Abstract
The world faces many challenges to keep pace with progress in this era. Enzymes are the mainstay in various application fields conducive to this progress. Enzymes are proteins that catalyze different reactions converting substrates to new products. While animals, plants and microbes produce them, fungi are characterized by their higher potency of enzymes production. Other important features of fungal enzymes are the more stability and more retaining of their activity, which allows their application. This chapter emphasizes on the many studies that are directed towards increasing fungal enzymes production with lower costs by developing the processes of fermentation, and improving the recombinant DNA and enzymes engineering technologies. Further insights into the most important applications in different disciplines are given. Applications of fungal enzymes can satisfy the industrial needs, the medicinal demands, and the environmental requests. For instance, among the current applications of fungal enzymes is the fuel industries as ethanol production and fungal microbial fuel cells, and in paper, leather and detergents manufacturing. They can be utilized in food industries as cheese and bread making, in beverages as wine and juices, and flavoring. Moreover, they are used in the biomedical field for their antiviral, antibacterial, antifungal and antitumor activities, and in treatment of neurological disorders. Furthermore, in the environmental field, they are used to degrade keratinaceous wastes, organic pollutants as diesel oil hydrocarbons, herbicides, insecticides, naphthalene and anthracene, decolorize dyes and detoxify ink.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Fungal enzymes
- Production
- Purification
- Industrial applications
- Biomedical applications
- Environmental applications
1 Introduction
In light of the many developments that are taking place around us, we cannot but always search for what is new to keep pace with this tremendous development of the era. Meanwhile, it is clear that enzymes play a pivotal role in many applications that keep pace with such enormous progress. There is always a rising demand for enzymes production to satisfy the need for them.
In general, enzymes are proteins in nature. All living organisms produce them to catalyze specific reactions. Enzymes are manipulated in fields of industry, medicine and environment. Enzymatic processes are more advantageous when compared with the conventional chemical ones. This appears in the gentler reaction conditions and the more advanced specificity which led to higher production of desired products and less production of byproducts (de Souza et al. 2020), moreover more efficient and cleaner processes thus contributing to the sustainable growth concept (Dhevagi et al. 2021).
While all living organisms are capable of producing enzymes, it is noticed that animals and plants cannot satisfy the industrial demands. This drew the attentions towards microbial enzymes (Guerrand 2018). Microbial enzymes can be produced at much higher rates. They are also cost-effective, scalable and more genetically compliant (Singh et al. 2019). Regarding fungal enzymes, they are more stable and more retaining of their activity (Verma et al. 2020). Fungal enzymes show higher production potency, easier purification steps, especially in case of filamentous fungi. Furthermore, since ancient times, fungi have been utilized for different purposes such as baking and brewing. From this perspective, fungi can be considered safe and thus justifying the continuity of their recent use in more than half of commercial enzymes. For all of the previous fungal enzymes are of more significance in various application fields (Kango et al. 2019). Some species belonging to genera of Aspergillus, Penicillium, Rhizopus and Trichoderma, and recently mushroom are fulfilling the enzyme market requirements. The rapid growth of this market led to continuous attempts to find novel enzymes producers satisfying the industrial characteristics (Kumla et al. 2020).
According to their mode of nutrition, fungi are considered chemo-organo-heterotrophs getting their nutrients via the breakdown of extracellular organic matter. They could be parasites if the source of organic matter used is from a living host or they could be saprophytes if the source is dead organic matter. (Devi et al. 2020; Suman et al. 2015). In either way, fungi produce an array of hydrolytic (glycolytic, proteolytic and lipolytic) and oxidative enzymes to breakdown the complex organic matters forming simple ones (Kour et al. 2019). Fungal enzymes are mainly produced during the log phase of growth. Extracellular enzymes are secreted to the outside of the cell for digestion of complex nutrients prior to being absorbed within the cells, then endocellular enzymes (found inside the cells) further assimilate the absorbed nutrients (Dhevagi et al. 2021). Extracellular enzymes could also participate in protection of fungi against the naturally existing hazardous materials or those resulting from substrates hydrolysis (Verma et al. 2020). Enzymes are classified where an enzyme could belong to a hydrolase, lyase, oxidoreductase, translocase, transferase, ligase or an isomerase group (Jeske et al. 2019). Hydrolases and oxidoreductases are the most commercially valuable fungal enzymes (Berbee et al. 2017).
There is a variety of enzymes secreted by fungi namely amylases, xylanases, cellulases, lipases, proteases, peroxidases, catalases and laccases (Marco et al. 2013). It is always desirable to use enzymes instead of corrosive chemicals to perform specific functions at the ambient temperatures. Fungal enzymes when purified, their application could be expensive due to the number of phases in the purification process. Nevertheless, their employment could be cost-effective if recyclable biocatalysts are utilized (Godfrey and Reichelt 1996; Gianfreda and Rao 2004).
This chapter discusses the production and purification of fungal enzymes with emphasis on their recent biotechnological applications. Such applications will be outlined in the industrial, biomedical and environmental fields.
2 Production of Fungal Enzymes
Large-scale production of enzymes was developed through the numerous researches conducted in the recent period using specific strains. Studies are concerned with developing fermentation processes, recombinant DNA cloning and enzymes engineering, introducing them to many application fields (Gurung et al. 2013).
2.1 Optimization of Medium
Economically important compounds with applications in various fields are produced by fermentation technology (Dubey et al. 2008). Studies for medium optimization are performed to enhance production of the desired yield. Many investigations were concerned with the microbial nutritional requirements for enhancing metabolites (e.g. enzymes) production (Shih et al. 2002; Singh et al. 2012). It must be taken into consideration that medium optimization should fulfil minimal microbial growth to obtain maximum production of the desired metabolite. This is to have maximum efficiency and minimum cost and wastes thus competing the traditional methods (Singh et al. 2017).
Various strategies are proposed for designing and optimizing the medium for highest efficiency for production. In the classical experimental technique for fermentation medium optimization, the one-factor-at-a-time (OFAT), one factor is changed at each experiment while the other factors are kept constant. Then the concentrations of each selected medium component is varied over a tested range. The OFAT is easy and convenient. Hence, many researchers prefer it (Gonzalez et al. 1995) and they still follow this method (Singh et al. 2017). On the other hand, using the statistical design of experiments (DOE) technique for optimizing the fermentation medium can overcome some of the limitations of the OFAT technique. In 1992, Fisher proposed the theory of experimental design. This theory describes that varying more than one of the medium factors at a time is more efficient than varying only one factor at a time (Fisher 1992).
Optimization of fermentation medium reached new dimensions with the advancements in the statistical methodology. There were improvements in the process efficiency, reduction in experimental time and cost, consequently contributing in the process economics. The microbial process is biological in nature containing relatively many natural variables. Microbial reactions are associated together in a complex network, where several factors influencing different parts in this network. Applying the rational experimental design statistically evaluating the results, leads to increasing the reliability of the obtained experimental data. Furthermore, using the experimental design reduces the number of experiments needed for obtaining reliable data (Elibol 2004).
The experimental design is considered a study plan for achieving certain objectives. Experiments have to be well-planned and the sample size should be enough for obtaining sufficient data so as to answer the objectives of the study. In the full factorial design, all factors, e.g. strain, medium constituents, temperature, pH etc. are studied. Meanwhile, in the partial factorial one, a few number of factors are chosen to be tested, which is usually done if the full factorial design cannot be applied due to little availability of knowledge about all the interactions of medium constituents (Singh et al. 2017).
