Abstract
Restoring the structural complexity and functional diversity of tropical rainforest is not possible in human time scales but knowledge of the process has significantly increased over the past three decades. Strategies to restore tropical forests must build on theories of community assembly and succession, as well as understanding of both the local ecological and human communities. In this chapter, we discuss three long-term tropical forest restoration case studies in Australia, Costa Rica, and Thailand, each using specific approaches tailored to overcome local ecological, cultural, and socioeconomic constraints. Differences are apparent in the intensity of restoration intervention adopted to manage ecological issues, and in the way local cultures, prevailing socioeconomic conditions, and therefore costs can influence outcomes. Based on the unifying threads identified, we detail key factors essential to recovering tropical biodiversity whilst protecting the livelihoods of landholders on whose land restoration is most likely to occur.
Lead authors of the case studies: Costa Rica: Karen D. Holl & Rakan A. Zahawi; Thailand: Stephen Elliott and Australia: Nigel I. J. Tucker.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Introduction
Complexity of structure, high species diversity, niche abundance and a myriad of ecological interactions combine to challenge the very human notion that tropical rainforests could ever be truly restored to their natural condition. Despite this, our understanding of the ongoing loss of tropical biodiversity, particularly the widespread intolerance of obligate forest species to fragmentation and loss of forest cover, has spurred global attempts to reverse the decline. Evidence of this support is seen in the Bonn Challenge, the UN Decade of Ecosystem Restoration and various ‘trillion tree planting’ initiatives (Brancalion & Holl, 2020). Tropical restoration is underpinned by ecological succession and community reassembly theory, where ecosystem recovery is largely driven by interactions between animals and the plants on which they depend (e.g., Howe, 2016). In this scenario, succession is neither uniform nor predictable (Norden et al., 2015), but in an ecosystem restoration context it provides a means to test traditional notions of sequential replacement and a framework to monitor development of ecosystem processes and function (Hobbs & Norton, 1996).
Typically, restoration interventions have been dichotomised as either ‘passive’ or ‘active’, the former meaning reliance solely on natural regeneration and the latter involving tree planting (DellaSala et al., 2003). However, an either/or approach is overly simplistic; anthropogenically modified landscapes impose both biological and socio-economic constraints, and restoration requires nuanced approaches that consider landscape context and prior land-use, as well as biophysical factors (Holl & Aide, 2011). Restoration ecologists respond by using various techniques, ranging from manipulation of natural regeneration through to planting increasingly diverse mixtures of species, and various terms describe these techniques, as discussed by McDonald et al. (Chap. 7, this volume).
Tropical restoration is mostly conducted in developing nations, on lands where agriculture provides the primary livelihood. This means that loss of agricultural land (which can be regarded as an opportunity cost) to forest restoration for the provision of global ecosystem services exposes lower socio-economic societies to additional economic stress, unless such services are fairly valued and paid for. Recognising this, restoration may embrace economically or culturally valuable species to encourage uptake, but this and other trade-offs also require a nuanced approach. As such, large-scale global restoration initiatives test the ability of restoration ecologists to ensure potential biodiversity benefits are realised, livelihoods are protected and appropriate restoration techniques are applied (Di Sacco et al., 2021).
In this chapter, we provide a brief overview of the ecological and socio-economic factors that influence tropical forest recovery, illustrating how these have been addressed under various ecological and socio-economic settings. We have used three long-term restoration case studies carried out in tropical Australia, Asia and Central America. Our studies encompass various levels of intervention used to achieve restoration outcomes that are relevant to both the level of degradation and landscape context. Despite inherent differences, common problems and challenges can be seen to emerge. We close by detailing key unifying lessons distilled from these case studies.
Key Constraints
Ecological Factors
Following human disturbance, autogenic recovery rates in tropical forest ecosystems vary tremendously. In some cases, biomass and species composition recover within a couple of decades (Marín-Spiotta et al., 2008; Letcher & Chazdon, 2009). Elsewhere land may remain in a state of arrested succession due to highly degraded soils or competition with aggressive ruderal species (Chazdon, 2003; Lamb et al., 2005). Rates of natural regeneration depend on a combination of the type, intensity, duration and sequencing of past disturbances, the ecology of the specific forest type (Holl & Aide, 2011) and crucially, the density and composition of incoming seed rain. Since the seeds of most tropical forest species are recalcitrant, few retain viability in the soil seed bank beyond 2–3 years post-clearing (Vázquez-Yanes & Orozco-Segovia, 1993). Consequently, long-term recovery of tree species richness and its accompanying biodiversity depends mostly on seed rain. This in turn is dependent, firstly, on the presence of seed sources near restoration sites and, secondly, on viable populations of seed-dispersing animals, given that 70–90% of wet forest tropical tree species are dispersed by animals (Howe & Smallwood, 1982).
Many studies demonstrate that animal-mediated dispersal is often a primary factor limiting tropical forest recovery (reviewed in Holl, 2007). Regeneration may also occur vegetatively from seedlings and/or re-sprouts from stumps, roots or stems already present when land was abandoned. The contribution of different modes of regeneration to ecosystem recovery depends on the nature an intensity of prior disturbance (e.g., low-intensity agriculture or selective logging) providing these modes of regeneration remain after human disturbance.
After seed arrival, several other factors may limit seed germination and seedling survival, as well as time to reproductive maturity. These include seed/seedling predation, competition from aggressive under-storey vegetation, stressful microclimatic conditions, limited availability of soil nutrients and diseases (Holl, 2012, Fig. 3.1). Seed/seedling predation by insects and mammals can be a major obstacle to the recovery of certain species on agricultural lands. On former pasture lands, aggressive exotic grasses (e.g., Imperata cylindrica, Urochloa spp., Megathyrsus spp., Pennisetum spp., Saccharum spontaneum) often form a monoculture which out-competes tree seedlings and elevates fire risk. Ferns (e.g., Dicranopteris spp., Pteridium spp.), shrubs and vines can rapidly overwhelm disturbed sites and impede the establishment and growth of forest trees (Zimmerman et al., 2007). Invasive species of both plants and animals are a particular obstacle to recovery in island ecosystems (Cordell et al., 2009).
Ecological factors affecting the rate of forest recovery. Square boxes illustrate stages in the dispersal, establishment and reproduction of vegetation. Circles illustrate ecological factors that affect the rate of transitions between the stages. (Holl et al., 2000)
Stressful microclimatic conditions may also limit seed germination and seedling survival and growth, particularly in seasonally dry forests (Vieira & Scariot, 2006). Light levels and air and soil temperatures are commonly much higher and humidity and soil moisture levels much lower in agricultural lands than in forests. Moreover, drier conditions in pastures and high grass biomass provide ideal fuel for fire, which kills seeds and seedlings of wet forest species, as most are not well adapted to fire (Janzen, 2002; Nepstad et al., 2008). Fires are becoming increasingly important with rising temperatures and more variable rainfall resulting from climate change, in addition to anthropogenic disturbances (Armenteras et al., 2021), and in some cases may lead to a transition towards savanna vegetation dominated by fire-tolerant species.
Soil nutrients and structure vary greatly across the tropics and as a function of land use history. In the large areas of the tropics covered by oxisols and ultisols, seedling growth is often limited by low nutrient levels, although the extent of nutrient limitation and the primary limiting nutrient vary by soil type and extent of degradation (Powers & Marín-Spiotta, 2017). After intensive human use, soils may become highly compacted, which impedes root growth and water-holding capacity. Many tropical trees form mycorrhizal associations, which facilitate phosphorus uptake, but agricultural land uses may substantially alter microbial communities (Carpenter et al., 2001; Allen et al., 2005), in turn affecting nutrient cycling.
The relative importance of each particular factor (Fig. 3.1) varies greatly from site to site depending on local-, landscape- and regional-scale factors. Surrounding land uses affect not only the abundance and composition of native flora and fauna that arrive at a site but also the abundance of potential seed and seedling predators, invasive plants and pathogens and the risk of fire spreading from adjacent land uses. If remnant trees are intentionally retained within agricultural lands, such as shade trees for coffee, cacao or for grazing animals in pastures, they can facilitate recovery (Guevara et al., 1986; Ramos et al., 2020). Higher within-site tree cover plays an important role in facilitating natural recovery by attracting seed-dispersing animals, ameliorating stressful microclimate conditions and shading out light-demanding vegetation (Holl, 2012). Recovery also tends to be faster in relatively warmer and wetter lower-elevation areas, which generally favour more rapid growth (Zarin et al., 2001).
