Abstract
Zingiberaceae, commonly known as ginger, is one of the largest families of the plant kingdom with 52 genera and over 1300 species and distributed widely throughout the tropics, particularly in Southeast Asia. The family consists of a large number of economically and medicinally important plants well known for their use in ethnomedicine. Various plant parts from these plants, such as rhizome and seeds, have been used as ingredients of many herbal preparations in Chinese, Thai, African, and Indian traditional medicinal systems including Ayurveda. Interestingly, many of these plant parts are also used as spices since ancient times, becoming an essential part of ethnic cuisine. Many plants from different genera of Zingiberaceae were studied for their medicinal usages, and a number of compounds were purified with potent bioactivities including antimicrobial, antifungal, anti-inflammatory, anticancer, antioxidant, antiviral, antidiabetic, antiarthiritic, larvicidal, neuroprotective, and heptoprotective activities. Phytochemical analyses of different genera of Zingiberaceae have revealed the presence of a wide range of pharmacologically active phytochemical groups which mainly include terpenoids, diarylheptanoids, phenylpropanoids, and flavanoids. Interestingly, there are a number of essential oils, plant extracts, and compounds from Zingiberaceae species known to possess potent cytotoxic / antiproliferative / anticancer activities. These extracts and compounds are capable of specifically targeting cancer-related proteins and signaling pathways to kill cancer cells without severe damage to normal healthy cells. This chapter attempts to provide a comprehensive review on anticancer potential and mechanistics of action of such plant-derived extracts and bioactive compounds reported from various genera of the family such as Curcuma, Zingiber, Kaempferia, Alpinia, Amomum, and Hedychium as evidenced by in vitro and in vivo studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Cancer, the second leading cause of human death worldwide, behind cardiovascular disease, might soon rank as the leading cause of death. It is the single most important barrier to increasing life expectancy in every country of the world in the 21st century [1]. Cancer is an abnormal growth of cells caused by multiple changes in gene expression which leads to dysregulation in balancing cell proliferation and cell death. As a consequence, a population of cells evolve at the site of origin, capable of invading into tissues at distant sites, causing significant morbidity and death of the host, if untreated [2,3,4,5]. Cancers are conventionally treated using a combination of three major modes - surgery, radiation, and chemotherapy. Chemotherapy involves treatment of cancer with one or more chemicals known to have cytotoxic, antineoplastic activity. Traditional chemotherapeutic agents, which act by killing rapidly dividing cancer cells, also harm cells that divide rapidly under normal circumstances, thereby resulting in side effects such as immunosuppression and mucositis among others. Newer anticancer drugs (targeted chemotherapy) are not indiscriminately cytotoxic, but rather target proteins that are abnormally expressed in cancer cells and are essential for their growth [6]. It is believed that anticancer effects of plants develop by suppressing pathways involved in cancer progression, DNA repair, increasing body immunity, and inducing antioxidant effects [7,8,9].
During the last few decades, ethnomedicinal plants have played a significant role in the development of anticancer drugs with fewer side effects in different continents of the world. Out of 121 prescription drugs that are being used today for cancer treatment, 90 are plant-based and 75% of them were discovered from folklore claims. Secondary metabolites from plants, including alkaloids, terpenoids, and polyphenolic compounds with promising anticancer potential, have been developed for clinical practice [10, 11]. Classical examples include Vinca alkaloids (vinblastine and vincristine), isolated from Catharanthus roseus G. Don. (Apocynaceae), paclitaxel and docetaxel - semisynthetic derivatives of Taxanes (diterpenoids) from the Pacific Yew tree, Taxus brevifolia and T. baccata respectively, irinotecan and topotecan - semisynthetic derivatives of Camptotheca alkaloids, isolated from Camptotheca acuminate and Podophyllum lignans from mayapple tree, Podophyllum peltatum and P. hexandrum [11,12,13].
Zingiberaceae is one of the largest families of the plant kingdom, distributed widely throughout the tropics, particularly in Southeast Asia [14, 15]. The family consists of a large number of economically and medicinally important plants well known for their use in ethnomedicine. The Zingiberaceae plants contain a number of volatile and essential oils including terpenoids, phenylpropanoids, flavonoids, and sesquiterpenes, which have been reported to possess anticancer activity [16,17,18,19]. Hence, these plants are considered to be excellent candidates for development of novel chemotherapeutics. Various extracts and pure compounds / secondary metabolites isolated from Curcuma, Zingiber, Kaempferia, Alpinia, Amomum, and Hedychium genera reportedly possess anticancer activity as evidenced by in vitro and in vivo studies [20,21,22,23,24]. Few notable examples for anticancer compound include curcumin/curcuminoids (Curcuma longa), zerumbone (Zingiber zerumbet), gingerol, and shogaol (Zingiber officinale) among many others [25,26,27,28,29]. Even though a number of review articles were available about various biological activities of different genera of Zingiberaceae family, a comprehensive scrutiny about anticancer potential of these plants was found lacking. Hence, this chapter aims to overview anticancer potential of organic solvent extracts and compounds identified therein belonging to different genera of Zingiberaceae plants.
2 Zingiberaceae
The taxonomic position of the Family Zingiberaceae is as follows:
Kingdom: | Plantae |
Subkingdom: | Trachebionta |
Superdivision: | Spermatophyta |
Division: | Magnoliophyta |
Subdivision: | Angiospermae |
Class: | Monocotyledonae (Liliopsida) |
Subclass: | Zingiberidae |
Order: | Zingiberales |
Family: | Zingiberaceae |
Zingiberaceae commonly known as ginger family is a one of the largest families of flowering plants comprising 52 genera divided into more than 1300 species. These aromatic herbs, with creeping horizontal or tuberous rhizomes, are distributed throughout tropical Africa, Asia, and the America [14, 15]. India is one of the richest regions displaying high diversity of Zingiberaceae with 21 genera and around more than 200 species, confined to northeastern, southern parts of India and Andaman-Nicobar Islands [15]. The members of Zingiberaceae are perennial rhizomatous herbs. The rhizome is sympodially branched and composed of distinct segments. The rhizomes are variously colored ranging from pale yellow, orange-yellow, deep yellow, blue, greenish blue, pink, or combinations of these in different species. The young rhizomes and axillary buds are protected by scale leaves. Leafy shoots are generally unbranched and true aerial stem is present in some genera and absent in others; yet others have very short true stem or pseudostem with clasping leaf sheaths. Other general characteristics of Zingiberaceae include distichous simple leaves, presence of terminal or lateral inflorescence on the leafy shoot, highly modified - ephemeral - delicate flower and capsular fruit [15, 30]. Members of the family are usually aromatic in all plant parts, have functions as natural sources of spices, herbal medicine, natural dyes, perfumes, and as multipurpose aesthetic compounds [31, 32].
Zingiberaceae is well known for its use in ethnomedicine. It constitutes a vital group characterized by the presence of volatile oils and oleoresins. Generally, the rhizomes and fruits are aromatic, tonic, stimulant, and occasionally nutritive. Some are used as food as they contain starch in large quantities, while others yield an astringent and diaphoretic juice. Some of the medicinally and economically important genera of Zingiberaceae include Alpinia, Amomum, Curcuma, and Zingiber, followed by Boesenbergia, Kaempferia, Elettaria, Hedychium, Elettariopsis, and Etlingera [30, 33, 34]. Detailed literature survey reveals that many species of the family are used for treatment of various ailments due to their unique medicinal values. They are part of many herbal preparations in Chinese, Thai, African, and Indian traditional medicinal systems including Ayurveda [33, 35,36,37,38]. Zingiberaceae species Alpinia, Amomum, Curcuma, Elettaria, Hedychium, Kaempferia, and Zingiber play a major role in the preparation of many Ayurvedic drugs [33]. Various species from Zingiberaceae reported with different biological activities include antimicrobial, antifungal, anti-inflammatory, anticancer, antioxidant, antiviral, antidiabetic, antiarthiritic, larvicidal, neuroprotective, and heptoprotective activities [25, 39,40,41,42,43,44,45,46,47,48,49,50,51,52].
3 Phytochemistry of Zingiberaceae
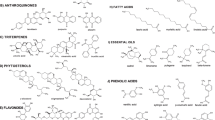
Various pharmacological activities of Zingiberaceae plants are credited to the presence of phytochemicals / secondary metabolites therein. Phytochemical analyses of different genera of Zingiberaceae have revealed the presence of a wide range of pharmacologically active phytochemical groups which mainly include terpenoids, diarylheptanoids, phenylpropanoids, and flavanoids [16]. Interestingly, these phytochemical groups have been considered to be potential candidates for chemotherapeutics development [17,18,19, 53]. Close to 100 terpenoid compounds have been identified from Zingiberaceae. Mono- and sesquiterpenoids such as α-pinene, β-pinene, 1,8-cineole, camphor, terpinen-4-ol, β-caryophyllene, and zingiberene are common chemical constituents of most of Zingiberaceae species, especially in essential oils extracted from rhizome [26, 39, 54]. Among diterpanoids, labdane-type diterpenes occur commonly in species of Alpinia, Amomum, Hedychium, Curcuma, and Zingiber [55,56,57,58,59] and pimarane diterpenes have been reported from Kaempferia species [60]. Diarylheptanoids is another pharmacologically prominent group of secondary metabolites from Zingiberaceae plants, commonly found in Curcuma, Alpinia, and Zingiber species. Curcuminoids, isolated from several Curcuma sp., is a good example for diarylheptanoids with remarkable biological activities [61]. Another group of bioactive phytochemicals are phenylpropanoids, mostly reported from Alpinia, Kaempferia, Curcuma, and Zingiber spp. [62,63,64,65,66]. Flavonoids and related derivatives are the constituents of plants such as Alpinia, Amomum, Boesenbergia, Kaempferia, and Zingiber genera [67,68,69,70,71]. Phenylbutanoids are yet another rare group in nature found only in the genus Zingiber [72, 73]. The molecular structures of some of the well known bioactive phytochemical compounds from Zingiberaceae plants reported with anticancer activity have been shown in Fig. 1.
4 Anticancer Activities Reported from Different Genera of Zingiberaceae Family
Anticancer activities reported from various Zingiberaceae species can be categorized according to different genera. This in itself reveals the hidden treasure trove within the Zingiberaceae plant family, which can contribute tremendously to chemotherapeutic drug development. Table 1 highlights the significant anticancer activities of different extracts, essential oils, and pure compounds from different genera of Zingiberaceae family.