Since not all medium constituents contribute in the production of the desired product, then the unimportant factors should be removed from the study. R.L. Plackett and J.P. Burman issued their study in 1946 about designing optimal factorial experiments to precisely set and select the major effects in any process. This is the Placket-Burman Design (PBD), which is a two-level design. It is economically useful in finding the main effectors when assuming that other interacting effects are negligible, to compare the important ones. In other words, the effect of a factor will be superior or will be underestimated if there are no interactions (Vaidya et al. 2003).
2.2 Genetic Approaches
Genetic engineering (transcriptomics, proteomics and designing recombinant strains) is used in analyzing and improving enzymes production with least alterations in strains genome (Meyer et al. 2010; Liu et al. 2013). For instance, in Saccharomyces cerevisiae, overexpression of several transcription factors (TFs) resulted in enhancing TF target genes expression, whether under inducing or non-inducing effects (Chua et al. 2006). While in Neurospora crassa, inducer-independent cellulases production was accomplished by the constitutive overexpression of clr-2 via the ccg-1 promoter (Coradetti et al. 2013). Aspergillus tamarii was subjected to Illumina RNA-seq transcriptome profiling to identify genes responsible for encoding proteins managing plant biomass degradation. There were 209 CAZyme (carbohydrate-active enzyme) genes identified. Another five genes belonging to AA9 (GH61) family and related to LPMO (lytic polysaccharide monooxygenase) were identified. It was noticed that there was up-regulation of transcription factor gene XlnR, responsible for hemicellulases induction, and ClrA gene, involved in regulating cellulases, as well as more than 150 transporter genes (Midorikawa et al. 2018). In Aspergillus niger, it was found that overexpression of gaaR via A. nidulans promotor, gpdA, lead to the constitutive expression of genes responsible for encoding pectinases (Alazi et al. 2018). Another study revealed that in order to achieve stable and safe cellulase gene (sestc) expression, clustered regularly interspaced short palindromic repeats-Cas9 (CRISPR-Cas9) approach was applied to integrate the sestc expression cassette, which contains Agaricus biporus gpd (glyceraldehyde-3-phosphate-dehydrogenase) gene promoter, in the chromosome of Saccharomyces cerevisiae. Ethanol production showed 37.7-fold increase in the engineered S. cerevisiae strain compared with the wild type (Yang et al. 2018).
Properties of fungal enzymes can be improved through protein engineering (Ribeiro and Ribeiro 2013). Work began in the field of protein engineering in the eighties of the last century. Protein engineering is concerned with constructing proteins that are modified via site directed mutagenesis. Researches extended to study the catalytic mechanisms of enzymes and the relationship between their structure and function (Brannigan and Wilkinson 2002). By conducting advanced gene manipulation techniques, the proteins macromolecular structure can be changed to allow the manipulation of the enzymes target functions (Fan et al. 2009).
Site directed mutagenesis is a traditional technique of rational enzyme designing. It is used for evaluating the impact of a certain amino acid or more on the characteristics of the studied enzyme. The thermostable endoglucanase of Humicola grisea Cel12A showed three uncommon free cysteines. These were Cys175, Cys206 and Cys216. They were used to construct mutants by site directed mutagenesis. The study demonstrated that these cysteines have a role in enzyme stability (Sandgren et al. 2005).
Another type of rational approaches is creation of multifunctional, chimera, enzymes. Such strategy aids in reducing costs when enzymes are economically used. When enzymes are engineered to have multi-domains along a single polypeptide chain, this would simplify production and purification processes. A natural linker was used to fuse a domain having laccase activity and obtained from Pycnoporus cinnabarinus with an Aspergillus niger CBM1 domain (Ravalason et al. 2009). The CBM1 domain is responsible for connection to molecules of cellulose, while the laccase domain manages lignin degradation around cellulose with good end results when applied in pulp and paper industries (Ibarra et al. 2006).
In the directed evolution approach, protein engineering employs the natural selection basis for the creation of novel characteristics of proteins and RNAs. Molecular diversity is generated here by random mutations using selective pressure. Survivors to these pressures are selected (Otten and Quax 2005). An example of the random mutagenesis methods is the error occurring in a PCR (polymerase chain reaction), where there is a controllable mis-incorporation of bases during amplification of genes (Cadwell and Joyce 1994). Another method for random mutagenesis is EP-RCA that employs rolling circle amplification (RCA) (Fujii et al. 2004). EP-RCA was used in the small DNA which encodes glucoamylase signal peptide in a recombinant Saccharomyces cerevisiae. This DNA was circularized and then it served as an EP-RCA template (Luhe et al. 2010). The technique of DNA shuffling was developed for random recombination mimicking natural evolution (Stemmer 1994a, b). An example for DNA shuffling technique is the staggered extension process (StEP). For instance, GOase (galactose oxidase) was obtained from Fusarium and then it was evolved by StEP and expressed in functional form inside E. coli. The evolved enzymes showed same substrate specificity and activity but showed more thermostbility and higher expression levels compared with native fungal oxidase (Sun et al. 2001).
In the semi-rational design, semi-rational mutagenesis can be considered a combination of directed and random mutagenesis. Here, hot spots can be defined by the structural or the functional information, which are then randomized for all the amino acids. This is to enhance the enzyme activity or change substrate specificity or the enantioselectivity mutations found close to the active site which are more important than those found on the enzyme surface (Bornscheuer and Pohl 2001). In this relation, an up-shift took place in the optimum pH of Trichoderma reesei endoglucanase II variants which occurred when a library was constructed by strand overlap extension (SOE) saturation mutagenesis technique (Qin et al. 2008). Another strategy with less number of cloning steps than SOE is in vivo overlap extension (IVOE) which was explored in ascomycetes and basidiomycetes (Mate et al. 2011). The Pycnoporus cinnabarinus laccase activity was 8000-fold increased using the error-prone PCR technique together with the in vivo shuffling and also the IVOE site directed mutation and recombination (Camarero et al. 2012).
3 Purification of Fungal Enzymes
It has become pivotal that researchers should try to find new methods replacing the traditional ones for fungal enzymes recovery and purification (Polizeli et al. 1991). We also cannot ignore that it is necessary to investigate the biochemical characteristics and the correlation between the structure and function of the purified enzyme (Gupta et al. 2003). Furthermore, its purity as well as its molecular weight are usually examined by SDS-PAGE (Patil and Chaudhari 2010).
Various procedures are employed for fungal enzymes purification. The purification process usually starts with precipitating proteins found in crude enzyme extract to concentrate them. In this step, ammonium sulfate or an organic solvent such as ethanol or acetone can be used. Next steps include dialysis and chromatographic techniques such as ion exchange or gel filtration (Kiiskinen et al. 2004). If the organic solvent step is only applied, where it is tested in different percentages to separate different protein types, the obtained precipitated protein in this case is only partially purified (Kumarevel et al. 2005; Yadav et al. 2019).
The liquid-liquid extraction technique surpasses the traditional ones in that several early stages can be cut short. This technique is based on the fact that when immiscible liquids are brought together, molecules transfer from phase to another. For instance, the ATPS (acqeuous two-phase system) technique avoids organic solvents use, but molecules are separated between two phases whether it is salt/salt or polymer/salt or polymer/polymer immiscible aqueous phases (Albertsson 1958). The ATPS method is preferred in extracting enzymes since high amounts of water are present (Freire et al. 2012), lower cost than when utilizing chromatography, more environmentally friendly and can be scaled up to reach higher purification folds (Naganagouda and Mulimani 2008; Schwienheer et al. 2015). In this relation, Penicillium candidum protease was purified using a system of PEG and sodium citrate and it was amended with sodium chloride to enrich the salt phase thus increasing the purification level (Alhelli et al. 2016).
