Abstract
Transcatheter aortic valve replacement (TAVR) is being extended to younger patients. However, TAVR-compatible bioprostheses are based on xenogeneic materials with limited durability. Off-the-shelf tissue-engineered heart valves (TEHVs) with remodeling capacity may overcome the shortcomings of current TAVR devices. Here, we develop for the first time a TEHV for TAVR, based on human cell-derived extracellular matrix and integrated into a state-of-the-art stent for TAVR. The TEHVs, characterized by a dense acellular collagenous matrix, demonstrated in vitro functionality under aortic pressure conditions (n = 4). Next, transapical TAVR feasibility and in vivo TEHV functionality were assessed in acute studies (n = 5) in sheep. The valves successfully coped with the aortic environment, showing normal leaflet motion, free coronary flow, and absence of stenosis or paravalvular leak. At explantation, TEHVs presented full structural integrity and initial cell infiltration. Its long-term performance proven, such TEHV could fulfill the need for next-generation lifelong TAVR prostheses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcatheter aortic valve replacement (TAVR) was introduced to address patients deemed to be ineligible to surgical aortic valve replacement, but it has tremendously evolved since then, becoming the standard of care for severe aortic stenosis in inoperable and high-risk patients [1]. Several randomized controlled clinical trials reported TAVR superiority for inoperable patients and non-inferiority in high-risk patients when compared to conventional surgical techniques [2, 3]. Recent data also demonstrated the efficacy and non-inferiority of TAVR in intermediate-risk patients with aortic stenosis at 2-year follow-up [4, 5]. Therefore, based on these encouraging outcomes, the indication for TAVR is expected to be further extended to lower-risk and younger patients in the near future and clinical trials are underway [6].
However, current TAVR stented devices are made of glutaraldehyde-fixed xenomaterials as used for surgical bioprostheses. Durability is the major concern of bioprostheses, and with the onset of calcification and structural failure of the bioprostheses within 10–15 years [7, 8], the application in younger patients is limited.
Few data are available on the lifespan of TAVR devices [9], but, so far, the reported failure modes were design-correlated or similar to those of surgically implanted bioprostheses [10, 11]. While the former will be presumably overcome with the design improvements of the next-generation devices [12] (e.g., introduction of outer skirts to prevent from paravalvular leakage, delivery systems that are retrievable and repositionable, low-profile frames to limit the risk of coronary ostia obstruction), the latter are mostly related to the valve material used for this approach. It is reasonable to assume that the long-term failure mode of TAVR prostheses will be prevalently a structural valve failure, similarly to current surgical bioprostheses [8, 13]. Since TAVR is now being extended to intermediate-risk and younger patients, the longevity of TAVR prostheses has become crucial.
To solve the issue of valve durability, tissue engineering has been repeatedly suggested as a potential solution in the past years [14]. Specifically, tissue-engineered heart valves (TEHVs) obtained from decellularized engineered extracellular matrix (ECM) represent a clinically relevant, off-the-shelf available option. The decellularization step is essential to inhibit the immune response to the implant and provide off-the-shelf availability, with minimal alterations of the mechanical or structural features of the matrix [15]. In addition, the combination of such TEHVs with minimally invasive implantation techniques has already successfully advanced into preclinical evaluation in the low-pressure system [16,17,18,19]. In these studies, such TEHVs showed good functionality as pulmonary valve replacement, together with remarkable host cell repopulation and tissue remodeling capacity towards native-like features in vivo. Due to this remodeling potential, such TEHVs may represent a valid option to solve the problem of long-term prosthesis durability.
However, so far, this promising transcatheter ECM-based TEHV concept has been investigated only in the pulmonary setting and by using ovine cells for the development of the engineered ECM [16,17,18]. The transition from pulmonary to aortic replacement is limited by the unique and complex anatomy of the aortic root and by the demanding hemodynamic loading of the systemic circulation. When coupling TEHV and TAVR approaches, the former challenge is addressed by using stents with low-profile frames and delivery systems capable of controlled valve deployment, to ensure open flow to the coronary arteries. On the other hand, the biomaterial properties have to be optimized to obtain a TEHV capable to withstand the aortic hemodynamic conditions.
In the present study, we describe the developmental process and in vivo validation of an off-the-shelf human cell-derived TEHV optimized for TAVR using a state-of-the-art anatomically orienting transcatheter system. With respect to our previous transcatheter TEHVs, investigated in the pulmonary circulation, we here (1) enhanced the translational potential of such approach by developing a novel protocol to manufacture TEHVs based on human cell-derived ECM, (2) systematically improved the biomechanical and structural properties of our valve to cope with the aortic high-pressure environment, and (3) developed a novel valve design adapted to state-of-the-art anatomically orienting TAVR system. Finally, we have validated in vivo our novel TAVR-compatible TEHV using a translational sheep model.
Methods
Patch Manufacturing and Characterization
Scaffold Preparation
Patches (n = 18) were produced with non-woven polyglycolic acid meshes (thickness 1.0 mm; specific gravity 70 mg/cm3; Cellon) coated with 1% poly-4-hydroxybutyrate (MW 1 × 106; TEPHA Inc.) in tetrahydrofuran (Fluka, Sigma-Aldrich). The scaffolds were sutured to stainless steel rings (external ∅ = 28 mm, Hasler) to counteract tissue retraction during culture and sterilized with 30-min incubation in 70% ethanol followed by 30-min incubation in PBS supplemented with 2% amphotericin (Lonza).