4.1 Genus Alpinia Roxb.
The tropical and subtropical genus, Alpinia Roxb., with about 230 species, is mainly distributed in the Indo-Pacific region [15]. The genus is generally called as ‘shell ginger’ and several species are cultivated as ornamentals. Alpinia species are well known medicinal herbs with incredible biopharmaceutical potential. The presence of bioactive substances such as terpenoids, diarylheptanoids, phenylpropanoids, and flavonoids is key to their therapeutic efficiency [175]. Many in vitro studies carried out in diverse cancer cell lines and in vivo studies with animal models reflect clearly the anticancer potential of Alpinia species. Antiangiogenic potential of n-hexane and ethyl acetate fractions from A. oxyphylla fruits has been tested against zebrafish model, human umbilical vein endothelial cells, and tumor cell lines [74]. Hexane and chloroform extract of A. galanga rhizome lead to the isolation of two compounds, viz. 1’(S)-1’-acetoxychavicol acetate and p-coumaryl alcohol γ-O-methyl ether, both of which were found to exhibit significant cytotoxicity against human cancer cell lines like A549, SNU638, HT1080, HL60, and HCT116 [75]. 1’S-1’-acetoxychavicol acetate (ACA) is a phenylpropanoid compound reported from various Alpinia spp. such as A. galanga and A. conchigera, and is known to induce apoptotic cell death in various cell lines [76, 176]. ACA was found to inhibit inflammatory transcription factor NF-κB, growth of oral squamous cell carcinoma, and potentiate effect in combination with cisplatin by modulating pro-inflammatory microenvironment [177, 178]. Flavonoid mixture as well as galangin (3, 5, 7-trihydroxyflavone) isolated from A. officinarum reportedly exhibits a broad absorption band at 270–290 nm related to the UV-B area, supporting that galangin could be a potential whitening agent capable of preventing skin cancer [77]. Recently, galangin was found to induce apoptosis via p53-dependent pathway in ovarian cancer cells, A2780/CP70 and OVCAR-3 [179]. A compound, 7-(3,4-dihydroxyphenyl)-1-(4-hydroxy-3methoxyphenyl)-4-en-3-heptanone isolated from A. officinarum, was found to possess remarkable cytotoxicity against HepG2, MCF-7, and SF-268 [78]. Diarylheptanoids isolated from A. officinarum have been shown to wield multiple antitumor effects in neuroblastoma cell lines [79]. A novel compound, pinostrobin chalcone, has been isolated from A. mutica which displays notable cytotoxic potential against various human carcinoma cell lines (KB, MCF-7, and Caski) with significant IC50 values [80]. A. purpurata ethyl acetate extract exhibited antioxidant and anticancer activity against OAW42 and HeLa cells [81, 82]. Crude extracts from A. pahangensis and A. murdochii, endemic to Malaysia, have shown cytotoxic activity against different cancer cell lines [83, 84]. A novel monoterpene-chalcone conjugate, sumadain, isolated from A. katsumadai showed potent cytotoxicity against HepG2, MCF7, and MDA-MB-435 cancer cell lines [85]. Dichromethane and methanol extracts from A. zerumbet flowers exhibit potent antitumor activity against Ehrlich Ascites Carcinoma (EAC) cells in vivo and 5,6,dehydrokawain (DK) isolated from the extract displayed potent antiproliferative activity against various human cancer cell lines, MCF7, HepG2, HEP-2 with noteworthy IC50 values [86]. Ethanol extracts prepared from A. nantoensis rhizome and leaf were reported to inhibit cell migration, invasion, and sphere formation in breast cancer cell lines, MCF-7 and MDA-MB-231. This study also revealed the extracts’ capability to inhibit signal transductions in EGFR as well as the PI3K/AKT and Ras-ERK pathways, which are crucial players of tumor cell migration and invasion [87].
4.2 Genus Amomum Roxb.
Amomum is the second largest genus after Alpinia within Zingiberaceae with about 150 -180 species, widely distributed in Southeast Asia. In India, the genus is represented by 22 species, mostly restricted to North-Eastern and southern India. A chalcone, namely, cardamonin (2′,4′-dihydroxy-6′-methoxychalcone), first isolated from A. subulatum (black cardamom) fruit, has been reported to affect cell growth by modulation of a variety of cell signaling pathways, including mammalian target of rapamycin (mTOR), NF-κB, cell surface receptors, and Wnt/β-catenin pathways [180, 181]. Cardamonin also potentiates TNF-related apoptosis-inducing ligand (TRAIL) for induction of apoptosis through ROS-CHOP (CCAAT/Enhancer-Binding Protein Homologous Protein)-mediated upregulation of death receptors (DRs), which makes TRAIL more effective as an anticancer therapy [91]. Hexane and ethyl acetate extracts of A. subulatum seeds exhibited cytotoxicity against MCF7 and HeLa cancer cell lines besides being immunosuppressive effect against peripheral blood mononuclear cells [92]. Activity-guided fractionation of hexane- and chloroform-soluble extracts of A. aculeatum leaves led to the isolation of aculeatin A and B, found to be cytotoxic against MCF7 breast cancer cell line (in vitro) and in vivo hollow fiber assay [93]. A. villosum and A. kravanh have cytotoxic effects against human hepatocellular carcinoma cell lines SMMC-7721 and Hep G2, respectively [46, 96, 182]. Essential oil and various pure compounds such as tsaokoarylone, isotsaokoin, hannokinol, 2,3-dihydro-2-(4′-hydroxy-phenylethyl)-6-[(3″,4″-dihydroxy-5″-methoxy)phenyl]-4-pyrone, and 4-dihydro-2-(4′-hydroxy-phenylmethyl)-6-[(3″,4″-dihydroxy-5″-methoxyphenyl)methylene]-pyran-3,5-dione isolated from A. tsao-ko fruit (part of traditional Chinese medicine) have been reported with cytotoxicity against various human cancer cell lines [94,95,96]. Ethanol extract from this plant was found to inhibit ovarian cancer and decrease angiogenesis in vivo, through endoplasmic reticulum (ER) stress-mediated interruption in p-STAT3/NF-κB/IL-6 and VEGF loop of angiogenesis regulation [97].
4.3 Genus Kaempferia L.
The genus includes about 70 species, two third of which are found in Asia and remaining one third in Africa [33]. The flavanone, pinostrobin, isolated from Kaempferia rotunda rhizome chloroform extract, has been shown to possess anticancer activity against human breast cancer in vitro (T47D cell line) and in vivo (Xenograft model). Repair of breast tissue and suppression of c-Myc expression were evident on mice with T47D breast cancer xenograft [105]. Lectin isolated from K. rotunda was found to inhibit proliferation of Ehrlich ascites carcinoma cells and induce mitochondrial apoptosis in colorectal cancer cells, SW48 and SW480 [106, 107]. Alcoholic extracts of K. galanga rhizome were reported to possess antineoplastic activities in both in vivo and in vitro model systems [108, 109]. Interestingly, water-soluble polysaccharides purified from K. galanga rhizome reportedly protect thymus and spleen of solid tumor bearing mice and also capable of enhancing immunoregulatory ability of CD4+ T cells, the cytotoxic effects of CD8+ T cells and NK cells, thereby inhibiting tumor [110]. Additionally, major constituent of volatile oil of K. galangal, Ethyl-p-methoxycinnamate, has been reported with anticancer potential against oral cancer HSC-3 and Ca922 cell lines [111]. Ethanol extract of K. parviflora rhizome, commonly known as Thai black ginger used in traditional medicine, showed dose-dependent inhibition of cell proliferation and induction of apoptosis in leukemic HL60 and U937 cell lines [112]. The ethanol extracts of K. Parviflora rhizome supercritical CO2 fluid extracts (SFEs) of K. parviflora have been contained in higher concentration of polymethoxyflavones (PMFs), which showed potent antiproliferative activity against both human cervical (HeLa) and gastric adenocarcinoma (AGS) cell lines [115]. It is also reported to possess anticancer properties as evidenced by suppression of growth and survival signaling pathways, inhibition of metalloproteinase 2 activity, inhibition of cell migration, and invasion and induction of apoptosis in cancer cell lines such as HeLa (cervical) and SKOV3 (ovarian) cells [113, 114]. Extracts and flavone derivatives from the rhizome of K. parviflora have been shown to suppress multidrug resistance-associated proteins (MRP) in A549 (lung cancer) cells, making it useful as modulators of drug resistance in cancer cells [183]. 5,7,4-trimethoxyflavone isolated from K. parviflora rhizome extract reportedly possesses anticancer activity against human cholangiocarcinoma HuCCA-1 and RMCCA-1 cell lines [116]. Methoxyflavones isolated from K. parviflora have been demonstrated to exhibit melanogenesis inhibition in theophylline-stimulated murine B16 melanoma 4A5 cells, without notable cytotoxicity to normal cells [23, 184]. K. angustifolia rhizome extracts and pure compounds abietene diterpene and kaempfolienol present therein were found to be cytotoxic against Leukemic HL-60 and breast cancer MCF-7 cell lines [117]. Labdane and clerodane diterpenoids isolated from K. elegans and K. pulchra found cytotoxic against leukemic HL60 cell line [118].
4.4 Genus Curcuma L.
The genus Curcuma L., with around 120 species, is distributed mainly in tropical and subtropical Asia. Curcuma longa, commonly known as turmeric and a major source of curcumin, has been consumed as a dietary spice and a cure for human ailments for thousands of years in Asian countries. The potential anticancer activity of turmeric and curcumin was demonstrated by Kuttan et al. 1985 [185] in both in vitro and in vivo models. Various crude extracts of C. longa were reported to have antiproliferative activity against different human cancer cell lines such as, U937 (myeloid leukemia), Molt4 (acute lymphoblastic leukemia), A549 (lung carcinoma), T98G (glioblastoma), HeLa (cervical cancer), MDA-MB-231 (breast cancer), and murine melanoma cell line (B164A5) [119,120,121,122]. The immunomodulatory activities of the polar fractions of C. longa hot water extracts were investigated using human peripheral blood mononuclear cells (PBMC). High polarity fraction containing polysaccharides exhibited stimulatory effects on PBMC proliferation, thereby attesting to its potential use as an adjuvant supplement for cancer patients with suppressed immunity due to exposure to chemotherapeutic drugs [186].
Other Curcuma sp. reported with antiproliferative potential include C. amada (mango ginger), C. aromatica (wild turmeric), C. caesia (black turmeric), C. zedoaria (white turmeric), and C. xanthorrhiza (Java turmeric). Supercritical CO2 extract of C. amada rhizome reported to have specific anticancer potential against human glioblastoma (U-87MG) cell line induces apoptosis or drug resistance in a dose-dependent manner [129]. Methanol extracts from C. amada leaves and rhizome also reportedly possess antiproliferative activity against breast cancer cell lines MCF7 and MDA-MB-231 [130].
Aqueous extract of C. aromatica inhibited LS-174-T (colon carcinoma) cell proliferation in a dose- and time-dependent manner, inducing extrinsic and intrinsic apoptosis by activation of caspase-8, -9, and -3 and G2/M phase arrest. Downregulation of cyclin B1 and CDK1 without the participation of p53 was also observed in a study by Hu et al. [131]. Treatment with C. aromatica oil also inhibited growth of implanted hepatoma in mice models which could be correlatable with suppression of PCNA (Proliferating cell nuclear antigen) protein [132,133,134]. Alcoholic extracts of C. aromatica and C. caesia rhizome have been found to possess potent antiangiogenic and proapoptotic activity under in vivo conditions [121, 135,136,137].
