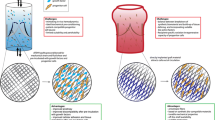
Abstract
A tissue-engineered heart valve (TEHV) could serve as an implantable valve replacement that would grow and adapt with the patient. A TEHV consists of a degradable scaffold material and relevant cells, either seeded onto or entrapped within the scaffold during in vitro culture or recruited in vivo upon implantation, which produce their own extracellular matrix components as the scaffold degrades. Because the valve consists of living tissue at or post implantation, it can grow and remodel as a patient ages, making it an especially attractive option for pediatric and young adult patients. Using various scaffolds, cell sources, and fabrication methods, several laboratories have produced TEHVs with suitable geometry and mechanics, which have been shown to allow for cell infiltration and remodeling once implanted. TEHVs implanted in both preclinical and clinical trials have shown promising short-term functionality, but many designs have failed to maintain consistently good performance after several months in vivo due to leaflet thickening or shortening, and in situ remodeling can vary from patient to patient. Future TEHV research will focus on optimization of TEHV composition and geometry for sustained function and growth, standardization of manufacturing and implantation procedures, and continued investigation into in situ remodeling processes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Mechanical and biological prosthetic heart valves and cryopreserved homograft valves have been used successfully to replace diseased and damaged heart valves in patients for several decades. However, drawbacks such as the limited long-term durability of bioprosthetic valves, the short supply of cryopreserved homografts, and the requirement of anticoagulation drug therapy for recipients of mechanical prosthetic valves motivate innovative improvement in heart valve replacement technologies [1]. In addition, mechanical and bioprosthetic valves are unable to grow with the patient, thus pediatric and young adult patients require multiple surgeries to replace the previously implanted prosthetic valves as they are outgrown. With these shortcomings in mind, researchers have begun work to develop a living TEHV that could be used as a replacement valve, particularly for young patients.
Design criteria for a TEHV include long-term durability, non-calcific, minimal regurgitation and systolic pressure drop, and the capacity to grow and adapt with the patient. The TEHV must also be non-thrombogenic and non-immunogenic to prevent clot formation and immune rejection, respectively. While the biomechanics of native heart valves are relatively well understood [2, 3], the goal of most TEHV researchers is to produce a tissue or regenerative scaffold at implantation that is much simpler than the tri-layer structure of the native leaflets [4] but is still functional. These design criteria are demanding, but researchers in the field of heart valve tissue engineering are becoming well-equipped to confront many of these issues.
In general, a TEHV is produced by forming a degradable scaffold material into the valve geometry then either seeding or entrapping a relevant cell type during in vitro culture or implanting the cell-free scaffold and recruiting cells to the scaffold in situ. Some approaches rely on seeded cells to produce extracellular matrix (ECM) components during an in vitro culture period, before removing these cells through a decellularization process and implanting the cell-free ECM [5,6,7,8,9,10,11]. The cell produced ECM components, specifically collagen, provide the mechanical strength necessary to maintain valve structure and function, and the TEHV undergoes further remodeling as cells repopulate the matrix in vivo. Many different combinations of scaffold materials, geometries, cells, and culture methods are possible, so research groups have developed different approaches to meet the TEHV design criteria. This chapter will provide an overview of the different methods currently employed for producing TEHVs. The chapter will conclude with results from recent preclinical and clinical studies and a discussion of the future directions and trends in TEHV research.
2 Current Methods of Heart Valve Tissue Engineering
Research groups around the world are working on developing TEHVs, using various types of cells, scaffolds, and culture methods. There are many possible combinations of materials and stimuli, and the interactions between the different components of tissue-engineered constructs are complex. Thus, the “optimal” TEHV fabrication and culture process has yet to be determined, and there is likely more than one way to produce an adequate TEHV, that is, one which is able to meet the aforementioned design criteria. Two main approaches will be discussed in this section: (i) TEHVs consisting of cell-produced ECM, requiring in vitro culture and (ii) TEHVs fabricated from bioresorbable synthetic polymeric scaffolds eliminating the need for in vitro culture. While there is also ongoing work investigating the use of decellularized valve homografts [12,13,14,15,16,17,18,19] and xenografts [20,21,22,23,24,25,26,27] for heart valve replacement, these approaches are considered out of the scope of this chapter.
2.1 Tissue-Engineered Matrix TEHVs
Several research groups utilize a tissue-engineered matrix (TEM) approach to fabricate TEHVs. In this approach, relevant cells are seeded on or entrapped within a degradable scaffold material in the correct geometry. Then, during a period of in vitro culture, this scaffold is degraded and replaced by cell-produced ECM, crucially collagen, which provides the mechanical strength for the valve function. The scaffold must degrade at a rate that balances with the rate of ECM production, so that the cells are always provided with sufficient mechanical support. While early approaches focused on using possible autologous cell sources during TEHV fabrication to create a patient-specific valve [28,29,30,31,32], more recent approaches have utilized allogeneic cells for matrix production and then decellularized the TEHVs prior to implantation [5,6,7,8,9,10,11]. This latter approach allows for the TEHVs to be utilized as “off-the-shelf” replacements, if the TEM induces recellularization post-implantation to achieve long-term durability.
Multiple groups are utilizing the TEM approach, but their choice of scaffold material, cell source, and culture conditions differ. One current approach to fabricating TEHVs is to seed cells onto a synthetic, degradable polymeric scaffold made from polyglycolic acid (PGA), polylactic acid (PLA), the PLGA copolymer, or polyhydroxyalkanoate polymers [6, 28, 33,34,35,36,37]. The synthetic polymer mesh is formed into a tri-leaflet valve geometry and vascular derived cells [6, 28, 33, 36], dermal fibroblasts [34, 35], or mesenchymal stem cells [37] are seeded onto the polymer mesh. The synthetic polymers provide the initial mechanical strength and stiffness and degrade in a period of weeks or months. The initial strength and stiffness of the synthetic polymer scaffolds are greater than the strength and stiffness of native heart valve tissue, but after several weeks of in vitro culture, TEHV mechanical properties become more similar to those of native heart valve tissue as the synthetic polymer degrades [28]. Upon completion of the in vitro culture process, these TEHVs can be decellularized using a detergent solution and can either be implanted cell-free [34,35,36] or re-seeded with a cell source such as autologous mesenchymal stem cells [6]. Figure 13.1 shows a decellularized TEHV created using this method.
