatomy, [66] and it may even be absent [67], making pacemaker lead placement challenging, but 3D-printed models have been used in such cases to plan and guide an alternative approach for successful lead placement.
3D-printed model of a patient with transposition of the great arteries who has undergone a Mustard procedure and required transcatheter ablation for atrial flutter with a tricuspid annular focus from lateral (a) and anterior (b) views. Because of the complex surgical intra-atrial venous rerouting, the inferior vena cava no longer communicated with the tricuspid valve annulus (dotted line) and transbaffle puncture was needed to perform the intervention. The 3D-printed model was used to plan the two puncture sites (*) needed to position the interventional catheters (represented by the blue line)
24.3.4 Simulation
Aside from procedural planning, groups have also been exploring the utility of 3D-printed models for various simulations. The most common use has been simulated surgical intervention [20]. Trainees and junior surgeons can learn complex congenital surgical interventions on life-size 3D-printed models made from thin, flexible materials, such as polyurethane resin, before sewing a stitch in a human [68, 69]. Several authors have published small series and proof-of-concept papers, but these works have lacked objective data to prove the utility of the models in improving surgical outcomes [70], aside from one objective assessment of successful simulated arterial switch procedures, to be able to better understand the utility of surgical simulation in improving outcomes [71]. Groups have also utilized 3D-printed models for simulated transcatheter interventions, such as intracardiac shunt occlusion [69] and interventions for pulmonary artery stenosis [72], but more assessment tools will need to be developed for other lesions to understand the potential benefit of 3D-printed models for clinical care.
Another innovative use of 3D-printed models is to simulate post-intervention physiology. One team 3D printed a series of different-sized simulated ASDs to allow for deployment of a transcatheter ASD occluder to better define and understand the deployed dimensions of different device sizes in different-sized defects [73]. Another group used 3D-printed models to predict adverse outcomes after surgical intervention on coronary arteries both for arterial switch procedures [74] and for repair of anomalous coronary artery origins [75]. 3D-printed models of right ventricular outflow anatomy (normal and tetralogy of Fallot after transannular patch repair) were used in hemodynamic simulators and 4D MRI modeling of flow to assess factors that can affect the longevity of implanted bioprosthetic valves [76]. Another group used 3D-printed mitral valves in hemodynamic simulators to predict hemodynamic changes after transcatheter edge-to-edge valve repair [77], a technique that could potentially be used in single-ventricle patients. Another team compared 3D-printed models of Fontan conduits using SLA, SLS, and FDM printers and assessed flow dynamics with hemodynamic simulators. Overall, each made a reasonable representation, but the internal support structures required in FDM models produced less accurate flow modeling [78]. The attractive low-cost FDM models will need to be balanced against the anatomic and physiologic accuracy of the model.
24.3.5 Education
Just as surgeons can learn to perform an arterial switch operation on a 3D model, students can learn anatomy of CHD lesions using models. An open-access library of 3D models of CHD that would be freely available for downloading and printing has been proposed [79]. Others have used cardiac CT of fetal hearts [80] and digital manipulation of cardiac CT scans of normal hearts [81] to create libraries of CHD for education.
There have been numerous studies that assess the subjective and objective benefits of incorporating 3D-printed models of CHD into didactic sessions. These have included multiple levels of learners (medical students, nursing staff, cardiology fellows, attending cardiologists and surgeons) and covered a variety of CHD, such as VSDs and tetralogy of Fallot [82,83,84,85,86,87,88,89,90], extracardiac vascular defects [84, 85, 88,89,90,91], crisscross hearts [92], and complex single ventricle lesions [85, 93]. These studies have shown subjective improvements in learner understanding, but the objective improvements have been less consistent. One innovation that improves understanding is the use of different colored materials to distinguish anatomic structures [85]. There is also probably more benefit for higher complexity lesions (tetralogy of Fallot versus an isolated VSD) [87] and for more junior learners [89, 92].
3D-printed models of CHD have also been used for hands-on surgical training which can allow for practice on pathological hearts without patient risk or having to follow the traditional training model of waiting for potential opportunities to present themselves [68, 94,95,96,97,98]. Hearts can be 3D printed with flexible, tissue-like material to allow for cutting and suturing and have been used for transposition of the great arteries, tetralogy of Fallot, and hypoplastic left heart syndrome, among other defects. Earlier studies found the material to be too different from human myocardium to be useful [94], but there have been advances in material development [68]. Cardiac valves were poorly recreated in the earlier models [94], but a newer technique for valve simulation using 3D echocardiographic imaging to print negative molds and then cast silicone valves has created more tissue-like valves for surgical practice [96]. Hussein and colleagues have shown a reduction in simulated operative time for the arterial switch procedure with repeated practice using 3D-printed models [97]. The same group has also created an objective assessment tool to define successful simulated arterial switch procedures to better understand the utility of surgical simulation in improving clinical outcomes beyond just shorter operative time [71]. Scientific proof of the utility of 3D-printed models for surgical education is challenging due to many confounders that affect outcome [99], and more tools will need to be developed for other CHD to truly understand the potential benefit of 3D-printed models for clinical care.