On the other hand, the emerging TPP (three-phase partitioning) technique is developed for proteins, especially enzymes, extraction. It is characterized by high potential for concentrating proteins from multi-component crude broths and exhibiting higher purification levels compared with conventional methods of protein concentration (Gagaoua and Hafid 2016). The basis of this rising tool is combining the crude protein extract with a solid salt, ammonium sulfate, and organic solvent (e.g. butanol) for obtaining three phases (Ketnawa et al. 2017). The major draw-back here is using an organic solvent, which limits the large-scale utilization of this technique (Alvarez-Guerra et al. 2014) since enzymes activity is reduced in presence of organic solvents (Ketnawa et al. 2017). However, butanol delivered a 7.2-fold of purity and a 184-recovery percentage for a laccase obtained from Pleurotus ostreatus (Kumar et al. 2011).
4 Recent Applications of Fungal Enzymes
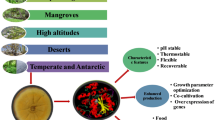
In this section, different fungal enzymes will be reviewed while elucidating their progress in industrial, biomedical and environmental fields (Fig. 1).
4.1 Industrial Applications
Fungal enzymes are widely used in various industrial applications (Table 1). In the biofuel field, Rice straw was used for the production of cellulase-hemicellulase consortium by Aspergillus niger P-19. This enzyme preparation caused saccharification (70 g/L reducing sugars) of rice straw pretreated with 0.25 N NaOH. Fermentation of reducing C6 sugars yielded 15.6 g/L ethanol, with possibility of increasing the yield by targeting C5 sugars (Kaur et al. 2020). On the other hand, the enzymes hydrolysate (lignin peroxidase, manganese peroxidase, cellulase, xylanase) obtained from Pycnoporus sanguineus MCA 16 achieved saccharification of sugarcane bagasse. This hydrolysate was utilized for the production of ethanol by Saccharomyces cerevisiae CAT-1 (Scarpa et al. 2019).
As for microbial fuel cells (MFCs), their performance is influenced by microbial growth and metabolism. Fungi are eukaryotic microorganisms characterized by complex cell organization. In fungi, electron transfer occurs in two pathways. While oxidation of the substrate glucose by glycolysis produces two molecules of NADH for each glucose molecule, we find that interaction of mediators as methylene blue with a constituent of the electron transport chain (ETC) results in continuous functioning of the ETC and generation of electrons from Krebs cycle. These two pathways are therefore crucial for the simultaneous electrons providing and waste removal from substrate. Basically, there are two designs for constructing MFCs; single and dual chambers MFCs (Sarma et al. 2021). An example of the single-chambered fungal MFC is the one constructed by Abdallah et al. (2019). In their work, Aspergillus sydowii NYKA 510 was utilized as a cathodic biocatalyst in an MFC, where its oxidoreductases were responsible for performance of the MFC at 2000 Ω, which achieved 160 mWm−2, 0.4 W, 0.76 V as well as 380 mAm−2. A project was designed for a lighting unit that was implemented by using a system of two sets of four MFCs each, and connected in series, to generate electricity. The scanning electron microscope image of the utilized A. sydowii NYKA 510 was used in algorithmic form generation equations to design the lighting unit. On the other hand, Shabani et al. (2021) constructed a dual-chambered fungal MFC with the pure culture of Trichoderma harzianum. Another MFC was constructed with a mixed culture of Trichoderma harzianum and Pseudomonas fluorescens, which were used as bioanodes as sources of oxidoreductases. The MFC recorded a 1.7 mW m−2 power density for the MFC system working with mixed biofilm, while that of the pure fungal biofilm achieved 0.13 mW m−2.
Beside the ability of xylanases to improve the overall utilization of lignocellulosic matters in generation of biofuels and chemicals, they have also attracted much attention in the paper and pulp technological industries. Fungi are broadly used as producers of xylanases more than bacteria. A xylanase was purified from Sclerotium rolfsii with high thermal and pH stabilities making it a good candidate for such industrial applications (Moussa et al. 2014).
Acrylamide has been encountered in some foods that are subjected to heat treatments, e.g. French fries, bread and coffee beans. One of its formation mechanisms is the Maillard reaction, where at highly elevated temperatures the amino group in the amino acid L-asparagine reacts with the carbonyl group in another compound (e.g. reducing sugar). L-asparaginase can be used to hydrolyze L-asparagine to L-aspartic acid and ammonia, thus contributing in decreasing acrylamide generation (Xu et al. 2016). A heterodimer L-asparaginase was purified from Penicillium crustosum NMKA 511 that was highly specific towards L-asparagine. The enzyme reduced the acrylamide levels up to 80.7% and 75.8% for light-roasted coffee beans and dark-roasted ones, respectively (Khalil et al. 2021).
Increasing the daily supply of dietary fibers is of great priority while searching for novel sources and production technologies. Lignocellulosic materials hydrolysis by enzyme cocktails from Aspergillus and Trichoderma could be efficiently improved after Trametes versicolor laccase action. The procured soluble dietary fibers exhibited a 20-fold increase in the antioxidant activity when compared with the untreated (Margetic et al. 2021). The endo- and exopolygalacturonases synergistic action ensures effective pectic substances hydrolysis. Exo-TePG28a and endo-TePG28b polygalacuronases from Talaromyces leycettanus JCM12802 were overexpressed in the yeast Pichia pastoris and it was then characterized. Both enzymes showed high pH (2–7) and thermal (70 °C) stabilities. They caused a 140% pectin degrading efficiency making them worthy to be applied in the juice industry (Li et al. 2017).
In starch industrial applications, such as bread making, α-amylase could be of great value. The α-amylase (AmyA1) gene from the fungus Geomyces pannorum was cloned and expressed in Aspergillus oryzae. The enzyme could increase bread cohesiveness and decrease gumminess. Furthermore, the immobilized AmyA1 enzyme displayed thermal and pH stabilities and reusability (He et al. 2017). On the other hand, in the winemaking industry, β-glucosidase breaks down the glycoside-terpene complexes releasing the terpene groups, which promote wine flavor and quality. The β-glucosidase obtained from Meyerozyma guilliermondii revealed ethanol-glucose tolerance which is important to be applied in final saccharification during winemaking (da Silva et al. 2019).
The lipase from Thermomyces lanuginosus was immobilized. It was then used in the interesterification process of fats procured from camel hump. This is to be a potential analogue of cocoa butter manufacture (Shekarchizadeh and Kadivar 2012). Another immobilized lipase, with high catalytic activity, from Thermomyces lanuginosus was exploited for the synthesis of ethyl valerate which is responsible for the green apple flavoring (Sadighi et al. 2017).
Owing to their high stable activity at acidic pHs and flavor enhancing property, it is becoming popular nowadays to use milk-clotting proteases as substitutes for calf rennin in cheese manufacture (Mamo et al. 2020). The protease secreted by Aspergillus oryzae is considered safe; accordingly, it can be applied as a milk-clotting agent in dairy industries (Kumura et al. 2017). In addition, Pleurotus albidus, the edible mushroom, was utilized as safe and efficient producer of milk-clotting enzyme (Abdel-Rahman et al. 2018).
Other applications of proteases are recognized for the alkaline protease produced by Aspergillus terreus. The purified protease was highly stable at wide temperature and alkaline pH ranges. It was also compatible with surfactants and detergents and exhibited good washing performance. Moreover, it showed a dehairing ability for animal hides without added chemicals thus it could be exploited in the leather industry (Abu-Tahon et al. 2020).
