Abstract
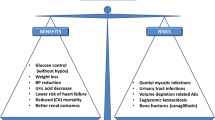
Sodium-glucose cotransporter 2 inhibitors are the latest medications to be approved and released for treatment of patients with type 2 diabetes. These include canagliflozin, dapagliflozin, and empagliflozin. Their mechanism of action is characterized by permissive glucosuria, which has significant effects on lowering HbA1c by up to 1%. These drugs are also beneficial for blood pressure control mainly due to its diuretic-like effects as well as weight loss secondary to caloric loss via glucosuria. Most impressive to these medications are the marked potential benefits observed in cardiovascular and renal outcomes noted with empagliflozin but yet to be assessed with canagliflozin and dapagliflozin. The use of these medications has introduced to the scientific and medical world the concept of ketones as a superfuel as well. Sodium-glucose cotransporter 2 inhibitors are promising drugs to the diabetic community, and the use of these has continued to increase.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sodium-glucose cotransporter 2 inhibitors
- SGLT-2 inhibitors
- Canagliflozin
- Dapagliflozin
- Empagliflozin
- Superfuel
- Cardiovascular benefits
Introduction
The concentration of glucose in plasma is held within narrow limits primarily to ensure fuel supply to the brain; the kidneys play a key role in glucose homeostasis by ensuring that glucose is not lost in the urine [1]. Contrary to common belief, the liver is not the only gluconeogenic organ although it does produce 80% of the endogenously derived glucose; the remaining 20% is produced by the kidney, which also contains the necessary gluconeogenic enzymes [2]. In nondiabetic individuals, the kidney filters approximately 180 mg of glucose daily [2]. Ninety percent of this is absorbed via the energy-dependent sodium-glucose cotransporter receptor moving from the tubular lumen to the arterioles via GLUT 4 glucose transport back into the circulation; the remaining 10% is reabsorbed in the distal collecting tubule leaving no glucose excreted into the urine [2]. Both at the liver and kidney, insulin is a potent inhibitor of gluconeogenesis; most of the filtered glucose is reabsorbed by the cotransporter enzyme SGLT2, and the remaining 10–20% is reabsorbed by the cotransporter SGLT1 [3]. Although the kidneys freely filter plasma glucose, none appears in the urine [1]. Glucose reabsorption from the glomerular filtrate by SGLT2 and SGLT1 occurs at different segments of the apical membrane of cells in the proximal tubule and from the passive exit of glucose through the basolateral membrane to the plasma via GLUT2, and at the expense of the extrusion of three sodium ions for every tw potassium ions entering the cell [1,2,3]. Glucose produced by renal gluconeogenesis is completely consumed by the kidney, but in patients with type 2 diabetes, insulin resistance increases the production of glucose in the kidney and liver despite high levels of fasting glucose [3].
One of the most important entries into the diabetes therapy armamentarium is the sodium-glucose cotransporter 2 inhibitors (SGLT 2 inhibitors), which first reached the US and European markets in early 2013. The idea for this mechanism of action is derived from the identification of an older drug, phlorizin, originally derived from the bark of an apple tree as a treatment for malaria [4]. Phlorizin caused marked increase in urinary glucose excretion through competitive inhibition of SGLT2, the principal transporter of renal glucose reabsorption and of SGLT1, a lesser glucose transporter in the kidney [3]. Phlorizin was useful for mechanistic studies in animal models but was too toxic for use in patients [5]. Additionally, there is a naturally occurring mutation in this co-transporter found in less than 1% of the population from the analysis of familial renal glucosuria, a rare genetic disorder of renal glucose transport [6, 7]. These patients have been known for decades since the original study of Hjärne of three generations of a single family [8, 9]. They have glucosuria with normal plasma glucose unless they also happen to have diabetes, which occurs rarely in this population. They seem to live perfectly normal lives except for increased risk of vaginal candidiasis related to glycosuria. Work began in the 1990s looking for less-toxic analogs of phlorizin, which led to the currently available marketed drugs with the discovery of dapagliflozin and canagliflozin [10, 11].
The Emergence of Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i)
These drugs have rapidly become extremely valuable tools in treating diabetes. Most of the published data has come from type 2 diabetes trials although in recent years an increasing number of studies about their use in type 1 diabetes have also been published. All the currently available SGLTi reduce both fasting and postprandial hyperglycemia and HbA1c between 0.6–1.0% [12,13,14]. There is also associated weight loss averaging 1–5 kg in most patients, presumably related primarily to caloric loss from excreted glucose [12]. The mechanism of action is independent of insulin itself and therefore should remain effective at all stages of the disease and work in a complementary fashion with other antidiabetics [12]. The risk of hypoglycemia with the use of SGLTi as monotherapy is similar to the use of other agents unless they are paired with sulfonylureas or insulin [15]. Several other interesting metabolic consequences have been identified including somewhat elevated plasma glucagon and ketone body production, which will be elaborated on further in this chapter, in addition to cardiorenal and pleiotropic effects [16,17,18,19,20,21].
As of December 2021, the FDA and European agencies have four agents for clinical use: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Six additional compounds have undergone clinical trials: tofogliflozin [22], luseogliflozin [23], bexagliflozin [24], sotagliflozin [25], remogliflozin [26], ipragliflozin [27], and recent basic research that has confirmed the SGLT2 inhibitor properties of swertisin, a novel islet cell differentiation inducer [28, 29]. Table 36.1 shows the current list of SGLT2 inhibitors including FDA-approved and non-FDA approved.