Besides medical professionals, 3D models can educate patients and their families about their cardiac defect. Children with CHD will require lifelong cardiology care, and a good understanding of the pathologic disease process is likely helpful in improving medical compliance. This can start as early as the initial diagnosis with fetal echocardiograms being used to 3D print models [18, 19]. Pre-operative 3D-printed models can also help families understand the indications for surgery and aid in operative consent [39, 100]. 3D-printed models have also been shown to help reduce patient and family anxiety before cardiac catheterizations [101]. Stressing the importance of lifelong cardiology follow-up, Liddle used virtual 3D models to teach adolescents with CHD about their disease and found improved understanding after the teaching sessions [102]. A meta-analysis found a small number of reports which suggest that 3D models are accurate and help with communication, but consistent with other studies, objective clinical utility has yet to be demonstrated [103].
24.4 Future of 3D Printing for Congenital Heart Disease
24.4.1 Machine Learning
There are several barriers to widespread adoption of 3D printing in CHD, and one of the most important is fast, accurate segmentation from the initial 3D dataset. This is currently mostly a manual, time-consuming process, and any inaccuracies that occur during segmentation will carry through the entire 3D printing process, which can lead to inappropriate clinical interpretations or plans [104]. There are only limited options for semi-automated segmentation of scans [105] and still no consistent method for model creation and printing across centers [106]. Machine learning has been proposed as a solution for improved automated segmentation [107]. The striking variation in CHD anatomy that occurs in a relatively small population of patients limits the datasets available for machine learning [107]. However, progress has been made with development of a machine learning technique to autosegment the left atrium and anomalous pulmonary veins for pre-operative planning in patients with total anomalous pulmonary venous return [108]. The authors report that using their method would decrease the radiologist’s workload from several hours per scan to only 400 ms. Expansion of this technique to other CHD will be critical to widespread and clinically useful adoption of 3D modeling and printing.
24.4.2 Bioprinting
An exciting area of future development for 3D printing in CHD is bioprinting of customized implants [109]. Work has been done to create cardiac valve replacements [110], bioprinted scaffolds seeded with a patient’s stem cells to create vascular grafts that can be used to replace stenotic or hypoplastic vessels [111], or implanted as Fontan conduits [112] that can grow with the patients. While tissue engineering has existed for some time, 3D printing can aid in more lifelike and anatomically accurate scaffold creation [113]. Another step toward growing implants is 4D bioprinting of materials that are designed to respond to specific stimuli, such as heat, to alter their shape which could allow for growth of implanted structures, such as intravascular stents [114]. In addition, implants printed from biodegradable material could grow with the patients, who would not need chronic anti-coagulation typically used after implantation of metallic devices [115].
Currently, there is no ideal printer available, but industry has taken notice and is working to develop printers that can provide optimal resolution and speed with low costs and high viability of the printed cells [113].
24.5 Limitations of 3D Printing for Congenital Heart Disease
As exciting as 3D-printed models are for CHD, there are still several limitations that hinder widespread adoption. The first was discussed earlier and relates to the time-consuming nature of the process of model generation and the steep learning curve [104, 106, 116]. There have already been steps made at reducing segmentation time by utilizing machine learning [108] and these will only continue to improve as larger datasets of CHD anatomy are created. There is still a lack of consistency in methods for segmentation and model generation [105, 106], but future work will continue to refine the techniques to aid in universal adoption.
The costs of 3D printing cannot be ignored, and are often cited as a reason to reject adoption [37]. However, several studies have looked at this issue and found that highly accurate and clinically useful models can be printed using free and open source software and commercially available FDM desktop printers, often for average costs of less than €100 per model [117, 118].
Another limitation is the difficulty in quantifying the clinical benefits of 3D-printed models of CHD. There are several reports of subjective improvements in procedural planning, but objective measures are still lacking [6, 119]. Work that has already been undertaken to develop objective outcome measures for CHD surgery [97] will continue to provide data to “prove” the utility of 3D-printed models in improving care for patients with CHD.
24.6 Conclusions
Incorporation of 3D printing into clinical care for patients with CHD has rapidly evolved in the past two decades and has helped increase understanding of the anatomy and physiology and allowed for the development of novel treatment approaches. As technology continues to improve and adoption becomes more widespread, there will be further optimization of the outcomes for this complex patient population.
Change history
12 December 2023
A correction has been published.
References
Hull CW. The birth of 3D printing. Res Technol Manag. 2015;58(6):25–30.