Decellularized synthetic polymer-based TEM valve in closed (a), open (b), and cross-section (c) views. Vascular-derived cells were seeded on PGA/P4HB synthetic polymer matrix and cultured in vitro to allow for the deposition of cell-produced matrix components. Following the in vitro culture period, the TEHV was decellularized prior to implantation. (Reprinted from Driessen-Mol et al. [36] with permission from Elsevier. This article was published in Driessen-Mol et al. [36], Copyright American College of Cardiology Foundation (2014))
In contrast to this synthetic polymer scaffold approach, fibrin, a biopolymer, can be used as a scaffold material. A fibrin gel is a highly hydrated network of entangled protein fibrils in which cells are entrapped, producing a completely biological TEHV [5, 7, 10, 11, 29, 31, 38,39,40,41,42,43]. Fibrin scaffolds are additionally advantageous, because the cell-mediated fibrin gel contraction can be used to achieve fiber alignment and anisotropy similar to that of native heart valve root and leaflets [11, 31]. With this method of TEHV fabrication, dermal fibroblasts are suspended in fibrinogen, and the addition of thrombin causes a fibrin gel to form. The suspension can be cast into a mold with the desired geometry, and the cells contract the fibrin gel around the mold surfaces. A fibrin gel is much weaker than native heart valve tissue, even after cell-mediated contraction of the fibril network. A significant challenge in the production of fibrin-based TEHVs is obtaining sufficient mechanical properties for in vivo function by inducing the cells to convert the aligned fibrin into aligned ECM of appropriate stiffness and sufficient strength, and optimized in vitro culture conditions and bioreactor conditioning are often utilized to accelerate this process. Similar to the PGA/P4HB-based TEHV discussed previously, the fibrin-based TEHVs can also be decellularized using a detergent solution, enabling “off-the- shelf” availability [5, 7, 10, 11].
Some fibrin-based approaches have utilized a mold for the fibrin gel that recreates the entire root and leaflet geometry [29, 31, 38,39,40,41], recently there has been increased focus on using this approach to create simpler geometries. For example, utilizing tubular tissues and sewing these tubes into a suitable valve geometry after the in vitro remodeling process is complete [5, 7, 10, 11, 42, 43]. Reimer et al. demonstrated the feasibility of a “tube-in-tube” design in which two of these engineered matrix tubes were sewn together to form the root and leaflet structures [7], and Syedain et al. utilized three tubes to create a tri-tube design (Fig. 13.2) with improved commissure stability [11]. There are no frames or stents present in these designs and because the sutures used are degradable, these TEHVs are intended to be suitable for a pediatric patient were growth is required.
Photograph of tri-rube decellularized fibrin-based TEM valve showing (a) side view of construction with suture lines and (b) top view with three coapting leaflets. Dermal fibroblasts entrapped within a fibrin scaffold were cultured in vitro to form the TEM. After in vitro culture, the tubes were decellularized and sewn into the configurations shown. (From Syedain et al. [11]. Reprinted with permission from AAAS)
In an alternative approach, researchers have developed a method of printing hydrogels in the geometry of a heart valve using a 3D printer [44,45,46,47,48,49,50]. By printing various compositions of the hydrogels and modifying photo-crosslinked molecules by UV light upon ejection from the print head, they are able to tune the stiffness of the printed hydrogels. This enables them to print a scaffold with spatially varying mechanical properties, although the fabrication process is more intensive than the commonly used techniques of synthetic polymer seeding or biopolymer casting approaches.
Hockaday et al. printed a hydrogel TEHV consisting of polyethylene glycol diacrylate (PEGDA) both with and without an interpenetrating collagen fibrillar network. The root and leaflet portions of the anatomically accurate aortic valve geometry were printed with different molecular weight PEGDA solutions, so that the two distinct regions had differing mechanical strength and stiffness. Porcine aortic valve interstitial cells cultured on these PEGDA and collagen/PEGDA scaffolds for up to 21 days were viable and exhibited spread morphology, demonstrating the feasibility of using a photo-crosslinked polymer scaffold for TEHV applications [45].
Duan et al. used a similar 3D printing process to print hybrid methacrylated hyaluronic acid (Me-HA) and methacrylated gelatin (Me-Gel) in a tri-leaflet valve geometry and found that by adjusting the composition of this hybrid solution thus altering the stiffness of the scaffold, the response of the encapsulated porcine aortic valve interstitial cells could be regulated [46]. While this study only utilized a short in vitro culture period of 7 days after printing, cell viability both inside the scaffold and on the surface was preserved, and there was early evidence of in vitro remodeling of the printed hydrogel scaffold. Recent work in the area of 3D printing for TEHV applications has investigated optimization and modification of hydrogel materials to promote cell attachment and attain desirable cell phenotypes [47, 48], optimizing cell viability during the photo-crosslinking process [49], and investigating in vivo remodeling of 3D printed hydrogel constructs [50].
2.2 In Vitro Culture of Tissue-Engineered Matrix TEHVs
The in vitro culture environment can profoundly affect the final TEHV properties for valves created using tissue-engineered matrix. Biochemical molecules such as growth factors, ascorbic acid, and insulin have been shown to have significant effects on the resulting TEHVs. Ramaswamy et al. were able to nearly double the amount of collagen produced per MSC in their synthetic polymer-based constructs by supplementing their standard growth medium with basic fibroblast growth factor and ascorbic acid-2-phosphate [37]. In fibrin-based constructs, Neidert et al. demonstrated that collagen deposition by human dermal fibroblasts could be increased 20-fold by supplementing their medium with transforming growth factor-beta, plasmin, and insulin [51]. Depending on the cell type and scaffold material involved, each laboratory uses different combinations of culture medium and supplements in an attempt to optimize ECM production and maturation.
Using a bioreactor for the mechanical conditioning of TEHVs is another approach that can optimize the in vitro culture environment. A TEHV is a complex, 3D structure that can be mechanically stimulated in multiple ways. Flow through a TEHV results in combinations of leaflet flexure and stretch, shear stress, and root distension. Because the system is so complex, the optimal stimulation protocol is still unknown, and research groups have developed several different types of bioreactors to improve the mechanical properties of their constructs.
Several groups have designed and implemented pulse duplicator systems to condition entire TEHVs using physiological pulsatile pressure waveforms. The details of the bioreactors differ, but all share some key features. These include a pump to induce pulsatile fluid motion, a medium reservoir to replenish the system, a section in which the TEHV can be mounted, a fluid capacitance for energy dissipation (to mimic arterial elasticity), and a tunable resistance element to control the pressure in the system [52]. In addition, these systems must be sterilizable, allow for gas exchange, fit in a cell culture incubator, and minimize the volume of culture medium used in order to reduce operational cost.
Hoerstrup et al. [53] developed a pulsatile bioreactor for conditioning their synthetic polymer-based TEHVs that consisted of an air chamber and a medium chamber separated by a silicone membrane. A ventilator was used to pump air into the air chamber, cyclically displacing the silicone membrane and thereby generating pulsatile flow in the medium chamber. The TEHV was mounted onto a tube in the medium chamber, with a minimal amount of culture medium above the valve and below. By changing the stroke volume and rate of the ventilator, they were able to vary the pressure drop and flow rate across the valve. With this system, they achieved pressures of 10–240 mm Hg and flow rates of 50–2000 mL/min. In one study [28], TEHVs made from myofibroblasts and endothelial cells (ECs) seeded on PGA/P4HB scaffolds were subjected to flow conditions gradually increasing from 35 to 50 mm Hg and 135 to 750 mL/min over 28 days. Compared to controls that were cultured statically, the bioreactor conditioned TEHVs were more robust, contained more collagen, had higher suture retention strengths, and maintained structural integrity throughout the culture period. An additional benefit of this system was the ability to test the TEHVs at physiological pressures immediately before implantation to ensure function.