The efficacy and safety data appear similar for the drugs studied to date, and very few head-to-head trials are available for direct comparison. The efficacy and side effects appear similar in most trials. In general, phase 3 trials have shown a HbA1c reduction of 0.7–1.0% as monotherapy or in addition to other antidiabetic agents including insulin.
Mechanism of Action
In diabetes, there is an apparently maladaptive increase in the tubular threshold from the normal of 180 mg up to 220–240 mg making it even harder to eliminate excess serum glucose. In the presence of SGLT 2 inhibitors, the threshold for glucose elimination is reduced to about 40 mg, allowing much more glucose loss. This tends to reduce both fasting and postprandial glucose levels [30]. As there is caloric loss from increased glucose excretion, weight loss is usually seen as well in the range of 2–3 kg in most studies. Approximately two thirds of the loss is secondary to fat loss and one third from fluid loss. A molecule of sodium is also excreted with each molecule of glucose resulting associated with a net loss of body sodium and to a small reduction in systolic blood pressure averaging about 5 mmHg. This may be beneficial since most patients tend to have some sodium excess. However, in patients somewhat sodium or volume depleted, this could result in excessive blood pressure reduction and dehydration. In the United States, the Food and Drug Administration (FDA) has reported approximately 100 cases of acute kidney injury to patients placed on these drugs. Many cases are seen in older patients with some renal dysfunction who are also taking loop diuretics. Therefore, cautious is advised in these patients, starting with lower doses and observing the initial response.
Individual Profiles
Canagliflozin was approved by the FDA in the United States in March of 2013 for use in patients with type 2 diabetes mellitus, and it was the first of the SGLT2 inhibitors to be released in the market. The initial dose is 100 mg daily and can be increased to 300 mg in those tolerating the medication if GFR is ≥60 mL/min/1.73 m2. Fixed doses of canagliflozin in combination with metformin are available in 50/500, 50/1000, 150/500, and 150/1000 mg [31]. Glucosuric effects are estimated to be an excretion of approximately 100 g of urinary glucose per day. It has the most largest glucosuric effect among approved SGLT2 inhibitors. In addition to its main effect as an SGLT2 inhibitor, canagliflozin induces weak inhibition of SGLT1, which is located in both the gut and renal tubules. SGLT1 inhibition is thought to have effects in lowering postprandial hyperglycemia by delaying intestinal glucose absorption, an observation from studies published in 2013 [32].
Comparative Efficacy and Safety of Canagliflozin with Oral Antidiabetics
The efficacy of canagliflozin has been studied as add-on therapy to metformin in comparison with other antihyperglycemic agents such as DPP4-inhibtors and sulfonylureas according to a randomized, double blinded trial was published in 2013 comparing the efficacy of canagliflozin with sitagliptin in patients on monotherapy with metformin ≥1500 mg daily. After 52 weeks, both sitagliptin 100 mg and canagliflozin 100 mg were effective in lowering HbA1C by an average of 0.73%, while canagliflozin at a dose of 300 mg/day decreased HbA1C by 0.88% [33]. Both canagliflozin doses were superior in weight reduction (3.8% and 4.2%) compared with a decrease of 1.3% in the sitagliptin group [33].
In 2015, canagliflozin was compared with glimepiride in a phase 3, randomized, double blinded, 104 week-long study as add-on therapy for diabetic patients already on therapeutic doses (≥1500 mg today daily) of metformin [34]. Canagliflozin decreased HbA1C by an average of 0.65% for the 100 mg dose and 0.74% for the 300 mg dose in comparison to glimepiride, which resulted in an average 0.55% reduction. The use of canagliflozin was associated with a lower risk of hypoglycemia, with a prevalence of 40% in the glimepiride group and only 6 and 8% in the canagliflozin 100 mg and 300 mg groups, respectively. Weight loss was observed with canagliflozin, as opposed to weight gain for patients on glimepiride, with an average loss of 4.1% (3.6 kg) of pretreatment body weight for the 100 mg and 4.2% (3.6 kg) for the 300 mg groups [34].
Comparative Efficacy of Canagliflozin with Insulin
Data about the use of canagliflozin in patients on insulin therapy were published in one of the reports of the CANVAS trial comparing canagliflozin and placebo to patients on basal or basal-bolus insulin for 18 weeks with a 52-week follow-up [35]. The addition of canagliflozin to insulin improved glycemic control: HbA1c was 8.3% in both groups; at 18 weeks, reductions in HbA1c of 0.62% and 0.73% for canagliflozin 100 mg and 300 mg, respectively, were observed in comparison to placebo with persisting differences in HbA1c after 52 weeks with a reduction of 0.58% in the 100 mg group and 0.73% in the 300 mg group in comparison to placebo [35]. There were differences in weight and blood pressure reduction as well. A weight loss of 1.9% and 2.4% was seen for each canagliflozin dose. Systolic blood pressure decreased by an average of 3.1 and 6.2 mmHg and diastolic blood pressure by 1.2 and 2.4 mmHg in each of the canagliflozin groups [35]. In another randomized controlled trial, the efficacy and safety of canagliflozin was compared with liraglutide in patients with type 2 diabetes previously controlled with multiple doses of insulin (MDD) [36]. Basal insulin was maintained, and bolus insulin was randomly switched to canagliflozin, 100 mg/day or liraglutide, 0.30.3–0.9 mg/day for 24 weeks [36]. Changes in HbA1c were comparable between treatments, and both treatments maintained HbA1c levels as baseline with stable glucose variability and no severe hypoglycemia at 24 weeks, with reduced total insulin doses and improvements in quality of life [36].