4.2 Biomedical Applications
Different biomedical activities are explored for fungal enzymes that vary between antimicrobial, antitumor, antioxidant, as well as therapeutic (Table 2). Fungal enzymes can cause cell membrane rupturing which results in losing cytoplasmic constituents. Moreover, they can inhibit synthesis of DNA, essential enzymes, or electron transport chain, in addition to blocking receptors of bacteria. This accounts for their antimicrobial activity (Fuglsang et al. 1995).
Chitinases acquired from fungi have potent antifungal activity, which enables their use in biocontrol applications (Le and Yang 2018). In this relation, the chitinase obtained from Trichoderma harzianum caused efficient retardation in growth of the phytopathogenic fungus Botrytis cinerea (Deng et al. 2019). The collagenase from Penicillium aurantiogriseum URM 4622 caused hydrolysis of collagen resulting in formation of peptides having molecular weights less than two kDa. These peptides showed antibacterial activities against Bacillus subtilis, Escherichia coli and Staphylococcus aureus, in addition to an antioxidant activity (Lima et al., 2015). Antioxidant compounds are of great importance since they augment in avoiding oxidative stresses generated by the harmful reactive oxygen species (ROSs). ROSs can cause cell damage by modifying structures of compounds like lipids, proteins and nucleic acids (Aklakur 2016). Fungal enzyme antioxidants can protect against severe actions of ROSs by transforming them into water and molecular oxygen (Rafi et al. 2016). Tyrosinase is a copper-containing monooxygenase, which is involved in the formation of the melanin pigment. The purified tyrosinase obtained from Saccharomyces cerevisiae showed an antioxidant activity. It also caused an increase in the viable count of MFB-4 cell line (normal skin melanocytes) before and after exposure to UV-irradiation indicating the protective and healing actions against UV (Abdel-Rahman et al. 2019).
The enzyme L-phenylalanine ammonia lyase (PAL) was purified from Rhodosporidium toruloides. The enzyme showed remarkable in vitro and in vivo antitumor activities against the cell lines MCF7 (breast cancer) and DU145 (prostate cancer), suggesting their potential application in cancer treatment (Babich et al. 2013). Meanwhile, the ribonuclease (RNase) purified from the fruiting bodies of the mushroom Hohenbuehelia serotina caused inhibition of reverse transcriptase of HIV-1 (human immunodeficiency virus type 1), in addition to decreasing the uptake of [3H-methyl]-thymidine by the leukemia cells L1210 and the lymphoma cells MBL2 (Zhang et al. 2014).
It is noted that normal as well as leukemia cells require asparagine amino acid for their proliferation. However, only normal cells are capable of synthesizing asparagine using asparagine synthetase, while leukemia cells lack this enzyme. Asparaginase administration to ALL (acute lymphoblastic leukemia) patients causes hydrolysis of serum asparagine, consequently, proliferation of leukemic cells will be prohibited (El-Naggar et al. 2014). The endophytic fungus Lasiodiplodia theobromae was used as a source of asparaginase which exhibited a potential to be utilized as a reliable anticancer agent against leukemic cell line M-NFS-60 (Moubasher et al. 2022).
On the other hand, extracellular β-glucosidases, BGL1 and BGL2, were isolated from Aspergillus sp. YDJ216. They presented a potential to be applied in pharmaceutical industries. The flavone glycosides hydrolysis showed an inhibitory action on monoamine oxidase. This suggests their possible application in treating neurological disorders (Oh et al. 2018).
It is worthy to point out that, lectins, non-immunogenic proteins, do not have the catalytic activity of enzymes. However, they can bind without catalysis to certain carbohydrates (Lam and Ng 2011). Fungi are important producers of lectins, where mushrooms constitute for 82% of fungal lectins (Diaz et al. 2011; Varrot et al. 2013). They have various applications regarding their antiproliferative, immune stimulating, antioxidant, antimicrobial and therapeutic potentials. In this regard, a lectin purified from Pleurotus ostreatus SS89 was stable over wide temperature and pH ranges. It showed significant antiproliferative activities towards the colorectal cancer cells HCT and the hepatic cancer cells HepG2. It also exhibited antibacterial activities towards Escherichia Coli, Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus and Streptococcus faecalis (Kamel et al. 2021).
4.3 Environmental Applications
The continuous growth of the world population along with employing environmental resources is offset by an increase in pollution levels of waste materials as well as xenobiotics in the environment, which could be hazardous (Moussa and Khalil 2022). Table 3 depicts some fungal enzymes exploited in the environmental field. In this regard, the alkaline keratinase of Scopulariopsis brevicaulis, obtained from Egyptian black sand, showed hydrolyzing activities towards different keratinaceous waste materials (human hair, human nails, chicken feathers). The highest degrading ability was achieved against chicken feathers (Sharaf and Khalil 2011). Marchut-Mikolajczyk et al. (2015) found that the immobilized enzymes, lipases, laccases and peroxidases of Mucor circinelloides enhanced the biodegradation efficiency of diesel oil hydrocarbons by 20–30%.
The pollutants polycyclic aromatic hydrocarbons (PAHs) are, due to their hydrophobicity, quite resistant to biodegradation (Antizar-Ladislao et al. 2006). They can be chemically, physically or biologically remediated (Wu et al., 2010). Fungal ligninolytic enzymes can degrade PAHs. A potent Aspergillus terreus isolate producing lignin peroxidase and manganese peroxidase, degraded efficiently naphthalene (98.5%) and anthracene (91%) in tested soil models (Ali et al. 2012).
Synthetic dyes are found in the effluents of textile, cosmetics, paper and leather industries (Rezaei et al. 2015). Laccases can decolorize these dyes. For instance, a laccase purified from Aspergillus flavus NG85, a Saint Catherine Protectorate isolate, showed remarkable decolorization efficiencies against different dyes especially malachite green. Moreover, laccase decolorized a real textile effluent (Khalil et al. 2016).
Sustainable energy is meeting today’s demands without jeopardizing the consumption of environmental resources for the future generations. In this context, wastes such as paper wastes can be used for production of bioethanol. However, this approach is obstructed due to presence of ink. A study was conducted where a cellulase from Aspergillus oryzae MDU-4 along with the laccase isozymes of Ganoderma lucidum MDU-7 showed significant effects in the toxic ink degradation. The CAZymes enzymatic consortium from Trichoderma citrinoviride MDU-1 caused solubilization of carbohydrate in the deinked papers. This was followed by fermentation of hexose sugars, which are free from the toxic ink to form bioethanol using Saccharomyces cerevisiae NCIM-3640 (Saini et al. 2020).
BDMMs (bentonite-derived mesoporous materials) were used to immobilize a laccase from Trametes versicolor to develop BDMMs-Lac. This was used for TC (tetracycline) removal. It showed 60% efficiency in TC removal (Wen et al. 2019).
Manganese peroxidase (MnP) has drawn the attention to be used in wastewater treatment. MnP was obtained from the fungus Anthracophyllum discolor and then immobilized on the nanocomposite Fe3O4/chitosan. It caused 96% ± 2% and 98% ± 2% removal of the dyes methylene blue and reactive orange 16, respectively, showing its potential in bioremediation of wastewater (Siddeeg et al. 2020).