Cell Expansion, Seeding, and Tissue Culture
After expansion in DMEM Advanced (Gibco, Invitrogen), supplemented with 10% fetal bovine serum (Gibco), 1% GlutaMax (Gibco), and 1% penicillin-streptomycin (Lonza), neonatal human dermal fibroblasts (CellSystems Biotechnologie Vertrieb GmbH, three donors) were seeded onto the patches (1 × 106 cells/cm2, passages 5–6) using fibrin as cell carrier [20]. The patches were then cultured at 37 °C on an orbital shaker for 6 weeks, with (n = 9, 5 ng/mL) or without transforming growth factor-beta1 (TGF-β1, Peprotech) (n = 9).
Decellularization
After tissue culture, the patches were decellularized adjusting a previously developed protocol [15]. Briefly, the constructs were washed in PBS and decellularized twice in a detergent solution (0.25% Triton X-100, sodium deoxycholate, and 0.02% ethylenediaminetetraacetic acid). To enzymatically degrade the remaining nucleic remnants, Benzonase (EMD Millipore) incubation steps (for 8 h, on a shaker at 37 °C) in decreasing concentrations of 100, 80, and 20 U/mL in 50 mM TRIS–HCl buffer solution were used, and followed by washing steps in PBS.
(Immuno)histology
After fixation in 4% formalin and paraffin embedding, 5-μm thick slices were cut longitudinally from all patches. The presence of cells and tissue morphology were analyzed with hematoxylin and eosin (H&E) staining, while the deposition of the collagenous matrix was examined with collagen-1 (Col-1, Abcam, ab34710) and collagen-3 (Col-3, Abcam, ab7778) stainings. Elastica van Gieson (ELVG) staining was included to detect the presence of elastic fibers and collagen. The stained samples were imaged with brightfield microscopy (Mirax Midi Microscope, Carl Zeiss GmbH) and assessed with Pannoramic Viewer software. The thickness of the patches (n = 9 per group) was manually determined from the histological images at ten random locations per leaflet and averaged. In order to correct the supposed loss of tissue volume due to tissue processing, a volume shrinkage correction factor of 1.15 was employed [21].
DNA and ECM Analyses
The composition of all patches was analyzed by means of biochemical assays as previously described [15] for the quantification of total content of deoxyribonucleic acid (DNA), sulfated glycosaminoglycans (GAGs), and hydroxyproline (Hyp), an indicator for collagen formation. Data are reported as total content normalized by the total dry weight of the samples.
Valve Manufacturing
Scaffold Preparation
Human ECM-based off-the-shelf TEHVs (n = 13) were manufactured using a newly developed protocol. Tubular scaffolds (n = 13) were produced from non-woven polyglycolic acid meshes coated with 1% poly-4-hydroxybutyrate in tetrahydrofuran. The scaffolds were integrated into cylindrical stents (28 mm diameter) to provide dimensional support and prevent from tissue retraction during culture, with an intermediate layer of manually punctured paraffin paper (Parafilm M, Sigma-Aldrich) to allow for medium exchange (Fig. 1a–c).
In vitro production of TEHVs. a The cylindrical support used for tissue culture is covered by a b manually punctured paraffin paper layer. c The scaffold is then secured around the support and d seeded with human dermal fibroblasts. e After 6 weeks of culture in a perfusion bioreactor, the tissue-engineered constructs are removed from the support, f inserted, and g sutured in a JenaValve stent. h The extra-tissue of the lower region is removed and i the leaflets are cut and shaped using a custom-made mold
Cell Expansion, Seeding, and Tissue Culture
As previously described, neonatal human dermal fibroblasts (four donors) were expanded in DMEM Advanced and then seeded onto the tubular scaffold (1 × 106 cells/cm2, passages 5–6) using fibrin as cell carrier [20] (Fig. 1d). Following seeding, the tubular scaffolds were placed into a Diastolic Pulse Duplicator system for culture [21], to ensure pulsatile medium perfusion for 6 weeks (Fig. 1e). Tissue culture medium was supplemented with l-ascorbic acid 2-phosphate (0.25 mg/mL; Sigma-Aldrich) and, from the second week of culture, with TGF-β1 (5 ng/mL) and replaced every 2–3 days. After tissue culture, the tubular constructs were decellularized as described above.
Valve Manufacturing
After removal of the cylindrical support, the tubular construct was subsequently sewn into JenaValve self-expanding nitinol stents (JenaValve Technology Inc.) (Fig. 1f), and trileaflet valves were obtained by folding, cutting, and pre-shaping the decellularized engineered tubes (Fig. 1g–i). The cutting of the cusps was optimized by using a mold to ensure reproducibility. Subsequently, the leaflets were pre-shaped with specific inserts [22]. The engineered valves were then sterilized by immersion in 70% ethanol and antibiotic treatment [16, 22] and stored at 4 °C in DMEM medium (Gibco) until further use.