Hull CWA, inventor; UVP, Inc. (San Gabriel, CA), assignee. Apparatus for production of three-dimensional objects by stereolithography. United States patent 4575330; 1986.
Ngan EM, Rebeyka IM, Ross DB, Hirji M, Wolfaardt JF, Seelaus R, et al. The rapid prototyping of anatomic models in pulmonary atresia. J Thorac Cardiovasc Surg. 2006;132(2):264–9.
Noecker AM, Chen JF, Zhou Q, White RD, Kopcak MW, Arruda MJ, et al. Development of patient-specific three-dimensional pediatric cardiac models. ASAIO J. 2006;52(3):349–53.
Parthasarathy J, Krishnamurthy R, Ostendorf A, Shinoka T. 3D printing with MRI in pediatric applications. J Magn Reson Imaging. 2020;51(6):1641–58.
Celi S, Gasparotti E, Capellini K, Vignali E, Fanni BM, Ali LA, et al. 3D printing in modern cardiology. Curr Pharm Des. 2021;27(16):1918–30.
Hadeed K, Guitarte A, Briot J, Dulac Y, Alacoque X, Acar P, et al. Feasibility and accuracy of printed models of complex cardiac defects in small infants from cardiac computed tomography. Pediatr Radiol. 2021;51(11):1983–90.
Lee S, Squelch A, Sun Z. Quantitative assessment of 3D printed model accuracy in delineating congenital heart disease. Biomolecules. 2021;11(2):270.
Dodge-Khatami J, Adebo DA. Evaluation of complex congenital heart disease in infants using low dose cardiac computed tomography. Int J Cardiovasc Imaging. 2021;37(4):1455–60.
Talanki VR, Peng Q, Shamir SB, Baete SH, Duong TQ, Wake N. Three-dimensional printed anatomic models derived from magnetic resonance imaging data: current state and image acquisition recommendations for appropriate clinical scenarios. J Magn Reson Imaging. 2022;55:1060.
Parimi M, Buelter J, Thanugundla V, Condoor S, Parkar N, Danon S, et al. Feasibility and validity of printing 3D heart models from rotational angiography. Pediatr Cardiol. 2018;39(4):653–8.
Seckeler MD, Boe BA, Barber BJ, Berman DP, Armstrong AK. Use of rotational angiography in congenital cardiac catheterisations to generate three-dimensional-printed models. Cardiol Young. 2021;31(9):1407–11.
Fetterly KA, Ferrero A, Lewis BR, Anderson JH, Hagler DJ, Taggart NW. Radiation dose reduction for 3D angiography images in pediatric and congenital cardiology. Catheter Cardiovasc Interv. 2021;97(4):E502–e9.
Kurup HK, Samuel BP, Vettukattil JJ. Hybrid 3D printing: a game-changer in personalized cardiac medicine? Expert Rev Cardiovasc Ther. 2015;13(12):1281–4.
Gosnell J, Pietila T, Samuel BP, Kurup HK, Haw MP, Vettukattil JJ. Integration of computed tomography and three-dimensional echocardiography for hybrid three-dimensional printing in congenital heart disease. J Digit Imaging. 2016;29(6):665–9.
Gomez A, Gomez G, Simpson J, Valverde I. 3D hybrid printed models in complex congenital heart disease: 3D echocardiography and cardiovascular magnetic resonance imaging fusion. Eur Heart J. 2020;41(43):4214.
Mowers KL, Fullerton JB, Hicks D, Singh GK, Johnson MC, Anwar S. 3D echocardiography provides highly accurate 3D printed models in congenital heart disease. Pediatr Cardiol. 2021;42(1):131–41.
Huang J, Shi H, Chen Q, Hu J, Zhang Y, Song H, et al. Three-dimensional printed model fabrication and effectiveness evaluation in fetuses with congenital heart disease or with a normal heart. J Ultrasound Med. 2021;40(1):15–28.
Veronese P, Bertelli F, Cattapan C, Andolfatto M, Gervasi MT, Vida VL. Three-dimensional printing of fetal heart with d-transposition of the great arteries from ultrasound imaging data. World J Pediatr Congenit Heart Surg. 2021;12(2):291–2.
Illmann CF, Ghadiry-Tavi R, Hosking M, Harris KC. Utility of 3D printed cardiac models in congenital heart disease: a scoping review. Heart. 2020;106(21):1631–7.
Valverde I, Gomez G, Gonzalez A, Suarez-Mejias C, Adsuar A, Coserria JF, et al. Three-dimensional patient-specific cardiac model for surgical planning in Nikaidoh procedure. Cardiol Young. 2015;25(4):698–704.
Valverde I, Gomez-Ciriza G, Hussain T, Suarez-Mejias C, Velasco-Forte MN, Byrne N, et al. Three-dimensional printed models for surgical planning of complex congenital heart defects: an international multicentre study. Eur J Cardiothorac Surg. 2017;52(6):1139–48.