Lignin peroxidase was procured from Pichia methanolica by heterologous expression. The enzyme was purified and immobilized on the nanoparticles Fe3O4@SiO2@polydopamine. The immobilized enzyme caused remarkable dissipation of the organic pollutants phenol, 5-chlorophenol, dibutyl phthalate, tetracycline, phenanthrene and fluoranthene. The dissipation that occurred was due to degradation, primarily, and adsorption (Guo et al. 2019).
An investigation was performed to study the importance of the cytochrome P450 (CYP450) system of Trametes versicolor in removing the herbicide diuron, the insecticides acetamiprid (ACE) and imidacloprid (IMI). Presence of 1-ABT, CYP450 inhibitor, in the culture retarded the degradation of diuron. In addition, the half-life of ACE and IMI markedly increased when 1-ABT was present. Accordingly, the authors concluded that the system of CYP450 takes part in the degradation of the tested pollutants (Hu et al. 2022).
5 Conclusion
The global industrial demands for enzymes increases daily. Animal and plant enzymes cannot fulfil these demands; hence, the attention is drawn to microbial enzymes due to their feasible production in high quantities and more stability. Among microbial enzymes, enzymes derived from fungi are produced at larger scales and are more easily purified. They are inevitable in the industrial, biomedical and environmental sectors, as they can perform many tasks with high efficiency in production of foods and beverages, generation of biofuels, manufacture of detergents, leather, paper, textile and pharmaceuticals, and management of wastes. More research should be focused on exploring novel fungal sources for production of enzymes with desired features.
References
Abdallah YK, Estevez AT, Tantawy DEDM et al (2019) Employing laccase-producing Aspergillus sydowii NYKA 510 as a cathodic biocatalyst in self-sufficient lighting microbial fuel cell. J Microbiol Biotechnol 29(12):1861–1872. https://doi.org/10.4014/jmb.1907.07031
Abdel-Rahman TM, Khalil NM, Abdel-Ghany MN et al (2018) Purification and characterization of milk-clotting enzyme from the edible mushroom Pleurotus albidus. Res J Pharm Biol Chem Sci 9:49–63
Abdel-Rahman TM, Khalil NM, Abdel-Ghany MN et al (2019) Purification, characterization and medicinal application of tyrosinase extracted from Saccharomyces cerevisiae. J Inn Pharm Biol Sci 6(1):1–11
Abu-Tahon MA, Arafat HH, Isaac GS (2020) Laundry detergent compatibility and dehairing efficiency of alkaline thermostable protease produced from Aspergillus terreus under solid-state fermentation. J Oleo Sci 69(3):241–254. https://doi.org/10.5650/jos.ess19315
Aklakur M (2016) Natural antioxidants from sea: a potential industrial perspective in aquafeed formulation. Rev Aquac 10:385–399. https://doi.org/10.1111/raq.12167
Alazi E, Knetsch T, Di Falco MD et al (2018) Inducer-independent production of pectinases in Aspergillus Niger by overexpression of the D-galacturonic acid responsive transcription factor gaaR. Appl Microbiol Biotechnol 102:2723–2736. https://doi.org/10.1007/s00253-018-8753-7
Albertsson PA (1958) Partition of proteins in liquid polymer-polymer two-phase systems. Nature 182:709–711. https://doi.org/10.1038/182709a0
Alhelli AM, Abdul Manap MY, Mohammed AS et al (2016) Response surface methodology modelling of an aqueous two-phase system for purification of protease from Penicillium candidum (PCA 1/TT031) under solid state fermentation and its biochemical characterization. Int J Mol Sci 17(11):1872. https://doi.org/10.3390/ijms17111872
Ali MIA, Khalil NM, Abd El-Ghany MN (2012) Biodegradation of some polycyclic aromatic hydrocarbons by Aspergillus terreus. Afr J Microbiol Res 6:3783–3790. https://doi.org/10.5897/AJMR12.411
Alvarez-Guerra E, Ventura SPM, Coutinho JA et al (2014) Ionic liquid-based three phase partitioning (ILTPP) systems: ionic liquid recovery and recycling. Fluid Phase Equilib 371:67–74. https://doi.org/10.1016/j.fluid.2014.03.009
Antizar-Ladislao B, Lopez-Real J, Beck A (2006) Bioremediation of polycyclic aromatic hydrocarbons (PAH) in an aged coal–tar-contaminated soil using different in-vessel composting approaches. J Hazard Mater 137:1583–1588. https://doi.org/10.1016/j.jhazmat.2006.04.056
Babich OO, Pokrovsky VS, Anisimova NY et al (2013) Recombinant l-phenylalanine ammonia lyase from Rhodosporidium toruloides as a potential anticancer agent. Biotechnol Appl Biochem 60(3):316–322. https://doi.org/10.1002/bab.1089
Berbee ML, James TY, Strullu-Derrien C (2017) Early diverging fungi: diversity and impact at the dawn of terrestrial life. Annu Rev Microbiol 71:41–60. https://doi.org/10.1146/annurev-micro-030117-020324
Bornscheuer UT, Pohl M (2001) Improved biocatalysts by directed evolution and rational protein design. Curr Opin Chem Biol 5(2):137–143. https://doi.org/10.1016/S1367-5931(00)00182-4
Brannigan JA, Wilkinson AJ (2002) Protein engineering 20 years on. Nature Rev Mol Cell Biol 3(12):964–970. https://doi.org/10.1038/nrm975
Cadwell RC, Joyce GF (1994) Mutagenic PCR. PCR Methods Appl 3(6):S136–S140. https://doi.org/10.1101/gr.3.6.s136
Camarero SI, Pardo AI, Canas P et al (2012) Engineering platforms for directed evolution of laccase from Pycnoporus cinnabarinus. Appl Env Microbiol 78(5):1370–1384. https://doi.org/10.1128/AEM.07530-11
Chua G, Morris QD, Sopko R et al (2006) Identifying transcription factor functions and targets by phenotypic activation. Proc Natl Acad Sci U S A 103:12045–12050. https://doi.org/10.1073/pnas.0605140103
Coradetti ST, Xiong Y, Glass NL (2013) Analysis of a conserved cellulase transcriptional regulator reveals inducer-independent production of cellulolytic enzymes in Neurospora crassa. Microbiol Open 2:595–609. https://doi.org/10.1002/mbo3.94
da Silva RR, da Conceicao PJP, de Menezes CLA et al (2019) Biochemical characteristics and potential application of a novel ethanol and glucose-tolerant β-glucosidase secreted by Pichia guilliermondii G1.2. J Biotechnol 294:73–80. https://doi.org/10.1016/j.jbiotec.2019.02.001
de Souza JB, Michelin M, Amancio FL et al (2020) Sunflower stalk as a carbon source inductive for fungal xylanase production. Ind Crop Prod 153:112368. https://doi.org/10.1016/j.indcrop.2020.112368
Deng JJ, Shi D, Mao HH et al (2019) Heterologous expression and characterization of an antifungal chitinase (Chit46) from Trichoderma harzianum GIM 3.442 and its application in colloidal chitin conversion. Int J Biol Macromol 134:113–121. https://doi.org/10.1016/j.ijbiomac.2019.04.177
Devi R, Kaur T, Kour D et al (2020) Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb Biosyst 5:21–47. https://doi.org/10.21608/MB.2020.32802.1016