Hildebrand et al. developed a pulse duplicator system that was similar to the system described above, but had several novel features including electronic control of all components and a precise pneumatic system for waveform generation. The system, shown in Fig. 13.3, consisted of a flow loop containing an atrial chamber that filled a ventricular chamber through a mechanical valve. The ventricular chamber was controlled by a pneumatic system which could generate physiological waveforms to eject culture medium through the TEHV mounted downstream. An electronically controlled resistance element was used to achieve the desired system pressure. Pressure and flow through the system was measured using a pressure transducer and ultrasonic flow probe, respectively [54].
In a study to validate the benefits of this system, TEHVs made from MSCs seeded onto synthetic polymer scaffolds were cultured statically for weeks 1–3 then subjected to physiological pulmonary valve pressure and flow conditions for weeks 4–6, and a four-fold increase in collagen content was observed compared to 6 week static culture samples [37]. It is clear that pulse duplicator systems can have beneficial effects for TEHV growth and remodeling. However, despite providing well-defined pressure waveforms, the applied mechanical stimuli are complex and difficult to control. The leaflet properties and deformation behavior change as the tissue remodels in vitro, the mechanical stimuli applied by these systems are time-dependent and poorly defined.
Instead of attempting to replicate physiological operating conditions, several research groups have developed bioreactors to apply well-defined mechanical stimulation to TEHVs. A Diastolic Pulse Duplicator (DPD) bioreactor (Fig. 13.4) was developed in which cyclic pulses of backpressure were applied resulting in coaptation of the TEHV leaflets by compressing and releasing silicone tubing containing culture medium [33]. The leaflet strain in this system depended on the mechanical properties of the tissue, which varied with time as the tissue matured, an inherent complication as noted above. The dynamic strain in the leaflets increased from 8% to 20% from weeks 2 to 4 in culture as the polymer scaffold degraded and the stiffness of the construct decreased. No significant improvements in collagen production or mechanical properties were observed in the dynamically strained samples compared to TEHVs which were exposed to constant, low flow rate at medium circulation in the DPD system. This lack of improvement may have been because the applied strains in the later weeks of the culture period were too large, as the applied load remained constant while the tissue stiffness decreased. However, Kortsmit et al. proposed a method of implementing a volumetric deformation-controlled feedback loop in which the applied load in the DPD bioreactor was automatically adjusted to achieve the desired deformation [55]. By controlling the overall volumetric deformation of the coapting leaflets, they were able to apply defined average cyclic strain to their TEHV leaflets.
Schematic of Diastolic Pulse Duplicator consisting of a bioreactor chamber (a) and a medium chamber (b). The location of the TEHV is indicated by the red arrow. The bioreactor and medium chambers are connected by tubing (c) and flow is driven by roller pumps (d). Part of the tubing is encased in a polycarbonate cylinder (e), and the injection of air into the cylinder through a magnet valve (f) compresses the tubing and applies cyclic back pressure to the TEHV. A syringe acts as compliance chamber (g), and pressure is monitored by sensors on either side of the TEHV (h). (Reprinted from Mol et al. [33] by permission from Springer Nature: Mol et al. [33], Copyright (2005))
In the Tranquillo research group, a cyclic stretch bioreactor was used to apply controlled pulsatile circumferential deformation to the fibrin-based tubular constructs used to create TEHVs [5, 7, 10, 11]. Previous studies demonstrated increased collagen content and improved mechanical strength and stiffness of fibrin-based vascular grafts subjected to cyclic distension in a similar system [56, 57], and this concept was adapted for the larger tubular constructs used to fabricate TEHVs. After an initial static culture period, the fibrin-based tube is placed over an elastic latex tube for support and mounted between two end pieces then placed in a jar containing culture medium. A reciprocating syringe pump injects medium into one end of the mounted tube, causing the elastic support and tubular construct to distend. The pumped fluid then flows out of a small hole in the other end piece and into the jar of medium surrounding the tubular structure [57, 58]. The distension magnitude can be controlled by the stroke volume of the syringe pump to apply controlled cyclic stretching throughout culture as shown in Fig. 13.5.
Cyclic stretch bioreactor for TEM tubes. Schematic of (a) bioreactor components and (b) reciprocating syringe pump. Culture medium is pumped down through the center of the latex support sleeve and out through the small efflux hole in the lower end piece resulting in cyclic distension of the latex. The TEM tube is placed outside the support sleeve and is cyclically stretched along with the latex. (Reprinted from Schmidt and Tranquillo [58] by permission from Springer Nature: Schmidt and Tranquillo [58], Copyright (2016))
In addition to the development of bioreactors to promote in vitro tissue formation, bioreactor devices can also be used to seed and dynamically condition seeded cells on TEHVs prior to implantation [59, 60]. Sierad et al. developed a bioreactor based on the pulse duplicator system of Hoerstrup et al. [28], which they used to dynamically condition porcine aortic endothelial cells that were seeded on decellularized porcine aortic valves. This resulted in viable and spread cells after 17 days of dynamic conditioning under physiological pulmonary conditions [59]. Using the same system, Kennamer et al. dynamically conditioned decellularized porcine aortic valves seeded with human adipose-derived stem cells. Although the bioreactor performed as intended, at the conclusion of the 24-day study, the majority of the seeded cells had died, highlighting the challenging task of determining the appropriate conditioning regimen for a particular cell type and scaffold combination [60]. As researchers strive to fabricate TEHVs reproducibly with properties suitable for implantation, bioreactor conditioning for both tissue formation and cell conditioning continues to play an important role during fabrication of engineered matrix TEHVs.
2.3 Bioresorbable Polymer TEHVs
Recently, there has been interest in using bioresorbable polymer scaffolds as a basis for “in situ tissue engineering.” In this approach, in vitro culture is not required to degrade the polymer scaffold and replace it with cell-produced ECM. Rather, the polymer scaffold is either implanted directly into the patient, cell-free [61,62,63,64,65,66] or pre-seeded with an autologous cell source such as bone marrow mononuclear cells [67]. Once implanted, the patient’s own cells are recruited to the scaffold and remodel it in situ as the scaffold is absorbed by the body and replaced with ECM.