In Vitro Valve Characterization
In Vitro Valve Functionality
TEHVs (n = 4) were employed for in vitro functionality assessment in a custom-made hydrodynamic pulsatile mock circulatory system (LifeTec Group) in conformity with ISO5840-1 for 1 h. The valves, placed in custom-made housing with an internal diameter of 23 mm, were subjected to normotensive aortic conditions (rate of 70 bpm, systolic duration of 35%, pressure of 80/120 mmHg) at 37 °C. The test fluid consisted of 0.05% xanthan gum solution. Flow and pressures were respectively measured by means of transonic flow sensors (LifeTec Group; Transonic Systems, and SonoTT Em-Tec) and pressure sensors (Becton Dickinson). In the first 2 min, after 30 min, and after 60 min, high-speed video acquisitions (MS40K, MegaSpeed) and hydrodynamic data measurement/calculation were performed, to extract the following parameters: effective orifice area (EOA), cardiac output (CO), regurgitant fraction (RF), leakage volume, and closing volume, in accordance with ISO5840-1.
Immunohistology
Representative samples from control and implanted TEHVs were cut longitudinally, fixed in 4% formalin, and qualitatively evaluated with (immuno)histology, in comparison with native ovine aortic valve leaflets obtained from slaughterhouse material. As described for the patches, the presence of cells and tissue morphology were analyzed with H&E staining, Col-1 staining, and ELVG staining. The stained samples were imaged with brightfield microscopy and assessed with Pannoramic Viewer software. The leaflet thickness of TEHVs (n = 18, two leaflets per nine valves) and native controls (n = 4, one leaflet per valve) was manually determined at ten random locations per leaflet, averaged, and corrected for volume shrinking [21].
DNA and ECM Analyses
DNA, GAGs, and Hyp were quantified in representative samples from TEHVs (n = 12) and native valvular tissue (n = 5) and normalized by the total dry weight of the samples as described in previous sections.
Mechanical Tests
The mechanical properties of the TEHVs were tested with suture retention strength (SRS) tests, in compliance with the ANSI/AAMI/ISO 7198 Standard [23]. Rectangular samples (n = 8, minimum dimensions of 10 × 20mm) were clamped on one of the short sides and sutured (5/0 suture threads, YAVO) at the opposite side, at a distance of 3 mm from the sample’s free edge, and pulled at a displacement speed of 0.2 mm/s until failure, in a custom-built setup endowed with 20 N load cells (MTS Systems). The force corresponding to crack propagation (break starting strength) and the peak force typically measured in the SRS tests were measured as previously described [23].
Transapical Aortic Implantation of TEHVs in Sheep
All animals received humane care in compliance with the “Principles of Laboratory Animal-Care” and the “Guide for the care and use of laboratory animals” of the National Institutes of Health. All procedures received the approval of the Cantonal Veterinary Office (license number ZH-048-2015) and performed in accordance with the European Union guidelines (86/609/EEC) and Swiss Federal animal protection law and ordinance. To evaluate the feasibility of using the newly developed TEHVs as aortic valve substitutes, the valves (n = 5) were implanted transapically in the orthotopic aortic position in sheep [24]. The animals were selected based on their annulus size (22.4 ± 1.5 mm), measured by echocardiography, and received a 27-mm stented valve. Prior to implantation, the anatomical site (n = 2) was reconstructed with three-dimensional computer tomography (3D-CT).
The TEHVs were crimped and loaded onto the capsule of a Cathlete transapical delivery system (JenaValve), having an outer diameter of 32Fr (Supplementary Fig. 1). After a mini-sternotomy and the insertion of 5/0 Prolene pledgeted, purse-string sutures, the apex of the left ventricle was punctured, and the TEHVs were delivered to the implantation site under fluoroscopy guidance (ALLURA FD 20/20, Koninklijke Philips Electronics N.V.) with a three-step deployment procedure. After full valve deployment, the positioning of the TEHVs was confirmed by fluoroscopy and contrast angiography. Postoperatively and before sacrifice, transesophageal echocardiography (TEE) was employed to evaluate valve functionality (Philips Healthcare iE33W xMATRIX Ultrasound). The animals were followed up for up to 6 h. Before explantation, postoperative 3D-CT reconstruction of the implantation site was performed to confirm appropriate TEHV positioning and orientation (n = 2).
After sacrifice, gross examination of the TEHVs was performed to assess the positioning and verify the structural integrity of the leaflets. Subsequently, representative samples from all TEHVs were analyzed with H&E staining and DNA quantification.
Statistics
Quantitative data concerning mechanical analyses, thickness measurement, and assessment of DNA, GAGs, and Hyp content were averaged per sample and subsequently per group, and they are provided here as the average ± standard deviation. One-way ANOVA with a post hoc Mann-Whitney test was implemented for comparing the TEHVs to the native tissue or comparing the control TEHVs with the explanted valves, with p < 0.05 considered statistically significant. To compare control and TGF-β1 supplemented patches, an unpaired non-parametric two-tailed t test with Kolmogorov-Smirnov test was employed. Statistics were elaborated with Prism software (v. 7, GraphPad).
Results
Patch Manufacturing and Characterization
After 6 weeks of in vitro culture all patches (n = 18) presented shiny, uniform tissue deposition.
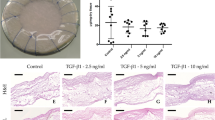
Qualitatively, the structure of the newly produced ECM was more compact in patches cultured with TGF-β1 than in the controls (Fig. 2a–d). The supplementation of TGF-β1 resulted in higher Col-1 and Col-3 expressions (Fig. 2e–h). Patch thickness between TGF-β1 and control groups was not statistically different (506.958 ± 92.925 μm and 438.534 ± 93.589 μm respectively, Fig. 2i). The addition of TGF-β1 resulted in significantly higher GAG deposition (p = 0.0667) and lower but more reproducible Hyp production (p = 0.0667) compared to control samples (Fig. 2j).