C. zedoaria rhizome termed Ezhu in Chinese is extensively used in traditional Chinese medicine to treat various ovarian and cervical cancers. Ethanolic extract of C. zedoaria rhizome is known to exhibit strong antiproliferative and invasive activities against human esophageal squamous carcinoma TE-8 cells and suppress tumor formation in mice. Upregulation of PTEN and downregulation of phosphorylated Akt, mTOR and STAT3 expressions, attenuation of FGFR1 and MMP-2, activation of caspase-9, -3 and PARP, and suppression of Bcl-2 leading to apoptosis were observed in the same study [138]. Hexane and chloroform extracts of C. zedoaria were found to have moderately potent cytotoxic activity on ovarian cancer cells (SKOV3) as well as umbilical vein endothelial cells [139]. Essential oil obtained from C. zedoaria, known as ‘zedoary’, possesses efficient cytotoxic effects on H1299 (non-small cell lung carcinoma) and AGS (gastric cancer) cells and can induce cell cycle arrest and apoptosis. Potential active compounds of Zedoary oil, detected using gas chromatography and mass spectrometry (GC-MS), were 8,9-dehydro-9-formyl-cycloisolongifolene, 6-ethenyl-4,5,6,7-tetrahydro-3,6-dimethyl-5-isopropenyl-trans-benzofuran, eucalyptol, and γ-elemene [141, 142]. Zedoary oil also reportedly exhibits antiangiogenic activity both in vitro and in vivo, resulting in suppressing melanoma growth and lung metastasis, associated with downregulating MMPs [140]. Isocurcumenol isolated from C. Zedoaria rhizome shows antiproliferative potential in KB (nasopharyngeal carcinoma), A549 (lung carcinoma), K562 (leukemic), and DLA (Daltons Lymphoma Ascites) cells [21].
Methanol extract of C. xanthorrhiza rhizome possesses cancer chemopreventive potential [187]. Xanthorrhizol is the most active and abundant compound isolated from the essential oil of C. xanthorrhiza, rhizomes. Studies have shown that xanthorrhizol is an attractive chemopreventive agent as it inhibited tumor nodules in a spontaneous mouse lung metastasis model and TPA (12-O tetradecanoylphorbol-13-acetate) - induced skin cancer promotion in mice, by decreasing phosphorylated ERK (pERK), JNK, and p38 expression [143, 188]. This compound is known to induce apoptosis via activation of p53-dependent mitochondrial pathway in HCT 116 (colon cancer), MCF 7, and MDA-MB-231 (breast cancer) cell lines [144, 145]. Xanthorrhizol also reported to induce caspase-independent apoptosis through ROS-mediated p38, MAPK, and JNK (c-jun N-terminal kinase) activation in SCC-15 (oral squamous cell carcinoma) cells [146]. A combination of xanthorrhizol with other compound(s) like tamoxifen, astaxanthine, and α-tocopherol showed more effective antiproliferatve activity against breast and esophageal cancer cell lines [189].
Dichloromethane extract from Javanese medicinal plant C. purpurascens rhizome induces apoptosis through mitochondrial-dependent pathway in colon cancer HT-29 cells [147]. C. mutabilis is an endemic Zingiberaceae plant confined to Western Ghats of India and the petroleum ether extract of this plant rhizome as well as a novel labdane diterpenoid isolated from this extract reported to be cytotoxic to various cancer cell lines and induce apoptosis in colorectal cancer HCT116 and leukemic K562 cells [149]. Essential oil isolated from various Curcuma species, such as C. elata, C. kwangsiensis, C. yunnanensis, C. nankunshanensis, C. sichuanensis, C. rubescens, C. purpurascens, and C. mutabilis, also exhibited cytotoxicity against various cell lines [148, 150, 190, 191].
Curcumin : Anticancer activity of curcumin need a special mention, as it’s a most studied compound from Curcuma species of Zingiberaceae family. Curcuminoids represent a major component of the phytoconstituents found in various Curcuma species [192]. Of the various curcuminoids known, curcumin (1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione), also called iferuloylmethane, deserves a special mention. It is one of the most studied curcuminoids displaying a wide spectrum of biological actions, including cholesterol-lowering, chemopreventive, antidiabetic, anti-inflammatory, antimicrobial, and antioxidant activities [193,194,195]. Other commonly found curcuminoids are derivatives of curcumin which are known as demethoxycurcumin and bisdemethoxycurcumin [196].
Curcumin has been studied in multiple human carcinomas including melanoma, head and neck, breast, colon, pancreatic, prostate, and ovarian cancers [123,124,125,126,127,128]. Curcumin’s potent antioxidant and free-radical quenching properties play an important role in the inhibitory effects of the compound on the initial stages of carcinogenesis as demonstrated by animal models of various tumor types [197]. NF-κB and AP-1 are two transcription factors intimately involved in the cellular pathways leading to tumorigenesis. NF-κB and AP-1 expression is induced by various stressful stimuli (tumor promoters including oxidative stress, UV irradiation and infectious antigens, pro-inflammatory cytokines such as TNF-α and IL-1), resulting in expression of genes involved in inflammation and cellular proliferation [198]. Curcumin has an inhibitory effect on both NF-κB and AP-1 activation. Its effect on NF-κB, is mediated through inhibition of IκK and results in inactive NF-κB remaining bound to IκBα in the cytoplasm leading to suppression of a variety of gene products involved in carcinogenesis and tumor growth including cyclin D1, VEGF (Vascular endothelial growth factor), COX-2 (cycloxygenase-2), c-myc, Bcl-2, ICAM-1, and MMP-9 (Matrix metalloproteinase-9) [199]. Curcumin also has a stimulatory effect on the extrinsic apoptotic pathway, which is triggered by the binding of ‘death activators’ such as TNF-α and Fas-ligand to their corresponding cell surface receptors. In addition to proapoptotic effect, curcumin also induces autophagic cell death in chronic myelogenous leukemia, esophageal cancer, and malignant glioma cells, mediated through inhibition of the Akt/mTOR/p70S6 kinase pathway and the ERK1/2 pathway [200, 201]. Curcumin has demonstrated antiangiogenic effect in vivo xenograft models, by regulating a variety of proangiogenic growth factors, enzymes, and transcription factors like bFGF (basic fibroblast growth factor), VEGF, angiopoetin-1 and 2, COX-2 MMP-9 [126, 202]. Its derivative, demethoxycurcumin (DMC), has been reported to affect a number of cellular adhesion molecules involved in the processes of metastasis [203].
Curcumin is known to target mTOR, which is recognized as a key therapeutic target for the prevention and / or treatment of cancer [204]. Curcumin has been shown to have numerous cytotoxic effects on cancer stem cells (CSCs) by suppressing the release of cytokines, particularly interleukin (IL)-6, IL-8, and IL-1, which stimulate CSCs [205]. It is an inhibitor of enzymes involved in epigenetic changes of chromatin such as DNA methyltransferase, histone acetyl transferase, and histone deacetylase (HDAC) leading to selective activation or inactivation of genes (oncogenes/tumor suppressors) implicated in cancer death and progression. Curcumin also modulates miRNAs (miR-15a, miR-16, miR-21, miR-22, miR-26, miR-101, miR-146, miR-200, miR-203, and let-7) and their multiple target genes. Altogether, curcumin is able to restore the epigenetic regulation balance and appears as an attractive preventive and/or therapeutic approach against human cancer [206, 207].
Although curcumin has long been used extensively to treat several inflammatory diseases including cancer, poor aqueous solubility and reduced bioavailability limit its efficacy as a promising therapeutic agent in cancer therapy. Various research groups have focused on increasing the bioavailability of curcumin by combining other phytochemicals as adjuvants. For instance, curcumin has often been used in combination with other phytochemicals such as resveratrol, quercetin, sulforaphane, retinoic acid, and folates in cancer treatment [208,209,210]. The chemosensitizing effect of curcumin has been reported in cancers of the breast, colon, pancreas, gut, liver, lung, prostate, brain, lymphoma, and leukemia [211, 212]. Various types of curcumin nanoparticles appropriate for cancer treatment have been developed, such as polymer nanoparticles, liposomes, micelles, solid lipid nanoparticles (SLNs), and polymer conjugates, with improved bioavailability, devoid of degradation and further metabolism and with enhanced targeting capacities [207, 213, 214].
4.5 Genus Zingiber Boehmer
The genus Zingiber represented by 141 species is distributed mainly in tropical Asia. Z. officinale rhizome extract and its major pungent components, 6-shogaol and 6-gingerol, have been reported to induce antiproliferative effects on several tumor cell lines [48]. Ginger extract significantly reduced the elevated expression of NF-κB and pro-inflammatory TNF-α in in vivo model with liver cancer, thereby acting as an anticancer and anti-inflammatory agent [215]. Ethanol extract of ginger is also reported to have potent anticancer activity against pancreatic cancer cells, inhibit cell cycle progression, and induce ROS-mediated apoptosis [151]. Experimental studies also showed that ginger extracts as well as the purified constituents therein such as 6-gingerol and 6-shogaol exerted anticancer activity against gastrointestinal cancer cells by modulating several signaling molecules like NF-κB, STAT3, MAPK, PI3K, ERK1/2, Akt, TNF-α, COX-2, cyclin D1, cdk, MMP-9, survivin, cIAP-1, XIAP, Bcl-2, caspases, and other cell growth regulatory proteins [22]. The remarkable increase of shogaols in steamed ginger contributed to its improved anticancer potential [216, 217]. 6-shogaol significantly inhibited cell proliferation in colon cancer cell lines HCT-116 and SW-480, with IC50 values of 7.5 and 10 μM, respectively, and can cause cell cycle arrest in G2/M phase by p53/p21-mediated pathway [152]. It is known to act through endoplasmic reticulum stress and mitochondrial pathways involved in apoptosis induction in HeLa (cervical cancer) cells [153]. Gingerol was also found to sensitize A549 cells to TRAIL-induced apoptosis by inhibiting the autophagy flux [155], inhibit cell proliferation, and induce apoptosis in SW-480 cells and HCT116 (colon cancer) cells [154]. Methanol extract of ginger leaves also reportedly induce apoptosis and reduction of cell viability in human colorectal cancer cells [156].
Various organic solvent extracts and bioactivity guided column chromatography subfractions of Z. zerumbet rhizome displayed strong antiproliferative effects on breast cancer MCF7 cells [157]. Zerumbone, a natural cyclic sesquiterpene from Z. zerumbet, reported to have a diverse range of biological activities, including anticancer and antitumor activities. Studies have demonstrated that zerumbone has little or no cytotoxic effect on normal human cells but induces apoptosis in many cancer cell lines [158,159,160]. Chloroform extract of Z. cassumunar rhizome and compounds therein cis-3-(3’, 4’-dimethoxyphenyl)-4-[(E)-3, 4 dimethoxystyryl] cyclo-hex-1-ene and 8-(13,14-dimethoxyphenyl)-2-methoxynaphto-1,4-quinone showed strong activity against human T-acute lymphoblastic leukemia (CEMss) and cervical (HeLa) cancer cell lines [161].
4.6 Other Zingiberaceae Plants with Anticancer Potential
Experimental evidence suggests that aqueous extracts of Elettaria cardamomum (cardamom) extracts exert anti-inflammatory roles (immunomodulatory). It is evident that black pepper and cardamom aqueous extracts together significantly enhance the cytotoxic activity of natural killer cells, thereby indicating their potential anticancer effects [162]. E. cardamomum extract also possesses potential chemopreventive effects evidenced by preventing diethylnitrosamine (DENA)-induced hepato-cellular carcinoma through blocking oxidative stress, decreasing pro-inflammatory cytokine, NF-κB, and ornithine decarboxylase (ODC) [218].