An advantage to this approach is the ability to reproducibly fabricate scaffolds with the desired geometry, porosity, mechanical properties, and chemical composition. Using this approach, Kluin et al. designed a bioresorbable polymer scaffold comprised of electrospun bis-urea-modified polycarbonate (PC-BU). The PC-BU electrospun tubes were sewn over a polyether ether ketone (PEEK) frame and coated with fibrin [61]. Coyan et al. similarly fabricated a bioresorbable polymer TEHV scaffold from electrospun polycarbonate urethane urea, which was mounted on a degradable magnesium stent [62]. Capulli et al. utilized a Rotary Jet Spinning system to deposit P4HB and gelatin composite fiber scaffolds, creating the bioresorbable JetValve, which supported valve interstitial cell infiltration in vitro [63]. The Xeltis Pulmonary Valved Conduit (XPV) shown in Fig. 13.6 is another example of a bioresorbable polymer valve, consisting of 2-ureido-4[1H]-pyrimidinone (U-Py), with different formulations of U-Py using the root and leaflet structures to give the desired properties [64,65,66].
Xeltis Pulmonary Valved Conduit (XPV). The XPV consists of a UPy synthetic biopolymer scaffold implanted without prior in vitro culture or cell seeding. (Reprinted from Bennink et al. [64] with permission from Elsevier. This article was published in Bennink et al. [64], Copyright American Association for Thoracic Surgery (2018))
Synthetic polymers offer the promise of consistency and scalability, making this approach attractive for “off-the-shelf” valve replacement technologies, however, this approach does rely heavily on the remodeling response in vivo, which can vary from patient to patient and are not yet fully understood.
3 In Vivo Results: Preclinical and Clinical Studies
The ovine model is the current gold standard for preclinical heart valve replacement studies [68]. In a 1995 study designed to explore the feasibility of using TEHVs as replacement heart valves, Shinoka et al. demonstrated that tissue-engineered single leaflets consisting of autologous cells seeded onto a synthetic polyglactin/PGA scaffold functioned well for up to 4 weeks in the pulmonary position in lambs [69]. While a single tissue-engineered leaflet has limited applications, this preliminary study demonstrated that it was possible to implant at least a portion of a TEHV for a short-term in vivo study and paved the way for future pre-clinical ovine model studies.
To date, several research groups utilizing both the TEM and bioresorbable polymer approaches have implanted their TEHVs into sheep to evaluate in vivo performance and remodeling. While the ovine model is the most commonly used, non-human primate and porcine models have also been utilized in TEHV preclinical trials. In the majority of these preclinical studies, TEHVs have been implanted in the pulmonary position, although several groups have studied performance in the aortic position as well. Both surgical and transcatheter approaches have been utilized, depending on the TEHV design.
Table 13.1 provides a summary of recent preclinical studies using TEM TEHVs grouped by approach. In an early study investigating the recellularization potential, Weber et al. implanted a TEM valve, fabricated using human vascular-derived fibroblasts seeded on a PGA/P4HB scaffold, in the pulmonary position in a non-human primate model [9]. Significant leaflet shortening was observed at 8 weeks, resulting in increasing pulmonary regurgitation. However, the TEM valve showed increased recellularization potential compared to decellularized human native heart valve controls, demonstrating the promise of a TEM scaffold for in vivo remodeling. Using a similar approach, Mol et al. implanted an ovine-derived TEM valve as a pulmonary valve replacement in sheep [36]. Although these TEHVs demonstrated good early performance and recellularization potential, by 16 weeks mild regurgitation had developed, progressing to moderate regurgitation at 24 weeks. This degraded valve performance was hypothesized to be caused by a reduction in leaflet mobility due to fusion of the leaflets with the valve wall.
In an effort to overcome the issues of leaflet retraction, Motta et al. utilized a more anatomically relevant valve geometry, incorporating Valsalva sinuses into their stented TEHV design [34, 70]. It was hypothesized that the inclusion of this feature would create hemodynamic conditions more similar to those in the native pulmonary valve and prevent abnormal loading that may promote a contractile phenotype of the infiltrating cells. Ovine-derived TEM valves with Valsalva sinuses were created using a PGA/P4HB scaffold and ovine vascular derived cells, and the resulting TEHVs were implanted in sheep. Although the Valsalva sinus TEHV design was able to be safely implanted, leaflet retraction and increasing regurgitation continued to be an issue, motivating additional optimization of TEHV geometry and stent design [34]. Emmert et al. utilized a computationally driven geometry, which incorporated leaflet belly curvature and an increased coaptation area in an ovine-derived TEM valve design, similarly aiming to produce a more physiological mechanical environment for cells as they repopulate and remodel the TEHV in vivo [8]. TEHVs fabricated in this geometry were implanted in the pulmonary position in sheep, and 9 of 10 implanted TEHVs maintained function at the 52-week follow-up point with trivial to mild insufficiency. The dependence of the cells’ remodeling response on the mechanical environment demonstrated in this study motivates continued investigation into in vivo loading conditions and the use of computational tools to optimize TEHV geometry.
In the Tranquillo laboratory, they have tested fibrin-based TEM TEHVs in several different geometries in the ovine model. This TEM is fabricated using ovine dermal fibroblasts entrapped in a sacrificial fibrin gel cast around a mandrel in a tubular mold and the resulting ovine-derived TEM tubes are decellularized prior to implantation. Syedain et al. implanted TEHVs consisting of a single TEM tube sutured over a Mitroflow® frame (Sorin Group) in the first long-term (6 month) study of a TEM valve in the aortic position [10]. TEHVs were repopulated with interstitial-like cells, and there was evidence of endothelialization and tissue remodeling after 6 months in vivo. No stenosis was observed, however, three of four TEHVs exhibited increasing aortic insufficiency at 3 months that stabilized and remained unchanged until explant. Gross pathology of the valve at explant showed small tears in two of the valves at the frame post, leading to leaflet prolapse, and the entire tube sagged downward around the frame in one of the valves. These frame attachment issues were likely the cause of the observed mild-to-moderate regurgitation.
Building on the demonstrated regenerative potential of this fibrin-based TEM, Reimer et al. surgically implanted their previously discussed “tube-in-tube” design TEHV in the pulmonary position in a growing lamb model [7]. These TEHVs functioned well up to 8 weeks, demonstrating fusion between the tubes after degradation of the suture, but after 8 weeks, pulmonary insufficiency increased. This insufficiency was likely a result of the combined effects of root growth in the growing lamb model without accompanying leaflet growth leading to leaflet shortening, as well as due to inadequate fusion between the two TEM tubes. To address the issue of commissure stability, Syedain et al. developed the previously mentioned “tri-tube” design, in which the loading under diastolic pressure was primarily carried by the matrix rather than the suture line [11]. TEHVs with the tri-tube design (Gen 1) were implanted in the pulmonary position in four growing lambs. Although the Gen 1 tri-tube valves functioned well immediately upon implantation, as the diameter of the valve root increased in the growing lambs (from ~19 to 25 mm over 1 year), tissue growth between the TEM tubes created gaps at commissures, resulting in regurgitation and degraded performance long-term, even though initial leaflet height was maintained.