Optimization of human cell-derived in vitro-grown extracellular matrix. a–h Histological evaluation of patches cultured with human dermal fibroblasts for 6 weeks without or with TGF-β1 showing qualitatively more abundant neotissue formation in patches supplemented with TGF-β1 (b, d, f, h). Scale bars indicate 100 μm. i Thickness measurement from histological images. j Quantitative extracellular matrix analysis, expressed as GAGs and Hyp amount in the control and TGF-β1-treated patches. Asterisk indicates p ≤ 0.05
Valve Manufacturing and Characterization In Vitro
Manufacturing, In Vitro Functionality, and Tissue Organization
After 6 weeks of in vitro culture in the bioreactor, the macroscopic examination of the decellularized tubes (n = 13) presented a shiny and uniform neo-tissue deposition through the entirety of the construct (Fig. 3a). Neither the subsequent cutting, integration within the stents, nor crimping induced any macroscopically visible damage or tear (Fig. 3b).
In vitro characterization of TEHVs. a Macroscopic appearance of the decellularized tissue-engineered tubular constructs and b of the final TEHV incorporated in the JenaValve stent (top, bottom, and side view). c Pressures and transvalvular flow (qa) after 1 h of in vitro functionality assessment in a diastolic pulse duplicator system for a representative TEHV; right side: blue and red indicate ventricular pressure (Pv) and aortic pressure (Pa) respectively. d Representative movie stills of the opening and closure behavior of the TEHV during the cardiac cycle simulated in the pulse duplicator system. Representative images of e H&E and f ELVG stainings of a TEHV show the presence of collagen (pink) and elastic network (black). Elastic fibers are sporadically detected in the i TEHV (black arrows). g Col-1 is abundant but not homogeneously distributed in the tissue. h Representative images of H&E staining performed on a native aortic valve. Scale bars indicate 100 μm. j Thickness cusp measurement for TEHVs and native ovine aortic valves. k Quantitative biochemical analysis to measure DNA, GAGs, and Hyp amount in the TEHVs and native ovine aortic valves (AVs). Single, double, or triple asterisks indicate p ≤ 0.05, p ≤ 0.01, or p ≤ 0.001 respectively
All tested TEHVs (n = 4) successfully withstood the hydrodynamic valve tester for 1 h under physiologic aortic conditions, showing complete and normal opening and closing behavior (Fig. 3c, d). No decrease in valve functionality was observed in any of the TEHVs over time. During valve opening, the TEHVs presented with EOA = 2.875 ± 0.263 cm2 under a CO of 5.050 ± 0.058 L/min. Values obtained for RF (87.750 ± 7.974%) and leakage volume (60.750 ± 5.737 mL) were considered not representative of valve functionality, being significantly affected by the geometrical mismatch between the cylindrical valve holder and the JenaClip technology of the stent. None of the valves showed evidence of leaflet rupture or tears in the commissure points after test.
Tissue Organization
Histological analyses of the decellularized TEHVs confirmed the macroscopic observations (Fig. 3e–g, Supplementary Fig. 2), showing a dense collagenous matrix. Negligible cell nuclear remnants were detected within the dense tissue-engineered matrix (Fig. 3e). The ELVG staining also confirmed the presence of differently dense collagen layers through the transversal plane (Fig. 3f) and indicated the presence of sparse elastic fibers (Fig. 3i). The ECM was abundant in Col-1 (Fig. 3g). The TEHVs were found to be significantly thicker than the native leaflets (p = 0.0424) (Fig. 3j).
Tissue Composition and Mechanical Properties
The quantitative measurement of DNA amount in the decellularized TEHVs was 0.093 ± 0.224 μg/mg dry tissue, confirming the histological findings and suggesting an almost complete decellularization prior to implantation [25]. The biochemical assays also confirmed the presence of GAGs and abundant Hyp (7.448 ± 1.628 and 28.834 ± 6.184 μg/mg dry tissue respectively, as shown in Fig. 3k), which were, however, lower than in the native valves (with p = 0.0194 and p = 0.0061 respectively). The break starting strength obtained from the loading curve of the samples during the SRS test was 0.6639 ± 0.2643 N; the peak force was 0.9915 ± 0.3473 N.
Transapical Aortic Implantation of TEHVs in Sheep
Valve Delivery and Acute Functionality
Transapical aortic delivery of TEHVs was successful in all animals (Fig. 4) except for one case (n = 4/5), in which due to technical problems during the stepwise deployment, one of the leaflets of the TEHV was entrapped by the corresponding native leaflet, affecting the functionality of that valve. Of the five implanted valves, four TEHVs could be correctly positioned in the orthotopic aortic position, excluding the native leaflets and showing appropriate positioning, valve functionality, and perfusion of the coronary arteries (Fig. 4i, j, Online Resource Video 1).
Transapical delivery of TEHVs into aortic position under fluoroscopy guidance. Schematic representation of the transapical implantation route for aortic valve replacement (a). Upon imaging the native aortic root (b), the JenaValve delivery system was inserted (c) and aligned to the implantation site (d). The stent feelers were therefore released (e, white arrows) and positioned in correspondence to the native leaflets (f), prior to expanding the stent in two subsequent steps (g, h). Fluoroscopy evaluation of the deployed valve confirmed proper positioning, valve functionality (i), and unobstructed coronary arteries (j, black arrows)
All animals remained stable during the entire implantation procedure, without experiencing any adverse events (i.e., thromboembolic events, bleeding, cardiac arrhythmia).