Labdane diterpenes (isocoronarin D, methoxycoronarin D, ethoxycoronarin D, and benzoyl eugenol) from Hedychium coronarium ethanol extract reported to possess chemopreventive effect [164]. Other labdane-type diterpenes reported from this plant showed moderate to potent cytotoxic activities against different cancer cell lines. They are reported with antiangiogenic activity, proved through inhibition of human vascular endothelial cells [165]. Coronarin D from H. coronarium induces significant G2/M arrest, apoptosis, and autophagy in various human cancer cell lines including nasopharyngeal carcinoma (NPC) cells [166, 219]. It is also reported to induce cell death through the upregulation of JNK/MAPK and caspase-dependent apoptosis pathways in human hepatocellular carcinoma (HCC) Huh7 and Sk-hep-1 cells [167]. H. coronarium rhizome ethanol extract can induce apoptosis-mediated G1 phase cell arrest, while inhibiting the migratory potential of cervical cancer HeLa cells [163].
Two novel labdane-diterpenes isolated from chloroform extract of Hedychium spicatum rhizomes have shown good cytotoxic activity against Colo-205 (Colon cancer), A-431 (skin cancer), MCF-7 (breast cancer), A549 (lung cancer), and Chinese hamster ovary cells (CHO) [168]. Six new sesquiterpenes, including two potent cytotoxic (against HeLa cells) compounds, have been isolated from this extract [169]. Essential oil isolated from Hedychium spicatum rhizome also reported cytotoxicity against various cancer cell lines [170].
5 Conclusion
To conclude, members of family Zingiberaceae continue to provide innumerable bioactive extracts and compounds reported with potent cytotoxic and anticancer activities. As a matter of fact, many of these plants are utilized either as ingredients of traditional food varieties or additives in time-tested, traditional ethnomedicinal herbal preparations, well known for their efficacious cure of a plethora of diverse ailments. Discovery of various anticancer compounds from these plants is emerging as a highly enriched, promising, and biocompatible bioresource for modern /complementary or alternative medicine systems. Compared to the notorious and undesirable side effects of chemotherapeutics used in the past decades, these compounds can be developed for effective and specific targeting of key proteins of cancer-related signaling pathways. Despite an impressive array of such compounds, the hunt needs to continue for hitherto unexplored, yet to be discovered drug candidates within the Zingiberaceae family of plants.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68:394–424. http://www.ncbi.nlm.nih.gov/pubmed/30207593
Ruddon RW (2007) Cancer biology, 4th edn. Oxford University Press
Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, Sung B, Aggarwal BB (2008) Cancer is a preventable disease that requires major lifestyle changes. Pharm Res 25:2097–2116
Cohen L, Jefferies A (2017) Comprehensive lifestyle change: Harnessing synergy to improve cancer outcomes. J Natl Cancer Inst. 2017:33–36
Weinberg RA (2014) The Biology of Cancer, 2nd edn. Garland Science, Taylor and Francis group
Ke X, Shen L (2017) Molecular targeted therapy of cancer: The progress and future prospect. Front Lab Med 1:69–75. https://doi.org/10.1016/j.flm.2017.06.001
Kooti W, Servatyari K, Behzadifar M, Asadi-Samani M, Sadeghi F, Nouri B, Zare MH (2017) Effective medicinal plant in cancer treatment. J Evidence-Based Complement Altern Med 22(4):982–995
Thapliyal A, Khar RK, Amrish ChandraChandra A (2018) Overview of cancer and medicinal herbs used for cancer therapy. Asian J Pharm 12:1–8. https://www.asiapharmaceutics.info/index.php/ajp/article/view/2033
Guerra B, Issinger O-G (2019) Natural compounds and derivatives as Ser/Thr protein kinase modulators and inhibitors. Pharmaceuticals 12:4. https://doi.org/10.3390/ph12010004
Tariq A, Sadia S, Pan K, Ullah I, Mussarat S, Sun F, Abiodun OO, Batbaatar A, Li Z, Song D, Xiong Q, Ullah R, Khan S, Basnet BB, Kumar B, Islam R, Adnan M (2017) A systematic review on ethnomedicines of anti-cancer plants. Phyther Res 31:202–264
Seca AML, Pinto DCGA (2018) Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int J Mol Sci 19(1):263
Cragg GM, Pezzuto JM (2016) Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract 25:41–59
Iqbal J, Abbasi BA, Kanwal S, Khalil AT, Mahmood T, Shah SA, Ali B (2017) Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Trop Biomed 7:1129–1150. https://doi.org/10.1016/j.apjtb.2017.10.016
Kress WJ, Prince LM, Williams KJ (2002) The phylogeny and a new classification of the gingers (Zingiberaceae): evidence from molecular data. Am J Bot 89(11):1682–1696
Sabu M (2006) Zingiberaceae and Costaceae of South India. Indian Association for Angiosperm Taxonomy
Pancharoen O, Prawat U, Tuntiwachwuttikul P (2000) Phytochemistry of the Zingiberaceae. Stud Nat Prod Chem 23:797–865
Fadilah F, Yanuar A, Arsianti A, Andrajati R (2017) Phenylpropanoids, eugenol scaffold, and its derivatives as anticancer. Asian J Pharm Clin Res 10:41–46
Ansari IA, Akhtar MS (2019) Chapter 3 - Current insights on the role of terpenoids as anticancer agents: a perspective on cancer prevention and treatment. In: Swamy MK, Akhtar MS (eds) Nature Bio-active Compound. Springer Nature Singapore, pp 53–80. https://doi.org/10.1007/978-981-13-7205-6_3
Kopustinskiene DM, Jakstas V, Savickas A, Bernatoniene J (2020) Flavonoids as anticancer agents. Nutrients 12:457. https://doi.org/10.3390/nu12020457
Basak S, Sarma GC, Rangan L (2010) Ethnomedical uses of Zingiberaceous plants of Northeast India. J Ethnopharmacol 132:286–296
Lakshmi S, Padmaja G, Remani P (2011) Antitumour effects of Isocurcumenol isolated from Curcuma zedoaria rhizomes on human and murine cancer cells. Int J Med Chem. https://doi.org/10.1155/2011/253962
Prasad S, Tyagi AK (2015) Ginger and its constituents: role in prevention and treatment of gastrointestinal cancer. Gastroenterol Res Pract. https://doi.org/10.1155/2015/142979
Chen D, Li H, Li W, Feng S, Deng D (2018) Kaempferia parviflora and its Methoxyflavones: chemistry and biological activities. Evidence-Based Complement Altern Med. https://doi.org/10.1155/2018/4057456
Alkandahri MY, Shafirany MZ, Rusdin A, Agustina LS, Pangaribuan F, Fitrianti F, Farhamzah KAH, Sugiharta S, Mardiana LA (2021) Amomum compactum: a review of pharmacological studies. Plant Cell Biotechnol Mol Biol 22:61–69
Kirana C, Record IR, McIntosh GH, Jones GP (2003) Screening for antitumor activity of 11 species of Indonesian Zingiberaceae using human MCF-7 and HT-29 cancer cells. Pharm Biol 41:271–276
Afzal A, Oriqat G, Khan AM, Jose J, Afzal M (2013) Chemistry and biochemistry of terpenoids from Curcuma and related species. J Biol Active Prod Nat 3:1–55. http://www.tandfonline.com/doi/abs/10.1080/22311866.2013.782757
Ghosh S, Rangan L (2013) Alpinia: the gold mine of future therapeutics. 3 Biotech 3:173–185
Hartati R, Suganda AG, Fidrianny I (2014) Botanical, phytochemical and pharmacological properties of Hedychium (Zingiberaceae) – a review. Procedia Chem 13:150–163. http://linkinghub.elsevier.com/retrieve/pii/S1876619614002095
Wallace D (2016) Natural products as a source of anti-cancer lead compounds: Ginger and breast cancer. J Pharmacol Clin Res 1(3):1–5
Joy PP, Thomas J, Mathew S, Skaria BP (1998) Zingiberaceous medicinal and aromatic plants. Aromatic and Medicinal Plants Research Station, Odakkali
Sirirugsa P (1998) Thai Zingiberaceae: Species diversity and their uses. Pure Appl Chem 70:23–27
Jatoi SA, Kikuchi A, Watanabe KN (2007) Genetic diversity, cytology, and systematic and phylogenetic studies in Zingiberaceae fleshy roots. Genes Genome Genom 1(1):56–62
Prabhu KKM, Asish G, Sabu M, Balachandran I (2013) Significance of gingers (Zingiberaceae) in indian system of medicine - Ayurveda: an overview. Anc Sci Life 32:253
Zahara M, Hasanah M, Zalianda R (2018) Identification of Zingiberaceae as medicinal plants in Gunung cut village, Aceh Barat Daya, Indonesia. J Trop Hortic. 1:24–28
Chhabra SC, Mahunnah RLA, Mshiu EN (1993) Plants used in traditional medicine in Eastern Tanzania. VI. Angiosperms (Sapotaceae to Zingiberaceae). J Ethnopharmacol 39:83–103
Peng L, Zou HQ, Bauer R, Liu Y, Tao O, Yan SR, Han Y, Li JH, Ren ZY, Yan YH (2014) Identification of Chinese herbal medicines from Zingiberaceae family using feature extraction and cascade classifier based on response signals from E-Nose. Evidence-based Complement Altern Med. https://doi.org/10.1155/2014/963035
Ujang Z, Subramaniam T, Nordin NI (2015) Ginger species and their traditional uses in modern applications. J Ind Technol 23:59–70
Kasarkar AR, Kulkarni DK (2016) Traditional knowledge of medicines belonging to family Zingiberaceae from South Western Maharashtra, India. Int J Bot Stud 1(4):20–23
Jantan IB, Yassin MSM, Chin CB, Chen LL, Sim NL (2003) Antifungal activity of the essential oils of nine Zingiberaceae species. Pharm Biol 41(5):392–397
Cheenpracha S, Karalai C, Ponglimanont C, Subhadhirasakul S, Tewtrakul S (2006) Anti-HIV-1 protease activity of compounds from Boesenbergia pandurata. Bioorganic Med Chem 14:1710–1714
Tewtrakul S, Subhadhirasakul S (2007) Anti-allergic activity of some selected plants in the Zingiberaceae family. J Ethnopharmacol 109:535–538
Chen IN, Chang CC, Ng CC, Wang CY, Shyu YT, Chang TL (2008) Antioxidant and antimicrobial activity of Zingiberaceae plants in Taiwan. Plant Foods Hum Nutr 63:15–20