Farooqi KM, Gonzalez-Lengua C, Shenoy R, Sanz J, Nguyen K. Use of a three dimensional printed cardiac model to assess suitability for biventricular repair. World J Pediatr Congenit Heart Surg. 2016;7(3):414–6.
Garekar S, Bharati A, Chokhandre M, Mali S, Trivedi B, Changela VP, et al. Clinical application and multidisciplinary assessment of three dimensional printing in double outlet right ventricle with remote ventricular septal defect. World J Pediatr Congenit Heart Surg. 2016;7(3):344–50.
Bhatla P, Tretter JT, Chikkabyrappa S, Chakravarti S, Mosca RS. Surgical planning for a complex double-outlet right ventricle using 3D printing. Echocardiography. 2017;34(5):802–4.
Bhatla P, Tretter JT, Ludomirsky A, Argilla M, Latson LA Jr, Chakravarti S, et al. Utility and scope of rapid prototyping in patients with complex muscular ventricular septal defects or double-outlet right ventricle: does it alter management decisions? Pediatr Cardiol. 2017;38(1):103–14.
Tiwari N, Ramamurthy HR, Kumar V, Kumar A, Dhanalakshmi B, Kumar G. The role of three-dimensional printed cardiac models in the management of complex congenital heart diseases. Med J Armed Forces India. 2021;77(3):322–30.
Yang DH, Park SH, Kim N, Choi ES, Kwon BS, Park CS, et al. Incremental value of 3D printing in the preoperative planning of complex congenital heart disease surgery. JACC Cardiovasc Imaging. 2021;14(6):1265–70.
Yıldız O, Köse B, Tanıdır IC, Pekkan K, Güzeltaş A, Haydin S. Single-center experience with routine clinical use of 3D technologies in surgical planning for pediatric patients with complex congenital heart disease. Diagn Interv Radiol. 2021;27(4):488–96.
Zhao L, Zhou S, Fan T, Li B, Liang W, Dong H. Three-dimensional printing enhances preparation for repair of double outlet right ventricular surgery. J Card Surg. 2018;33(1):24–7.
Taqatqa AS, Vettukattil JJ. Atrioventricular septal defects: pathology, imaging, and treatment options. Curr Cardiol Rep. 2021;23(8):93.
Najm HK, Karamlou T, Ahmad M, Hassan S, Yaman M, Stewart R, et al. Biventricular conversion in unseptatable hearts: “ventricular switch”. Semin Thorac Cardiovasc Surg. 2021;33(1):172–80.
Elmaghraby A, Ghareep AN, Alkuwari M, Hijazi ZM, Salustri A. Patient-specific 3-dimensional printing of tetralogy of fallot with major aortopulmonary collaterals. JACC Case Rep. 2019;1(4):535–7.
Averkin II, Grehov EV, Pervunina TM, Komlichenko EV, Vasichkina ES, Zaverza VM, et al. 3D-printing in preoperative planning in neonates with complex congenital heart defects. J Matern Fetal Neonatal Med. 2022;35:2020.
Yokoyama S, Fukuba R, Mitani K, Uemura H. Preoperative simulation for complex transposition of great arteries using a three-dimensional model. Cardiol Young. 2020;30(2):278–80.
Xu J, Tian Y, Yin J, Wang J, Xu W, Shi Z, et al. Utility of three-dimensional printing in preoperative planning for children with anomalous pulmonary venous connection: a single center experience. Quant Imaging Med Surg. 2019;9(11):1804–14.
Zhu Y, Zhang XE, Li Q, Yao H. Three-dimensional printing in a patient with pulmonary artery pseudoaneurysm and complex congenital heart disease-a case report. Clin Case Rep. 2020;8:2107–10.
Carberry T, Murthy R, Hsiao A, Petko C, Moore J, Lamberti J, et al. Fontan revision: presurgical planning using four-dimensional (4D) flow and three-dimensional (3D) printing. World J Pediatr Congenit Heart Surg. 2019;10(2):245–9.
Vettukattil JJ, Mohammad Nijres B, Gosnell JM, Samuel BP, Haw MP. Three-dimensional printing for surgical planning in complex congenital heart disease. J Card Surg. 2019;34(11):1363–9.
Kappanayil M, Koneti NR, Kannan RR, Kottayil BP, Kumar K. Three-dimensional-printed cardiac prototypes aid surgical decision-making and preoperative planning in selected cases of complex congenital heart diseases: early experience and proof of concept in a resource-limited environment. Ann Pediatr Cardiol. 2017;10(2):117–25.
Miller JR, Singh GK, Woodard PK, Eghtesady P, Anwar S. 3D printing for preoperative planning and surgical simulation of ventricular assist device implantation in a failing systemic right ventricle. J Cardiovasc Comput Tomogr. 2020;14:e172.