Safety and efficacy of canagliflozin has been evaluated in patients with preexisting chronic kidney disease with GFRs between ≥30 and ≤50 mL/min/1.73 m2. Placebo-subtracted differences in A1c values were seen for the 100 mg and 300 mg groups from baseline (0.27% and 0.41%). Lower body weight and blood pressure for both doses in comparison with placebo were also documented [37].
Dapagliflozin was approved for treatment in patients with type 2 diabetes mellitus in the United States in 2014 as an adjunct to diet and exercise. It is a highly selective SGLT2 inhibitor. The initial dose is 5 mg, which can be increased to 10 mg orally daily. It is available in combination with metformin as well [31].
Dapagliflozin has been observed to be non-inferior to sulfonylureas and superior to DPP-4 inhibitors as add-on therapy to metformin. Monotherapy comparing metformin and dapagliflozin has been evaluated in treatment naïve patients. Results from this study demonstrated non-inferiority between metformin and dapagliflozin. Dapagliflozin as monotherapy decreased HbA1C by an average range of 0.55–0.9% in comparison to 0.73% with metformin [38]. Dapagliflozin is also effective in lowering HbA1c when added to metformin. A 52-week double-blinded trial with patients having HbA1c values between 8% and 12% at baseline showed significant improvement [39]. Dapagliflozin added to metformin decreased HbA1C an average of 1.2%, which was significantly lower than the combination of saxagliptin with metformin (0.9%). This study also compared triple therapy with all three agents and found superiority to dual therapy by reducing HbA1c by up to 1.5%. Weight loss was superior in the dual therapy dapagliflozin and metformin group with an average loss of 2.8% (2.1 kg) and in the triple therapy group, which lost an average of 2.4% (2.1 kg) compared to the saxagliptin and metformin group (no significant change seen) [39]. The efficacy of dapagliflozin has been compared to sulfonylureas: glipizide was compared to dapagliflozin and resulted in non-inferiority at 52 weeks [40]. This trial was extended for 2 years, and a sustained decrement in HbA1C was observed with dapagliflozin compared with glipizide (0.32% vs 0.14%) [40]. An additional 52-week, randomized trial compared the efficacy and safety of dapagliflozin as monotherapy or combined with saxagliptin versus glimepiride in patients with type 2 diabetes previously receiving metformin [41]. Mean HbA1c change from baseline was −0.82 with dapagliflozin alone, −1.20% with dapagliflozin plus saxagliptin, and −0.99% with glimepiride [49]. Fasting blood glucose decreased significantly with dapagliflozin plus saxagliptin compared with glimepiride and was similar when not in combination [41]. Both dapagliflozin regimens decreased body weight and systolic blood pressure; the combined incidence of hypoglycemia was lower with dapagliflozin, and genital infections were more frequent [41].
Empagliflozin the FDA-approved empagliflozin as an antihyperglycemic agent to be used in patients with type 2 diabetes mellitus in the United States in 2014. It is available in a starting dose of 10 mg, which can be increased to 25 mg daily in patients with a GFR ≥ 45 mL/min/1.73 m2. Its glucosuric effects are estimated to be 78 g of glucose per day. Like canagliflozin and dapagliflozin, it also has weight loss and blood pressure-lowering effects.
Empagliflozin has been studied as add-on therapy to metformin in comparison to sulfonylureas as well as triple therapy with DPP-4 inhibitors and metformin. A double -phase 3, 104-week long study in patients with poor diabetes control on monotherapy with metformin was randomized to either glimepiride or empagliflozin therapy [42]. At baseline HbA1c levels between 7% and 10%, empagliflozin 25 mg significantly decreased HbA1c a mean of 0.11% more than glimepiride. Adverse events were similar in both groups, but there was a marked difference in the frequency of hypoglycemia between the empagliflozin and glimepiride groups (2% vs 24%) [42]. A 208-week extension of this trial, the adjusted mean difference in change from baseline in HbA1c with empagliflozin versus glimepiride, was statistically significant, and hypoglycemic episodes occurred in 3% of patients on empagliflozin and 28% on patients receiving glimepiride [43]. Addition of empagliflozin 10 mg and 25 mg was compared to placebo in a 24-week long, double-blinded trial with poorly controlled type 2 diabetic patients on linagliptin and metformin combination therapy [44]. By comparison with placebo, the empagliflozin 10 mg and 25 mg groups were observed to have a −0.79% and −0.7% difference in HbA1c from baseline. Addition of empagliflozin to linagliptin and metformin had no added adverse effects. Weight loss and blood pressure benefits were seen in both empagliflozin groups. Hypoglycemia occurred more frequently in the empagliflozin 25 mg group versus the placebo group in this trial (2.7% vs 0.9%) [44]. Positive outcomes and improvement in glycemic control have been observed with the use of empagliflozin with other agents including sitagliptin, pioglitazone, and insulin therapy (both basal and basal/bolus regimens) [45,46,47,48]. Recent comparisons between 25 mg empagliflozin and one-weekly 1 mg oral semaglutide have shown significant differences on HbA1c and body weight versus empagliflozin [49, 50].