Dhevagi P, Ramya A, Priyatharshini S et al (2021) Industrially important fungal enzymes: productions and applications. In: Yadav AN (ed) Recent trends in mycological research. Fungal biology. Springer, Cham. https://doi.org/10.1007/978-3-030-68260-6_11
Diaz EM, Vicente-Manzanares M, Sacristan M et al (2011) Fungal lectin of Peltigera canina induces chemotropism of compatible Nostoc cells by constriction-relaxation pulses of cyanobiont cytoskeleton. Plant Signal Behav 6(10):1525–1536. https://doi.org/10.4161/psb.6.10.16687
Dubey KK, Ray A, Behera B (2008) Production of demethylated colchicine through microbial transformation and scale-up process development. Process Biochem 43:251–257. https://doi.org/10.1016/j.procbio.2007.12.002
Elibol M (2004) Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology. Process Biochem 39:1057–1062. https://doi.org/10.1016/S0032-9592(03)00232-2
El-Naggar NEA, El-Ewasy SM, El-Shweihy NM (2014) Microbial l-asparaginase as a potential therapeutic agent for the treatment of acute lymphoblastic leukemia: the pros and cons. Int J Pharmacol 10(4):182–199. https://doi.org/10.3923/ijp.2014.182.199
Fan ZM, Werkman JR, Yuan L (2009) Engineering of a multifunctional hemicellulase. Biotechnol Lett 31(5):751–757. https://doi.org/10.1007/s10529-009-9926-3
Fisher RA (1992) The arrangement of field experiments. In: Kotz S, Johnson NL (eds) Breakthroughs in statistics. Springer-Verlag Inc, New York, NY, pp 82–91. https://doi.org/10.1007/978-1-4612-4380-9_8
Freire MG, Cláudio AFM, Araújo JMM et al (2012) Aqueous biphasic systems: a boost brought about by using ionic liquids. Chem Soc Rev 41:4966–4995. https://doi.org/10.1039/C2CS35151J
Fuglsang CC, Johansen C, Christgau S et al (1995) Antimicrobial enzymes: applications and future potential in the food industry. Trends Food Sci Technol 6:390–396. https://doi.org/10.1016/S0924-2244(00)89217-1
Fujii R, Kitaoka M, Hayashi K (2004) One-step random mutagenesis by error-prone rolling circle amplification. Nucleic Acids Res 32(19):e145. https://doi.org/10.1093/nar/gnh147
Gagaoua M, Hafid K (2016) Three phase partitioning system, an emerging non-chromatographic tool for proteolytic enzymes recovery and purification. Biosensors J, OMICS 5(1):100134
Gianfreda L, Rao MA (2004) Potential of extra-cellular enzymes in remediation of polluted soils: a review. Enzym Microb Technol 35:339–354. https://doi.org/10.1016/j.enzmictec.2004.05.006340L
Godfrey T, Reichelt J (1996) Industrial enzymology. The application of enzymes in industry, 2nd edn. Macmillan New York Nature Press. https://onlinelibrary.wiley.com/doi/abs/10.1002/star.19840360111
Gonzalez R, Islas L, Obregon AM et al (1995) Gentamicin formation in Micromonospora purpurea: stimulatory effect of ammonium. J Antibiot 48:479–483. https://doi.org/10.7164/antibiotics.48.479
Guerrand D (2018) Economics of food and feed enzymes: status and prospectives. In: Nunes CS, Kumar V (eds) Enzymes in human and animal nutrition: principles and perspectives. Academic Press, London, pp 487–514. https://doi.org/10.1016/B978-0-12-805419-2.00026-5
Guo J, Liu X, Zhang X et al (2019) Immobilized lignin peroxidase on Fe3O4@SiO2@polydopamine nanoparticles for degradation of organic pollutants. Int J Biol Macromol 138:433–440. https://doi.org/10.1016/j.ijbiomac.2019.07.105
Gupta R, Gigras P, Mohapatra H et al (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38(11):1599–1616. https://doi.org/10.1016/S0032-9592(03)00053-0
Gurung N, Ray S, Bose S et al (2013) A broader view: microbial enzymes and their relevance in industries, medicine, and beyond. Biomed Res Int 2013:1–18. https://doi.org/10.1155/2013/329121
He L, Mao Y, Zhang L et al (2017) Functional expression of a novel α-amylase from Antarctic psychrotolerant fungus for baking industry and its magnetic immobilization. BMC Biotechnol 17(1):22. https://doi.org/10.1186/s12896-017-0343-8
Hu K, Barbieri MV, Lopez-Garcia E et al (2022) Fungal degradation of selected medium to highly polar pesticides by Trametes versicolor: kinetics, biodegradation pathways, and ecotoxicity of treated waters. Anal Bioanal Chem 414(1):439–449. https://doi.org/10.1007/s00216-021-03267-x
Ibarra DJ, Romero MJ, Martinez AT et al (2006) Exploring the enzymatic parameters for optimal delignification of eucalypt pulp by laccase-mediator. Enzym Microb Technol 39(6):1319–1327. https://doi.org/10.1016/j.enzmictec.2006.03.019
Jeske L, Placzek S, Schomburg I et al (2019) BRENDA in 2019: a European ELIXIR core data resource. Nucleic Acids Res 47:D542–D549. https://doi.org/10.1093/nar/gky1048
Kamel IS, Khalil NM, Atalla SMM et al (2021) Purification, molecular and biochemical characterization and biological applications of hemagglutinating lectin with anticancer activities from Pleurotus ostreatus. Plant Arch 21(S1):416–431. https://doi.org/10.51470/PLANTARCHIVES.2021.v21.S1.065
Kango N, Jana UK, Choukade R (2019) Fungal enzymes: sources and biotechnological applications. In: Satyanarayana T, Deshmukh SK, Deshpande MV (eds) Advancing frontiers in mycology & mycotechnology: basic and applied aspects of fungi. Springer, Singapore, pp 515–538. https://doi.org/10.1007/978-981-13-9349-5_21
Kaur J, Chugh P, Soni R et al (2020) A low-cost approach for the generation of enhanced sugars and ethanol from rice straw using in-house produced cellulase-hemicellulase consortium from A. Niger P-19. Bioresour Technol Rep 11:100469. https://doi.org/10.1016/j.biteb.2020.100469
Ketnawa S, Rungraeng N, Rawdkuen S (2017) Phase partitioning for enzyme separation: an overview and recent applications. Int Food Res J 24(1):1–24
Khalil NM, Ali MIA, Ouf SA et al (2016) Characterization of aspergillus flavus NG 85 laccase and its dye decolorization efficiency. Res J Pharm Biol Chem Sci 7:818–829
Khalil NM, Rodríguez-Couto S, El-Ghany MNA (2021) Characterization of Penicillium crustosum l-asparaginase and its acrylamide alleviation efficiency in roasted coffee beans at non-cytotoxic levels. Arch Microbiol 203(5):2625–2637. https://doi.org/10.1007/s00203-021-02198-6
Kiiskinen LL, Kruus K, Bailey M et al (2004) Expression of Melanocarpus Albomyces laccase in Trichoderma reesei and characterization of the purified enzyme. Microbiol 150:3065–3074. https://doi.org/10.1099/mic.0.27147-0
Kour D, Rana KL, Kaur T et al (2019) Extremophiles for hydrolytic enzymes productions: biodiversity and potential biotechnological applications. In: Molina G, Gupta VK, Singh B et al (eds) Bioprocessing for biomolecules production. Wiley, pp 321–372. https://doi.org/10.1002/9781119434436.ch16
Kumar VV, Sathyaselvabala V, Kirupha SD et al (2011) Application of response surface methodology to optimize three phase partitioning for purification of laccase from Pleurotus ostreatus. Separation Sci Technol 46:1922–1930. https://doi.org/10.1080/01496395.2011.583306