Farooqi KM, Saeed O, Zaidi A, Sanz J, Nielsen JC, Hsu DT, et al. 3D printing to guide ventricular assist device placement in adults with congenital heart disease and heart failure. JACC Heart Fail. 2016;4(4):301–11.
Sodian R, Weber S, Markert M, Loeff M, Lueth T, Weis FC, et al. Pediatric cardiac transplantation: three-dimensional printing of anatomic models for surgical planning of heart transplantation in patients with univentricular heart. J Thorac Cardiovasc Surg. 2008;136(4):1098–9.
Smith ML, McGuinness J, O’Reilly MK, Nolke L, Murray JG, Jones JFX. The role of 3D printing in preoperative planning for heart transplantation in complex congenital heart disease. Ir J Med Sci. 2017;186(3):753–6.
Blitzer D, Nguyen S, Farooqi KM, Takeda K. Orthotopic heart transplantation and concomitant aortic arch replacement in an adult Fontan patient with hypoplastic left heart syndrome. Interact Cardiovasc Thorac Surg. 2021;32(2):325–7.
Aroney N, Putrino A, Scalia G, Walters D. 3D printed cardiac fistula: guiding percutaneous structural intervention. Catheter Cardiovasc Interv. 2018;92(7):E478–e80.
Aroney N, Markham R, Putrino A, Crowhurst J, Wall D, Scalia G, et al. Three-dimensional printed cardiac fistulae: a case series. Eur Heart J Case Rep. 2019;3(2):ytz060.
Chamberlain RC, Ezekian JE, Sturgeon GM, Barker PCA, Hill KD, Fleming GA. Preprocedural three-dimensional planning aids in transcatheter ductal stent placement: a single-center experience. Catheter Cardiovasc Interv. 2020;95(6):1141–8.
Hassler KR, Stephens EH, Miranda WR, Foley TA, Dearani JA. Intra-atrial pulmonary venous conduit leak in criss-cross heart: role of three-dimensional modeling. World J Pediatr Congenit Heart Surg. 2022;13:113.
Jivanji SGM, Qureshi SA, Rosenthal E. Novel use of a 3D printed heart model to guide simultaneous percutaneous repair of severe pulmonary regurgitation and right ventricular outflow tract aneurysm. Cardiol Young. 2019;29(4):534–7.
Kern MC, Janardhanan R, Kelly T, Fox KA, Klewer SE, Seckeler MD. Multimodality imaging for diagnosis and procedural planning for a ruptured sinus of Valsalva aneurysm. J Cardiovasc Comput Tomogr. 2020;14(6):e139–e42.
Aregullin EO, Mohammad Nijres B, Al-Khatib Y, Vettukattil J. Transcatheter Fontan completion using novel balloon and stent system. Catheter Cardiovasc Interv. 2021;97(4):679–84.
Lee DT, Venkatesh P, Bravo-Jaimes K, Lluri G, Yang EH, Tan W, et al. Using a 3-dimensional printed model to plan percutaneous closure of an unroofed coronary sinus. Circ Cardiovasc Imaging. 2021;14:e013018.
Seckeler MD, Pineda J, Lotun K. Successful transcatheter recanalization of a chronically occluded left pulmonary artery due to fibrosing mediastinitis. JACC Cardiovasc Interv. 2021;14(16):e215–e6.
Alshawabkeh L, Mahmud E, Reeves R. Percutaneous mitral valve repair in adults with congenital heart disease: report of the first case-series. Catheter Cardiovasc Interv. 2021;97(3):542–8.
Olivieri L, Krieger A, Chen MY, Kim P, Kanter JP. 3D heart model guides complex stent angioplasty of pulmonary venous baffle obstruction in a Mustard repair of D-TGA. Int J Cardiol. 2014;172(2):e297–8.
Valverde I, Gomez G, Coserria JF, Suarez-Mejias C, Uribe S, Sotelo J, et al. 3D printed models for planning endovascular stenting in transverse aortic arch hypoplasia. Catheter Cardiovasc Interv. 2015;85(6):1006–12.
Pluchinotta FR, Giugno L, Carminati M. Stenting complex aortic coarctation: simulation in a 3D printed model. EuroIntervention. 2017;13(4):490.
Yan C, Wang C, Pan X, Li S, Song H, Liu Q, et al. Three-dimensional printing assisted transcatheter closure of atrial septal defect with deficient posterior-inferior rim. Catheter Cardiovasc Interv. 2018;92(7):1309–14.
He L, Cheng GS, Du YJ, Zhang YS. Feasibility of device closure for multiple atrial septal defects with an inferior sinus venosus defect: procedural planning using three-dimensional printed models. Heart Lung Circ. 2020;29:914.