Socio-economic Factors
Restoring tropical forest ecosystems involves both direct and indirect costs, regardless of the particular methods employed. Whilst ecologists have delivered the technical means to restore self-sustaining ecosystems, the long-term socio-economic sustainability of restored ecosystems is assured only when the value of their benefits outweigh restoration costs and where restoration outcomes are valued higher than alternative land uses. Although there is a growing body of literature showing this to be true in many situations (Abram et al., 2016; Bradbury et al., 2021; Mappin et al., 2021), no reliable mechanisms exist to convert benefit values into cash incentives for local people, who bear the brunt of the financial and social costs of restoration.
The level of cost depends on the extent of restoration intervention needed and the needs of the local economy. Even where restoration is achieved solely through natural regeneration, costs remain for site protection, including fire prevention, livestock exclusion, prevention of logging and for assisting regeneration by weeding, fertilizer application and mulching. Where natural regeneration potential is insufficient, tree planting becomes necessary, and this requires seed collection and tree nurseries, in addition to planting, maintenance and monitoring costs. If start-up funds are obtained as loans, interest payments must be added to the ancillary overheads, which also include costs for planning, training, legal services and verification. Finally, lost opportunity costs (defined as income forgone from the most likely alternative land-uses) must also be considered. Labour is the greatest cost component (Brancalion et al., 2019), and since labour costs depend on the local economy, total restoration cost varies enormously among countries.
As expected, assessing the economic value of ecosystem services is challenging. The Economics of Ecosystems and Biodiversity study (TEEB 2009) determined that the average value of tropical forest ecosystem services was $US6,120 ha/year ($US7732/ha/year today, adjusted for inflation, based on 109 studies). Watershed-related services accounted for 38.8% of that value, climate regulation (mostly carbon storage) 35.9% and forest products 11.2%, with cultural services and genetic resources comprising the remainder. All these values depend on biomass accumulation and biodiversity recovery—both core goals of restoration.
Economists and social scientists have made minimal progress with realizing these benefits to local communities in financial terms. For estimating the value of carbon sequestration, the UN’s REDD+Footnote 1 scheme offers some hope in this respect. However, the scheme has been criticized for subverting local forest management practices to meet global demands and for failing to deliver adequate income to local people. Furthermore, forest-related CO2 emissions in most of the countries where REDD+ has been implemented have not declined as expected (Duchelle et al., 2018; Elliott, 2018). Although forest-carbon value often exceeds revenue from the main drivers of deforestation (Abram et al., 2016; Mappin et al., 2021; Jantawong et al., 2022), REDD+ has largely failed to provide financial incentives for restoration due to cultural factors, inadequate governance and unfavorable socio-economic conditions. Another problem is the unpredictability of fluctuations in carbon credit prices, relative to the profitability of alternative land uses, which constitutes a considerable financial risk.
Although the value of non-timber forest products (NTFPs) in restored forests is low compared with other benefits, start-up investment is often not needed and local people can directly market the products to customers (de Souza et al., 2016). NTFPs also provide security when other income sources decline (Guariguata et al., 2009), and their diversity provides a buffer against fluctuating market prices. However, sustainable harvesting is essential for the long-term viability of slow-growing species, and such a strategy requires careful monitoring. If yields begin to decline, community-level agreement on self-regulation needs to be introduced.
Financial realization of watershed services is also problematic. Efforts in this respect mostly consist of estimating the cost of ‘avoided detrimental impacts’, such as preventing flooding, landslides or mitigating declines in agricultural productivity arising from drought or siltation of irrigation infrastructure. These issues are mostly unpredictable in time and place. Furthermore, inhabitants of upper catchments often bear the brunt of restoration costs, whereas many of the water-related benefits accrue to downstream users. This suggests that watershed services should be regarded as a public good rather than a commodity, and in this respect, payments for them have increasingly been derived by governments through taxation, with successful schemes well-documented in Latin America and China (Porras et al., 2008).
Attainment of all these income streams from forest restoration depends on competent governance, particularly as it relates to land tenure, taxation and the absence of corruption (Mansourian, 2020). Another key requirement is extensive capacity-building, to mentor stakeholders in the skills, initiative and integrity, needed to implement these financial mechanisms. Innovative marketing will also be essential, because both investors and the public are largely unfamiliar with environmental services, and assistance will be needed in assembling support to sustain these services.
Case Studies
Strategies to overcome the ecological and socio-economic constraints associated with tropical forest restoration are detailed in the following case studies. They illustrate three different approaches, with each based on a specific landscape and social context. Whilst these studies all involve various levels of active restoration, each ultimately relies on natural regeneration to complete the recovery of forest structural complexity, biodiversity and ecological function. Each example also demonstrates that successful restoration involves meaningful engagement with all stakeholders and instituting a concomitant obligation to ensure that community and landholder needs and expectations are met. Importantly, each Case Study details projects established over 20+ years ago, allowing comprehensive insight into the processes of community reassembly, external support, on-going financial issues and the challenges which have been faced.
Case Study 1: Applied Nucleation in Costa Rica
Achieving restoration at scale is a major challenge for practitioners, and a key factor is cost, particularly for active restoration methods (Holl & Aide, 2011). Developing active restoration approaches that facilitate forest recovery, as much as or more than plantation-style planting, while reducing implementation costs is the key to scaling up tropical forest restoration. Trees can be planted in spatial patterns (Shaw et al., 2020), such as strip-planting (i.e., rows of seedlings between unplanted areas allowed to regenerate naturally), planting patches of trees in applied nucleation (Corbin & Holl, 2012) or focusing plantation-style planting in areas where regeneration is impeded.
The Islas Project, established between 2004 and 2006 in southern Costa Rica (Holl et al., 2020), is the longest-running tropical restoration experiment designed to directly compare the effectiveness of applied nucleation to plantation-style planting and natural regeneration. Forests in this region are at the boundary between Tropical Premontane Wet and Rain Forest zones. They range in elevation from 1100 to 1430 m and receive a mean annual rainfall of 3500–4000 mm, with a dry season from December to March. Restoration treatments were replicated across 15 ~ 1-ha sites, each separated by >700 m and spread across a >100-km2 area (Fig. 3.2). At each replicate site, three 0.25-ha (50 × 50 m) plots were established, receiving one of three treatments: natural regeneration, applied nucleation or plantation. Plantation treatments were uniformly planted with trees, whereas applied nucleation plots were planted with six patches of trees of three different sizes: two each of 4 × 4, 8 × 8 and 12 × 12 m (Fig. 3.3). No planting was done in the natural regeneration plots. Four tree species, widely used in a range of agroforestry practices in the region, were inter-planted in alternating rows, each separated by 2.8 m. Species included two later-successional species, Terminalia amazonia (Combretaceae) and Vochysia guatemalensis (Vochysiaceae), and two fast-growing N-fixing species, Erythrina poeppigiana and Inga edulis (Fabaceae). A range of surrounding forest cover (~8–80% within 500 m) was integrated into the experimental design. Results from this study are detailed in more than 55 publications to date (http://www.holl-lab.com/islas-project.html) as well as in a recent synthesis paper (Holl et al., 2020). Here we highlight core findings that are most relevant to the theme of this chapter.
Study area (a) and the 15 study sites from which data were collected in southern Costa Rica (b). (Forest cover data are from Mendenhall et al., 2011)
Top panels show planting design and bottom panels illustrate the plots after 15 years, showing both planted and naturally recruited vegetation. In top panels, grey areas were planted with E Erythrina poeppigiana. I Inga edulis, T Terminalia amazonia and V Vochysia guatemalensis. Sm small, Med medium. (Artist credit: Michelle Pastor)
Applied nucleation, where only a quarter of the number of seedlings was planted as compared to plantations, is effective in restoring a range of ecological metrics but costs less than more extensive planting and promotes ecological heterogeneity over time (Holl et al., 2020). Most floral and faunal groups quantified had similar abundance and/or species richness by the end of the first decade of recovery in both applied nucleation and plantation restoration treatments (Fig. 3.4). Applied nucleation and plantation treatments attracted similar abundances of seed-dispersing birds and bats (Fig. 3.4a, b, Reid et al., 2014, 2015b), resulting in similar abundance and species richness measures of animal-dispersed seed deposition and germination and seedling recruitment (Fig. 3.4c, d, Reid et al., 2015a; Holl et al., 2017; Werden et al., 2020). Furthermore, both active restoration treatments had consistently greater recovery compared with natural regeneration (Holl et al., 2020). A critical factor in the vegetation recovery of both active restoration treatments was probably the role played by large-seeded dispersers such as toucans (Ramphastidae), in the recruitment of late-successional species (Reid et al., 2021); active restoration treatments overall had close to two-fold the proportion of large-seeded species arriving into treatments compared with natural regeneration sites (Werden et al., 2021).