Fluoroscopy and 2D and 3D TEE confirmed good acute functionality of the TEHVs in vivo (Fig. 4 and 5a–f, Online Resource Videos 1–4).
Echocardiographic and 3D-CT evaluation of implanted TEHVs. Echocardiographic assessment of valve functionality in vivo, showing leaflet motion and functionality (2D: a, d, 3D: c, f) in systole (b) and diastole (e). Pre-operative (g) and postoperative (h) 3D-CT reconstruction confirming the correct positioning of the TEHV
Immediately upon implantation, the TEHVs demonstrated to withstand the aortic hemodynamic loading, with normal leaflet motion, sufficient opening/closing pattern, and a good coaptation area. No acute structural leaflet failure or tearing was observed. The mean transvalvular gradient was 3 ± 0.8 mmHg (Table 1). In presence of optimal prostheses sizing, the central insufficiency was none-to-trivial, while two animals showed mild-to-moderate central aortic regurgitation due to suboptimal prosthesis-to-native annulus size match. One animal revealed severe eccentric aortic regurgitation, due to the entrapment of one leaflet of the TEHV in the native aortic valve (Table 1). Neither paravalvular leakage, stent migration or dislocation, or affection of the mitral valve were observed for any of the animals confirming good positioning, orientation, and sizing of the valve (Table 1). The TEHVs were explanted after planned animal sacrifice (follow-up for up to 6 h).
Postoperative 3D-CT, Gross Examination, and Histology
Postoperative CT reconstruction of the aortic root, performed before explantation, showed correct TEHV positioning and orientation (Fig. 5h).
At explantation, the gross examination of the TEHVs showed intact leaflets, without signs of thrombi, tears or ruptures, and free native coronary arteries, and confirmed appropriate positioning within the aortic root (Fig. 6b, c). No crimping-associated tissue damages were observed macroscopically.
Macroscopic and histological appearance of explanted TEHVs. a TEHV prior to implantation (side and top view), b upon harvesting in situ (aortic and ventricle side view) and c upon explantation (side and aortic view). d, e H&E staining revealed minimal fibrin coverage and the presence of infiltrated cells within the matrix (top: aortic side, bottom: ventricular side. D: whole leaflet - scale bar 2 mm; E: scale bar 200 μm)
Histology demonstrated negligible fibrin deposition onto the leaflet surface (Fig. 6d, e and Supplementary Fig. 2) and preserved tissue structure, together with substantial early cell infiltration (Fig. 6e). This was further supported by biochemical analysis in the explanted valves which showed significantly higher amounts of DNA when compared to the non-implanted control valves (0.596 ± 0.682 versus 0.093 ± 0.224 μg/mg dry tissue respectively; p = 0.0268).
Discussion
TAVR is in the process to be extended to lower-risk, younger patients in the near future [26]. Compared to the standard of care for valve replacement, TAVR is less invasive for the patient and requires shorter hospitalization, being an attractive alternative to open-heart surgery [27]. However, the prostheses currently employed for TAVR are known to undergo continuous structural degeneration and calcification [11, 13]. Hence, with the prospect to extend TAVR to younger patients, the demand for lifelong-durable valve substitutes, able to self-repair and self-regenerate, has become urgent.
Here, for the first time, we successfully applied in vitro-grown ECM-based TEHVs as aortic valve replacement by using a clinically relevant, anatomically orienting transcatheter delivery system [28]. We firstly implemented a new protocol for the culture and manufacturing of TEHVs based on human in vitro-grown ECM. The developed TEHVs successfully withstood the aortic hemodynamics and demonstrated good in vitro and in vivo functionality with normal leaflet motion. The high values of RF and leakage volume measured during in vitro functionality assessment resulted from geometrical mismatch between the TEHVs and the valve holder, and were not observed in vivo. This underlines the relevance of animal models for a correct evaluation of valve functionality, without the influence of artifacts associated to in vitro mock circulation loop setups. Notably, in our study, we also proved that our ECM-based valve technology is fully compatible with a state-of-the-art catheter system, which further enhances the clinical relevance of our ECM-based TEHV technology. Taken together, these findings suggest that the concept of decellularized ECM-based TEHV is transferable to the high-pressure circulation.
Initially proposed to decreases the logistical complexity of classical TE concepts [15], decellularized in vitro-grown ECM-based TEHVs have shown a substantial self-repair and remodeling potential as pulmonary valve replacements. Such valves, implanted with transcatheter techniques, showed rapid cell repopulation and significant remodeling in sheep and primates [16,17,18]. In line with these results, the TEHVs used in our current study, optimized for high-pressure applications, demonstrated fast host cell infiltration under aortic conditions, suggesting their potential as regenerative therapy option for next-generation TAVR. Recently, surgical aortic valve replacement using decellularized ECM-based TEHVs showed encouraging results, comprising infiltration of interstitial-like cells, endothelialization, and tissue remodeling with elastic fibers formation at a 6-month follow-up [29]. Although this study utilized a surgical approach and ovine cell sources, it strongly supports the general notion of feasibility and safety of an ECM-based TEHV approach. In this context, to increase the clinical relevance of our TEHV, we transitioned from animal-derived cells to human cell sources for the in vitro production of the ECM, achieving another major step towards clinical applicability.