In an effort to slow the “commissure separation,” a sleeved tri-tube TEHV (Gen 2) was developed, in which a fourth tube was placed around the tri-tube design acting as a sleeve to counteract the faster root growth compared to leaflet growth. Two of the three Gen 2 TEHVs had only trivial to mild regurgitation up to 1 year even as the pulmonary artery grew from 19 to 25 mm, and the third Gen 2 valve developed moderate regurgitation due to a larger-than-expected increase in diameter of the pulmonary artery, again attributed to tissue growth between TEM tubes creating a gap at one commissure. As shown in previous studies, this TEM was suitable for recellularization in vivo, as all explanted TEHVs showed substantial repopulation by interstitial cell types and the presence of an endothelium throughout the length of the root and progressing from the base of the leaflet. Although sparse evidence of microcalcification was present in some leaflet regions at explant, it was noted that both Gen 1 and Gen 2 valves had less calcification and improved function compared to bioprosthetic controls (Hancock 150 valved conduit and Contegra 200 valved conduit) implanted in the same growing lamb model. Figure 13.7 shows representative images of the explanted Gen 2 TEHVs after 12 months in vivo in sheep.
Explanted Gen 2 tri-tube TEHV after 12-month implantation in a growing lamb. (a) Distal view and (b) cut open view showing the three leaflets. (From Syedain et al. [11]. Reprinted with permission from AAAS)
There have been multiple preclinical studies utilizing bioresorbable polymer TEHVs as well. Recent studies using this approach are summarized in Table 13.2. Early efforts investigating the bioresorbable polymer approach utilized PGA/P4HB scaffolds seeded with autologous bone marrow mononuclear cells [71,72,73,74]. Although these early studies had relatively short follow-up periods of 1 month or less, they demonstrated the feasibility of implanting a bioresorbable polymer scaffold to serve as a platform for in situ remodeling. Even in these short-term in vivo studies, there was evidence of cell infiltration and remodeling of the matrix, although mild leaflet shortening resulting in progressive regurgitation was observed [74]. Coyan et al. [62] and Capulli et al. [63] also demonstrated promising acute function in their bioresorbable polymer TEHVs, although additional studies are necessary to investigate long-term function and in situ remodeling.
In a long-term study, bioresorbable polymer valves based on PC-BU scaffolds have been implanted for up to 1 year in sheep with and without pre-seeding with autologous bone marrow mononuclear cells. Unseeded PC-BU TEHVs demonstrated acceptable function up to 1 year, with extensive recellularization with both interstitial-like cells and endothelial cells [61]. The deposited matrix components consisted of collagen, glycosaminoglycans (GAGs), and elastin, including mature elastin fibers. Although some absorption of the biopolymer scaffold had occurred 1 year post implant, scaffold material still remained in the explanted valves, especially in the less cellular regions. In a follow-up study, Fioretta et al. found that pre-seeding this same PC-BU scaffold with autologous bone marrow mononuclear cells was detrimental to long-term function [67]. Pre-seeded TEHVs demonstrated maladaptive remodeling including calcification and leaflet fusion which ultimately led to degraded performance after only 24 weeks in vivo. In addition, Fioretta et al. observed differences in remodeling of the leaflets, even within the same valve and concluded that further investigation into the in situ remodeling process is necessary to ensure the safety of this approach prior to clinical translation. Seeking to better understand the cell–scaffold interaction in PC-BU valves, Uiterwijk et al. implanted unseeded PC-BU TEHVs with randomly oriented or circumferentially oriented polymer fibers [75]. Contrary to their hypothesis, the circumferentially oriented fibers in the initial scaffold did not result in circumferentially oriented collagen deposition, and in fact, explanted scaffolds at 6 and 12 months exhibited isotropic properties regardless of the initial scaffold orientation. The authors additionally noted that there was heterogeneous remodeling of TEHVs in both study groups, again highlighting the need for a better understanding of the in situ remodeling process for bioresorbable polymer TEHVs.
Several preclinical studies have been performed with the Xeltis Pulmonary Valved Conduit in adult sheep, using both transcatheter [76] and surgical [64, 66] approaches. Bennink et al. surgically implanted XPVs in the pulmonary position in sheep for up to 1 year [64]. Degradation and remodeling of the UPy bioresorbable polymer scaffold began around 2 months and continued throughout the 1-year study. XPVs demonstrated acceptable function up to 1 year, and compared with Hancock bioprosthetic valve controls, XPVs showed reduced calcification and neointimal thickening. In a study by Soliman et al., XPVs were shown to be functional with only trace-to-mild regurgitation in most cases 2 years after surgical implantation into sheep [66].
Building on the promising results in the preclinical studies, the first clinical trial with the XPV in children is currently ongoing [65, 77]. Two designs of the XPV were used in this study, with the second design being a modification of the first to address issues observed during early follow up. The original XPV-1 design was surgically implanted in the pulmonary position in 12 children (median age 5) and followed up to 12 months with echocardiography. The modified XPV-2 design with a more homogeneous leaflet thickness was implanted in an additional six children (median age 5) and followed up to 12 months with echocardiography for comparison with the original design. At 12 months post operation, all 18 children were in New York Heart Association (NYHA) functional class 1, with no limitation of physical activity. At 12 months, five patients with the XPV-1 design developed severe regurgitation caused by leaflet prolapse, while only one patient with the XPV-2 design developed more than trace-to-mild regurgitation. One patient with XPV-2 required reoperation due to stenosis that was attributed to a hyperinflammatory response.
Children with the XPV-1 design have completed their 24-month follow up [77]. At 24 months, 9 of 12 children are in NYHA class I and the remaining three children are in NYHA class 2. None of the patients have required reoperation, although five patients have severe pulmonary valve regurgitation due to the leaflet prolapse previously mentioned. While the outcome of this first human study with bioresorbable polymer TEHVs is promising, future work must continue to address valve regurgitation and investigate the growth potential of these grafts in longer term studies.
4 Future Directions
TEHVs are an exciting and attractive alternative to traditional mechanical and bioprosthetic replacement heart valves. Although great progress has been made, there are still challenges that must be overcome before TEHVs are suitable for routine clinical use. The in situ remodeling process is not yet fully understood even in a healthy patient and the remodeling response may be species and age dependent and affected by the disease state of the recipient. Work is ongoing to investigate the variable remodeling observed in both preclinical and clinical trials, as obtaining a predictable and consistent remodeling result will be necessary for the clinical translation of TEHVs [78].
Although longer term preclinical and clinical trials with TEHVs are being conducted, currently full regeneration of the TEHV scaffold with native cells and ECM components has not been demonstrated, in part due to slower recellularization of the leaflets compared to the TEHV root. The challenging task of inducing and accelerating recellularization of leaflets, both for repair and durability in adult applications and for growth in pediatric applications, remains to be solved. Substantial work is still required to understand the factors contributing to the in situ recellularization and remodeling process. Computational models can predict the initial valve function well and such models should be used to optimize design with respect to typical factors like valve performance metrics, solid stress concentration, and hemodynamic factors affecting endothelial phenotype [8, 11]. However, accurate patient-specific prediction of remodeling, yet alone growth, of the implanted material requires further understanding of complex regenerative processes.