Responses of ecological variables to forest restoration treatments. (a) Frugivorous bird abundance in 2016 (n = 11 sites); (b) frugivorous bat abundance in 2009 and 2012 (n = 10, Reid et al., 2015b); (c) abundance of animal-dispersal seeds >5 mm in 2012–2013 (n = 10, Reid et al., 2015a); (d) abundance of recruits with animal-dispersal seeds >5 mm in 2015 (n = 13, Holl et al., 2017); (e) estimated species richness of epiphytes in 2015 based on sample-based accumulation curves (n = 13, Reid et al., 2016); and (f) leaf litter arthropods in 2012 (n = 4, Cole et al., 2016). Values are means ±1 se. Means with the same letter do not differ significantly using Tukey’s multiple-comparison test among treatments
Applied nucleation costs less to implement than plantation-style planting because of the lower cost of planting and the maintenance of fewer trees (in this case 27% of trees planted in plantations). This is a key benefit that enables its use for scaling-up restoration, to achieve ambitious global targets (Wilson et al., 2021). Applied nucleation also promotes heterogeneity. This applies in the structural sense, as there is a gradient of canopy cover from the interior of planted tree nuclei to natural regeneration areas (Holl et al., 2013). It also applies to seed dispersal, as vertebrate-dispersed seeds were more heterogeneous in applied nucleation than in the plantation treatments (Werden et al., 2021). Moreover, growth of later-successional saplings was 39% higher in applied nucleation plots than in plantations, probably due to greater light availability (Kulikowski et al., 2023). As such, applied nucleation promotes recovery that more closely mimics natural regeneration, but at an accelerated rate.
Over the 17 years of this case study, recovery patterns have been seen to shift rapidly. For example, Inga edulis quickly became the dominant planted tree across all sites in the first few years of the study (Holl et al., 2011), but it has since been displaced by Vochysia guatemalensis (Lanuza et al., 2018). In turn, while it is not surprising that aboveground biomass was greater in the plantation treatments after a decade of recovery, litterfall rates at the onset of the second decade were comparable in plantation and applied nucleation restoration strategies (Lanuza et al., 2018). This indicates that productivity in applied nucleation can reach similar levels to the more expensive plantation option within just a decade. Successional dynamics of recruiting species have also shifted rapidly, from dominance by early-successional species in the first few years to a marked increase of later-successional species in active restoration treatments (but not in natural regeneration), during the second decade (Holl et al., 2017; Werden et al., 2021). Such rapid changes underscore the importance of long-term monitoring of recovering systems to increase understanding of the implications of different restoration interventions.
Recovery across sites was highly variable, consistent with most multi-site restoration studies. For example, above-ground biomass varied ~10-fold across sites (Holl & Zahawi, 2014). Whereas near complete canopy cover in plantation plots was established at some sites within 3–5 years, other plantation plots still have only partial canopy cover, even after >15 years. We have determined that a few important baseline measures can help predict whether or not a site is going to recover rapidly. First, we found a strong positive relationship between the initial rate of change in planted tree height in the first 2 years and the above-ground biomass of those same sites 6–8 years later (Holl & Zahawi, 2014). As such, early indicators of growth represent benchmarks for regeneration capacity. Second, we found that the number of tree recruits that establish within the first year and a half and their canopy cover are good indicators of the number of recruits and canopy over upcoming years (Holl et al., 2018). As such, leaving a targeted restoration site for a year or two to document natural recovery, before deciding whether to enact active restoration measures, is strategic. Finally, while it is important to quantify general trends that guide our ability to implement restoration at scale, a key management lesson is that selection of restoration strategies must be tailored to local site conditions.
Local restoration strategy was consistently more important, in the first few years, than was the percentage of surrounding forest cover, in driving recruitment patterns. Whereas surrounding percent forest cover was consistently a weak predictor of recovery (Reid et al., 2015a; Holl et al., 2017), the composition of surrounding remnants was key (Zahawi et al., 2021). Presence of a potential ‘mother tree’ within 100 m of a restoration plot resulted in a 10-fold increase in conspecific recruit abundance on average; proximity of adult ‘mother trees’ was also important, as was their abundance, where abundance of ‘mother trees’ was strongly correlated with the amount of forest cover. As such, results underscore the importance of assessing not only the amount of surrounding forest cover to predict the potential for recovery but also the species composition of that forest.
Whereas the ecological and economic advantages to less-intensive restoration approaches (such as applied nucleation and natural regeneration) are clear, limitations and social obstacles exist (Zahawi et al., 2014; Holl et al., 2020). First, most practitioners are accustomed to the widespread practice of plantation-style tree planting. Furthermore, many funding agencies measure success as numbers of trees planted. Whereas there are clearly other factors to consider as metrics of success, moving ingrained perceptions away from the ‘need’ for monoculture plantations will be difficult. Second, less uniform approaches tend to look ‘messy’ or ‘unkempt’. This transitional successional stage is a hindrance; local residents and investors may perceive it as abandoned land or project failure. As such, clear guidelines and sharing of information about proposed restoration approaches are essential to overcome both of these factors. Spatially patterned approaches are likely to be most appropriate where large land holdings are being restored with limited resources (Holl et al., 2020; Wilson et al., 2021).
Finally, central to restoration success is the assumption that what is set aside for recovery can persist in a regenerating forested state for a prolonged period of time (i.e., for several decades and ideally longer). However, experiences from our study, as well as assessments of the longevity of secondary forest patches in the region and elsewhere in Latin America (Reid et al., 2019; Schwartz et al., 2020), paint a somewhat challenging picture. Even within the confines of a formal study framework with year-round vigilance and monitoring, incursions and damage to some of our plots have occurred multiple times. This low-grade degradation can come in the form of livestock (e.g., cattle or goats released to graze in plots), opportune harvesting of trees for firewood or other timber purposes, and, in the worst situation, the wholesale conversion of land-use by an owner who no longer wished to participate in the project. Such incidents underscore the importance of understanding local socio-economic drivers of deforestation and developing effective socio-economic tools to counteract them (Brancalion & Holl, 2020; Di Sacco et al., 2021). To not do so increases the probability for long-term project failure and squanders the limited financial resources that are available for restoration.
Case Study 2: Testing the Framework Species Method of Forest Restoration in Northern Thailand
Chiang Mai University’s Forest Restoration Research Unit (FORRU-CMU) was established in 1994 to develop effective techniques to restore the tropical forest ecosystems of northern Thailand, with a particular focus on biodiversity conservation and environmental protection. At that time, colonial-era logging and subsequent agricultural conversion had left remnants of both primary and degraded forests scattered across landscapes, which were consequently undergoing rapid deforestation. This was exacerbated by continued disturbance including (i) fire, (ii) hunting of large seed-dispersing animals and (iii) land use encroachment. A national logging ban in 1989 prompted the instigation of a policy to convert many cancelled logging concessions into protected areas. This created a demand to restore forest ecosystems to near-natural conditions, encouraging the return of maximal biomass, structural complexity, biodiversity and ecological functioning by means of harnessing regenerative potential at both landscape and site levels. This trend towards restoration, primarily for conservation, was boosted substantially in 1993 when the Plook Pah Chalermphrakiat project was launched to celebrate His Majesty King Bhumibol Adulyadej’s Golden Jubilee. The goal was to plant diverse mixtures of native tree species on sites totaling more than 8000 km2 nationwide.