Hanish Singh JC, Alagarsamy V, Diwan PV, Sathesh Kumar S, Nisha JC, Narsimha RY (2011) Neuroprotective effect of Alpinia galanga (L.) fractions on Aβ(25–35) induced amnesia in mice. J Ethnopharmacol 138:85–91
Kalaivani K, Senthil-Nathan S, Murugesan AG (2012) Biological activity of selected Lamiaceae and Zingiberaceae plant essential oils against the dengue vector Aedes aegypti L. (Diptera: Culicidae). Parasitol Res 110:1261–1268
Salama SM, Abdulla MA, AlRashdi AS, Ismail S, Alkiyumi SS, Golbabapour S (2013) Hepatoprotective effect of ethanolic extract of Curcuma longa on thioacetamide induced liver cirrhosis in rats. BMC Compl Alter Med 13:56. http://www.biomedcentral.com/1472-6882/13/56
Lu CL, Zhao HY, Jiang JG (2013) Evaluation of multi-activities of 14 edible species from Zingiberaceae. Int J Food Sci Nutr 64(1):28–35
Al-Nahain A, Jahan R, Rahmatullah M (2014) Zingiber officinale: A potential plant against rheumatoid arthritis. Evidence-Based Complement Altern Med. https://doi.org/10.1155/2014/159089
Danciu C, Vlaia L, Fetea F, Hancianu M, Coricovac DE, Ciurlea SA, Şoica CM, Marincu I, Vlaia V, Dehelean CA, Trandafirescu C (2015) Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biol Res 48:1–9. http://www.biolres.com/content/48/1/1
Lakhan SE, Ford CT, Tepper D (2015) Zingiberaceae extracts for pain: a systematic review and meta-analysis. Nutr J 14:50. https://doi.org/10.1186/s12937-015-0038-8
Nithya R, Jayshree N (2017) A review on herbs of the Zingiberaceae family with beneficial effects on cardiovasular diseases. World J Pharm Pharm Sci 6:635–643
Aghasi M, Ghazi-Zahedi S, Koohdani F, Siassi F, Nasli-Esfahani E, Keshavarz A, Qorbani M, Khoshamal H, Salari-Moghaddam A, Sotoudeh G (2018) The effects of green Cardamom supplementation on blood glucose, lipids profile, oxidative stress, Sirtuin-1 and Irisin in type 2 diabetic patients: a study protocol for a randomized placebo-controlled clinical trial. BMC Complement Altern Med 18:1–6. https://doi.org/10.1186/s12906-017-2068-6
Raju R, Singh A, Gunawardena D, Reddell P, Münch G (2019) Diarylheptanoids with anti-inflammatory activity from the rhizomes of Pleuranthodium racemigerum (Zingiberaceae). Phytochem Lett 30:10–13
Ganapathy G, Preethi R, Moses JA, Anandharamakrishnan C (2019) Diarylheptanoids as nutraceutical: a review. Biocatal Agric Biotechnol 19:101109
Gurib-Fakim A, Maudarbaccus N, Leach D, Doimo L, Wohlmuth H (2002) Essential oil composition of Zingiberaceae species from Mauritius. J Essent Oil Res 14:271–273
Abe M, Nakamura Y, Yamada Y, Osawa T, Morimitsu Y, Uda Y (2003) Labdane-type diterpene dialdehyde, pungent principle of Myoga, Zingiber mioga Roscoe. Biosci Biotechnol Biochem 66(12):2698–2700
Chimnoi N, Sarasuk C, Khunnawutmanotham N, Intachote P, Seangsai S, Saimanee B, Pisutjaroenpong S, Mahidol C, Techasakul S (2009) Phytochemical reinvestigation of labdane-type diterpenes and their cytotoxicity from the rhizomes of Hedychium coronarium. Phytochem Lett 2:184–187
Manse Y, Ninomiya K, Nishi R, Kamei I, Katsuyama Y, Imagawa T, Chaipech S, Muraoka O, Morikawa T (2016) Melanogenesis inhibitory activity of a 7-O-9’-linked neolignan from Alpinia galanga fruit. Bioorganic Med Chem 24:6215–6224. https://doi.org/10.1016/j.bmc.2016.10.001
Win NN, Ito T, Ngwe H, Win YY, Prema OY, Tanaka M, Asakawa Y, Abe I, Morita H (2017) Labdane diterpenoids from Curcuma amada rhizomes collected in Myanmar and their antiproliferative activities. Fitoterapia 122:34–39
Ji KL, Fan YY, Ge ZP, Sheng L, Xu YK, Gan LS, Li JY, Yue JM (2019) Maximumins A-D, rearranged Labdane-type diterpenoids with four different carbon skeletons from Amomum maximum. J Org Chem 84:282–288
Sematong T, Pongprayoon U, Tuchinda P, Claeson P, Reutrakul V, Nahar N (1996) Topical antiinflammatory activity of two pimarane diterpenes from Kaempferia pulchra. Phyther Res 10:534–535
Alberti Á, Riethmüller E, Béni S (2018) Characterization of diarylheptanoids: an emerging class of bioactive natural products. J Pharm Biomed Anal. 147:13–34
Kim NJ, Byun SG, Cho JE, Chung K, Ahn YJ (2008) Larvicidal activity of Kaempferia galanga rhizome phenylpropanoids towards three mosquito species. Pest Manag Sci 64:857–862
Kuddus R, Rumi F, Kaisar A, Hasan CM (2010) Sesquiterpene and phenylpropanoids from Curcuma longa. Bangladesh Pharm J 13(2):31–34
Hong SS, Oh JS (2012) Phenylpropanoid ester from Zingiber officinale and their inhibitory effects on the production of nitric oxide. Arch Pharm Res 35:315–320
Samarghandian S, Hadjzadeh MAR, Afshari JT, Hosseini M (2014) Antiproliferative activity and induction of apoptotic by ethanolic extract of Alpinia galanga rhizhome in human breast carcinoma cell line. BMC Complement Altern Med 14:192. http://www.biomedcentral.com/1472-6882/14/192
Chouni A, Paul S (2018) A review on phytochemical and pharmacological potential of Alpinia galanga. Pharmacogn J 10(1):9–15
Rao CH, Namosiva T, Suryaprakasam S (1976) Cardamonin and Alpinetin from the seeds of Amomum subulatum. Planta Med 29:391–392
Jang DS, Han A-R, Park G, Jhon G-J, Seo E-K (2004) Flavonoids and aromatic compounds from the rhizomes of Zingiber zerumbet. Arch Pharm Res 27(4):386–389
Ching AYL, Wah TS, Sukari MA, Lian GEC, Rahmani M, Khalid K (2007) Characterization of flavonoid derivatives from Boesenbergia rotunda (L.). Malay J Anal Sci 11:154–159
Sutthanut K, Sripanidkulchai B, Yenjai C, Jay M (2007) Simultaneous identification and quantitation of 11 flavonoid constituents in Kaempferia parviflora by gas chromatography. J Chromatogr A 1143:227–233
Liu D, Qu W, Liang JY (2013) Flavonoids and other constituents from Alpinia sichuanensis Z.Y. Zhu. Biochem Syst Ecol 46:127–129
Sabulal B, Dan M, John JA, Kurup R, Purushothaman CS, George V (2007) Phenylbutanoid-rich rhizome oil of Zingiber neesanum from Western Ghats, Southern India. Flavour Fragr J 22:521–524
Taechowisan T, Suttichokthanakorn S, Phutdhawong WS (2018) Antibacterial and cytotoxicity activities of phenylbutanoids from Zingiber cassumunar Roxb. J Appl Pharm Sci 8:121–127
He ZH, Ge W, Yue GGL, Lau CBS, He MF, But PPH (2010) Anti-angiogenic effects of the fruit of Alpinia oxyphylla. J Ethnopharmacol 132:443–449
Nam JW, Kim SJ, Han RM, Lee SK, Seo EK (2005) Cytotoxic phenylpropanoids from the rhizome of Alpinia galanga. J Appl Pharm 13:263–266
Awang K, Nurul Azmi M, Lian Aun LI, Nazif Aziz A, Ibrahim H, Hasima NN (2010) The apoptotic effect of 1’S-1’-Acetoxychavicol acetate from Alpinia conchigera on human cancer cells. Molecules 15:8048–8059
Lu Y, Wang Z, Wei D, Xiang H (2007) Mechanism and inhibitory effect of galangin and its flavonoid mixture from Alpinia officinarum on mushroom tyrosinase and B16 murine melanoma cells. J Enz Inhibit Med Chem 22(4):433–438
An N, Zou Z, Tian Z, Luo X, Yang S, Xu L (2008) Diarylheptanoids from the rhizomes of Alpinia officinarum and their anticancer activity. Fitoterapia 79:27–31
Tabata K, Yamazaki U, Okada M, Fukumura K, Shimada A, Sun Y, Yasukawa K, Suzuki T (2009) Diarylheptanoids derived from Alpinia officinarum induce apoptosis, S-phase arrest and differentiation in human neuroblastoma cells. Anticancer Res 29:4981–4988
Malek ANS, Phang CW, Ibrahim H, Wahab NA, Sim KS (2011) Phytochemical and cytotoxic investigations of Alpinia mutica rhizomes. Molecules 16:583–589. https://doi.org/10.3390/molecules16010583
Raj CA, Ragavendran P, Sophia D, Rathi MA, Gopalakrishnan VK (2012) Evaluation of in-vitro antioxidant and anticancer activity of Alpinia purpurata. Chin J Nat Med 10(4):263–268
Oirere EK, Anusooriya P, Malarvizhi D, Raj CA, Gopalakrishnan VK (2016) Antioxidant, cytotoxic and apoptotic activities of crude extract of Alpinia purpurata on cervical cancer cell line. Int J Pharm Sci Rev Res 36(2):28–34
Phang C, Nurestri S, Malek A, Ibrahim H (2013) Antioxidant potential, cytotoxic activity and total phenolic content of Alpinia pahangensis rhizomes. BMC Complement Altern Med 13:243. http://www.biomedcentral.com/1472-6882/13/243
Sim KS, Ibrahim H, Malek ANS, Syamsir DR, Awang K (2014) Cytotoxic activity of Alpinia murdochii Ridl: a mountain ginger species from Peninsular Malaysia. Pharmaco Mag 10:70–72
Hua SZ, Luo JG, Wang XB, Wang JS, Kong LY (2009) Two novel monoterpene-chalcone conjugates isolated from the seeds of Alpinia katsumadai. Bioorganic Med Chem Lett 19:2728–2730. https://doi.org/10.1016/j.bmcl.2009.03.117
Zahra MH, Salem TAR, El-Aarag B, Yosri N, EL-Ghlban S, Zaki K, Marei AH, EL-Wahed AA, Saeed A, Khatib A, AlAjmi MF, Shathili AM, Xiao J, Khalifa SAM, El-Seedi HR (2019) Alpinia zerumbet (Pers.): Food and medicinal plant with potential in vitro and in vivo anti-cancer activities. Molecules 24:2495. https://doi.org/10.3390/molecules24132495
Kuo C-Y, Teng-Song Weng T-S, Senthil Kumar KJ, Tseng Y-H, Tung T-W, Wang S-Y, Wang H-C (2019) Ethanol Extracts of Dietary Herb, Alpinia nantoensis, exhibit anticancer potential in human breast cancer cells. Integr Cancer Ther 18:1–12. https://doi.org/10.1177/153473541986692
Reddy AS, Abd Malek SN, Ibrahim H, Sim KS (2013) Cytotoxic effect of Alpinia scabra (Blume) Náves extracts on human breast and ovarian cancer cells. BMC Complement Altern Med 13:314. https://doi.org/10.1186/1472-6882-13-314
Ali MS, Banskota AH, Tezuka Y, Saiki I, Kadota S (2001) Antiproliferative activity of diarylheptanoids from the seeds of Alpinia blepharocalyx. Biol Pharm Bull 24(5):525–528
Yang HL, Chen SC, Chen CS, Wang SY, Hseu YC (2008) Alpinia pricei rhizome extracts induce apoptosis of human carcinoma KB cells via a mitochondria-dependent apoptotic pathway. Food Chem Toxicol 46:3318–3324