Cardiovascular Benefits of SGLT2 Inhibitors
Approvals for most new antidiabetic agents in the United States have included a requirement for generally large-scale cardiovascular outcome trials primarily to be certain they do not increase cardiovascular risk. The first of these for this new class was presented at the European Association for the Study of Diabetes (EASD) in September 2015. In contrast to previous studies, this one for empagliflozin (EMPA-REG Trial) showed striking benefit particularly in cardiovascular mortality (38% relative risk reduction), hospitalizations for congestive heart failure (35% relative risk reduction), and death from any cause (32% relative risk reduction). Death from any cause was reduced by 32% [51]. This was much less striking for myocardial infarctions and nonexistent for stroke benefit. The median duration of this trial was 3.1 years. Remarkably, the survival curves began to diverge within about 3 months of beginning the trial. Although there was an expected reduction in plasma glucose, it seems unlikely that this effect could result in a benefit of this magnitude so quickly. A proposed mechanism for such rapid benefits has been reduction in arterial stiffness. Sodium and glucose loss reduces extracellular fluid volume and blood pressure. This reduces cardiac pre and after load and myocardial metabolism, improving both systolic and diastolic function. All of this may play a role in the observed rapid reduction in hospitalizations for heart failure and cardiac death. The majority of subjects were treated with platelet inhibitors, statins, and adequate blood pressure control. Therefore, the benefits appear to be over and above these standard therapies [52]. In addition to their established efficacy as antidiabetics, clinical trials comparing the use of empagliflozin with GLP-1 agonists have shown that the use of SGLT2 inhibitors is associated with consistent reductions in hospitalization for heart failure among type 2 patients with and without cardiovascular disease (CVD), although the absolute reduction is greater in patients with CVD (Fig. 36.1) [53].
Cardiovascular outcomes and death from any cause. Shown are the cumulative incidence of the primary outcome (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) (Panel a), cumulative incidence of death from cardiovascular causes (Panel b), the Kaplan–Meier estimate for death from any cause (Panel c), and the cumulative incidence of hospitalization for heart failure (Panel d) in the pooled empagliflozin group and the placebo group among patients who received at least one dose of a study drug. Hazard ratios are based on Cox regression analyses (Zinman NEJM 2015)
What could account for these remarkable improvements? The known effects of the drug are unlikely to account for the magnitude of this effect. Reduction in arterial stiffness had been observed with these drugs verified by arterial ultrasound compression [54]. The onset of heart failure sets in motion a cascade of effects, which may lead to a vicious cycle of vasoconstriction with activation of the adrenergic nervous system and the renin-angiotensin-aldosterone system including the tubular glomerular feedback in the kidney, which may alter this adverse sequence of events. The sum total of these changes likely reduce cardiac preload and afterload and improve myocardial oxygen supply [55].
The first report of the results of the cardiovascular and renal outcomes CANVAS trial for canagliflozin was published in 2017 [56]. The rate of the primary outcome, a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke was lower with canagliflozin than with placebo, occurring in 26.9% versus 31.5 participants (P < 0.001) [56]. Renal outcomes were not statistically significant, but the results showed possible benefits of canagliflozin on the progression of albuminuria and the composite outcome of a sustained reduction in glomerular filtration rate, the need for renal replacement therapy, or death from renal causes. Adverse reactions showed and increased risk for amputations at the level of toe or metatarsal [56]. The first report of the DECLARE-TIMI 58 dapagliflozin cardiovascular outcome trial was published in 2019 [57]. In the primary safety analysis, dapagliflozin met the prespecified criterion for noninferiority to placebo with respect to major adverse cardiovascular events (MACE) defined by cardiovascular death, myocardial infarction, or ischemic stroke. Patients in the dapagliflozin group had lower rates of cardiovascular death or hospitalization for heart failure, without between-group difference in cardiovascular death [57]. Diabetic ketoacidosis was more common with dapagliflozin than with placebo (0.3% vs 0.1%) as was the rate of genital infections leading to discontinuation (0.9% vs 0.1%) [57]. The first report to assess cardiovascular outcomes with ertugliflozin from the VERTIS CV trial showed equal rates of major cardiovascular events (11.9%) in the ertugliflozin group and with placebo [58]. Death from cardiovascular causes or hospitalization for heart failure occurred in 8.1% of patients in the ertugliflozin group and 9.1% of patients in the placebo group, and the hazard ratio for death from cardiovascular causes was 0.92 [58]. Amputations were performed in 2.0% of patients who received the 2 mg dose and 2.1% of patients who received the 15 mg dose, as compared with 1.6% of patients who received placebo [58].
Renal Effects
Renoprotective effects of SGLT2 inhibitors were also analyzed in the EMPA-REG, CANVAS, DECLARE-TIMI 58, and VERTIS trials [53, 56,57,58,59]. In a subsequent preplanned sub-study (EMPA_REG Renal), significant benefits were observed in those having renal dysfunction with estimated glomerular filtration rates (GFR) of 30–60 mL/min [59]. There is a transient small drop in GFR seen on initiating these drugs that is possibly related to diuresis and volume contraction. However, as can be readily seen from Figs. 36.2 and 36.3, the net result was positive for preservation of renal function compared to the placebo treated arm in which there was small continuing loss of eGFR. Renal endpoints of newly appearing or worsening nephropathy and progression to macroalbuminuria were reduced by 29% and 38%, respectively. Hard renal endpoints of doubling of serum creatinine and need for renal replacement were reduced by 44% and 55%, respectively, although the latter endpoint occurred in relatively few subjects. These subjects were treated with standard of care with 79–85% receiving angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Therefore, the benefits are additive and over and above those seen with treatments known to be effective [59]. A recent sub-analysis from the VERTIS trial in patients with an estimated glomerular filtration rate (GFR) 30 to < 60 mL/min showed that in addition to reduction in HbA1c, body weight, and blood pressure, patients receiving ertugliflozin maintained baseline GFR levels [60]. The prospect of significantly reducing the decline of GFR in chronic diabetic chronic kidney disease is exciting [61].