One restoration technique in line with the above criteria, which had emerged at that time, was the framework species method (FSM) (Fig. 3.5), which was first conceived to restore forest to degraded sites in the Wet Tropics of Queensland, Australia (Goosem & Tucker, 2013). This method complements natural regeneration by densely planting open sites, close to natural forest, with woody species characteristic of the reference ecosystem (sensu, Gann et al., 2019) selected for their ability to accelerate ecological succession. To begin the process, a rapid site assessment first determines the existing density of natural regeneration, based on a count of saplings >50-cm tall, live stumps or remnant mature trees. Framework species are then planted to raise the stocking density to that capable of closing the canopy within 2–3 years (3100 trees per ha in upland northern Thailand).
Framework tree species are selected from the indigenous tree flora of the reference forest for their high survival and growth rates on exposed sites, ability to inhibit herbaceous weeds and attractiveness to seed-dispersing wildlife. Only a small fraction of species from the reference-forest ecosystem are established, but planted trees attract frugivorous birds and mammals, dispersing seeds of many other tree species from nearby remnants. Planted trees also create suitable ground-level microclimate and weed-free conditions, which support establishment of the seedlings that germinate from the incoming seeds by providing a moist, shaded microclimate, free of weed competition (Fig. 3.5).
Following training with the originator of the technique, Nigel Tucker, at Lake Eacham National Park, Queensland, FORRU-CMU staff, adapted and tested the concept to restore upland evergreen forest in northern Thailand as the first reference-forest target (EGF, sensu Maxwell, 2001).
A survey of evergreen forest (EGF) trees in Doi Suthep-Pui National Park recorded more than 250 species (FORRU-CMU, 2005). Thereafter, multiple individuals of 100 identified species were tagged for a phenology study to determine optimal seed-collection times. Nursery experiments were performed to develop effective germination techniques, which produce potted saplings (30–50 cm tall) by the start of the rainy season—the optimum planting time (Blakesley et al., 2002). These experiments led to the development of production schedules, detailing the treatments and timings required for efficient planting-stock production of each species (Elliott et al., 2002; Elliott & Kuaraksa, 2008).
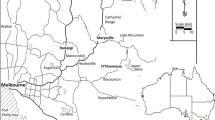
Field trials were established annually (1996–2013), forming a chronosequence and a wildlife corridor, covering 33 ha of a watershed 1200–1325 m above sea level. The plot system overlooked the Hmong community of Ban Mae Sa Mai, in the upper Mae Sa Valley of Doi Suthep-Pui National Park (DSPNP), Chiang Mai Province (Fig. 3.6, 18°51′46.62′′ N, 98°50′58.81′′ E). Plot details and locations may be viewed at www.restor.eco.
Plots ranging in size from 0.48 to 6.4 ha, were planted annually with various mixes of 20–30 framework species. The initial density of planted trees was calculated as 3100 per ha, less the estimated density of pre-existing natural regeneration, the latter being determined by surveys using circular sample plots of 5 m radius. Weeds were cut 6 weeks before planting, followed by a single application of glyphosate, which provided the planted trees with weed-free conditions for 6–8 weeks, before further hand-weeding became necessary. Planting stock comprised saplings 30–50 cm tall, grown from locally collected seeds. Trees were grown in plastic bags (22.8 × 6.3 cm) in a medium of forest soil, peanut husk and coconut husk in the ratio of 50:25:25.
Planting spots were marked with bamboo canes, spaced 1.8 m apart. A triplicated field trial in 1999, which compared spacings of 1.5–2.5 m on subsequent species recruitment (Sinhaseni, 2008), confirmed that a spacing of 1.8 m was optimal. Wider spacing delayed canopy closure, which resulted in weed persistence and fire. Closer spacing resulted in higher tree mortality and lower tree species recruitment. Approximately 50–100 g of fertilizer (NPK 15:15:15) was added around the base of each tree stem (Elliott et al., 2000), along with a mulch of cut weeds or corrugated cardboard.
This basic planting protocol was varied each year, in order to test the relative performance of different tree species and the effectiveness of various silvicultural treatments, including spacing, fertilizer types and dosages, weeding frequency, pre-plant pruning, bare-rooted vs. potted plant stock and cardboard mulch mats.
Hand weeding and fertiliser application, applied to both planted trees and natural regeneration, were performed three times in both the first and second rainy seasons after planting. Fire breaks were cut in mid-January at the start of the hot-dry season. Subseqeuntly, until mid-April (the start of the rainy season), fire prevention teams manned observation points to detect and extinguish any fires approaching the study area.
Standard data-collection protocols were developed, specifically to determine which tree species met framework-species-qualifying criteria. We monitored survival and growth 2 weeks after planting and at the end of the first, second and, sometimes, third rainy seasons. Survival and relative growth-rate data were combined into a relative performance index, allowing comparison among both the species and the treatments tested each year (see Elliott et al., 2013). Plots planted in 1998, 1999 and 2000 were also monitored over 6 years to determine first flowering and fruiting events and to assess their attractiveness to wildlife.
A key outcome of this work was an effective FSM for restoring evergreen forest on Stage-3 degraded land (sensu Elliott et al., 2013) (Fig. 3.7). Top-performing framework tree species were identified (Elliott et al., 2003) and the silvicultural treatments that maximized post-planting performance were determined (Elliott et al., 2000).
Deforested, over-cultivated and repeatedly burnt, this site in the upper Mae Sa Valley supported very little natural regeneration (a). Within 1 year of planting framework species in 2000, several of the planted trees over-topped weeds and began site recapture (b). By 2012, a structurally complex and biodiverse forest had re-established, with many trees germinating from incoming animal-dispersed seeds (c)
Rapid biomass and carbon accumulation were achieved by design, since framework species were deliberately selected for high survival and rapid growth and planted to achieve high initial stocking density. Jantawong et al. (2017) reported that tree-carbon stocks in the FSM plot system exceeded those of nearby old-growth forest remnants after 16–17 years. Above-ground tree-carbon accumulation was 106 ton C/ha over 14 years—almost double the pan-tropical average for natural forest regeneration (58 ton C/ha) over 20 years (Silver et al., 2000) and substantially higher than that achieved by 17-year-old teak plantations in western Thailand (16-ton C/ha) (Chayaporn et al., 2021).
Partitioning of the accumulating biomass resulted in rapid recovery of forest structural complexity (Fig. 3.8). Using the best-performing species and maintenance regimes, canopy closure can now be achieved routinely within 2–3 years. After 6 years, pioneer species form an upper canopy of 16–18 m above ground, with planted climax tree species creating a dense under-story 8–10 m high (Fig. 3.8). Tree seedlings and saplings form a dense ground layer growing in a deep layer of leaf litter, with litterfall reaching rates typical of old growth forest in 14–16 years (Kavinchan et al., 2015). The last structural components to return were vascular epiphytes and woody climbers, which appeared 18–20 years after tree planting.
Structural complexity created the niches required for biodiversity recovery. Species richness of the bird community increased from about 30 before planting to 88 after 6 years, representing about 54% of bird species recorded in nearby mature forest (Toktang, 2005). Sinhaseni (2008) documented 73 species of non-planted trees re-colonizing the plot system (0.46 ha sampled) within 8–9 years, most having germinated from seeds dispersed from nearby forest by birds (particularly bulbuls), fruit bats and civets. Species richness of mycorrhizal fungi (Nandakwang et al., 2008), lichens (Phongchiewboon, 2006) and bryophytes (Chawengkul, 2019) also increased, often exceeding the levels found in natural forest.
Recovery of ecological functioning, particularly those plant-animal interactions that enable pollination and seed dispersal, led to the return of natural forest dynamics, as evidenced by the density and diversity of regeneration ready to replace the planted trees (Sangsupan et al., 2018), particularly pioneers, which live for only 20–25 years.
In addition to an effective restoration procedure for EGF in northern Thailand, the project generated information for generic research methodologies, which were needed to devise framework species approaches suited to the ecological and social circumstances of almost any tropical forest type. This culminated in the publication of a guide for research students in 2008 (Forest Restoration Research Unit, 2008). The manual included standardised protocols for nursery and field experiments, data collection, presentation and analysis. Building on lessons learned from the EGF plots, FORRU-CMU devised equally effective FSMs for lowland deciduous forest in northern Thailand, bamboo-deciduous forest in Kanchanburi Province (Sapanthuphong et al., 2011) and lowland evergreen forest in Krabi Province (Elliott et al., 2008).