The first attempts to utilize TEHVs for TAVR are based on the use of autologous bone marrow cells pre-seeding on polymer-based TEHVs, confirming the principal feasibility and safety of this approach [24, 30, 31]. However, despite encouraging data from these first experiences of tissue-engineered TAVR, such approaches utilized a more classical cell-based TE approach comprising cell harvesting and seeding before implantation which adds further complexity to the entire procedure and limits the opportunity for cost-effective off-the-shelf solutions.
Few other approaches of in situ TEHVs for aortic valve replacement are currently undergoing in-depth investigation, including decellularized allografts [32] and fully synthetic TEHV concepts [33]. However, when compared to the poorly available allografts, our approach allows unlimited availability and high, rapid host cell infiltration upon implantation, which is crucial for long-term tissue remodeling. On the other hand, synthetic TEHVs based on supramolecular biodegradable polymers come with an abundant availability and have demonstrated safety and good hemodynamic performance as TAVR in a proof-of-concept large-animal study [33]. While such a TEHV concept indeed allows to significantly abrupt the costs and logistics of valve production, it is to recognize that its long-term performance and safety completely depend on the recipients’ regenerative potential and the degradation profile of the initial polymeric matrix. Recent data from a chronic preclinical study of such valves in the pulmonary circulation showed slow resorption of the scaffold after 1 year in vivo [34]. This highlights the necessity of further long-term in vivo validation until complete scaffold resorption is achieved before any final conclusion on the safety profile of this interesting technology can be drawn. Conversely, our TEHVs are based on in vitro-produced ECM which is already competent upon implantation and which therefore conceptually provides an enhanced safety profile particularly when used in recipients with low regenerative potential. Despite the lack of in vitro or in vivo durability data for these valves under aortic conditions, we have recently proved preserved long-term functionality (up to 1 year) of similar transcatheter TEHVs based on decellularized in vitro-grown ECM as pulmonary valve replacement in adult sheep [35]. These valves demonstrated integration and remodeling potential upon implantation, preventing the development of leaflet retraction and valvular insufficiency, which are the main cause of early failure of TEHVs [16,17,18, 36, 37]. Based on comparable leaflet geometry and heart valve TE technology, the TEHV here developed for aortic valve replacement might ensure long-term valve functionality and remodeling outcomes, thereby allowing to produce TAVR prostheses available for the young [35, 38].
Limitations
Our study has several limitations. First, the in vitro characterization of the ECM can be further improved with deeper analysis of the matrix composition (e.g., with mass spectroscopy), structure (e.g., via morphometric characterization of the collagen network), and maturation (e.g., by quantitatively assessing the collagen crosslinks). Second, the suboptimal prostheses sizing impacted TEHV functionality in two animals, resulting in under-expansion of the valve in vivo, asymmetrical leaflet motion, and mild-to-moderate central regurgitation. These results emphasize the crucial and delicate role of a correct prosthesis sizing and of correct positioning in determining the success of TAVR devices, even in an acute setting. Next, long-term functionality of our TEHV in the aortic setting needs to be confirmed to validate its safety and long-term efficacy profile. However, this was beyond the scope of this study, and systematic and longitudinal evaluation of the remodeling process in the aortic circulation will be addressed in the near future. Finally, further design improvement in regard to valve geometry [35], ECM-stent interface [39], and sizing is needed to optimize TEHV performance.
Conclusions
This study demonstrates for the first time the feasibility and safety of using off-the-shelf available human cell-derived TEHVs as aortic valve replacement in an ovine model using a clinically relevant TAVR system. Considering the strong regenerative potential of this concept, as recently proven in vivo in the setting of pulmonary valve replacement [35], such valves could serve as a basis for the development of next-generation lifelong TAVR prostheses with self-repair and remodeling capacities which is particularly beneficial for the young.
Abbreviations
- 3D-CT:
-
Three-dimensional computer tomography
- Col-1:
-
Collagen-1
- Col-3:
-
Collagen-3
- DNA:
-
Deoxyribonucleic acid
- ECM:
-
Extracellular matrix
- ELVG:
-
Elastica van Gieson
- GAGs:
-
Glycosaminoglycans
- H&E:
-
Hematoxylin and eosin
- Hyp:
-
Hydroxyproline
- PBS:
-
Phosphate-buffered saline
- SRS:
-
Suture retention strength
- TAVR:
-
Transcatheter aortic valve replacement
- TEE:
-
Transesophageal echocardiography
- TEHV:
-
Tissue-engineered heart valve
- TGF-β1:
-
Transforming growth factor-beta1
References
Nishimura, R. A., Otto, C. M., Bonow, R. O., Carabello, B. A., Erwin 3rd, J. P., Guyton, R. A., et al. (2014). 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. The Journal of Thoracic and Cardiovascular Surgery, 148(1), e1–e132.
Kapadia, S. R., Leon, M. B., Makkar, R. R., Tuzcu, E. M., Svensson, L. G., Kodali, S., et al. (2015). 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet, 385(9986), 2485–2491.
Mack, M. J., Leon, M. B., Smith, C. R., Miller, D. C., Moses, J. W., Tuzcu, E. M., et al. (2015). 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet, 385(9986), 2477–2484.
Leon, M. B., Smith, C. R., Mack, M. J., Makkar, R. R., Svensson, L. G., Kodali, S. K., et al. (2016). Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. The New England Journal of Medicine, 374(17), 1609–1620.