Potential pathway linking empagliflozin (and possibly other SGLT2 inhibitors) with lower risks for HFH (and, linked to this, death due to cardiovascular disease). By increasing fluid losses via urinary glucose and sodium losses (1), intravascular volumes and systolic blood pressure are reduced and there is a significant rise in hematocrit (2). These latter effects may also be, to a small extent, assisted by weight loss. These changes in turn lessen cardiac stressors (pre- and afterload) and may also help improve myocardial oxygen supply (3). The net result is a likely improvement in cardiac systolic and diastolic function, lessening chances of pulmonary congestion, thus lowering risks of HFH and fatal arrhythmias. These cardiac function benefits will, in turn, feed back to improve renal blood flow and function (4). In this way, the cardio-renal axis is improved at a number of levels with SGLT2 inhibitor therapy (Sattar dibetologia 2016)
Kaplan–Meier analysis of two key renal outcomes. Shown are estimates of the probability of a first occurrence of a prespecified real composite outcome of incident or worsening nephropathy (Panel a) and of a post hoc renal composite outcome (a doubling of the serum creatinine level, the initiation of renal-replacement therapy, or death from renal failure (Wanner NEJM 2016 panel a & b and Panel a)
The Canagliflozin and Renal Events in Diabetes and Established Clinical Evaluation (CREDENCE) compared canagliflozin versus placebo in patients with type 2 diabetes and chronic kidney disease [62]. Canagliflozin significantly reduced the risk of the primary composite outcome: end-stage renal disease, doubling of serum creatinine, renal, or cardiovascular death. The CREDENCE trial was stopped early for efficacy after an interim analysis and recommendation from the independent Data Monitoring Committee [63]. Overall, the results of the CREDENCE trial showed that canagliflozin could be safely administered to patients with diabetic nephropathy, despite an initial drop in glomerular filtration rate. Ongoing kidney disease-focused outcome trials including DAPA-CKD and EMPA-KIDNEY will provide further information about the use of SGLT2 inhibitors in patients with type 2 diabetes and different stages of chronic kidney disease [64].
Other Metabolic Effects: Increased Ketogenesis a “Superfuel?”
SGLT2 inhibitors are known to increase glucagon, beta-hydroxybutyrate, and ketone body production. In this sense, they appear to shift metabolism from glucose to fat oxidation. Ketone bodies are readily taken up by the myocardial cells as fuel. Myocardium has the highest myocardial oxygen consumption at 8 mL O2/100 g of tissue followed by 5 mL O2 for kidney and 3 mL for brain tissue. It is postulated that an increased availability and use of ketone bodies could be beneficial to metabolically stressed organs [65, 66]. There is experimental evidence that this may result in more efficient oxygen sparing and cardiac work for any given level of demand. This could provide another mechanism for more rapid cardiac benefit in addition to a variety of potential pleiotropic effects beyond glucose lowering on a variety of diseases, including nonalcoholic fatty liver disease and obesity [14, 21].
Safety and Tolerability
SGLT2 are safe and well tolerated [12]. Rates of discontinuation in clinical trials are low, but according to their mechanism of action, they cause osmotic diuresis, volume depletion, and dehydration [12, 14]. Patients at risk, including those with frailty, the elderly, or taking diuretics, should be monitored and advised about these effects.
Genitourinary Infections
The most common adverse events is a higher risk for lower urinary tract infections, vulvovaginitis and vaginitis of bacterial and mycotic origin, which was documented in clinical trials and case reports [12, 14, 16]. A study by Lega et al. reported a five times higher risk of genital mycotic infections in patients treated with SGLT2 inhibitors, increasing in the first month of therapy and enduring for the duration of treatment [67]. Glycosuria resulting from diabetes provides a favorable substrate for microorganism growth, which is enhanced by the pharmacologic glycosuria induced by SGLT2 inhibitors [68]. Incidence rates of mycotic genital infections in clinical trials are 6.0% and more common in women [68]. The incidence of bacterial infections ranges from 4.0% to 9.0%, while severe infections occur in 0.4% of patients [68]. Severe forms of genitourinary infections include pyelonephritis, emphysematous pyelonephritis, and Fournier gangrene, a perineal disease of acute onset and rapid progression [69].
Ketoacidosis
There has been concern about increased ketone body production particularly in very insulin-deficient patents such as type 1 diabetics. There are several case series raising this concern of ketoacidosis. The rate appears to be low in type 2 diabetes. The mechanism of action could be related to decreased insulin levels, which leads to unopposed glucagon production and lipolysis, which leads to ketogenesis. Risk factors and precipitants for diabetic ketoacidosis related to SGLT-2 inhibitors are sepsis, dehydration, surgeries, decrease in insulin dose administration (for those on insulin), and a low carbohydrate diet [69]. The risk of ketoacidosis could be minimized by educating all patients upon initiation of therapy that nausea, vomiting, and dehydration require checking for ketones, and such symptoms should prompt them to seek medical attention [70].
Additional side effects include amputations and bone fractures.
Conclusion
SGLT-2 inhibitors are the newest pharmacologic resource for management of type 2 diabetes. Since their approval and release into the market in the United States in 2013, multiple studies have proven both efficacy and positive cardiovascular and renal outcomes. Their use has also enhanced our knowledge on fuel metabolism and the use of ketones as a source of energy. Although generally well tolerated, clinicians should be on alert for possible adverse effects of dehydration and even normoglycemic diabetic ketoacidosis. The use of these agents is expected to rise given their marked improvements in HbA1c in addition to beneficial effects on weight, blood pressure, and cardiovascular outcomes.