From the outset, education and outreach, based on the research outputs of the project, were essential activities of the unit. Educational events were implemented for school children and their teachers, workshops for NGO’s, government officers and community groups and training courses for professionals. Text books were produced in multiple languages, enabling outreach to extend to most south-east Asian countries. Units, based on the FORRU-CMU model, were replicated in China (Weyerhaeuser & Kahrl, 2006) and Cambodia (Kim, 2012), assisting forest authorities in those countries to interpret and establish FSMs, suited to their local forest types and socio-economic conditions.
Since FORRU-CMU is in a science faculty, our primary role has been to overcome the technical barriers to forest restoration. However, when establishing field trials, close collaboration with local communities was essential, inevitably involving us in addressing socio-economic aspects of restoration. Consequently, we developed procedures to perform participatory site surveys, project planning and collaborative costing and management protocols to run community-based tree nurseries (Table 3.1). This led to FORRU-CMU’s subsequent involvement in managing tree planting for Thailand’s first model PES (Payments for Ecological Services) project, which linked restoration financing to private-sector bottled water production (Elliott et al., 2018).
Case Study 3: Ecological Function in a Restored Wildlife Corridor
Whilst Australia has a well-developed economy, tropical forest restoration is subject to the same ecological and socioeconomic constraints present throughout the tropics. North Queensland’s Wet Tropics, the anthropogenic fragments of a Gondwanan remnant, have been impacted by typical patterns of human settlement, resulting in loss of habitat concentrated in areas of favourable climate, topography and high soil fertility. Some rainforest communities having been reduced to 2% of their pre-European colonisation extent. Lowland forests have been largely cleared for sugar cane and banana production, with only narrow strips of riparian forest remaining along major rivers. Most habitat below 40 m asl is limited to swamp and mangrove complexes, whereas upland fragments above 400 m asl, including many protected areas, are surrounded by a highly modified cropping and grazing matrix of private lands which imposes strict boundaries on the movements of many obligate rainforest species.
Donaghy’s Corridor was established to address this movement by restoring habitat to reconnect a 489-ha isolated reserve at Lake Barrine to the 80,000-ha block at Wooroonooran. These two National Park reserves were previously separated by ca.1-km of privately owned grazing lands. Intervening cattle pasture had been in place since the 1940s, and the banks of Toohey Creek, which flows through the property from Barrine into Wooroonooran, were severely eroded and compacted by livestock. In addition, large vertebrates such as the endangered southern cassowary (Casuarius casuarius johnsonii), a key species in the dispersal of fruits greater than 30-mm diameter, are now absent from Barrine and the overall loss of genetic variability has been documented in ubiquitous, but rainforest-dependent species at Barrine (Campbell, 1995).
A baseline survey recorded all vascular plants and mammals on the site before treatment, including vegetation along the creek, isolated paddock trees and other vegetation within 100-m of re-planted areas, but excluding forests at either end of the site. Permanent stock-exclusion fencing was erected around existing riparian vegetation and intervening pasture areas were re-planted. This ensured protection of higher-quality habitat resources so they could continue to attract seed-dispersing wildlife from adjacent areas. The corridor was established in four blocks over a period of 4 years, planting around 1.2 ha per year, with stems 1.7-m apart. Between 1995 and 1998, 16,800 selected seedlings from 100 reference ecosystem species (McDonald et al., 2016) were planted. Monitoring commenced on completion, focusing on colonisation by plants, reptiles and small mammals. Project design, establishment and monitoring parameters are discussed in Tucker (2000), and early post-establishment outcomes are detailed in Tucker and Simmons (2009) and Paetkau et al. (2009). Utilising both genetic and mark-recapture techniques, these studies demonstrated that within 5 years, planted areas functioned as both a movement conduit and habitat for some small mammals.
This restoration procedure at Donaghy’s Corridor was designed to establish a complex forest structure which would encourage rapid faunal utilisation and movement. In comparison to case studies 1 and 2, this project used a larger number of species, of about 55 on average per year, with selection based on functional traits. In addition to 30–40 framework species (Goosem & Tucker, 2013), narrow endemics, threatened species, large-fruited species and food plants of targeted vertebrates such as cassowaries were also planted. Such diverse plantings may be considered ‘maximum diversity’ approaches (Goosem & Tucker, 2013; Florentine et al., 2016), to be used where ecological connectivity is a primary goal of restoration. In this case study, we document changes in vegetation composition and structure that occurred over a 26-year period, and their relationship to ecosystem function, as seen through the prism of vertebrate seed dispersal.
Species Diversity and Composition
Baseline surveys recorded 132 native plants existing on site prior to treatment. These occurred along the creek and within 100-m of the corridor edge; trees, vines and shrubs, mostly concentrated in two small fragments totaling 1.75 ha. In 2000, 3 years after planting had been completed, transect surveys of naturally regenerating species revealed 115 native plants (4472 records from 180 × 5 m × 3 m plots), 25 of these being sourced from forests outside the corridor. In 2021, a re-survey of these 180 plots revealed 153 regenerating native species (4501 records), where coincidentally, 25 were again sourced from forests outside the corridor. Eleven regenerating species had disappeared in the intervening period. In 2000, average diversity of regenerating species at ground level was 6.9 per 15 m2. By 2021, average diversity at ground level foe species with stems less than 1 m, had increased to 15.4 per 15 m2.
Regenerating species comprised 59 plant families. Lauraceae was the most common (17 species), followed by Sapindaceae (13 species). Both are characteristic families in well-developed rainforests, producing fleshy fruits which are dispersed by many birds and mammals. Families of other basal lineages, for example, Annonaceae, Aristolochiaceae, Monimiaceae, Myristicaceae and Piperaceae, were represented by a number of regionally endemic species such as Galbulimima baccata – Himantandraceae, both inside and outside transects.
Changes in average seed size were less apparent. The number of large-seeded species (>30 mm diameter) increased from 7 to 14. Species diversity increased slightly but consistently across all fruit sizes.
Regenerating species were an admixture of pre-existing and planted species. However, 16% of regenerating species were neither planted nor pre-existing, and they had clearly originated from elsewhere. Many species have multiple dispersal vectors (Tucker & Murphy, 1997), but birds are responsible for most dispersal (89% of species). At the same time, species dispersed by both mammals and birds accounted for 31% whilst 25% were wind-dispersed.
This percentage of wind-dispersed species was largely attributable to reproduction of planted genera from Rutaceae and Proteaceae. Other species are dispersed by water, insects and gravity. Of 14 large-seeded species present in 2021, two of these (Gardenia ovularis seed dimensions 40 mm × 20–38 mm, and Beilschmiedia bancroftii 65–70 mm × 50–60 mm) were neither pre-existing nor planted. They have been introduced from outside the immediate vegetation matrix, emphasizing the potential diversity effect of vegetation proximity and presence of vertebrate dispersers.
Over a 20-year period, shifts occurred in the typical successional stage of regenerating vegetation (Fig. 3.9) and by 2021, late-successional and gap phase species occurred in equal proportions. In the intervening period, gap-phase species marginally declined, intermediate and late-intermediate groups remained relatively stable, but numbers of late-successional species increased.
Structural Development
In 2000, plantings displayed an even canopy of 3–5 m in height, whilst regenerating seedling heights were 25–150 mm, but this simple structure was sufficient to suppress weed growth and attract seed-dispersing wildlife. By 2021, a taller canopy (up to 32 m) with under-storey elements was in place, in addition to a diverse ground storey. In this community, regeneration was composed of a range of life forms. Canopy trees (40 spp.) and under-storey trees (42 spp.) were dominant, but vines, scramblers and rattans (25 spp.) were conspicuous elements of the under-storey, in some instances reaching canopy level.
Planted trees largely comprised the canopy and under-storey layers, even though regenerated vegetation was increasingly conspicuous in the under-storey. Buttresses were common on canopy trees which also hosted small numbers of epiphytic orchids and ferns. Figure 3.10 compares the forest structure in a 26-year section of the corridor and a forest reference site at Barrine. Whilst the number of stems greater than 1 cm diameter at breast height and the number of individuals is similar in the two sites, basal area in the reference site is higher than the corridor transect.
