Abstract
Real-time detection of airborne infection agents present in human breath and environmental airways, such as the human respiratory Coronavirus, is important for public health. For this, a model label-free immunosensor, based on multi-walled nanotubes (MWNT)-based screen-printed graphite electrodes (SPEs), was proposed and studied. For sensing applications, MWNTs have many advantages such as small size with larger surface area, excellent electron transfer promoting ability when used for antibody immobilization, with retention of its selectivity for potential immunosensors development. In order to verify the selectivity of the selected primary antibody (anti-CoV 229E antibody) and the effective immunocomplex formation (antigen-antibody), an in-depth voltammetric characterization of MWNT-SPEs interface was carried out during the multistep fabrication of CoV immunosensor using [Fe(CN)6]3−/4− as an electroactive probe. After that, the analytical robustness of the performances of these immunosensing platforms was estimated and verified. Indeed, a nanomolar range detection limit (180 TCID50/mL)g/mL) with excellent reproducibility (RSD% = 8%) was obtained.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Food quality and safety monitoring together with the clinical diagnosis are issues of utmost importance for human health [1].
In modern livestock production, veterinary drugs, pesticides, hormones and other additives are used on a large scale. The massive use of these compounds may leave residues in edible products negatively affecting human health [2]. In addition, inadequate storage conditions could lead to the formation of various toxins and the diffusion of pathogens [3]. For the detection and quantification of these analytes, several conventional methods such as chromatography coupled to mass spectrometry, ultraviolet, or fluorescence spectroscopy, among others, are utilized [4]. These methods are laborious, expensive, time-consuming, and require large sample volumes and highly trained personnel. To overcome these limitations, electrochemical, and in particular label-free biosensing platforms, offer a complementary alternative by allowing fast screening of samples on-site [5]. The combination between immunochemistry and electrochemistry offers the possibility to develop a huge number of simple-to-use devices with a low-cost, high selectivity, fast and sensitive response [6]. These characteristics make them especially well-suited for on-field screening analyses, as they are easily coupled to portable devices, especially when screen-printed electrodes (SPEs) are used as a cost-effective alternative to traditional solid electrodes.
In this work, developed in the frame of the project “SFIDE”, we developed a label-free immunosensor for the real-time detection of Coronaviridae (CoV) presence in the aerosol. The family Coronaviridae is part of the order Nidovirales [7] and includes a substantial number of pathogens of mammals and birds that individually cause a remarkable variety of diseases (i.e. pneumonia, enteritis, polyserositis, hepatitis, encephalomyelitis, nephritis, and various other disorders). Coronavirus-like infections have been described in different animals (i.e. swine, cattle, horses, cats, dogs, etc.) and in humans. In the last case, coronaviruses may induce pathologies in the spectrum between the common cold and severe acute respiratory syndrome (SARS) [7].
Herein, we report on an immunosensor assembled on multiwall (MWNT)-modified platforms able to detect the presence of coronaviruses in the nanomolar range. The nanomaterials employed are among the most versatile and customizable enabling surface coatings with a plethora of bio/materials for the construction of a wide variety of electrochemical sensing platforms [8]. A carefully voltammetric characterization was conducted in order to study the layer-by-layer construction of the immunosensor.
2 Experimental Section
2.1 Materials and Reagents
All chemicals were of analytical grade. Anti-mouse IgG antibody was purchased from Sigma-Aldrich (Steinheim, Germany). Rabbit anti-CoV-229E spike S1 was purchased from DBA SRL (Milano, Italy), whereas inactivated CoV-229E was provided by Istituto Superiore di Sanità (Roma, Italy). Potassium ferricyanide and potassium ferrocyanide were purchased from Sigma-Aldrich (Steinheim, Germany). Buffers: a) 0.05 M phosphate buffer saline + 0.1 M KCl, pH = 7.4 (PBS); b) 0.05 M phosphate buffer saline (PBS-T), 0.05% Tw20 (v/v).
2.2 Apparatus and Techniques
In-house produced screen-printed devices were used as transducing platforms for our immunosensors developments. A PalmSens 4.0 potentiostat (PalmSens, Netherlands) was employed for the electrochemical measurements (cyclic voltammetry, CV, and square wave voltammetry, SWV).
2.3 Theoretical Methods
The heterogeneous electron transfer constant (k0), the standard curves (using non-linear 4 parameter logistic calibration plots), the limit of detection (LOD), and the limit of quantification (LOQ) were calculated as reported in our previous work [5].
2.4 Fabrication of CoV-229E Label-Free Electrochemical Immunosensor
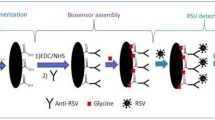
Initially, screen printed electrodes (SPEs) were modified via drop-casting with a dispersion of MWNT [8]. After that, 6 μL of IgG (10 μg/ml) prepared in PBS was drop-casted onto the working electrode (WE) and stored overnight at 4 ℃. 6 μL of 5% polyvinyl alcohol (PVA) (incubation of 20 min) was used to block the surface of WE remained free, and after that 6 μL of anti-CoV-229E antibody (5 μg/ml) in PBS was deposited onto the WE. This binding procedure lasted 120 min at room temperature using an orbital shaker. At this point, 6 μL of CoV (several concentrations) was added and left reacting for 60 min. The immunological complex formation was monitored using 0.01 M [Fe(CN)6]4−/3− solution as an electroactive probe and square wave voltammetry (SWV) as analytical techniques (Fig. 1).