Reardon, M. J., Van Mieghem, N. M., Popma, J. J., Kleiman, N. S., Sondergaard, L., Mumtaz, M., et al. (2017). Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. The New England Journal of Medicine, 376(14), 1321–1331.
Rogers, T., Torguson, R., Bastian, R., Corso, P., & Waksman, R. (2017). Feasibility of transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis: rationale and design of the Low Risk TAVR (LRT) study. American Heart Journal, 189, 103–109.
Manji, R. A., Lee, W., & Cooper, D. K. (2015). Xenograft bioprosthetic heart valves: past, present and future. International Journal of Surgery, 23(Pt B), 280–284.
Siddiqui, R. F., Abraham, J. R., & Butany, J. (2009). Bioprosthetic heart valves: modes of failure. Histopathology, 55(2), 135–144.
Arora, S., Ramm, C. J., Strassle, P. D., Vaidya, S. R., Caranasos, T. G., & Vavalle, J. P. (2017). Review of major registries and clinical trials of late outcomes after transcatheter aortic valve replacement. The American Journal of Cardiology, 120(2), 331–336.
Arsalan, M., & Walther, T. (2016). Durability of prostheses for transcatheter aortic valve implantation. Nature Reviews. Cardiology, 13(6), 360–367.
Mylotte, D., Andalib, A., Theriault-Lauzier, P., Dorfmeister, M., Girgis, M., Alharbi, W., et al. (2015). Transcatheter heart valve failure: a systematic review. European Heart Journal, 36(21), 1306–1327.
Metaxa, S., Ioannou, A., & Missouris, C. G. (2017). Transcatheter aortic valve implantation: new hope in the management of valvular heart disease. Postgraduate Medical Journal, 93(1099), 280–288.
Barbanti, M., & Tamburino, C. (2016). Late degeneration of transcatheter aortic valves: pathogenesis and management. EuroIntervention, 12(Y), Y33–Y36.
Dijkman, P. E., Fioretta, E. S., Frese, L., Pasqualini, F. S., & Hoerstrup, S. P. (2016). Heart valve replacements with regenerative capacity. Transfusion Medicine and Hemotherapy, 43(4), 282–290.
Dijkman, P. E., Driessen-Mol, A., Frese, L., Hoerstrup, S. P., & Baaijens, F. P. (2012). Decellularized homologous tissue-engineered heart valves as off-the-shelf alternatives to xeno- and homografts. Biomaterials, 33(18), 4545–4554.
Driessen-Mol, A., Emmert, M. Y., Dijkman, P. E., Frese, L., Sanders, B., Weber, B., et al. (2014). Transcatheter implantation of homologous “off-the-shelf” tissue-engineered heart valves with self-repair capacity: long-term functionality and rapid in vivo remodeling in sheep. Journal of the American College of Cardiology, 63(13), 1320–1329.
Weber, B., Dijkman, P. E., Scherman, J., Sanders, B., Emmert, M. Y., Grunenfelder, J., et al. (2013). Off-the-shelf human decellularized tissue-engineered heart valves in a non-human primate model. Biomaterials, 34(30), 7269–7280.
Schmitt, B., Spriestersbach, H., O H-Icí, D., Radtke, T., Bartosch, M., Peters, H., et al. (2016). Percutaneous pulmonary valve replacement using completely tissue-engineered off-the-shelf heart valves: six-month in vivo functionality and matrix remodelling in sheep. EuroIntervention, 12(1), 62–70.
Schmidt, D., Dijkman, P. E., Driessen-Mol, A., Stenger, R., Mariani, C., Puolakka, A., et al. (2010). Minimally-invasive implantation of living tissue engineered heart valves: a comprehensive approach from autologous vascular cells to stem cells. Journal of the American College of Cardiology, 56(6), 510–520.
Mol, A., van Lieshout, M. I., Dam-de Veen, C. G., Neuenschwander, S., Hoerstrup, S. P., Baaijens, F. P., et al. (2005). Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials, 26(16), 3113–3121.
Mol, A., Driessen, N. J., Rutten, M. C., Hoerstrup, S. P., Bouten, C. V., & Baaijens, F. P. (2005). Tissue engineering of human heart valve leaflets: a novel bioreactor for a strain-based conditioning approach. Annals of Biomedical Engineering, 33(12), 1778–1788.
Sanders, B., Loerakker, S., Fioretta, E. S., Bax, D. J. P., Driessen-Mol, A., Hoerstrup, S. P., et al. (2016). Improved geometry of decellularized tissue engineered heart valves to prevent leaflet retraction. Annals of Biomedical Engineering, 44, 1061–1071.
Pensalfini, M., Meneghello, S., Lintas, V., Bircher, K., Ehret, A. E., & Mazza, E. (2018). The suture retention test, revisited and revised. Journal of the Mechanical Behavior of Biomedical Materials, 77, 711–717.
Emmert, M. Y., Weber, B., Behr, L., Sammut, S., Frauenfelder, T., Wolint, P., et al. (2014). Transcatheter aortic valve implantation using anatomically oriented, marrow stromal cell-based, stented, tissue-engineered heart valves: technical considerations and implications for translational cell-based heart valve concepts. European Journal of Cardio-Thoracic Surgery, 45(1), 61–68.
Crapo, P. M., Gilbert, T. W., & Badylak, S. F. (2011). An overview of tissue and whole organ decellularization processes. Biomaterials, 32(12), 3233–3243.