Profile diagrams of (a) reference forest site at Barrine and (b) Donaghy’s Corridor. Key: Aaci Acronychia acidula, Aper Argyrodendron peralatum, Apet Alphitonia petriei, Asti Austrosteenisia stipularis, Calp Castanospora alphandii, Csub Cardwellia sublimis, Ctri Cryptocarya triplinervis, Dmol Dysoxylum mollissimum, Egra Elaeocarpus grandis, Erum E. ruminatus, Fhis Ficus hispida, Fpim Flindersia pimenteliana, Fsch Flindersia schottiana, Glas Guioa lasioneura, Lfaw Litsea fawcettiana, Mell Melicope elleryana, Msub Macaranga subdentata, Ndea Neolitsea dealbata, Opan Olea paniculata, Pcle Phaleria clerodendron, Scry Syzygium cryptophlebium, Slan Sloanea langii, Tcil Toona ciliata; Xwhi Xanthostemon whitei
Vertebrate Dispersal
The observed mechanisms of effective dispersal, germination and persistence indicate the existence of suitable plant niches within the forest and the presence of vertebrates capable of moving variously sized fruits. In this instance, dispersal of large-fruited Lauraceae such as Endiandra insignis (50–90 mm × 50–100 mm) and Beilschmiedia bancroftii almost certainly resulted from scatter-hoarding behaviour by giant white-tailed rats (Uromys caudimaculatus). Other Lauraceae appearing since 2000, including Beilschmiedia tooram and Endiandra sankeyana bear fruits of 35–55 mm dia. It is probable that white-tailed rats were also responsible for their dispersal, generally highlighting the important role of rodents in dispersal (Jansen et al., 2012). These plants and animals are all Wet Tropics endemics, characteristic of well-developed upland rainforests.
Thirty-one bird species were recorded in the corridor in 2021 (Tucker and Freeman, unpublished data). Twelve birds were mixed forest species and 19 were rainforest-dependent species, including four Wet Tropics endemics – the grey-headed robin (Heteromyias cinereifrons), Victoria’s riflebird (Lophorina victoriae), pied monarch (Arses kaupi) and tooth-billed bowerbird (Scenopoeetes dentirostris). In addition, six obligate frugivores were present – the Australasian figbird (Sphecotheres viridis), the black-eared catbird (Ailuroedus melanotis), the wompoo fruit-dove (Ptilinopus magnificus), the topknot pigeon (Lopholaimus antarcticus), the tooth-billed bowerbird and the migratory channel-billed cuckoo (Scythrops novaehollandiae). Other species, including Lewin’s honeyeater (Meliphaga lewinii), have mixed diets and are also important seed-dispersers in regenerating forest. Gape widths of all these species vary between 10 and 30 mm, accommodating the most common seed-size classes of regenerating vegetation.
Whilst figbirds, pigeons and channel-billed cuckoos are characteristically nomadic, other birds such as grey-headed robins and black-eared catbirds are confined to smaller territories, suggesting that the corridor contained resources that are used by both sedentary and wider-ranging species. Similarly, white-tailed rats are large and highly mobile rainforest rodents and have been recorded moving through, and residing within, the corridor after 3 years (Tucker & Simmons, 2009). Remote camera surveys in 2021 recorded white-tailed rats throughout the corridor. Dispersal of large fruits from within and outside the plantings confirmed their dispersal abilities and suggested that for this species, restored vegetation is used as both habitat and a movement conduit. In a study of bush rats (Rattus fuscipes), genetic exchange occurred in the corridor within 3 years (Paetkau et al., 2009), demonstrating that the corridor helped to overcome prior genetic isolation in the Barrine fragment.
Socio-economic Context
Locally, high land prices generally preclude the availability of large areas of cleared land for restoration by any method, especially in productive agricultural areas where native vegetation cover is very low and connectivity is most needed. Conversion of agricultural land to rainforest therefore requires targeted use of private lands to create such corridors, carried out with considerable community support. Donaghy’s Corridor typifies this situation since close contact and open negotiation with the Donaghy family (the main landholder) were the key factors influencing project outcomes. In this instance, the landholder wished to increase shade cover for grazing cattle to reduce heat loads during humid summer months (Lees et al., 2019). The establishment of the corridor vegetation provided significant shade, but a 3-row shelter-belt of hoop pine (Araucaria cunninghamiana) was additionally established outside the corridor to supply extra shade and also to provide an additional source of farm income. Hoop pine is an indigenous species commonly established in commercial timber plantations, and thus has significant value. Most hoop pines are now of equal height to corridor canopy vegetation, and the rows are favoured resting areas for stock (Fig. 3.11).
Community volunteers raised all seedlings and completed all plantings to establish the corridor; funding for fencing and off-stream stock watering points was provided by State and Commonwealth agencies. Initial monitoring was done by scientists and staff from the Queensland Parks and Wildlife Service’s Lake Eacham Nursery and a number of academic institutions, which demonstrated a cooperative effort across a range of stakeholders. Of particular importance is the community expectation that such public investment on private land was protected from future disturbance. Stakeholder engagement was therefore critical from conception to completion, and when Donaghy’s Corridor was ultimately protected under a Nature Refuge Agreement, it was provided with the same level of legislative protection as the adjacent National Park, thus securing its long-term future.
Reflection and Key Summary Points
Whilst these foregoing case studies are from three continents with markedly different social, economic and cultural settings, some common unifying threads are evident. Below we discuss six key points, derived from these threads, which we consider essential to the success of any tropical rainforest restoration project. Although it is obvious that no two restoration sites are the same, we feel that most projects would benefit by incorporating these general concepts into their project planning and implementation.
Prioritize Protection of Existing Forest
Despite the importance of tropical forests for conserving biodiversity, sequestering carbon, maintaining hydrologic cycling and supporting human wellbeing, rapid tropical deforestation continues with forest losses exceeding gains in many regions (Sloan et al., 2019). These three case studies clearly illustrate that although active restoration can accelerate tropical forest recovery, it is impossible to precisely recreate the diverse forests that were originally cleared. Substantial differences in species composition between the restoration sites and their respective reference forest ecosystems remained for all the case studies, (even after 15–26 years) and significant regeneration input. In particular, late-successional and large-seeded tree species were poorly represented. Hence, it is clear that the first priority must be to protect existing forests (Brancalion & Holl, 2020; Di Sacco et al., 2021) which means that restoration practitioners must accurately address the most important drivers of forest loss and degradation, which vary greatly depending on the socioeconomic and political context.
Preventing forest clearance and sensitively managing existing forest fragments are the most cost-effective forest conservation strategies. Moreover, undamaged existing forests provide significant contemporary benefits. Recovering habitats take many years for biomass to accumulate and for biodiversity to recover to the point of yielding substantial ecosystems services and forest products (Moreno-Mateos et al., 2017). Finally, our case studies illustrate that even small fragments of forest in agricultural landscapes, if managed well, can serve as reference ecosystems for establishing restoration goals and are therefore important biological reservoirs for the recolonization of restoration sites.
Match Management with Degradation Level
The intensity of degradation, the distance to remnant forests and the availability of seed dispersers are issues which are directly correlated with the nature of the restoration approach. Elliott et al. (2013) outlined five stages of forest degradation for which levels of restoration intensity and cost correspondingly increase (Fig. 3.12 and Table 3.2).
Matching restoration approach with level of degradation. (Adapted from Elliott et al., 2013)
Degradation Stage 1 is exemplified by selective logging. In such cases, sources of natural regeneration at a site remain varied and dense. If the site is protected from agricultural and exotic vegetation encroachment, wildfire and livestock grazing, it is likely the forest will recover without any further intervention. This is known as spontaneous or natural regeneration (Chazdon & Guariguata, 2016).
Degradation Stage 2 is similar, except that tree removal has been more intense, and reduced canopy cover allows weeds to colonise and suppress regeneration. In consequence, and in addition to the protective measures described above, other interventions are needed to tip the competitive balance in favour of regeneration, including weed control and fertiliser application. This is known as assisted natural regeneration (ANR) (FAO, 2019), and at Stages 1 and 2, the density of regenerating woody plants is sufficient to rapidly close the canopy, usually within 2–3 years.