Yadav VR, Prasad S, Aggarwal BB (2012) Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP- mediated up-regulation of death receptors and down- regulation of survival. Brit J Pharma 165:741–753
Sharma V, Lohia N, Handa V, Baranwal M (2017) Amomum subulatum seed extract exhibit antioxidant, cytotoxic and immune-suppressive effect. Indian J Biochem Biophys 54:135–139
Chin YW, Salim AA, Su BN, Mi Q, Chai HB, Riswan S, Kardono LBS, Ruskandi A, Farnsworth NR, Swanson SM, Kinghorn AD (2008) Potential anticancer activity of naturally occurring and semisynthetic derivatives of aculeatins A and B from Amomum aculeatum. J Nat Prod 71:390–395
Moon SS, Cho SC, Lee JY (2005) Tsaokoarylone, a cytotoxic diarylheptanoid from Amomum tsao-ko fruits. Bull Korean Chem Soc 26:447–450
Yang Y, Yue Y, Runwei Y, Guolin Z (2010) Cytotoxic, apoptotic and antioxidant activity of the essential oil of Amomum tsao-ko. Bioresour Technol 101:4205–4211
Zhang T-T, Lu C-L, Jiang J-G (2015) Antioxidant and anti-tumour evaluation of compounds identified from fruit of Amomum tsaoko Crevost et Lemaire. J Funct Foods. 18:423–431
Chen C, You F, Wu F, Luo Y, Zheng G, Xu H, Liu Y (2020) Antiangiogenesis Efficacy of Ethanol Extract from Amomum tsaoko in Ovarian Cancer through Inducing ER Stress to Suppress p-STAT3/NF-kB/IL-6 and VEGF Loop. Evidence-based Complement Altern Med:2390125. https://doi.org/10.1155/2020/2390125
Zhang D, Li S, Xiong Q, Jiang C, Lai X (2013) Extraction, characterization and biological activities of polysaccharides from Amomum villosum. Carbohydr Polym 95:114–122
Tangjitjaroenkun J, Tangchitcharoenkhul R, Yahayo W, Supabphol S, Sappapan R, Supabphol R (2020) Chemical compositions of essential oils of Amomum verum and Cinnamomum parthenoxylon and their in vitro biological properties. J Herbmed Pharmacol 9(3):223–231
Choi JW, Kim KH, Lee IK, Choi SU, Lee KR (2009) Phytochemical constituents of Amomum xanthioides. Nat Prod Sci 15(1):44–49
Kim KH, Choi JW, Choi SU, Lee KR (2010a) Terpene glycosides and cytotoxic constituents from the seeds of Amomum xanthioides. Planta Med 76(5):461–464
Kim KH, Choi JW, Choi SU, Seo EK, Lee KR (2010b) Amoxantin A: a new bisnorlabdane diterpenoid from Amomum xanthioides. Bull Kor Chem Soc 31(4):1035–1037
Kim KH, Choi JW, Choi SU, Lee K (2011) Cytotoxic sesquiterpenoid from the seeds of Amomum xanthioides. Nat Prod Sci 17(1):10–13
Luo JG, Yin H, Fan BY, Kong LY (2014) Labdane diterpenoids from the roots of Amomum maximum and their cytotoxic evaluation. Helv Chim Acta 97(8):1140–1145
Atun S, Arianingrum R (2015) Anticancer activity of bioactive compounds from Kaempferia rotunda rhizome against human breast cancer. Int J Pharmacogn Phytochem Res 7:262–269
Kabir SR, Reza MA (2014) Antibacterial activity of Kaempferia rotunda rhizome lectin and its induction of apoptosis in Ehrlich ascites carcinoma cells. Appl Biochem Biotechnol 172:2866–2876
Islam F, Gopalan V, Lam AKY, Kabir SR (2019) Kaempferia rotunda tuberous rhizome lectin induces apoptosis and growth inhibition of colon cancer cells in vitro. Int J Biol Macromol 141:775–782. https://doi.org/10.1016/j.ijbiomac.2019.09.051
Amuamuta A, Plengsuriyakarn T, Na-Bangchang K (2017) Anticholangiocarcinoma activity and toxicity of the Kaempferia galanga Linn. Rhizome ethanolic extract. BMC Complement Altern Med 17:213. https://doi.org/10.1186/s12906-017-1713-4
Ali H, Yesmin R, Satter Mohammed A, Habib R, Yeasmin T (2018) Antioxidant and antineoplastic activities of methanolic extract of Kaempferia galanga Linn. Rhizome against Ehrlich ascites carcinoma cells. J King Saud Univ Sci 30:386–392
Yang X, Ji H, Feng Y, Yu J, Liu A (2018) Structural characterization and antitumor activity of polysaccharides from Kaempferia galanga L. Oxid Med Cell Longev:9579262. https://doi.org/10.1155/2018/9579262
Ichwan SJA, Husin A, Suriyah WH, Lestari W, Omar MN, Kasmuri AR (2019) Anti-neoplastic potential of ethyl-p-methoxycinnamate of Kaempferia galanga on oral cancer cell lines. Mater Today Proc 16:2115–2121. https://doi.org/10.1016/j.matpr.2019.06.100
Banjerdpongchai R, Chanwikruy Y, Rattanapanone V, Sripanidkulchai B (2009) Induction of apoptosis in the human leukemic U937 cell line by Kaempferia parviflora Wall.Ex.Baker extract and effects of Paclitaxel and Camptothecin. Asian Pac J Cancer Prev 10:1137–1140
Potikanond S, Sookkhee S, Takuathung MN, Mungkornasawakul P, Wikan N, Smith DR, Nimlamool W (2017) Kaempferia parviflora extract exhibits anti-cancer activity against HeLa cervical cancer cells. Front Pharmacol 8:630. https://doi.org/10.3389/fphar.2017.00630
Paramee S, Sookkhee S, Sakonwasun C, Takuathung MN, Mungkornasawakul P, Nimlamool W, Potikanond S (2018) Anti-cancer effects of Kaempferia parviflora on ovarian cancer SKOV3 cells. BMC Complement Altern Med 18:178. https://doi.org/10.1186/s12906-018-2241-6
Wongsrikaew N, Kim H, Vichitphan K, Cho SK, Han J (2012) Antiproliferative activity and polymethoxyflavone composition analysis of Kaempferia parviflora extracts. J Korean Soc Appl Biol Chem 55:813–817
Leardkamolkarn V, Tiamyuyen S, Sripanidkulchai BO (2009) Pharmacological activity of Kaempferia parviflora extract against human bile duct cancer cell lines. Asian Pac J Cancer Prev 10:695–698
Tang SW, Sukari MA, Neoh BK, Yeap YSY, Abdul AB, Kifli N, Cheng Lian Ee G (2014) Phytochemicals from Kaempferia angustifolia Rosc. and their cytotoxic and antimicrobial activities. Biomed Res Int. https://doi.org/10.1155/2014/417674
Chawengrum P, Boonsombat J, Kittakoop P, Mahidol C, Ruchirawat S, Thongnest S (2018) Cytotoxic and antimicrobial labdane and clerodane diterpenoids from Kaempferia elegans and Kaempferia pulchra. Phytochem Lett 24:140–144
Kaneshiro T, Suzui M, Takamatsu R, Murakami A, Fujino T, Yoshimi N (2005) Growth inhibitory activities of crude extracts obtained from herbal plants in the Ryukyu Islands on several human colon carcinoma cell lines. Asian Pac J Cancer Prev 6:353–358
Ahmad R, Srivastava AN, Khan MA (2016) Evaluation of in-vitro anticancer activity of rhizome of Curcuma longa against human breast cancer and Vero cell lines. Int J Bot Stud 1:1–6
Hadem KLH, Sen A (2017) Curcuma species: a source of anticancer drugs. J Tumor Med Prev 1(5):1–7
Kukula-Koch W, Grabarska A, Jarogniew Ł, Czernicka L, Nowosadzka E, Gumbarewicz E, Jarzab A, Audo G, Upadhyay S, Głowniak K, Stepulak A (2018) Superior anticancer activity is demonstrated by total extract of Curcuma longa L. as opposed to individual curcuminoids separated by centrifugal partition chromatography. Phytother Res 32:933–942
Liu D, Chen Z (2013) Breast cancer the effect of curcumin on breast cancer cells. J Breast Can 16(2):133–137
Mukhopadhyay A, Bueso-ramos C, Chatterjee D, Pantazis P, Aggarwal BB (2001) Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene 20:7597–7609
Hanif R, Qiao L, Shiff SJ, Rigas B (1997) Curcumin, a natural plant phenolic food additive, inhibits cell proliferation and induces cell. J Lab Clin Med 130(6):576–584
Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han L, Armaiz-pena GN, Kamat AA, Spannuth W, Gershenson DM, Lutgendorf SK, Aggarwal BB, Sood AK (2007) Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the Nuclear Factor-κB pathway. Clin Cancer Res 13(11):3423–3431
Siwak DR, Shishodia S, Aggarwal BB, Kurzrock R (2005) Curcumin-induced antiproliferative and proapoptotic effects in melanoma cells are associated with suppression of IκB kinase and Nuclear Factor-κB activity and are independent of the B-Raf / mitogen- activated / extracellular signal-regulated protein kinase pathway and the Akt pathway. Cancer 104(5):879–890
Wang D, Veena MS, Stevenson K, Tang C, Ho B, Suh JD, Duarte VM, Faull KF, Mehta K, Srivatsan ES, Wang MB (2008) Liposome-encapsulated Curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of Nuclear Factor-κB by an AKT-independent pathway. Clin Cancer Res 14(19):6228–6237
Ramachandran C, Lollett IV, Escalon E, Quirin K, Melnick SJ (2015) Anticancer potential and mechanism of action of Mango ginger (Curcuma amada Roxb.) supercritical CO2 extract in human glioblastoma cells. J Evid-Based Compl Altern Med 20(2):109–119
Sivaprabha J, Dharani B, Padma PR, Sumathi S (2016) Apoptosis-induced in-vitro anticancer activity of methanolic extract of leaves and rhizomes of Curcuma amada Roxb. against breast cancer cells. Int J Green Pharm 10(2):98–103
Hu B, Shen K-P, An H-M, Wu Y, Du Q (2011) Aqueous extract of Curcuma aromatica induces apoptosis and G2/M arrest in human colon carcinoma LS-174-T cells independent of p53. Can Biother Radiopharm 26:97–104
Wu WY, Xu Q, Shi LC, Zhang WB (2000) Inhibitory effects of Curcuma aromatica oil on proliferation of hepatoma in mice. World J Gastroentero 6(2):216–219
Li Y, Wo JM, Ms QL, Li X, Martin RCG (2009) Chemoprotective effects of Curcuma aromatica on esophageal carcinogenesis. Ann Surg Oncol 16:515–523
Li Y, Shi X, Zhang J, Zhang X, Martin RCG (2014) Hepatic protection and anticancer activity of Curcuma: a potential chemopreventive strategy against hepatocellular carcinoma. Int J Oncol 44:505–513
Thippeswamy G, Salimath BP (2006) Curcuma aromatica extract induces apoptosis and inhibits angiogenesis in Ehrlich Ascites tumor cells in-vivo. mySCIENCE 1(1):79–92
Karmakar I, Dolai N, Kumar RBS, Kar B, Roy SN, Haldar PK (2013) Antitumor activity and antioxidant property of Curcuma caesia against Ehrlich’s ascites carcinoma bearing mice. Pharm Biol 51(6):753–759