In designing a TEHV for pediatric applications, only the growth potential of the root has been clearly demonstrated in the growing lamb model [11, 79, 80]. Although Syedain et al. showed indications of leaflet growth in a growing lamb model (i.e., increase of leaflet-free edge length), the leaflet growth lagged root growth resulting in commissure separation. However, it should be noted that the growth rate of lambs is faster than what would be expected in a pediatric patient [11]. The challenging task of balancing the rate of growth of the patient with the recellularization and growth rate of TEHV leaflets must be addressed in future pediatric TEHV designs. To date, longer-term studies of TEHVs with growth potential have used surgically implanted valves, and while there has also been investigation into the use of biodegradable stents for transcatheter delivery of TEHVs for pediatric applications, these designs have only been tested in acute preclinical studies and regeneration of the protein nanofiber scaffold has yet to be proven [62].
Another area of future investigation is the hemocompatibility and necessary anti-coagulation regimen for both TEM and bioresorbable polymer TEHVs. Although endothelization was reported after implantation in most previously discussed studies, all preclinical and clinical trials discussed used some form of anti-coagulation regimen at implantation, and it is not yet known when or if these treatments can be reduced or terminated. The best strategy to ensure hemocompatibility is likely specific to a particular TEHV material and design. Overall, the long-term outcome for TEHVs is not yet certain, and future studies will seek to determine when regeneration is “complete” and assess the long-term durability, biocompatibility, and hemocompatibility of each TEHV design.
The new approach of 3D bioprinting enables the fabrication of TEHVs with more complex architectures that better mimic native valves and valve mechanics. This approach can create TEHVs with spatially varying mechanical properties and size a TEHV specifically for an individual patient. Despite the potential advantages of this approach, the regenerative capacity of printed TEHVs with the current bio-inks has not yet been demonstrated [44,45,46,47,48,49,50].
In addition to these design-related challenges, there are manufacturing, economic, and regulatory hurdles that must be overcome before TEHVs are available for routine clinical use. As discussed previously, TEHVs can be fabricated using a variety of scaffold materials (bioresorbable polymers or TEM) and cell types (cell free, autologous cells entrapped, or pre-seeded). Especially for cell-based materials, there are significant challenges related to reproducibility and quality control during manufacturing. While bioresorbable polymer TEHVs have an advantage here, standardized manufacturing procedures must be developed to ensure a consistent and safe end product.
The regulatory pathway for TEHVs with regenerative potential, even acellular TEHVs, is unclear, and if TEHVs are classified and regulated as a biological product rather than a medical device, this pathway may be more challenging. Currently, preclinical testing is directed by ISO-5840 from the International Organization for Standardization [81, 82], which was initially designed for traditional mechanical and bioprosthetic valves. Unlike currently available heart valve replacement options, TEHVs undergo extensive in vivo remodeling, and this process will likely result in variability in performance among patients. Factors such as genetic variation, co-morbidities, and medications may lead to heterogeneity in the remodeling response and thus differences in device performance. This patient-specific interaction between device and patient may require additional study and regulatory principles beyond those required for traditional replacement heart valves [83]. In addition, attention must be paid to determining appropriate durability standards for TEHVs in light of the scaffold degradation and remodeling process. The ISO-5840 guidelines require heart valves to last 200 million cycles, but it is unclear whether this standard is appropriate for TEM or synthetic polymer valves where substantial remodeling is expected well before that many cycles.
In terms of cost-effectiveness, as TEHVs are not currently commercially available and fabrication methods vary widely, it is difficult to obtain a good estimate of TEHV cost. Market value depends on performance, unproven yet clinically relevant for TEHVs. From a cost standpoint, it will be difficult for TEHVs to compete with bioprosthetic valves, but they may be able to displace mechanical valves if 20+ year durability without the need for sustained anticoagulation therapy is proven. For pediatric patients, a TEHV would become the dominant valve if growth potential is demonstrated (at least of the root) and at least 5-year function and durability are proven, even if sustained anticoagulation is needed, as the number of cardiopulmonary bypass procedures would be reduced by at least one [84].
5 Summary
The development of a TEHV as a living heart valve replacement offers great promise, particularly for pediatric patients who require a prosthetic valve that can grow and adapt. This chapter provided a brief overview of the methods currently employed for TEHV fabrication, including both the tissue-engineered matrix approach and the bioresorbable polymer approach. Preclinical studies have demonstrated good short-term function and in vivo remodeling capability of THEVs, and an initial clinical trial with a bioresorbable polymer TEHV is currently ongoing. However, despite promising early results, these studies have also elucidated several key issues that must be addressed in future work before TEHVs are suitable for clinical use, and there are a number of manufacturing, regulatory, and economic hurdles that must be overcome.
References
Senthilnathan V, Treasure T, Grunkemeier G et al (1999) Heart valves: which is the best choice? Cardiovasc Surg 7:393–397
Sacks MS, Merryman WD, Schmidt DE (2009) On the biomechanics of heart valve function. J Biomech 42:1804–1824
Sacks MS, Yoganathan AP (2007) Heart valve function: a biomechanical perspective. Philos Trans R Soc Lond Ser B Biol Sci 362:1369–1391
Hinton RB, Yutzey KE (2010) Heart valve structure and function in development and disease. Annu Rev Physiol 17:29–46
Syedain ZH, Meier LA, Reimer JM et al (2013) Tubular heart valves from decellularized engineered tissue. Ann Biomed Eng 41:2645–2654
Dijkman PE, Driessen-Mol A, Frese L et al (2012) Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials 33:4545–4554
Reimer JM, Syedain ZH, Haynie BH et al (2015) Pediatric tubular pulmonary heart valve from decellularized engineered tissue tubes. Biomaterials 62:88–94
Emmert MY, Schmitt BA, Loerakker S et al (2018) Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 10:eaan4587