Degradation Stage 3 occurs where sapling density falls below that needed to achieve canopy closure within a reasonable desired time frame. At this point, protective measures and ANR must be complemented by tree planting, with obvious cost increases, as seed collection programs and tree nurseries become necessary (Table 3.2). Where restoration sites are close to forest remnants, the framework species method works well. Framework tree species may be planted to complement ANR in small nuclei (case study 1), in larger plots (case study 2) or to form wildlife corridors (case study 3), depending on local ecological and economic conditions. They are selected specifically to enhance regeneration through weed suppression and animal seed-dispersal from nearby intact forest (Fig. 3.12).
Degradation Stage 4 occurs when seed-dispersal at the landscape level is insufficient to achieve acceptable rates of regeneration because forest remnants are too distant, seed-dispersing animals have been extirpated or ecological connectivity is required at more rapid temporal scales. Under such conditions, forest ecosystem restoration can only be achieved by planting most of the characteristic tree species of the reference ecosystem. This is the “maximum diversity” approach to forest restoration discussed in case study 3.
Degradation Stage 5 is reached when soil and microclimatic conditions have deteriorated beyond the point at which tree seedlings can establish without substrate amelioration. This is characteristic of open cut mined surfaces. Necessary procedures to improve the substrate’s physical structure can include topsoil addition, deep ripping and mounding to improve drainage and aeration. Adding fertilizer, organic materials and green mulching can improve nutrient status and promote recovery of soil fauna and microbiota (Sansupa et al., 2021). Planting Ficus spp. and legumes as nurse trees can also improve soil structure and nutrient status respectively. Once the soil conditions have been improved, applied nucleation, the framework species method, or maximum diversity approaches can be implemented, depending on distances to seed sources and disperser availability.
Determining which level of degradation has been reached need not be complicated. A rapid site-assessment protocol is available, using simple participatory techniques to measure pre-existing natural regeneration, weed cover and soil conditions to guide stakeholders towards the most appropriate restoration strategy (Elliott et al., 2013). To assist in this work, several online tools are now available to advise on species selection for sites at Degradation Stages 3–5 that require tree planting (Fremout et al., 2022).
Encourage Dispersal
Conservation of large frugivores is essential for effective seed dispersal, just as seed dispersal is crucial to maintain diversity, connectivity and colonisation. Since large frugivores depend on mature forest, conserving this forest is a necessary precursor to maintain their dispersal services. Dispersal is also conditional on the configuration and composition of remnant forest patches and individual trees across the landscape and the behavioural responses of different dispersers (González-Varo et al., 2017). Many large frugivores do not cross open areas between forest patches. Moreover, species such as primates, tapirs, fruit bats, hornbills and cassowaries are rare, threatened or in decline throughout the tropics and this has myriad negative effects (Galetti et al., 2013; Boissier et al., 2020). In this context, restoration potentially plays a dual role.
First, by re-establishing habitat islands between fragments, it can enhance the mobility of large frugivores and the likelihood of maintaining dispersal at the landscape scale. This is the so-called stepping stone concept. The case studies in this chapter demonstrate the catalytic effect of such habitat establishment and vertebrate-mediated dispersal on ecosystem recovery at a range of scales. Clearly, where and how habitats are restored will depend on site- and species-specific parameters and objectives (McDonald et al. Chap. 7, this volume) but site-patch-to-remnant proximity, and the size and composition of both remnant and restored sites are additional key factors affecting dispersal success (Zahawi et al., 2021).
Second, habitat restoration provides additional resources that sustain frugivore populations and increases the likelihood of their persistence. Maintaining frugivore populations and the dispersal services they provide is essential to restore the structural complexity, species diversity and ecological functioning that typify mature tropical forests. As such, a more heterogeneous and ecologically connected landscape favours large frugivore persistence and the probability that dispersal will continue to aid natural development of functionality and resilience in restored areas. Restored forest may not closely resemble intact forest for decades or centuries, but these case studies show that strategic placement of restoration sites, as well as their species composition, can rapidly encourage effective dispersal across landscapes (Fig. 3.13).
Selection of restoration strategies tailored to local site conditions. The size, composition and location of remnant vegetation affect restoration strategy choice. In sites proximal to remnant forest (green outline) and some scattered trees, applied nucleation (purple outline) is an effective strategy to foster regeneration and recover large areas when it is compatible with stakeholder restoration goals. In larger open areas, frugivore-attracting framework species (brown outline) can be planted adjacent to remnant forest or as patches that form stepping stones between remnants. Reconnecting patches of remnant vegetation through corridors (gold outline) permits the flow of genetic material across the landscape. Using riparian zones to re-establish ecological connectivity confers additional benefits to soil stability and water quality. (Illustrator: Tim Parker)
Selecting which tree species to plant should be based on previously recorded performance, or on the functional traits that predict performance, to maximise ecological and social benefits (Rodrigues et al., 2009; Meli et al., 2014). Survival of planted trees is paramount, and using local species from the reference ecosystem confers the benefits of local adaptation. Many rainforest species, including shade-tolerant late-successional species, grow well in open, degraded sites. This ecological plasticity allows for direct establishment of late-successional species, circumventing existing barriers to establishment and the time lag associated with natural seed dispersal. Where degradation is severe, Leguminosae or other N-fixing groups should be planted to improve soil condition and fertility. This includes fast-growing species to shade out weeds, since it is key to reducing competition with newly established trees.
Because seed dispersal is crucial, selecting species that attract seed-dispersing wildlife is immensely beneficial. Fleshy fruits or arillate seeds with a 3–10-mm diameter attract many bird species with various gape sizes. Pioneer trees which fruit within a few years of plantings are important in this regard (Camargo et al., 2020). Furthermore, their early mortality (often within 20–30 years) creates light gaps and provides coarse woody debris, both of which to habitat structure and biodiversity recovery.
Across the tropics, several plant families are consistently associated with frugivorous seed dispersal. Some of these are Annonaceae, Arecaceae, Burseraceae, Lauraceae, Moraceae, Sapotaceae and Sapindaceae. Species from these families are likely to attract many frugivore guilds. Similarly, including a suite of local Ficus increases food availability for frugivores during seasonal scarcity, contributing to the continuity of dispersal and regeneration throughout the year (Zahawi & Reid, 2018).
Design Trials to Learn from Experience
These case studies demonstrate the value of using trials to assess the effectiveness of proposed restoration techniques and species choices locally, and draw attention to the considerable length of time needed for trials to yield sound advice. Therefore, attending to pre-existing knowledge is important for project initiation. Data from regular monitoring, accumulated as projects mature, is used later for ‘adaptive management’ – a central tenet of ecological restoration (Gilmour, 2007). International standards can provide broad guidance (Pedrini & Dixon, 2020), but surveys of reference forest and restoration sites involving all local stakeholders are essential to yield locally relevant information. Indigenous and local knowledge is invaluable for identifying the tree species that thrive on deforested sites, for locating seed trees, and for selecting species that local stakeholders value (Wangpakapattanawong et al., 2010).
We recommend that monitoring be carried out in three locations. These are: (i) the origin or control (part of the degraded site where no restoration interventions are applied), (ii) the treatment (where restoration interventions are applied) and (iii) the target (usually a nearby remnant of the reference ecosystem). Before any restoration interventions are applied, starting site conditions (baseline data) should be measured at permanent sampling points across all three locations (Viani et al., 2018), and measures should be repeated annually, at least until canopy closure. Comparing monitoring steps (i) and (ii) determines the effectiveness of restoration interventions relative to natural regeneration. Comparing steps (ii) and (iii) determines the extent of progress towards restoration goals and how restoration practices can be improved (Viani et al., 2018).
Variables recorded should relate to the fundamental restoration goals of maximizing the recovery of biomass, increasing forest structural complexity, recovering biodiversity and achieving sustained ecological functioning (Elliott et al., 2013). As we have previously indicated, these goals should also be consistent with social variables indicating improved human livelihoods (Viani et al., 2017). To establish a data bank, the size and condition of each tree should be recorded. Simple confidence limits can then be applied to estimate changes in tree density and size over time, with biomass and carbon accumulation derived from allometric equations (Pothong et al., 2021). For this task, drones now offer cost-effective and non-intrusive alternatives to conventional, labour-intensive field work to monitor tree survival and growth and canopy closure (de Almeida et al., 2020).