Hadem KLH, Sharan RN, Kma L (2016) Phytochemicals of Aristolochia tagala and Curcuma caesia exert anticancer effect by Tumor Necrosis Factor-α mediated decrease in Nuclear Factor κ B binding activity. J Basic Clin Pharma 7:1–11
Hadisaputri YE, Miyazaki T, Suzuki S, Kubo N, Zuhrotun A (2015) Molecular characterization of antitumor effects of the rhizome extract from Curcuma zedoaria on human esophageal carcinoma cells. Int J Oncol 47:2255–2263
Khaing SL, Omar SZ, Looi CY, Arya A, Mohebali N, Mohd A (2017) Identification of active extracts of Curcuma zedoaria and their real- time cytotoxic activities on ovarian cancer cells and HUVEC cells. Biomed Res 28(18):9182–9187
Shi H, Tan B, Ji G, Lu L, Cao A, Shi S, Xie J (2013) Zedoary oil (Ezhu You) inhibits proliferation of AGS cells. Chin Med 8:13. http://www.cmjournal.org/content/8/1/13
Chen C-C, Chen Y, His Y-T, Chang C-S, Huang L-F, Ho C-T, Way T-D, Kao J-Y (2013) Chemical constituents and anticancer activity of Curcuma zedoaria Roscoe essential oil against non-small cell lung carcinoma cells in-vitro and in-vivo. J Agric Food Chem 61:11418–11427
Chen W, Lu Y, Gao M, Wu J, Wang A, Shi R (2011) Anti-angiogenesis effect of essential oil from Curcuma zedoaria in-vitro and in-vivo. J Ethnopharmacol 133:220–226
Choi M, Kim SH, Chung W, Hwang J, Park K (2005) Xanthorrhizol, a natural sesquiterpenoid from Curcuma xanthorrhiza, has an anti-metastatic potential in experimental mouse lung metastasis model. Biochem Biophys Res Commun 326:210–217
Cheah YH, Nordin FJ, Tee TT, Azimahtol HL, Abdullah NR, Ismail Z (2008) Antiproliferative property and apoptotic effect of Xanthorrhizol on MDA-MB-231 breast cancer cells. Antican Res 28:3677–3690
Kang Y, Park K, Chung W, Hwang J, Lee SK (2009) Xanthorrhizol, a natural sesquiterpenoid , induces apoptosis and growth arrest in HCT116 human colon cancer cells. J Pharmacol Sci 111:276–284
Kim JY, An JM, Chung W, Park K, Hwang JK, Kim DS, Seo SR, Seo JT (2012) Xanthorrhizol induces apoptosis through ROS- mediated MAPK activation in human oral squamous cell carcinoma cells and inhibits DMBA-induced oral carcinogenesis in Hamsters. Phytother Res 27:493–498
Rouhollahi E, Zorofchian Moghadamtousi S, Paydar M, Fadaeinasab M, Zahedifard M, Hajrezaie M, Abdalla Ahmed Hamdi O, Yeng Looi C, Ameen Abdulla M, Awang K, Mohamed Z (2015) Inhibitory effect of Curcuma purpurascens BI. rhizome on HT-29 colon cancer cells through mitochondrial-dependent apoptosis pathway. BMC Complement Altern Med 15:15. https://doi.org/10.1186/s12906-015-0534-6
Hong SL, Lee GS, Syed Abdul Rahman SN, Ahmed Hamdi OA, Awang K, Aznam Nugroho N, Abd Malek SN (2014) Essential oil content of the rhizome of Curcuma purpurascens Bl. (Temu Tis) and its antiproliferative effect on selected human carcinoma cell lines. Sci World J:397430. https://doi.org/10.1155/2014/397430
Soumya T, Lakshmipriya T, Klika KD, Jayasree PR, Manish Kumar PR (2021) Anticancer potential of rhizome extract and a labdane diterpenoid from Curcuma mutabilis plant endemic to Western Ghats of India. Sci Rep 11:552. https://doi.org/10.1038/s41598-020-79414-8
Zhang L, Yang Z, Huang Z, Zhao M, Li P, Zhou W, Zhang K, Zheng X, Lin L, Tang J, Fang Y, Du Z (2017) Variation in essential oil and bioactive compounds of Curcuma kwangsiensis collected from natural habitats. Chem Biodivers 14:e1700020. https://doi.org/10.1002/cbdv.201700020
Akimoto M, Iizuka M, Kanematsu R, Yoshida M, Takenaga K (2015) Anticancer effect of Ginger extract against pancreatic cancer cells mainly through reactive oxygen species-mediated autotic cell death. PLoS ONE 10(5):e0126605. https://doi.org/10.1371/journal.pone.0126605
Qi L, Zhang Z, Zhang C, Anderson S, Liu Q, Yuan C, Wang C (2015) Anti-colon cancer effects of 6-Shogaol through G2/M cell cycle arrest by p53/p21-cdc2/cdc25A crosstalk. Am J Chin Med 43(4):743–756
Liu Q, Peng Y, Qi L, Cheng X, Xu X, Liu L, Liu E, Li P (2012) The cytotoxicity mechanism of 6-Shogaol-treated HeLa human cervical cancer cells revealed by label-free shotgun proteomics and bioinformatics analysis. Evidence-Based Complement Altern Med. https://doi.org/10.1155/2012/278652
Radhakrishnan EK, Bava SV, Narayanan SS, Nath LR, Thulasidasan AKT, Soniya EV, Ruby JA (2014) Prevents PMA-induced proliferation in colon cancer cells by inhibiting MAPK / AP-1 signaling. PLOS ONE 9(8):e104401. https://doi.org/10.1371/journal.pone.0104401
Nazim U, Jeong J, Seol J, Hur J, Eo S, Lee J, Park S (2015) Inhibition of the autophagy flux by Gingerol enhances TRAIL-induced tumor cell death. Oncol Reports 33:2331–2336
Park GH, Park JH, Song HM, Eo HJ, Kim MK, Lee JW, Lee MH, Cho K, Lee JR, Cho HJ, Jeong JB (2014) Anti-cancer activity of Ginger (Zingiber officinale) leaf through the expression of activating transcription factor 3 in human colorectal cancer cells. BMC Complement Altern Med 14:408. http://www.biomedcentral.com/1472-6882/14/408
Rashid RA, Pihie AHL (2005) The antiproliferative effect of Zingiber zerumbet extracts and fractions on the growth of human breast carcinoma cell lines. Malay J Pharm Sci 3(1):45–52
Rajan I, Jayasree PR, Kumar PRM (2015) Zerumbone induces mitochondria-mediated apoptosis via increased calcium, generation of reactive oxygen species and upregulation of soluble histone H2AX in K562 chronic myelogenous leukemia cells. Tumor Biol 36(11):8479–8489
Prasannan R, Kalesh KA, Shanmugam MK, Nachiyappan A, Ramachandran L, Nguyen AH, Prem A, Lakshmanan M, Seok K, Sethi G (2012) Key cell signaling pathways modulated by Zerumbone: role in the prevention and treatment of cancer. Biochem Pharmacol 84:1268–1276
Koga AY, Beltrame FL, Pereira AV (2016) Several aspects of Zingiber zerumbet: a review. Braz J Pharmacogn 26:385–391
Zulkhairi AM, Aspollah SM, Lian EGC, Bustamam AA (2017) Phytochemicals and cytotoxic studies of Zingiber cassumunar phytochemicals and cytotoxic studies of Zingiber cassumunar Roxb. J Trop Agric Food Sci 45(2):187–197
Majdalawieh AF, Carr RI (2010) In-vitro Investigation of the potential immunomodulatory and anti-cancer activities of Black Pepper (Piper nigrum) and Cardamom (Elettaria cardamomum). J Med Food 13(2):371–381
Ray A, Jena S, Dash B, Sahoo A, Kar B, PatnaikJ PPC, Nayak S, Mahapatra N (2019) Hedychium coronarium extract arrests cell cycle progression, induces apoptosis, and impairs migration and invasion in HeLa cervical cancer cells. Cancer Manage Res 11:483–500
Endringer DC, Taveira FSN, Kondratyuk TP, Pezzuto JM, Bragaa FC (2014) Cancer chemoprevention activity of labdane diterpenes from rhizomes of Hedychium coronarium. Braz J Pharmacogn 24:408–412
Zhana Z-J, Wena Y-T, Rena F-Y, Raob G-W, Shana W-G, Li C-P (2012) Diterpenoids and a diarylheptanoid from Hedychium coronarium with significant anti-angiogenic and cytotoxic activities. Chem Biodivers 9:2754–2760
Chen J, Hsieh M-C, Lin S, Lin C, Hsi Y-T, Lo Y-S, Chuang Y-C, Hsieh M-J, Chen MK (2017) Coronarin D induces reactive oxygen species-mediated cell death in human nasopharyngeal cancer cells through inhibition of p38 MAPK and activation of JNK. Oncotarget 8(64):108006–108019
Lin H, Hsieh M, Hsieh Y, Yeh C, Hsueh K, Yang S (2018) Coronarin D induces apoptotic cell death through the JNK pathway in human hepatocellular carcinoma. Environ Toxicol 33(9):946–954
Reddy PP, Ranga Rao R, Shashidhar J, Sastry BS, Madhusudana Rao J, Suresh Babu K (2009) Phytochemical investigation of labdane diterpenes from the rhizomes of Hedychium spicatum and their cytotoxic activity. Bioorg Med Chem Lett 19:6078–6081
Suresh G, Poornima B, Babu KS, Yadav PA, Rao MSA, Siva B, Prasad KR, Nayak VL, Ramakrishna S (2013) Cytotoxic sesquiterpenes from Hedychium spicatum: isolation, structure elucidation and structure-activity relationship studies. Fitoterapia 86:100–107
Mishra T, Pal M, Meena S, Datta D, Dixit P, Kumar A, Meena B, Rana TS, Upreti DK (2016) Composition and in-vitro cytotoxic activities of essential oil of Hedychium spicatum from different geographical regions of western Himalaya by principal components analysis. Nat Prod Res 30:1224–1227
Jing LJ, Bakar MFA, Mohamed M, Rahmat A (2011) Effects of selected Boesenbergia species on the proliferation of several cancer cell lines. J Pharmacol Toxicol 6:272–282
Kirana C, Jones GP, Record IR, McIntosh GH (2007) Anticancer properties of panduratin A isolated from Boesenbergia pandurata (Zingiberaceae). J Nat Med 61:131–137. https://doi.org/10.1007/s11418-006-0100-0
Isa NM, Abdelwahab SI, Mohan S, Abdul AB, Sukari MA, Taha MME, Syam S, Narrima P, Cheah SC, Ahmad S, Mustafa MR (2012) In vitro anti-inflammatory, cytotoxic and antioxidant activities of boesenbergin A, a chalcone isolated from Boesenbergia rotunda (L.) (fingerroot). Braz J Med Biol Res 45(6):524–530. https://doi.org/10.1590/S0100-879X2012007500022
Break MKB, Chiang M, Wiart C, Chin C-F, Khoo ASB, Khoo T-J (2020) Cytotoxic Activity of Boesenbergiarotunda Extracts against nasopharyngeal carcinoma cells (HK1). Cardamonin, a Boesenbergiarotunda constituent, inhibits growth and migration of HK1 cells by inducing caspase-dependent apoptosis and G2/M–Phase arrest. Nutr Cancer. https://doi.org/10.1080/01635581.2020.1751217
Ma XN, Xie CL, Miao Z, Yang Q, Yang XW (2017) An overview of chemical constituents from Alpinia species in the last six decades. RSC Adv 7:14114–14144
Sok SPM, Arshad NM, Azmi MN, Awang K, Ozpolat B, Nagoor NH (2017) The apoptotic effect of 1′S-1′-Acetoxychavicol Acetate (ACA) enhanced by inhibition of non-canonical autophagy in human non-small cell lung cancer cells. PLoS One 12(2):e0171329. https://doi.org/10.1371/journal.pone.0171329