Yan C, Li S, Song H, Jin J, Zheng H, Wang C, et al. Off-label use of duct occluder in transcatheter closure of secundum atrial septal defect with no rim to right pulmonary vein. J Thorac Cardiovasc Surg. 2019;157(4):1603–8.
Riahi M, Velasco Forte MN, Byrne N, Hermuzi A, Jones M, Baruteau AE, et al. Early experience of transcatheter correction of superior sinus venosus atrial septal defect with partial anomalous pulmonary venous drainage. EuroIntervention. 2018;14(8):868–76.
Velasco Forte MN, Byrne N, Valverde I, Gomez Ciriza G, Hermuzi A, Prachasilchai P, et al. Interventional correction of sinus venosus atrial septal defect and partial anomalous pulmonary venous drainage: procedural planning using 3D printed models. JACC Cardiovasc Imaging. 2018;11(2 Pt 1):275–8.
Hansen JH, Duong P, Jivanji SGM, Jones M, Kabir S, Butera G, et al. Transcatheter correction of superior sinus venosus atrial septal defects as an alternative to surgical treatment. J Am Coll Cardiol. 2020;75(11):1266–78.
Batteux C, Azarine A, Karsenty C, Petit J, Ciobotaru V, Brenot P, et al. Sinus venosus ASDs: imaging and percutaneous closure. Curr Cardiol Rep. 2021;23(10):138.
Kanawati J, Kanawati AJ, Rowe MK, Khan H, Chan WK, Yee R. Utility of 3-D printing for cardiac resynchronization device implantation in congenital heart disease. HeartRhythm Case Rep. 2020;6:754–6.
Seckeler MD, White SC, Klewer SE, Ott P. Transjugular transseptal approach for left ventricular pacing lead in an adult with criss-cross heart. JACC Clin Electrophysiol. 2019;5(8):998–9.
Hoashi T, Ichikawa H, Nakata T, Shimada M, Ozawa H, Higashida A, et al. Utility of a super-flexible three-dimensional printed heart model in congenital heart surgery. Interact Cardiovasc Thorac Surg. 2018;27(5):749–55.
Pushparajah K. Non-invasive imaging in the evaluation of cardiac shunts for interventional closure. Front Cardiovasc Med. 2021;8:651726.
Hussein N, Honjo O, Haller C, Hickey E, Coles JG, Williams WG, et al. Hands-on surgical simulation in congenital heart surgery: literature review and future perspective. Semin Thorac Cardiovasc Surg. 2020;32(1):98–105.
Hussein N, Lim A, Honjo O, Haller C, Coles JG, Van Arsdell G, et al. Development and validation of a procedure-specific assessment tool for hands-on surgical training in congenital heart surgery. J Thorac Cardiovasc Surg. 2020;160(1):229–40.e1.
Tomov ML, Cetnar A, Do K, Bauser-Heaton H, Serpooshan V. Patient-specific 3-dimensional-bioprinted model for in vitro analysis and treatment planning of pulmonary artery atresia in tetralogy of fallot and major aortopulmonary collateral arteries. J Am Heart Assoc. 2019;8(24):e014490.
O’Halloran CP, Qadir A, Ramlogan SR, Nugent AW, Tannous P. Deployed dimensions of the GORE® CARDIOFORM ASD occluder as function of defect size. Pediatr Cardiol. 2021;42(5):1209–15.
Batteux C, Abakka S, Gaudin R, Vouhé P, Raisky O, Bonnet D. Three-dimensional geometry of coronary arteries after arterial switch operation for transposition of the great arteries and late coronary events. J Thorac Cardiovasc Surg. 2021;161(4):1396–404.
Parthasarathy J, Hatoum H, Flemister DC, Krull CM, Walter BA, Zhang W, et al. Assessment of transfer of morphological characteristics of anomalous aortic origin of a coronary artery from imaging to patient specific 3D Printed models: a feasibility study. Comput Methods Programs Biomed. 2021;201:105947.
Schiavone NK, Elkins CJ, McElhinney DB, Eaton JK, Marsden AL. In vitro assessment of right ventricular outflow tract anatomy and valve orientation effects on bioprosthetic pulmonary valve hemodynamics. Cardiovasc Eng Technol. 2021;12(2):215–31.
Mashari A, Knio Z, Jeganathan J, Montealegre-Gallegos M, Yeh L, Amador Y, et al. Hemodynamic testing of patient-specific mitral valves using a pulse duplicator: a clinical application of three-dimensional printing. J Cardiothorac Vasc Anesth. 2016;30(5):1278–85.
Medero R, Garcia-Rodriguez S, Francois CJ, Roldan-Alzate A. Patient-specific in vitro models for hemodynamic analysis of congenital heart disease - additive manufacturing approach. J Biomech. 2017;54:111–6.