Multiple Choice Questions
-
1.
In patients with diabetes, the tubular threshold for the excretion of glucose:
-
(a)
Is decreased
-
(b)
Is adapted and increased
-
(c)
Is maladapted and increased
-
(d)
Is not different from people without diabetes
-
(e)
Is able to eliminate excess serum glucose
-
(a)
-
2.
In the presence of SGLT 2 inhibitors, the threshold for glucose elimination is reduced:
-
(a)
Approximately 10 mg
-
(b)
Approximately 20 mg
-
(c)
Approximately 40 mg
-
(d)
Approximately 80 mg
-
(e)
Approximately 100 mg
-
(a)
-
3.
Weight loss with the use of SGLT 2 inhibitors is estimated in the range of:
-
(a)
1–3 kg
-
(b)
2–3 kg
-
(c)
3–4 kg
-
(d)
4–5 kg
-
(e)
5–6 kg
-
(a)
-
4.
Weight loss from the use of SGLT 2 inhibitors is secondary:
-
(a)
To fat loss
-
(b)
To muscle loss
-
(c)
To fluid loss
-
(d)
All of the above
-
(e)
None of the above
-
(a)
-
5.
Inhibition of SGLT 1, located in the gut and renal tubules, results in:
-
(a)
Lowering postprandial hyperglycemia
-
(b)
Lowering fasting blood glucose
-
(c)
Lowering blood pressure
-
(d)
Increasing glucose uptake
-
(e)
Increasing intestinal glucose absorption
-
(a)
-
6.
Range doses of canagliflozin:
-
(a)
5–10 mg daily
-
(b)
10–25 mg daily
-
(c)
50–100 mg daily
-
(d)
100–300 mg daily
-
(e)
150–200 mg daily
-
(a)
-
7.
Range doses of dapagliflozin:
-
(a)
5–10 mg daily
-
(b)
10–25 mg daily
-
(c)
50–100 mg daily
-
(d)
100–300 mg daily
-
(e)
150–200 mg daily
-
(a)
-
8.
Range doses of empagliflozin:
-
(a)
5–10 mg daily
-
(b)
10–25 mg daily
-
(c)
50–100 mg daily
-
(d)
100–300 mg daily
-
(e)
150–200 mg daily
-
(a)
-
9.
The results of the EMPA-REG Trial showed that the use of empagliflozin was associated:
-
(a)
With a 38% relative risk reduction in cardiovascular mortality
-
(b)
With a 35% relative risk reduction for congestive heart failure
-
(c)
With a 32% relative risk reduction of death from any cause
-
(d)
All of the above
-
(e)
None of the above
-
(a)
-
10.
Cardiovascular benefits from the use of SGLT 2 inhibitors have been attributed to:
-
(a)
The effect of additional medications standard cardiovascular therapies
-
(b)
Reduction in arterial stiffness
-
(c)
Regression of atherosclerotic plaques
-
(d)
Their anti-hypertensive effects
-
(e)
Inhibition synthesis of advanced glycation products (AGEs)
-
(a)
References
Ghezzi C, Loo DD, Wright EM. Physiology of renal glucose handling via SGLT1, SGLT2 and GLUT2. Diabetologia. 2018;61:2087–97.
Mudaliar S, Polidori D, Zambrowicz B, Henry R. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport. Diabetes Care. 2015;38:2344–53.
Gronda E, Jessup M, Iacoviello M, Palazzuoli A, Napoli C. Glucose metabolism in the kidney: neurohumoral activation and heart failure development. J Am Heart Assoc. 2020;9:e018889.
De Koninck L. Ueber das phlorizin (phlorrizin). Annalen der Pharmacie. 1835;15:75–7.
Leslie BR, Gerwin LE, Taylor SI. Sodium-glucose cotransporter-2 inhibitors: lack of a complete history delays diagnosis. Ann Intern Med. 2019;171:421–6.
Elsas LJ, Rosenberg LE. Familial renal glycosuria: a genetic appraisal of hexose transport by kidney and intestine. J Clin Invest. 1969;48:1845–54.
Calado J, Soto K, Clemente C, Correia P, Rueff J. Gene symbol: SLC55A2. Disease: familial renal glucosuria. Hum Genet. 2004;115:170.
Hjärne V. A study of orthoglycaemic glycosuria with particular reference to its hereditability. Acta Med Scand. 1927;67:422–5.
Brown MS, Poleshuck R. Familial renal glycosuria. J Lab Clin Med. 1935;20:605–10.
Meng W, Ellsworth BA, Nirschl AA, McCann PJ, Patel M, Girotra RN, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose transporter 2 (SGLT-2) inhibitor for the treatment of type 2 diabetes. J Med Chem. 2008;51:1145–9.
Nomura S, Sakamaki S, Hongu M, Kawanishi E, Koga Y, Sakamoto T, et al. Discovery of canagliflozin, a novel C-glucoside with thiopene ring, as sodium-depedent glucose cotransporter 2 inhibitor for the treatment of type 2 diabetes mellitus. J Med Chem. 2010;53:6355–60.
Katz PM, Leiter LA. The role of the kidney and SGLT inhibitors in type 2 diabetes. Can J Diabetes. 2015;39:S167–75.
Brown E, Rajeev SP, Cuthbertson DJ, Wilding JPH. A review of the mechanism of action, metabolic profile and haemodynamic effects of sodium-glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019;21(Suppl 2):9–18.
Simes BC, MacGregor GG. Sodium-glucose cotransporter-2 (SGLT2) inhibitors: a clinician’s guide. Diabetes Metab Syndr Obes. 2019;12:2125–36.