Weber B, Dijkman PE, Scherman J et al (2013) Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials 34:7269–7280
Syedain Z, Reimer J, Schmidt J et al (2015) 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials 73:175–184
Syedain ZH, Haynie B, Johnson SL et al (2021) Pediatric tri-tube valved conduits made from fibroblast-produced extracellular matrix evaluated over 52 weeks in growing lambs. Sci Transl Med 13:eabb7225
Tudorache I, Horke A, Cebotari S et al (2016) Decellularized aortic homografts for aortic valve and aorta ascendens replacement. Eur J Cardiothorac Surg 50:89–97
Sarikouch S, Horke A, Tudorache I et al (2016) Decellularized fresh homografts for pulmonary valve replacement: a decade of clinical experience. Eur J Cardiothorac Surg 50:281–290
Cebotari S, Tudorache I, Ciubotaru A et al (2011) Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation 124:S115–S123
Boethig D, Horke A, Hazekamp M et al (2019) A European study on decellularized homografts for pulmonary valve replacement: initial results from the prospective ESPOIR Trial and ESPOIR Registry data†. Eur J Cardiothorac Surg 56:503–509
da Costa FD, Costa AC, Prestes R et al (2010) The early and midterm function of decellularized aortic valve allografts. Ann Thorac Surg 90:1854–1860
Helder MR, Kouchoukos NT, Zehr K et al (2016) Late durability of decellularized allografts for aortic valve replacement: a word of caution. J Thorac Cardiovasc Surg 152:1197–1199
Burch PT, Kaza AK, Lambert LM et al (2010) Clinical performance of decellularized cryopreserved valved allografts compared with standard allografts in the right ventricular outflow tract. Ann Thorac Surg 90:1301–1305
da Costa FDA, Etnel JRG, Torres R et al (2017) Decellularized allografts for right ventricular outflow tract reconstruction in children. World J Pediatr Congenit Heart Surg 8:605–612
Zafar F, Hinton RB, Moore RA et al (2015) Physiological growth, remodeling potential, and preserved function of a novel bioprosthetic tricuspid valve: tubular bioprosthesis made of small intestinal submucosa-derived extracellular matrix. J Am Coll Cardiol 66:877–888
van Rijswijk JW, Talacua H, Mulder K et al (2020) Failure of decellularized porcine small intestinal submucosa as a heart valved conduit. J Thorac Cardiovasc Surg 160:e201–e215
Mosala Nezhad Z, Poncelet A, de Kerchove L et al (2017) CorMatrix valved conduit in a porcine model: long-term remodelling and biomechanical characterization. Interact Cardiovasc Thorac Surg 24:90–98
Gilbert CL, Gnanapragasam J, Benhaggen R et al (2011) Novel use of extracellular matrix graft for creation of pulmonary valved conduit. World J Pediatr Congenit Heart Surg 2:495–501
Perri G, Polito A, Esposito C et al (2012) Early and late failure of tissue-engineered pulmonary valve conduits used for right ventricular outflow tract reconstruction in patients with congenital heart disease. Eur J Cardiothorac Surg 41:1320–1325
Simon P, Kasimir MT, Seebacher G et al (2003) Early failure of the tissue engineered porcine heart valve SYNERGRAFT in pediatric patients. Eur J Cardiothorac Surg 23:1002–1006
Rüffer A, Purbojo A, Cicha I, Cesnjevar RA et al (2010) Early failure of xenogenous de-cellularised pulmonary valve conduits--a word of caution! Eur J Cardiothorac Surg 38:78–85
Gonzalez BA, Pour Issa E, Mankame OV et al (2020) Porcine small intestinal submucosa mitral valve material responses support acute somatic growth. Tissue Eng Part A 26:475–489
Hoerstrup SP, Sodian R, Daebritz S et al (2000) Functional living trileaflet heart valves grown in vitro. Circulation 102:III44–III49
Flanagan TC, Cornelissen C, Koch S et al (2007) The in vitro development of autologous fibrin-based tissue-engineered heart valves through optimised dynamic conditioning. Biomaterials 28:3388–3397
Mol A, Rutten MC, Driessen NJ et al (2006) Autologous human tissue-engineered heart valves: prospects for systemic application. Circulation 114:I152–I158
Robinson PS, Johnson SL, Evans MC et al (2008) Functional tissue-engineered valves from cell-remodeled fibrin with commissural alignment of cell-produced collagen. Tissue Eng Part A 14:83–95
Sutherland FW, Perry TE, Yu Y et al (2005) From stem cells to viable autologous semilunar heart valve. Circulation 111:2783–2791
Mol A, Driessen NJB, Rutten MCM et al (2005) Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Ann Biomed Eng 33:1778–1788
Motta SE, Lintas V, Fioretta ES et al (2019) Human cell-derived tissue-engineered heart valve with integrated Valsalva sinuses: towards native-like transcatheter pulmonary valve replacements. NPJ Regen Med 4:14
Lintas V, Fioretta ES, Motta SE et al (2018) Development of a novel human cell-derived tissue-engineered heart valve for transcatheter aortic valve replacement: an in vitro and in vivo feasibility study. J Cardiovasc Transl Res 11:470–482
Driessen-Mol A, Emmert MY, Dijkman PE et al (2014) Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. J Am Coll Cardiol 63:1320–1329
Ramaswamy S, Gottlieb D, Engelmayr GC Jr et al (2010) The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells. Biomaterials 31:1114–1125
Flanagan TC, Sachweh JS, Frese J et al (2009) In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Eng Part A 15:2965–2976
Syedain ZH, Tranquillo RT (2009) Controlled cyclic stretch bioreactor for tissue-engineered heart valves. Biomaterials 30:4078–4084
Syedain ZH, Lahti MT, Johnson SL et al (2011) Implantation of a tissue-engineered heart valve from human fibroblasts exhibiting short term function in the sheep pulmonary artery. Cardiovasc Eng Technol 2:101–112
Weber M, Gonzalez de Torre I, Moreira R et al (2015) Multiple-step injection molding for fibrin-based tissue-engineered heart valves. Tissue Eng Part C Methods 21:832–840
Weber M, Heta E, Moreira R et al (2014) Tissue-engineered fibrin-based heart valve with a tubular leaflet design. Tissue Eng Part C Methods 20:265–275
Moreira R, Velz T, Alves N et al (2015) Tissue-engineered heart valve with a tubular leaflet design for minimally invasive transcatheter implantation. Tissue Eng Part C Methods 21:530–540
Filho ALL, Cheung PYC, Noritomi PY et al (2010) Construction and adaptation of an open source rapid prototyping machine for biomedical research purposes-a multinational collaborative development. In: Bartolo PJ et al (eds) Innovative developments in design and manufacturing: advanced research in virtual and rapid prototyping. CRC Press Taylor & Francis Group, London, pp 469–474
Hockaday LA, Kang KH, Colangelo NW et al (2012) Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication 4:035005
Duan B, Kapetanovic E, Hockaday LA et al (2014) Three-dimensional printed trileaflet valve conduits using biological hydrogels and human valve interstitial cells. Acta Biomater 10:1836–1846
Ma N, Cheung DY, Butcher JT (2022) Incorporating nanocrystalline cellulose into a multifunctional hydrogel for heart valve tissue engineering applications. J Biomed Mater Res A 110(1):76–91