3 Results and Discussion
3.1 Electrochemical Characterization of the Immunosensor Build-Up
Initially, an in-depth electrochemical characterization of the layer-by-layer construction (Fig. 1) of our CoV-immunosensor was undertaken. In particular, the Cyclic Voltammetry (CV) was used to study the fabrication of the immunological chain and thus the effect of the layer coating on the electrodic surface. A visual inspection of the voltammograms reported in Fig. 2 reveals the effects of the different chemical and biological layers over the electrical conductivity of the electrode/electrolyte interface, influencing the SPE performance. In particular, the construction of the immunological chain, first with mAb-IgG and then with mAb-CoV, produces a significant decrease in the magnitude of voltammetric peaks, as reported in Table 1. This is confirmed by the increase of the peak-to-peak separation, with a consequent deflection of [Fe(CN)6]3−/4− to its ideal reversible electrochemical behavior. These phenomena are ascribable to the layer coating of the electrode surface, which became thicker with the assembly procedure, whereby reducing [Fe(CN)6]3−/4− permeability through the immobilization of the layers on the WE [9].
3.2 Preliminary Analytical Performances
Once anti-IgG was immobilized, the ideal anti-CoV Ab concentration to be immobilized were investigated. To this purpose, the electrochemical response (SWV faradic current) of different anti-CoV Ab concentrations (0, 0.5, 1, 2, 5, 10 µg/mL), incubated for 1 h on anti-IgG-MWNT-modified SPE (Fig. 3a) were tested. By increasing the antibody concentration, a decrease in the recorded faradic current values is observed that can be explained by the following mechanism: the higher the antibody concentration, the higher the molecular crowding on the electrode surface due to the antibody molecular complementarity. The proper antibody concentration to be applied can be obtained considering the 75–80% of the maximum value of the association curve that for anti-CoV Ab is equal to 5 μg/mL. From the dose-response curve reported in Fig. 3b a LoD of 180 TCID50/mL [5] with a good reproducibility of 13% (RSD%) are observed.
a) Binding curves (dilution of antibody solution) obtained using different concentrations of anti-CoV Ab and b) the dose-response curve, obtained incubating different antigen concentrations. The results were obtained by making use of SWV and 0.05 M [Fe(CN)6]4−/3− solution, in 0.05 M PBS + 0.01 M KCl, pH 7.4.
4 Conclusion
In this work, a disposable, simple, low-cost, label-free voltammetric immunosensor for the determination of 229E CoV was successfully preliminary characterized. The serigraphic sensor, modified with MWNTs, exhibited a low detection limit and good sensitivity paving the way to its potential exploitation for the detection of 229E CoV in aerosol and environment airways.
References
Fung, F., Wang, H.-S., Menon, S.: Food safety in the 21st century. Biomed. J. 41, 88–95 (2018). https://doi.org/10.1016/j.bj.2018.03.003
Patel, M., Kumar, R., Kishor, K., Mlsna, T., Pittman, C.U., Mohan, D.: Pharmaceuticals of emerging concern in aquatic systems: chemistry, occurrence, effects, and removal methods. Chem. Rev. 119, 3510–3673 (2019). https://doi.org/10.1021/acs.chemrev.8b00299
Mitchell, M.J., Billingsley, M.M., Haley, R.M., Wechsler, M.E., Peppas, N.A., Langer, R.: Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021). https://doi.org/10.1038/s41573-020-0090-8
Alahi, M., Mukhopadhyay, S.: Detection methodologies for pathogen and toxins: a review. Sensors 17, 1885 (2017). https://doi.org/10.3390/s17081885
Cancelliere, R., et al.: Electrochemical and morphological layer-by-layer characterization of electrode interfaces during a label-free impedimetric immunosensor build-up: the case of ochratoxin A. Appl. Surf. Sci. 567, 150791 (2021). https://doi.org/10.1016/j.apsusc.2021.150791
Zhang, Y., et al.: Label-free electrochemical immunosensor based on enhanced signal amplification between Au@Pd and CoFe2O4/graphene nanohybrid. Sci. Rep. 6, 23391 (2016). https://doi.org/10.1038/srep23391
Coronaviridae. In: Fenner’s Veterinary Virology, pp. 393–413. Elsevier (2011). https://doi.org/10.1016/B978-0-12-375158-4.00024-9
Cancelliere, R., Tinno, A.D., Cataldo, A., Bellucci, S., Micheli, L.: Powerful electron-transfer screen-printed platforms as biosensing tools: the case of uric acid biosensor. Biosensors 12, 2 (2021). https://doi.org/10.3390/bios12010002
Cancelliere, R., Di Tinno, A., Di Lellis, A.M., Contini, G., Micheli, L., Signori, E.: Cost-effective and disposable label-free voltammetric immunosensor for sensitive detection of interleukin-6. Biosens. Bioelectron. 114467 (2022). https://doi.org/10.1016/j.bios.2022.114467
Funding
This work was partially supported by the Project “SFIDE A SMART FRAMEWORK FOR VIRUS DETECTION”, funded by Italian Ministry of University and Research FISR DD 562/2020 - FONDO INTEGRATIVO SPECIALE PER LA RICERCA, grant # FISR2020IP_02585.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Cancelliere, R. et al. (2023). Coronavirus Label-Free Immunosensor: Preliminary Results. In: Di Francia, G., Di Natale, C. (eds) Sensors and Microsystems. AISEM 2022. Lecture Notes in Electrical Engineering, vol 999. Springer, Cham. https://doi.org/10.1007/978-3-031-25706-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-25706-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25705-6
Online ISBN: 978-3-031-25706-3
eBook Packages: EngineeringEngineering (R0)