Ito K, Nakazato T, Xian MJ, Yamada T, Hozumi N, Murakami A, Ohigashi H, Ikeda Y, Kizaki M (2005) 1′-acetoxychavicol acetate is a novel nuclear factor κB inhibitor with significant activity against multiple myeloma in vitro and in vivo. Cancer Res 65:4417–4424
In LLA, Arshad NM, Ibrahim H, Azmi MN, Awang K, Nagoor NH (2012) 1’-Acetoxychavicol acetate inhibits growth of human oral carcinoma xenograft in mice and potentiates cisplatin effect via proinflammatory microenvironment alterations. BMC Complement Altern Med 12:179. http://www.biomedcentral.com/1472-6882/12/179
Huang H, Chen AY, Ye X, Guan R, Rankin GO, Chen YC (2020) Galangin, a flavonoid from lesser galangal, induced apoptosis via p53-dependent pathway in ovarian cancer cells. Molecules 25:1579. https://doi.org/10.3390/molecules25071579
Kim Y, Ko H, Park J, Han I, Amor EC, Wha J, Ok H (2010) International immunopharmacology Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking NF-κB p65 activation. Int Immunopharmacol 10:1127–1134
Liao Q, Shi DH, Zheng W, Xu XJ, Yu YH (2010) Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur J Pharmacol 641:179–186. https://doi.org/10.1016/j.ejphar.2010.05.024
Machana S, Weerapreeyakul N, Barusrux S, Nonpunya A, Sripanidkulchai B, Thitimetharoch T (2011) Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma. Chin Med 6:39. http://www.cmjournal.org/content/6/1/39
Patanasethanont D, Nagai J, Matsuura C, Fukui K, Sutthanut K, Sripanidkulchai B, Yumoto R, Takano M (2007) Modulation of function of multidrug resistance associated-proteins by Kaempferia parviflora extracts and their components. Euro J Pharma 566:67–74
Ninomiya K, Chaipech TMS, Katsuyama SMY (2016) Simultaneous quantitative analysis of 12 methoxyflavones with melanogenesis inhibitory activity from the rhizomes of Kaempferia parviflora. J Nat Med 70:179–189
Kuttan R, Bhanumathy P, Nirmala K, George MC (1985) Potential anticancer activity of Turmeric (Curcuma longa). Cancer Lett 29:197–202
Yue GGL, Chan BCL, Hon P, Kennelly EJ, Yeung SK, Cassileth BR, Fung K, Leung P, Lau CBS (2010) Immunostimulatory activities of polysaccharide extract isolated from Curcuma longa. Int J Biol Macromol. 47:342–347
Park JH, Park KK, Kim MJ, Hwang JK, Park SK, Chung WY (2008) Cancer chemoprotective effects of Curcuma xanthorrhiza. Phytother Res 22:695–698
Chung WY, Park JH, Kim MJ, Kim HO, Hwang JK, Lee SK, Park KK (2007) Xanthorrhizol inhibits 12- O-tetradecanoylphorbol-13-acetate-induced acute inflammation and two-stage mouse skin carcinogenesis by blocking the expression of ornithine decarboxylase , cyclooxygenase-2 and inducible nitric oxide synthase through mitogen-activated protein kinases and / or the nuclear factor- kB. Carcinogenesis 28:1224–1231
Oon SF, Nallappan M, Tee TT, Shohaimi S, Kassim NK, Sa’ariwijaya MSF, Cheah YH (2015) Xanthorrhizol: a review of its pharmacological activities and anticancer properties. Cancer Cell Int 15:100. https://doi.org/10.1186/s12935-015-0255-4
Xiang H, Zhang L, Xi L, Yang Y, Wang X, Lei D, Zheng X, Liu X (2018) Phytochemical profiles and bioactivities of essential oils extracted from seven Curcuma herbs. Ind Crops Prod 111:298–305
Soumya T, Jayasree PR, Deepak M, Manish Kumar PR (2019) Chemical composition, antioxidant and antiproliferative activities of essential oil from rhizome and leaves of Curcuma mutabilis Škorničk., M. Sabu & Prasanthk., endemic to Western Ghats of India. Nat Prod Res 34(16):2336–2340. https://doi.org/10.1080/14786419.2018.1533826
Itokawa H, Shi Q, Akiyama T, Morris-natschke SL, Lee K (2008) Recent advances in the investigation of curcuminoids. Chin Med 3:11. https://doi.org/10.1186/1749-8546-3-11
Hatcher H, Planalp R, Chob J, Tortia FM, Torti SV (2008) Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci 65:1631–1652
Wilken R, Veena MS, Wang MB, Srivatsan ES (2011) Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Can 10:12. http://www.molecular-cancer.com/content/10/1/12.
Gupta SC, Patchva S, Koh W, Aggarwal BB (2012) Discovery of Curcumin, a component of golden spice, and its miraculous biological activities. Clini Exp Pharmacol Physiol 39:283–299
Amalraj A, Pius A, Sreerag G, Sreeraj G (2017) Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives - A review. J Trad Chinese Med Sci 7:205–233
Collett GP, Robson CN, Mathers JC, Campbell FC (2001) Curcumin modifies Apcmin apoptosis resistance and inhibits 2-amino 1-methyl-6-phenylimidazo [4,5-b] pyridine (PhIP) induced tumour formation in Apcmin mice. Carcinogenesis 22:821–825
Hsu T-C, Young MR, Cmarik J, Colburn NH (2000) Activator protein 1 (AP-1) and Nuclear Factor κB (NF-κB) – dependent transcriptional events in carcinogenesis. Free Radical Biol Med 28(9):1338–1348
Kunnumakkara AB, Diagaradjane P, Anand P, Kuzhuvelil HB, Deorukhkar A, Gelovani J, Guha S, Krishnan S, Aggarwal BB (2009) Curcumin sensitizes human colorectal cancer to Capecitabine by modulation of cyclin D1, COX-2, MMP-9, VEGF and CXCR4 expression in an orthotopic mouse model. Int J Cancer 125:2187–2197
Bush JA, Cheung KJ, Li G (2001) Curcumin induces apoptosis in human melanoma cells through a Fas receptor/Caspase-8 pathway independent of p53. Exper Cell Res 271:305–314
Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB (2007) Evidence that curcumin suppresses the growth of malignant gliomas in-vitro and in-vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol 72:29–39
Gururaj AE, Belakavadi M, Venkatesh DA, Marm D, Salimath BP (2002) Molecular mechanisms of anti-angiogenic effect of Curcumin. Biochem Biophysi Res Commun 297:934–942
Yodkeeree S, Ampasavate C, Sung B, Aggarwal BB, Limtrakul P (2010) Demethoxycurcumin suppresses migration and invasion of MDA-MB-231 human breast cancer cell line. Eur J Pharmacol 627:8–15
Beevers CS, Chen L, Liu L, Luo Y, Webster NJG (2009) Curcumin disrupts the mammalian target of rapamycin- raptor complex. Cancer Res 69(3):1000–1009
Sordillo PP, Helson L (2015) Curcumin and cancer stem cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer Res 35:599–614
Teiten M-H, Dicato M, Diederich M (2013) Curcumin as a regulator of epigenetic events. Mol Nutr Food Res 57:1–11. https://doi.org/10.1002/mnfr.201300201
Allegra A, Innao V, Russo S, Gerace D, Alonci A, Musolino C (2016) Anticancer activity of Curcumin and its analogues: Preclinical and clinical studies. Cancer Invest. https://doi.org/10.1080/07357907.2016.1247166
Narayanan NK, Nargi D, Randolph C, Narayanan BA (2009) Liposome encapsulation of Curcumin and Resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 125:1–8
Liu Y, Wu Y, Yu Y, Cao C, Zhang J, Li K, Zhang P (2014) Curcumin and Resveratrol in combination modulate drug-metabolizing enzymes as well as antioxidant indices during lung carcinogenesis in mice. Human Exp Toxicol. https://doi.org/10.1177/0960327114551396
Zeng X, Cai D, Zeng Q, Chen Z, Zhong G, Zhuo J, Gan H, Huang X, Zhao Z, Yao N, Huang D, Zhang C, Sun D, Chen Y (2017) Selective reduction in the expression of UGTs and SULTs, a novel mechanism by which Piperine enhances the bioavailability of Curcumin in rat. Biopharm Drug Dispos 38(1):3–19
Siddique RA, Harvey KA, Walker C, Altenburg J, Xu Z, Terry C, Camarillo I, Jones-hall Y, Mariash C (2013) Characterization of synergistic anti-cancer effects of docosahexaenoic acid and curcumin on DMBA-induced mammary tumorigenesis in mice. BMC Cancer 13:418. http://www.biomedcentral.com/1471-2407/13/418
Bordoloi D, Kunnumakkara AB (2018) Chapter 2 - The potential of Curcumin: a multitargeting agent in cancer cell chemosensitization. In: Role of nutraceuticals in cancer chemosensitization. Vol 2, pp. 31-60. Academic Press, Elsevier Inc.
Yallapu MM, Jaggi M, Chauhan SC (2012) Curcumin nanoformulations: a future nanomedicine for cancer. Drug Discov Today 17:71–80
Panda AK, Chakraborty D, Sarkar I, Khan T, Gaurisankar SA (2017) New insights into therapeutic activity and anticancer properties of Curcumin. J Exp Pharm 9:31–45
Habib SHM, Makpol S, Aini N, Hamid A, Das S, Ngah WZW, Yusof YAM (2008) Ginger extract (Zingiber officinale) has anti-cancer and anti-inflammatory effects on ethionine-induced hepatoma rats. Clinics 63(6):807–813
Cheng XL, Liu Q, Peng YB, Qi LW, Li P (2011) Steamed ginger (Zingiber officinale): changed chemical profile and increased anticancer potential. Food Chem 129:1785–1792
Ghasemzadeh A, Jaafar HZE, Rahmat A (2015) Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med 15:258. https://doi.org/10.1186/s12906-015-0718-0
Elguindy NM, Yacout GA, El-Azab EF, Maghraby HK (2016) Effect of Elettaria cardamomum against chemically induced hepatocellular carcinoma in rats by inhibiting NF-κB, oxidative stress, and activity of ornithine decarboxylase. South African J Bot 105:251–258
Bailly C (2020) Anticancer activities and mechanism of action of the labdane diterpene coronarin D. Pathol Res Pract 216:152946. https://doi.org/10.1016/j.prp.2020.152946
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Soumya, T., Jayasree, P.R., Manish Kumar, P.R. (2023). Zingiberaceae Plants: A Cornucopia of Promising Chemotherapeuticals for Cancer Cure. In: Arunachalam, K., Yang, X., Puthanpura Sasidharan, S. (eds) Bioprospecting of Tropical Medicinal Plants. Springer, Cham. https://doi.org/10.1007/978-3-031-28780-0_16
Download citation
DOI: https://doi.org/10.1007/978-3-031-28780-0_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28779-4
Online ISBN: 978-3-031-28780-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)