Bramlet M, Olivieri L, Farooqi K, Ripley B, Coakley M. Impact of three-dimensional printing on the study and treatment of congenital heart disease. Circ Res. 2017;120(6):904–7.
Sandrini C, Lombardi C, Shearn AIU, Ordonez MV, Caputo M, Presti F, et al. Three-dimensional printing of fetal models of congenital heart disease derived from microfocus computed tomography: a case series. Front Pediatr. 2019;7:567.
Hopfner C, Jakob A, Tengler A, Grab M, Thierfelder N, Brunner B, et al. Design and 3D printing of variant pediatric heart models for training based on a single patient scan. 3D Print Med. 2021;7(1):25.
Costello JP, Olivieri LJ, Krieger A, Thabit O, Marshall MB, Yoo SJ, et al. Utilizing three-dimensional printing technology to assess the feasibility of high-fidelity synthetic ventricular septal defect models for simulation in medical education. World J Pediatr Congenit Heart Surg. 2014;5(3):421–6.
Costello JP, Olivieri LJ, Su L, Krieger A, Alfares F, Thabit O, et al. Incorporating three-dimensional printing into a simulation-based congenital heart disease and critical care training curriculum for resident physicians. Congenit Heart Dis. 2015;10(2):185–90.
Anwar S, Singh GK, Varughese J, Nguyen H, Billadello JJ, Sheybani EF, et al. 3D printing in complex congenital heart disease: across a spectrum of age, pathology, and imaging techniques. JACC Cardiovasc Imaging. 2017;10(8):953–6.
Biglino G, Capelli C, Koniordou D, Robertshaw D, Leaver LK, Schievano S, et al. Use of 3D models of congenital heart disease as an education tool for cardiac nurses. Congenit Heart Dis. 2017;12(1):113–8.
Loke YH, Harahsheh AS, Krieger A, Olivieri LJ. Usage of 3D models of tetralogy of Fallot for medical education: impact on learning congenital heart disease. BMC Med Educ. 2017;17(1):54.
White SC, Sedler J, Jones TW, Seckeler M. Utility of three-dimensional models in resident education on simple and complex intracardiac congenital heart defects. Congenit Heart Dis. 2018;13(6):1045–9.
Awori J, Friedman SD, Chan T, Howard C, Seslar S, Soriano BD, et al. 3D models improve understanding of congenital heart disease. 3D Print Med. 2021;7(1):26.
Hon NWL, Hussein N, Honjo O, Yoo SJ. Evaluating the impact of medical student inclusion into hands-on surgical simulation in congenital heart surgery. J Surg Educ. 2021;78(1):207–13.
Karsenty C, Guitarte A, Dulac Y, Briot J, Hascoet S, Vincent R, et al. The usefulness of 3D printed heart models for medical student education in congenital heart disease. BMC Med Educ. 2021;21(1):480.
Jones TW, Seckeler MD. Use of 3D models of vascular rings and slings to improve resident education. Congenit Heart Dis. 2017;12(5):578–82.
Valverde I, Gomez G, Byrne N, Anwar S, Silva MA, Martin Talavera M, et al. Criss-cross heart three-dimensional printed models in medical education: a multi-center study on their value as a supporting tool to conventional imaging. Anat Sci Educ. 2022;15:719.
Lee C, Lee JY. Utility of three-dimensional printed heart models for education on complex congenital heart diseases. Cardiol Young. 2020;30(11):1637–42.
Yoo SJ, Spray T, Austin EH III, Yun TJ, van Arsdell GS. Hands-on surgical training of congenital heart surgery using 3-dimensional print models. J Thorac Cardiovasc Surg. 2017;153(6):1530–40.
Chen SA, Ong CS, Malguria N, Vricella LA, Garcia JR, Hibino N. Digital design and 3D printing of aortic arch reconstruction in HLHS for surgical simulation and training. World J Pediatr Congenit Heart Surg. 2018;9(4):454–8.
Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A, et al. Comparison of 3D echocardiogram-derived 3d printed valve models to molded models for simulated repair of pediatric atrioventricular valves. Pediatr Cardiol. 2018;39(3):538–47.
Hussein N, Honjo O, Haller C, Coles JG, Hua Z, Van Arsdell G, et al. Quantitative assessment of technical performance during hands-on surgical training of the arterial switch operation using 3-dimensional printed heart models. J Thorac Cardiovasc Surg. 2020;160(4):1035–42.
Nam JG, Lee W, Jeong B, Park EA, Lim JY, Kwak Y, et al. Three-dimensional printing of congenital heart disease models for cardiac surgery simulation: evaluation of surgical skill improvement among inexperienced cardiothoracic surgeons. Korean J Radiol. 2021;22(5):706–13.
Yoo SJ, Hussein N, Peel B, Coles J, van Arsdell GS, Honjo O, et al. 3D modeling and printing in congenital heart surgery: entering the stage of maturation. Front Pediatr. 2021;9:621672.