McGill JB, Subramanian S. Safety of sodium-glucose co-transporter 2 inhibitors. Am J Cardiol. 2019;124:S45–52.
Augusto GA, Cassola N, Dualib PM, Saconato H, Melnik T. Sodium-glucose cotransporter-2 inhibitors for type 2 diabetes mellitus in adults: an overview of 46 systematic reviews. Diabetes Obes Metab. 2021;23:2289–302.
Brown E, Wilding JP, Alam U, Barber TM, Karalliede J, Cuthbertson DJ. The expending role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2021;53:2072–89.
Giugliano D, Longo M, Caruso P, Malorino MI, Bellastella G, Esposito K. Sodium-glucose co-transporter-2 inhibitors for the prevention of cardiorenal outcomes in type 2 diabetes: an updated meta-analysis. Diabetes Obes Metab. 2021;23:1672–6.
Verma S. Potential mechanism of sodium-glucose co-transporter 2 inhibitor-related cardiovascular benefits. Am J Cardiol. 2019;124:S36–44.
McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes. A meta-analysis. JAMA Cardiol. 2021;6:148–58.
Kaneto H, Obata A, Kimura T, Shimoda M, Kinoshita T, Matsuoka T, et al. Unexpected pleiotropic effects of SGLT2 inhibitors: pearls and pitfalls of this novel antidiabetic class. Int J Mol Sci. 2021;22:3062.
Ikeda S, Takano Y, Cynshi O, Tanaka R, Christ AD, Boerlin V, et al. A novel and selective sodium-glucose cotransporter-2 inhibitor, tofogliflozin, improves glycaemic control and lowers body weight in patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2015;17:984–93.
Sakai S, Kaku K, Seino Y, Inagaki N, Haneda M, Sasaki T, et al. Efficacy and safety of the SGLT2 inhibitor Luseogliflozin in Japanese patients with type 2 diabetes mellitus stratified according to baseline body mass index: pooled analysis of data from 52-week phase trials. Clin Ther. 2016;38:843–62.
Halvorsen Y-D, Walford G, Thurber T, Russell H, Massaro M, Freeman MW. A 12-week, randomized, double-blind, placebo-controlled, dour-arm dose-finding phase 2 study evaluating bexagliflozin as monotherapy for adults with type 2 diabetes. Diabetes Obes Metab. 2019;22:566–73.
Avgerinos I, Karagiannis T, Kakotrichi P, Michailidis T, Liakos A, Mathews DR, et al. Sotagliflozin for patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2021;24:106–14.
Mohan V, Mithal A, Joshi SR, Aravind SR, Chowdhuri S. Remoglifozin etabonate in the treatment of type 2 diabetes: design, development, and place in therapy. Drug Des Devel Ther. 2020;14:2487–501.
Aikabbani W, Gamble J-M. Profile of Ipragliflozin, an Oral SGLT-2 inhibitor for the treatment of type 2 diabetes: the evidence to date. Drug Des Devel Ther. 2021;15:3057–69.
Dadeech N, Srivastava A, Paranjape N, Gupta S, Dave A, Shah GM, et al. Swertisin and antidiabetic compound facilitate islet neogenesis from pancreatic stem/progenitor cells via P-38 MAP-kinase SMAD pathway: an in-vitro and in-vivo study. PLoS One. 2021;10:e0128244. https://doi.org/10.1371/journal.pone.0128244.
Bhardwaj G, Vakani M, Srivastava A, Patel D, Pappachan A, Murumkar P, et al. Swertisin, a novel SGLT2 inhibitor, with improved glucose homeostasis for effective diabetes therapy. Arch Biochem Biophys. 2021;710:108995.
Argento NB, Nakamura K. Glycemic effects of SGLT-2 inhibitor canagliflozin in type 1 diabetic patients using the DexCom G4 platinum CGM. Endocr Pract. 2016;22(3):315–22. https://doi.org/10.4158/EP151016.OR.
Plodkowski RA, McGarvey ME, Huribal HM, Reisinger-Kindle K, Kramer B, Solomon M, et al. SGLT-2 inhibitors for the treatment of type 2 diabetes mellitus. Fed Pract. 2015;32(Suppl. 11):10S–7S.
Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, et al. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013;36:2154–61.
Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–92.
Leiter LA, Yoon KH, Arias P, Langslet G, Xie J, Balis DA, et al. Canagliflozin provides durable glycemic improvements and body weight reduction over 104 weeks versus glimepiride in patients with type 2 diabetes on metformin: a randomized, double-blind, phase 3 study. Diabetes Care. 2015;38:355–64.
Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Ways K, et al. CANVAS Trial Collaborative Group. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Diabetes Care. 2015;38:403–11.
Ando Y, Shigiyama F, Hirose T, Kumashiro N. Simplification of complex insulin regimens using canagliflozin or liraglutide in patients with well-controlled type 2 diabetes: a 24-week randomized controlled trial. J Diabetes Investig. 2021;12:1816–26.
Yale JF, Bakris G, Cariou B, Nieto J, David-Neto E, Yue D, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–27.
List JF, Woo V, Morales E, Tang W, Fierdorek F. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650–7.
Rosenstock J, Hansen L, Zee P, Li Y, Cook W, Hirshberg B, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: a randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin. Diabetes Care. 2015;38(3):376–83. https://doi.org/10.2337/dc14-1142.
Nauck MA, Del Prato S, Meier JJ, Durán-García S, Rohwedder K, Elze M, et al. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin. Diabetes Care. 2011;34:2015–22.