Hockaday LA, Duan B, Kang KH et al (2014) 3D-printed hydrogel technologies for tissue-engineered heart valves. 3D Print Addit Manuf 1:122–136
Kang LH, Armstrong PA, Lee LJ et al (2017) Optimizing photo-encapsulation viability of heart valve cell types in 3D printable composite hydrogels. Ann Biomed Eng 45:360–377
Maxson EL, Young MD, Noble C et al (2019) In vivo remodeling of a 3D-bioprinted tissue engineered heart valve scaffold. Bioprinting 16:e00059
Neidert MR, Lee ES, Oegema TR et al (2002) Enhanced fibrin remodeling in vitro with TGFbeta 1, insulin and plasmin for improved tissue-equivalents. Biomaterials 23:3717–3731
Berry JL, Steen JA, Koudy Williams J et al (2010) Bioreactors for development of tissue engineered heart valves. Ann Biomed Eng 38:3272–3279
Hoerstrup SP, Sodian R, Sperling JS et al (2000) New pulsatile bioreactor for in vitro formation of tissue engineered heart valves. Tissue Eng 6:75–79
Hildebrand DK, Wu ZJ, Mayer JE Jr et al (2004) Design and hydrodynamic evaluation of a novel pulsatile bioreactor for biologically active heart valves. Ann Biomed Eng 32:1039–1049
Kortsmit J, Rutten MC, Wijlaars MW et al (2009) Deformation-controlled load application in heart valve tissue engineering. Tissue Eng Part C Methods 15:707–716
Syedain ZH, Weinberg JS, Tranquillo RT (2008) Cyclic distension of fibrin-based tissue constructs: evidence of adaptation during growth of engineered connective tissue. Proc Natl Acad Sci U S A 105:6537–6542
Syedain ZH, Meier LA, Bjork JW et al (2011) Implantable arterial grafts from human fibroblasts and fibrin using a multi-graft pulsed flow-stretch bioreactor with noninvasive strength monitoring. Biomaterials 32:714–722
Schmidt JB, Tranquillo RT (2016) Cyclic stretch and perfusion bioreactor for conditioning large diameter engineered tissue tubes. Ann Biomed Eng 44:1785–1797
Sierad LN, Simionescu A, Albers C et al (2010) Design and testing of a pulsatile conditioning system for dynamic endothelialization of polyphenol-stabilized tissue engineered heart valves. Cardiovasc Eng Technol 1:138–153
Kennamer A, Sierad L, Pascal R et al (2016) Bioreactor conditioning of valve scaffolds seeded internally with adult stem cells. Tissue Eng Regen Med 13:507–515
Kluin J, Talacua H, Smits AI et al (2017) In situ heart valve tissue engineering using a bioresorbable elastomeric implant – from material design to 12 months follow-up in sheep. Biomaterials 125:101–117
Coyan GN, D’Amore A, Matsumura Y et al (2019) In vivo functional assessment of a novel degradable metal and elastomeric scaffold-based tissue engineered heart valve. J Thorac Cardiovasc Surg 157:1809–1816
Capulli AK, Emmert MY, Pasqualini FS et al (2017) JetValve: rapid manufacturing of biohybrid scaffolds for biomimetic heart valve replacement. Biomaterials 133:229–241
Bennink G, Torii S, Brugmans M et al (2018) A novel restorative pulmonary valved conduit in a chronic sheep model: mid-term hemodynamic function and histologic assessment. J Thorac Cardiovasc Surg 155:2591–2601.e3
Morales DL, Herrington C, Bacha EA et al (2021) A novel restorative pulmonary valve conduit: early outcomes of two clinical trials. Front Cardiovasc Med 7:583360
Soliman OI, Miyazaki Y, Abdelghani M et al (2017) Midterm performance of a novel restorative pulmonary valved conduit: preclinical results. EuroIntervention 13:e1418–e1427
Fioretta ES, Lintas V, Mallone A et al (2019) Differential leaflet remodeling of bone marrow cell pre-seeded versus nonseeded bioresorbable transcatheter pulmonary valve replacements. JACC Basic Transl Sci 5:15–31
Iaizzo PA (2009) Handbook of cardiac anatomy, physiology, and devices, 2nd edn. Springer, New York, p 398
Shinoka T, Breuer CK, Tanel RE et al (1995) Tissue engineering heart valves: valve leaflet replacement study in a lamb model. Ann Thorac Surg 60:S513–S516
Motta SE, Fioretta ES, Dijkman PE et al (2018) Development of an off-the-shelf tissue-engineered sinus valve for transcatheter pulmonary valve replacement: a proof-of-concept study. J Cardiovasc Transl Res 11:182–191
Emmert MY, Weber B, Behr L et al (2014) Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. Eur J Cardiothorac Surg 45:61–68
Emmert MY, Weber B, Wolint P et al (2012) Stem cell-based transcatheter aortic valve implantation: first experiences in a pre-clinical model. JACC Cardiovasc Interv 5:874–883
Emmert MY, Weber B, Behr L (2011) Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves: first experiences in the systemic circulation. JACC Cardiovasc Interv 4:822–823
Weber B, Scherman J, Emmert MY et al (2011) Injectable living marrow stromal cell-based autologous tissue engineered heart valves: first experiences with a one-step intervention in primates. Eur Heart J 32:2830–2840
Uiterwijk M, Smits AIPM, van Geemen D et al (2020) In situ remodeling overrules bioinspired scaffold architecture of supramolecular elastomeric tissue-engineered heart valves. JACC Basic Transl Sci 5:1187–1206
Miyazaki Y, Soliman OII, Abdelghani M et al (2017) Acute performance of a novel restorative transcatheter aortic valve: preclinical results. EuroIntervention 13:e1410–e1417
Prodan Z, Mroczek T, Sivalingam S et al (2022) Initial clinical trial of a novel pulmonary valved conduit. Semin Thorac Cardiovasc Surg 34(3):985–991
Fioretta ES, Motta SE, Lintas V et al (2021) Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat Rev Cardiol 18:92–116
Syedain Z, Reimer J, Lahti M et al (2016) Tissue engineering of acellular vascular grafts capable of somatic growth in young lambs. Nat Commun 7:12951
Tudorache I, Theodoridis K, Baraki H et al (2016) Decellularized aortic allografts versus pulmonary autografts for aortic valve replacement in the growing sheep model: haemodynamic and morphological results at 20 months after implantation. Eur J Cardiothorac Surg 49:1228–1238
International Organization for Standardization (2021) Cardiovascular implants – cardiac valve prostheses – part 1: general requirements (ISO 5840-1)
International Organization for Standardization (2021) Cardiovascular implants – cardiac valve prostheses – part 2: surgically implanted heart valve substitutes (ISO 5840-2)
Zhang BL, Bianco RW, Schoen FJ (2019) Preclinical assessment of cardiac valve substitutes: current status and considerations for engineered tissue heart valves. Front Cardiovasc Med 6:72
Huygens SA, Rutten-van Molken M, Noruzi A et al (2019) What is the potential of tissue engineered pulmonary valves in children? Ann Thorac Surg 107:1845–1853
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schmidt, J.B., Syedain, Z.H., Tranquillo, R.T. (2023). Tissue-Engineered Heart Valves. In: Iaizzo, P.A., Iles, T.L., Griselli, M., St. Louis, J.D. (eds) Heart Valves. Springer, Cham. https://doi.org/10.1007/978-3-031-25541-0_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-25541-0_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25540-3
Online ISBN: 978-3-031-25541-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)