Deng X, He S, Huang P, Luo J, Yang G, Zhou B, et al. A three-dimensional printed model in preoperative consent for ventricular septal defect repair. J Cardiothorac Surg. 2021;16(1):229.
Boyer PJ, Yell JA, Andrews JG, Seckeler MD. Anxiety reduction after pre-procedure meetings in patients with CHD. Cardiol Young. 2020;30:991.
Liddle D, Balsara S, Hamann K, Christopher A, Olivieri L, Loke YH. Combining patient-specific, digital 3D models with tele-education for adolescents with CHD. Cardiol Young. 2022;32:912.
Lau IWW, Sun Z. Dimensional accuracy and clinical value of 3D printed models in congenital heart disease: a systematic review and meta-analysis. J Clin Med. 2019;8(9):1483.
Meier LM, Meineri M, Qua Hiansen J, Horlick EM. Structural and congenital heart disease interventions: the role of three-dimensional printing. Neth Heart J. 2017;25(2):65–75.
Byrne N, Velasco Forte M, Tandon A, Valverde I, Hussain T. A systematic review of image segmentation methodology, used in the additive manufacture of patient-specific 3D printed models of the cardiovascular system. JRSM Cardiovasc Dis. 2016;5:2048004016645467.
Cantinotti M, Valverde I, Kutty S. Three-dimensional printed models in congenital heart disease. Int J Cardiovasc Imaging. 2017;33(1):137–44.
Huff TJ, Ludwig PE, Zuniga JM. The potential for machine learning algorithms to improve and reduce the cost of 3-dimensional printing for surgical planning. Expert Rev Med Devices. 2018;15(5):349–56.
Li J, Chen H, Zhu F, Wen C, Wang L. Automatic pulmonary vein and left atrium segmentation for TAPVC preoperative evaluation using V-net with grouped attention. Annu Int Conf IEEE Eng Med Biol Soc. 2020;2020:1207–10.
Kiraly L, Vijayavenkataraman S. Biofabrication in congenital cardiac surgery: a plea from the operating theatre, promise from science. Micromachines (Basel). 2021;12(3):332.
Kehl D, Weber B, Hoerstrup SP. Bioengineered living cardiac and venous valve replacements: current status and future prospects. Cardiovasc Pathol. 2016;25(4):300–5.
Fukunishi T, Best CA, Sugiura T, Opfermann J, Ong CS, Shinoka T, et al. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg. 2017;153(4):924–32.
Best C, Strouse R, Hor K, Pepper V, Tipton A, Kelly J, et al. Toward a patient-specific tissue engineered vascular graft. J Tissue Eng. 2018;9:2041731418764709.
Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3(2):144–56.
Kwok JKS, Lau RWH, Zhao ZR, Yu PSY, Ho JYK, Chow SCY, et al. Multi-dimensional printing in thoracic surgery: current and future applications. J Thorac Dis. 2018;10(Suppl 6):S756–S63.
Hadeed K, Acar P, Dulac Y, Cuttone F, Alacoque X, Karsenty C. Cardiac 3D printing for better understanding of congenital heart disease. Arch Cardiovasc Dis. 2018;111(1):1–4.
Pluchinotta FR, Sturla F, Caimi A, Giugno L, Chessa M, Giamberti A, et al. 3-Dimensional personalized planning for transcatheter pulmonary valve implantation in a dysfunctional right ventricular outflow tract. Int J Cardiol. 2020;309:33–9.
Perens G, Chyu J, McHenry K, Yoshida T, Finn JP. Three-dimensional congenital heart models created with free software and a desktop printer: assessment of accuracy, technical aspects, and clinical use. World J Pediatr Congenit Heart Surg. 2020;11(6):797–801.
Gómez-Ciriza G, Gómez-Cía T, Rivas-González JA, Velasco Forte MN, Valverde I. Affordable three-dimensional printed heart models. Front Cardiovasc Med. 2021;8:642011.
Han F, Co-Vu J, Lopez-Colon D, Forder J, Bleiweis M, Reyes K, et al. Impact of 3D printouts in optimizing surgical results for complex congenital heart disease. World J Pediatr Congenit Heart Surg. 2019;10(5):533–8.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Seckeler, M.D., Guerrero, C.E., Hoyer, A.W. (2023). 3D Printing in Congenital Heart Disease. In: Syed, M.A., Mohiaddin, R.H. (eds) Magnetic Resonance Imaging of Congenital Heart Disease. Springer, Cham. https://doi.org/10.1007/978-3-031-29235-4_24
Download citation
DOI: https://doi.org/10.1007/978-3-031-29235-4_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-29234-7
Online ISBN: 978-3-031-29235-4
eBook Packages: MedicineMedicine (R0)