Müller-Wieland D, Kellerer M, Cyprik K, Skripova D, Rohwedder K, Johnsson E, et al. Efficacy and safety of dapagliflozin or dapagliflozin plus saxagliptin versus glimepiride as add-on to metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:2598–607.
Ridderstråle M, Andersen KR, Zeller C, Kim G, Woerle HJ, Broedl UC, et al. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:691–700.
Ridderstrale M, Rosenstock J, Andersen KR, Woerle HJ, Salsali A. Empagliflozin compared with glimepiride in metformin-treated patients with type 2 diabetes: 208-week data from a masked randomized controlled trial. Diabetes Obes Metab. 2018;20:2768–77.
Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40:201–9.
Ferrannini EI, Berk A, Hantel S, Pinnetti S, Hach T, Woerle HJ, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;36:4015–21.
Kovacs CS, Seshiah V, Merker L, Christiansen AV, Roux F, Salsali A, et al. Empagliflozin as add-on therapy to pioglitazone with or without metformin in patients with type 2 diabetes mellitus. Clin Ther. 2015;37:1773–88.
Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ. Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78-week randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2015;17:936–48.
Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care. 2014;37:1815–23.
Rodbard HW, Rosenstock J, Canani LH, Deerochanawong C, Gumprecht J, Ostergaard Lindberg S, et al. Oral Semaglutide versus Empagliflozin in patients with type 2 diabetes uncontrolled on metformin: the Pioneer 2 trail. Diabetes Care. 2019;42:2272–81.
Lingvay I, Capehorn MS, Catarig A-M, Johansen P, Lawson J, Sandberg A, et al. Efficacy on once-weekly Semaglutide vs Empagliflozin added to metformin in type 2 diabetes: patient-level meta-analysis. J Clin Endocrinol Metab. 2020;105:e4593–604.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–8.
Zaccardi F, Webb DR, Htike ZZ, Youssef D, Khunti K, Davies MJ. Efficacy and safety of sodium-glucose co-2 inhibitors in type 2 diabetes mellitus: systematic review and network meta-analysis. Diabetes Obes Metab. 2016;18:783–94. https://doi.org/10.1111/dom.12670.
Zinman B, Wanner C, Lachin J, Fitchett D, et al. Empagliflozin, cardiovascular outcones and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–27.
Chilton R, Tikkanen I, Cannon CP, Crowe S, Woerle HJ, Broedl UC, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180–93. https://doi.org/10.1111/dom.12572.
Sattar N, McLaren J, Fristen SL, Preiss D, McMurray JJ. SGLT2 inhibition and cardiovascular events: why did EMPA-REG outcomes surprise and what are the likely mechanisms? Diabetologia. 2016;59:1333–9.
Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–57.
Wiviott SD, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–57.
Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with Ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–35.
Wanner C, Inzucchi S, Lachin J, Fitchett D, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:1801–12.
Dagogo-Jack S, Pratley RE, Cherney DZI, McGuire DK, Cosentino F, Shih W, et al. Glycemic efficacy and safety of the SGLT2 inhibitor ertugliflozin in patients with type 2 diabetes and stage 3 chronic disease: an analysis from the VERTIS CV randomized trial. BMJ Open Diabetes Res Care. 2021;9:e002484.
Tang H, Li D, Zhang J, Li Y, Wang T, Zhai S. Sodium-glucose transporter-2 inhibitors and risk of adverse renal outcomes in patients with type 2 diabetes: a network meta-analysis of randomized control trials. Diabetes Obes Metab. 2017;19:142–7.
Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–306.
Brown E, Wilding JPH, Alam U, Barber TM, Karalliedde J, Cuthbertson DJ. The expending role of SGLT2 inhibitors beyond glucose-lowering to cardiorenal protection. Ann Med. 2020;53:2072–89.
Rhee JJ, Jardine MJ, Chertow GM, Mahaffey KW. Dedicated kidney disease-focused outcome trials with sodium-glucose cotransporter-2 inhibitors: lessons from CREDENCE and expectations from DAPA-HF, DAPA-CKD, and EMPA-KIDNEY. Diabetes Obes Metab. 2020;22(suppl 1):46–54.
Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108–14. https://doi.org/10.2337/dc16-0330.
Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115–22. https://doi.org/10.2337/dc16-0542.
Lega IC, Bronskill SE, Campitelli MA, Guan J, Stall NM, Lam K, et al. Sodium glucose cotransporter 2 inhibitors and risk of genital mycotic and urinary tract infection: a population-based study of older women and men with diabetes. Diabetes Obes Metab. 2019;21:2394–404.
Chandrashekar M, Philip S, Nesbit A, Joshi A, Perera M. Sodium glucose-linked transport protein 2 inhibitors: an overview of genitourinary and perioperative implications. Int J Urol. 2021;28:984–90.
Bersoff-Matcha SJ, Chamberlain C, Cao C, Kortepeter C, Chong WH. Fournier gangrene associated with sodium-glucose cotransporter-2 inhibitors. A review of spontaneous postmarketing cases. Ann Intern Med. 2019;170:764–9.
Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38:1638–42.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Dailey, G., Rodriguez-Saldana, J. (2023). Sodium-Glucose Cotransporter 2 Inhibitors. In: Rodriguez-Saldana, J. (eds) The Diabetes Textbook. Springer, Cham. https://doi.org/10.1007/978-3-031-25519-9_36
Download citation
DOI: https://doi.org/10.1007/978-3-031-25519-9_36
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25518-2
Online ISBN: 978-3-031-25519-9
eBook Packages: MedicineMedicine (R0)