Kumarevel TS, Gopinath SCB, Hilda A et al (2005) Purification of lipase from Cunninghamella verticillata by stepwise precipitation and optimized conditions for crystallization. World J Microbiol Biotechnol 21:23–26. https://doi.org/10.1007/s11274-004-1005-2
Kumla J, Suwannarach N, Sujarit K et al (2020) Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 25:2811. https://doi.org/10.3390/molecules25122811
Kumura H, Saito C, Taniguchi Y (2017) Adjunctive application of solid-state culture products from Aspergillus oryzae for semi-hard cheese. Adv Dairy Res 5(3):1–7. https://doi.org/10.4172/2329-888X.1000188
Lam SK, Ng TB (2011) Lectins: production and practical applications. Appl Microbiol Biotechnol 89(1):45–55. https://doi.org/10.1007/s00253-010-2892-9
Le B, Yang SH (2018) Characterization of a chitinase from Salinivibrio sp. BAO-1801 as an antifungal activity and a biocatalyst for producing chitobiose. J Basic Microbiol 58:848–856. https://doi.org/10.1002/jobm.201800256
Li Y, Wang Y, Tu T et al (2017) Two acidic, thermophilic GH28 polygalacturonases from Talaromyces leycettanus JCM 12802 with application potentials for grape juice clarification. Food Chem 237:997–1003. https://doi.org/10.1016/j.foodchem.2017.06.037
Lima CA, Campos JF, Filho JL et al (2015) Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. J Food Sci Technol 52(7):4459–4466. https://doi.org/10.1007/s13197-014-1463-y
Liu G, Zhang L, Qin Y et al (2013) Long-term strain improvements accumulate mutations in regulatory elements responsible for hyper-production of cellulolytic enzymes. Sci Rep 3:1–7. https://doi.org/10.1038/srep01569
Luhe AL, Ting ENY, Tan L et al (2010) Engineering of small sized DNAs by error-prone multiply-primed rolling circle amplification for introduction of random point mutations. J Mol Catal B Enzym 67(1–2):92–97. https://doi.org/10.1016/j.molcatb.2010.07.011
Mamo J, Getachew P, Kuria MS et al (2020) Application of milk-clotting protease from Aspergillus oryzae DRDFS13 MN726447 and Bacillus subtilis SMDFS 2B MN715837 for Danbo cheese production. J Food Qual 1:1–12. https://doi.org/10.1155/2020/8869010
Marchut-Mikolajczyk O, Kwapisz E, Wieczorek D et al (2015) Biodegradation of diesel oil hydrocarbons enhanced with Mucor circinelloides enzyme preparation. Int Biodeterior Biodegrad 104:142–148. https://doi.org/10.1016/j.ibiod.2015.05.008
Marco E, Font X, Sanchez A et al (2013) Co-composting as a management strategy to reuse the white–rot fungus Trametes versicolor after its use in a biotechnological process. Int J Environ Waste Manag 11:100–108. https://doi.org/10.1504/IJEWM.2013.050637
Margetic A, Stojanovic S, Ristovic M et al (2021) Fungal oxidative and hydrolyzing enzymes as designers in the biological production of dietary fibers from triticale. LWT-Food Science and Technology 145:111291. https://doi.org/10.1016/j.lwt.2021.111291
Mate D, Garcia-Ruiz E, Camarero S et al (2011) Directed evolution of fungal laccases. Curr Genomics 12(2):113–122. https://doi.org/10.2174/138920211795564322
Meyer V, Ram AFJ, Punt PJ (2010) Genetics, genetic manipulation, and approaches to strain improvement of filamentous fungi. In: Baltz R, Demain A, Davies J et al (eds) Manual of industrial microbiology and biotechnology. ASM Press, Washington, DC, pp 318–329. https://doi.org/10.1128/9781555816827.ch22
Midorikawa GEO, Correa CL, Noronha EF et al (2018) Analysis of the transcriptome in aspergillus tamarii during enzymatic degradation of sugarcane bagasse. Front Bioeng Biotechnol 6:123. https://doi.org/10.3389/fbioe.2018.00123
Moubasher HA, Balbool BA, Helmy YA et al (2022) Insights into asparaginase from endophytic fungus Lasiodiplodia theobromae: purification, characterization and antileukemic activity. Int J Environ Res Public Health 19(2):680. https://doi.org/10.3390/ijerph19020680
Moussa TAA, Khalil NM (2022) Extremozymes from extremophilic microorganisms as sources of bioremediation. In: Kuddus M (ed) Microbial extremozymes. Academic Press, pp 135–146. https://doi.org/10.1016/B978-0-12-822945-3.00005-1
Moussa TAA, Khalil NM, Ali DMI, Mostafa FA (2014) Purification and biochemical characterization of xylanase from Sclerotium rolfsii. J Pure Appl Microbiol 8(6):4727–4733
Naganagouda K, Mulimani VH (2008) Aqueous two-phase extraction (ATPE): an attractive and economically viable technology for downstream processing of Aspergillus oryzae α-galactosidase. Process Biochem 43:1293–1299. https://doi.org/10.1016/j.procbio.2008.07.016
Oh JM, Lee JP, Baek SC et al (2018) Characterization of two extracellular β-glucosidases produced from the cellulolytic fungus aspergillus sp. YDJ216 and their potential applications for the hydrolysis of flavone glycosides. Int J Biol Macromol 111:595–603. https://doi.org/10.1016/j.ijbiomac.2018.01.020
Otten LG, Quax WJ (2005) Directed evolution: selecting today’s biocatalysts. Biomol Eng 22(1–3):1–9. https://doi.org/10.1016/j.bioeng.2005.02.002
Patil NP, Chaudhari BL (2010) Production and purification of pectinase by soil isolate Penicillium sp. and search for better agro-residue for its SSF. Rec Res Sci Technol 2:36–42
Polizeli MDLTM, Jorge JA, Terenzi HF (1991) Pectinase production by Neurospora crassa: purification and biochemical characterization of extracellular polygalacturonase activity. J Gen Microbiol 137:1815–1823. https://doi.org/10.1099/00221287-137-8-1815
Qin YQ, Wei XM, Song X et al (2008) The role of the site 342 in catalytic efficiency and pH optima of endoglucanase II from Trichoderma reesei as probed by saturation mutagenesis. Biocatal Biotransformation 26(5):378–382. https://doi.org/10.1080/10242420802249299
Rafi S, Shoaib A, Awan ZA et al (2016) Chromium tolerance, oxidative stress response, morphological characteristics, and FTIR studies of phytopathogenic fungus Sclerotium rolfsii. Folia Microbiol 62:207–219. https://doi.org/10.1007/s12223-016-0489-0
Ravalason H, Herpoel-Gimbert I, Record E et al (2009) Fusion of a family 1 carbohydrate binding module of Aspergillus Niger to the Pycnoporus cinnabarinus laccase for efficient softwood Kraft pulp biobleaching. J Biotechnol 142(3–4):220–226. https://doi.org/10.1016/j.jbiotec.2009.04.013
Rezaei S, Tahmasbi H, Mogharabia M et al (2015) Efficient decolorization and detoxification of reactive orange 7 using laccase isolated from Paraconiothyrium variabile, kinetics and energetics. J Taiwan Inst Chem Eng 56:113–121. https://doi.org/10.1016/j.jtice.2015.04.008
Ribeiro LF, Ribeiro LFC (2013) Improving fungal enzyme properties through protein engineering. In: Polizeli MDLTM, Rai M (eds) Fungal enzymes, 1st edn. CRC Press, pp 341–366. https://doi.org/10.1201/b15247