Arora, S., & Vavalle, J. P. (2017). Transcatheter aortic valve replacement in intermediate and low risk patients-clinical evidence. Ann Cardiothorac Surg, 6(5), 493–497.
Alsara, O., Alsarah, A., & Laird-Fick, H. (2014). Advanced age and the clinical outcomes of transcatheter aortic valve implantation. Journal of Geriatric Cardiology: JGC, 11(2), 163–170.
Treede, H., Rastan, A., Ferrari, M., Ensminger, S., Figulla, H. R., & Mohr, F. W. (2012). JenaValve. EuroIntervention, 8 Suppl Q, Q88–Q93.
Syedain, Z., Reimer, J., Schmidt, J., Lahti, M., Berry, J., Bianco, R., et al. (2015). 6-month aortic valve implantation of an off-the-shelf tissue-engineered valve in sheep. Biomaterials, 73, 175–184.
Emmert, M. Y., Weber, B., Behr, L., Frauenfelder, T., Brokopp, C. E., Grünenfelder, J., et al. (2011). Transapical aortic implantation of autologous marrow stromal cell-based tissue-engineered heart valves. JACC: Cardiovascular Interventions, 4(7), 822–823.
Emmert M.Y., Weber B., Wolint P., Behr L., Sammut S., Frauenfelder T., et al. (2012). Stem cell-based transcatheter aortic valve implantation: first experiences in a pre-clinical model. JACC Cardiovascular Interventions, 5(8), 874–883.
Neumann, A., Cebotari, S., Tudorache, I., Haverich, A., & Sarikouch, S. (2013). Heart valve engineering: decellularized allograft matrices in clinical practice. Biomedizinische Technik. Biomedical Engineering, 58(5), 453–456.
Miyazaki, Y., Soliman, O. I. I., Abdelghani, M., Katsikis, A., Naz, C., Lopes, S., et al. (2017). Acute performance of a novel restorative transcatheter aortic valve: preclinical results. EuroIntervention, 13(12), e1410–e1417.
Kluin, J., Talacua, H., Smits, A. I., Emmert, M. Y., Brugmans, M. C., Fioretta, E. S., et al. (2017). In situ heart valve tissue engineering using a bioresorbable elastomeric implant - from material design to 12 months follow-up in sheep. Biomaterials, 125, 101–117.
Emmert, M. Y., Schmitt, B. A., Loerakker, S., Sanders, B., Spriestersbach, H., Fioretta, E. S., et al. (2018). Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Science Translational Medicine, 10(440).
Reimer, J., Syedain, Z., Haynie, B., Lahti, M., Berry, J., & Tranquillo, R. (2016). Implantation of a tissue-engineered tubular heart valve in growing lambs. Annals of Biomedical Engineering, 45(2), 439–451.
Flanagan, T. C., Sachweh, J. S., Frese, J., Schnoring, H., Gronloh, N., Koch, S., et al. (2009). In vivo remodeling and structural characterization of fibrin-based tissue-engineered heart valves in the adult sheep model. Tissue Engineering. Part A, 15(10), 2965–2976.
Loerakker, S., Ristori, T., & Baaijens, F. P. (2016). A computational analysis of cell-mediated compaction and collagen remodeling in tissue-engineered heart valves. Journal of the Mechanical Behavior of Biomedical Materials, 58, 173–187.
Motta, S. E., Fioretta, E. S., Dijkman, P. E., Lintas, V., Behr, L., Hoerstrup, S. P., et al. (2018). Development of an off-the-shelf tissue-engineered sinus valve for transcatheter pulmonary valve replacement: a proof-of-concept study. Journal of Cardiovascular Translational Research, 11(3), 182–191.
Funding
This work was supported by the Swiss Heart Foundation (grant agreement no. 75010) and by the Forschungskredit Candoc program of the University of Zurich (grant agreement no. F-43010-02-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval for Research Involving Animals
All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Ethical Approval for Research Involving Humans
This article does not contain any studies with human participants performed by any of the authors.
Clinical Relevance of the Study
Nowadays, all TAVR prostheses are based on non-regenerative glutaraldehyde-fixed xenomaterials and are susceptible to structural tissue degeneration. The short lifespan of current TAVR devices requires repeated interventions over the patient’s lifetime and hence questioning the expansion of TAVR to younger patients. Given proof of their long-term remodeling and functionality as aortic valve substitutes, off-the-shelf, TAVR-compatible TEHVs with self-repair capacity would overcome the shortcomings of current TAVR devices and offer durable, regenerative prostheses for treating adult and young patients.
Additional information
Associate Editor Enrique Lara-Pezzi oversaw the review of this article
Electronic Supplementary Material
Supplementary Figure 1
(PNG 4999 kb)
Supplementary Figure 2
(PNG 2140 kb)
Video 1
(MOV 6444 kb)
Video 2
(AVI 12742 kb)
Video 3
(AVI 11818 kb)
Video 4
(AVI 4433 kb)
ESM 5
(DOCX 80 kb)
Rights and permissions
About this article
Cite this article
Lintas, V., Fioretta, E.S., Motta, S.E. et al. Development of a Novel Human Cell-Derived Tissue-Engineered Heart Valve for Transcatheter Aortic Valve Replacement: an In Vitro and In Vivo Feasibility Study. J. of Cardiovasc. Trans. Res. 11, 470–482 (2018). https://doi.org/10.1007/s12265-018-9821-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-018-9821-1