Sadighi A, Motevalizadeh SF, Hosseini M et al (2017) Metal-chelate immobilization of lipase onto polyethylenimine coated MCM-41 for apple flavor synthesis. Appl Biochem Biotechnol 182:1371–1389. https://doi.org/10.1007/s12010-017-2404-9
Saini S, Chutani P, Kumar P et al (2020) Development of an eco-friendly deinking process for the production of bioethanol using diverse hazardous paper wastes. Renew Energy 146:2362–2373. https://doi.org/10.1016/j.renene.2019.08.087
Sandgren M, Stahlberg J, Mitchinson C (2005) Structural and biochemical studies of GH family 12 cellulases: improved thermal stability, and ligand complexes. Prog Biophys Mol Biol 89(3):246–291. https://doi.org/10.1016/j.pbiomolbio.2004.11.002
Sarma H, Bhattacharyya PN, Jadhav DA et al (2021) Fungal-mediated electrochemical system: prospects, applications and challenges. Curr Res Microb Sci 2:100041. https://doi.org/10.1016/j.crmicr.2021.100041
Scarpa JDCP, Marques NP, Monteiro DA et al (2019) Saccharification of pretreated sugarcane bagasse using enzymes solution from Pycnoporus sanguineus MCA 16 and cellulosic ethanol production. Ind Crop Prod 141:111795. https://doi.org/10.1016/j.indcrop.2019.111795
Schwienheer C, Prinz A, Zeiner T et al (2015) Separation of active laccases from Pleurotus sapidus culture supernatant using aqueous two-phase systems in centrifugal partition chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 1002:1–7. https://doi.org/10.1016/j.jchromb.2015.07.050
Shabani M, Pontié M, Younesi H et al (2021) Biodegradation of acetaminophen and its main by-product 4-aminophenol by Trichoderma harzianum versus mixed biofilm of Trichoderma harzianum/Pseudomonas fluorescens in a fungal microbial fuel cell. J Appl Electrochem 51:581–596. https://doi.org/10.1007/s10800-020-01518-w
Sharaf EF, Khalil NM (2011) Keratinolytic activity of purified alkaline keratinase produced by Scopulariopsis brevicaulis (Sacc.) and its amino acids profile. Saudi. J Biol Sci 18(2):117–121. https://doi.org/10.1016/j.sjbs.2010.12.011
Shekarchizadeh H, Kadivar M (2012) A study on parameters of potential cocoa butter analogue synthesis from camel hump by lipase-catalysed interesterification in supercritical CO2 using response surface methodology. Food Chem 135(1):155–160. https://doi.org/10.1016/j.foodchem.2012.04.033
Shih I, Van Y, Chang Y (2002) Application of statistical experimental methods to optimize production of poly (γ-glutamicacid) by Bacillus licheniformis CCRC12826. Enzym Microb Technol 31:213–220. https://doi.org/10.1016/S0141-0229(02)00103-5
Siddeeg SM, Tahoon MA, Mnif W et al (2020) Iron oxide/chitosan magnetic nanocomposite immobilized manganese peroxidase for decolorization of textile wastewater. PRO 8:5. https://doi.org/10.3390/pr8010005
Singh N, Rai V, Tripathi C (2012) Production and optimization of oxytetracycline by a new isolate Streptomyces rimosus using response surface methodology. Med Chem Res 21:3140–3145. https://doi.org/10.1007/s00044-011-9845-4
Singh V, Haque S, Niwas R et al (2017) Strategies for fermentation medium optimization: an in-depth review. Front Microbiol 7:2087. https://doi.org/10.3389/fmicb.2016.02087
Singh R, Singh T, Pandey A (2019) Microbial enzymes—an overview. In: Singh RS, Singhania RR, Pandey A et al (eds) Advances in enzyme technology. Elsevier, Amsterdam, pp 1–40. https://doi.org/10.1016/B978-0-444-64114-4.00001-7
Stemmer WPC (1994a) DNA shuffling by random fragmentation and reassembly in vitro recombination for molecular evolution. Proc Natl Acad Sci U S A 91(22):10747–10751. https://doi.org/10.1073/pnas.91.22.10747
Stemmer WPC (1994b) Rapid evolution of a protein in vitro by DNA shuffling. Nature 370(6488):389–391. https://doi.org/10.1038/370389a0
Suman A, Verma P, Yadav AN et al (2015) Bioprospecting for extracellular hydrolytic enzymes from culturable thermotolerant bacteria isolated from Manikaran thermal springs. Res J Biotechnol 10:33–42
Sun LH, Petrounia IP, Yagasaki M et al (2001) Expression and stabilization of galactose oxidase in Escherichia coli by directed evolution. Protein Eng 14(9):699–704. https://doi.org/10.1093/protein/14.9.699
Vaidya R, Vyas P, Chhatpar H (2003) Statistical optimization of medium components for the production of chitinase by Alcaligenes xylosoxydans. Enzym Microb Technol 33:92–96. https://doi.org/10.1016/S0141-0229(03)00100-5
Varrot A, Basheer SM, Imberty A (2013) Fungal lectins: structure, function and potential applications. Curr Opin Struct Biol 5:678–685. https://doi.org/10.1016/j.sbi.2013.07.007
Verma ML, Thakur M, Randhawa JS et al (2020) Biotechnological applications of fungal enzymes with special reference to bioremediation. In: Gothandam K, Ranjan S, Dasgupta N et al (eds) Environmental biotechnology Vol 2. Environmental chemistry for a sustainable world, vol 45. Springer, Cham. https://doi.org/10.1007/978-3-030-38196-7_10
Wen X, Zeng Z, Du C et al (2019) Immobilized laccase on bentonite-derived mesoporous materials for removal of tetracycline. Chemosphere 222:865–871. https://doi.org/10.1016/j.chemosphere.2019.02.020
Wu ML, Nie MQ, Wang XC et al (2010) Analysis of phenanthrene biodegradation by using FTIR, UV and GC-MS. Spectrochim Acta A Mol Biomol Spectrosc 75(3):1047–1050. https://doi.org/10.1016/j.saa.2009.12.051
Xu F, Oruna-Concha M, Elmore J (2016) The use of asparaginase to reduce acrylamide levels in cooked food. Food Chem 210:163–171. https://doi.org/10.1016/j.foodchem.2016.04.105
Yadav M, Bista G, Maharjan R et al (2019) Secretory laccase from Pestalotiopsis species CDBT-F-G1 fungal strain isolated from high altitude: optimization of its production and characterization. Appl Sci 9:340. https://doi.org/10.3390/app9020340
Yang P, Wu Y, Zheng Z et al (2018) CRISPR-Cas9 approach constructing cellulase sestc-engineered Saccharomyces cerevisiae for the production of orange peel ethanol. Front Microbiol 9:2436. https://doi.org/10.3389/fmicb.2018.02436
Zhang R, Zhao L, Wang H et al (2014) A novel ribonuclease with antiproliferative activity toward leukemia and lymphoma cells and HIV-1 reverse transcriptase inhibitory activity from the mushroom, Hohenbuehelia serotina. Int J Mol Med 33:209–214. https://doi.org/10.3892/ijmm.2013.1553
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Khalil, N.M. (2023). Recent Progress on Fungal Enzymes. In: Rashad, Y.M., Baka, Z.A.M., Moussa, T.A.A. (eds) Plant Mycobiome. Springer, Cham. https://doi.org/10.1007/978-3-031-28307-9_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-28307-9_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-28306-2
Online ISBN: 978-3-031-28307-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)