Encourage Stakeholder Participation Throughout the Restoration Process
Successful restoration depends on involving stakeholders at all stages, from planning and implementation to maintenance and monitoring (Mansourian & Vallauri, 2014; Holl, 2017). It is important to understand that restoration often fails because planted trees are not maintained, local people convert the land back to agricultural production, or less frequently, clear trees as a political protest (Brancalion & Holl, 2020). In order to avoids such pitfalls, the inclusion of all stakeholders (including those likely to legally or illegally use the land for other purposes) in the setting of project aims and its subsequent development, together with clarification of land tenure and usufruct rights, will increase the likelihood of long-term socio-economic sustainability of restoration projects (Guariguata & Brancalion, 2014; Chang & Andersson, 2019). These community stakeholders should be involved in planning to ensure that the project is transparently designed to address their needs and concerns. They should also be meaningfully engaged throughout the implementation, maintenance and monitoring phases of projects (Holl, 2020).
In some cases, restoration projects can be undertaken on publicly owned lands, but to meet the ambitious restoration targets of the Bonn Challenge and the UN Decade on Ecosystem Restoration, it is likely that most of them will have to occur on private lands. Since most landowners depend on income from their land, restoration projects must monetize the benefits of restoration. This may take the form of cash payments for environmental services to encourage landowner participation (Pirard et al., 2014). It also means that restoration projects must be designed to meet community needs, such as selecting tree species that will ultimately provide shade, timber, honey, firewood or other product with values for the community (Meli et al., 2014) or, alternatively, choosing a plantation-style planting design to accommodate landowner aesthetic preferences (Zahawi et al., 2014).
Equally important to successful restoration is incorporating local and indigenous knowledge into the project and ensuring that landowners are trained in best practices for restoration and site maintenance, to provide extra technical capacity. Moreover, giving landowners management responsibility over the project is a key to project success (Gregorio et al., 2020; Hagazi et al., 2020) and engaging stakeholders in participatory monitoring is a powerful way to encourage social learning and to promote adaptive management (Case study 2, Evans et al., 2018).
Our case studies focused largely on the ecological aspects of forest restoration, given our expertise as ecologists and our focus on tropical forest restoration for biodiversity conservation. We close by reiterating that achieving the ambitious forest restoration targets proposed internationally will require undertaking forest restoration for a range of reasons, including improvement of ecosystem functions and human livelihoods (Brancalion & Holl, 2020; Di Sacco et al., 2021). Key to the success of these efforts will be in (i) clearly stating the goals of specific projects, (ii) tailoring restoration approaches to be consistent with the stated goals and with local ecological and economic conditions, (iii) carefully monitoring whether goals have been achieved and, (iv) engaging stakeholder participation and support of the project (Brancalion & Holl, 2020).
Notes
- 1.
Policies and incentives, developed under the UN Framework Convention on Climate Change, to finance restoration and conservation of forests as carbon sinks.
References
Abram, N. K., MacMillan, D. C., Xofis, P., Ancrenaz, M., Tzanopoulos, J., Ong, R., Goossens, B., Koh, L. P., Del Valle, C., Peter, L., Morel, A. C., Lackman, I., Chung, R., Kler, H., Ambu, L., Baya, W., & Knight, A. T. (2016). Identifying where REDD+ financially out-competes oil palm in floodplain landscapes using a fine-scale approach. PLoS One, 11, e0156481.
Allen, M. F., Allen, E. B., & Gomez-Pompa, A. (2005). Effects of mycorrhizae and nontarget organisms on restoration of a seasonal tropical forest in Quintana Roo, Mexico: Factors limiting tree establishment. Restoration Ecology, 13, 325–333.
Armenteras, D., Dávalos, L. M., Barreto, J. S., Miranda, A., Hernández-Moreno, A., Zamorano-Elgueta, C., González-Delgado, T. M., Meza-Elizalde, M. C., & Retana, J. (2021). Fire-induced loss of the world’s most biodiverse forests in Latin America. Science Advances, 7, eabd3357.
Bagong Pagasa Foundation. (2011). Cost comparison analysis ANR compared to conventional reforestation. FAO Regional Office for Asia and the Pacific.
Blakesley, D., Elliott, S., Kuarak, C., Navakitbumrung, P., Zangkum, S., & Anusarnsunthorn, V. (2002). Propagating framework tree species to restore seasonally dry tropical forest: Implications of seasonal seed dispersal and dormancy. Forest Ecology and Management, 164, 31–38.
Boissier, O., Feer, F., Henry, P.-Y., & Forget, P.-M. (2020). Modifications of the rain forest frugivore community are associated with reduced seed removal at the community level. Ecological Applications, 30, e02086.
Bradbury, R. B., Butchart, S. H. M., Fisher, B., Hughes, F. M. R., Ingwall-King, L., MacDonald, M. A., Merriman, J. C., Peh, K. S. H., Pellier, A.-S., Thomas, D. H. L., Trevelyan, R., & Balmford, A. (2021). The economic consequences of conserving or restoring sites for nature. Nature Sustainability, 4, 602–608.
Brancalion, P. H. S., & Holl, K. D. (2020). Guidance for successful tree planting initiatives. Journal of Applied Ecology, 57, 2349–2361.
Brancalion, P. H. S., Meli, P., Tymus, J. R. C., Lenti, F. E. B., Benini, R. M., Silva, A. P. M., Isernhagen, I., & Holl, K. D. (2019). What makes ecosystem restoration expensive? A systematic cost assessment of projects in Brazil. Biological Conservation, 240, 108274.
Camargo, P. H. S. A., Pizo, M. A., Brancalion, P. H. S., & Carlo, T. A. (2020). Fruit traits of pioneer trees structure seed dispersal across distances on tropical deforested landscapes: Implications for restoration. Journal of Applied Ecology, 57, 2329–2339.
Campbell, N. J. H. (1995). Mitochondrial control region variation in two genera of Australian rodents; Melomys and Uromys: Application to phylogenetics, phylogeography and conservation. Southern Cross University.
Carpenter, F. L., Mayorga, S. P., Quintero, E. G., & Schroeder, M. (2001). Land-use and erosion of a Costa Rican ultisol affect soil chemistry, mycorrhizal fungi and early regeneration. Forest Ecology and Management, 144, 1–17.
Catterall, C. P., & Harrison, D. A. (2006). Rainforest restoration activities in Australia’s tropics and subtropics rainforest. Cooperative Research Centre for Tropical Rainforest Ecology and Management Rainforest CRC.
Chang, K., & Andersson, K. P. (2019). Contextual factors that enable forest users to engage in tree-planting for forest restoration. Land Use Policy, 104, 104017.
Chawengkul, P. (2019). Effects of forest restoration age on species diversity of epiphytic bryophyte community. Naresuan University.
Chayaporn, P., Sasaki, N., Venkatappa, M., & Abe, I. (2021). Assessment of the overall carbon storage in a teak plantation in Kanchanaburi province, Thailand – Implications for carbon-based incentives. Cleaner Environmental Systems, 2, 100023.
Chazdon, R. L. (2003). Tropical forest recovery: Legacies of human impact and natural disturbances. Perspectives in Plant Ecology Evolution and Systematics, 6, 51–71.
Chazdon, R. L., & Guariguata, M. R. (2016). Natural regeneration as a tool for large-scale forest restoration in the tropics: Prospects and challenges. Biotropica, 48, 716–730.
Cole, R. J., Holl, K. D., Zahawi, R. A., Wickey, P., & Townsend, A. R. (2016). Leaf litter arthropod responses to tropical forest restoration. Ecology and Evolution, 6, 5158–5168.
Corbin, J. D., & Holl, K. D. (2012). Applied nucleation as a forest restoration strategy. Forest Ecology and Management, 265, 37–46.
Cordell, S., Ostertag, R., Rowe, B., Sweinhart, L., Vasquez-Radonic, L., Michaud, J., Cole, T. C., & Schulten, J. R. (2009). Evaluating barriers to native seedling establishment in an invaded Hawaiian lowland wet forest. Biological Conservation, 142, 2997–3004.
de Almeida, D. R. A., Stark, S. C., Valbuena, R., Broadbent, E. N., Silva, T. S. F., de Resende, A. F., Ferreira, M. P., Cardil, A., Silva, C. A., Amazonas, N., Zambrano, A. M. A., & Brancalion, P. H. S. (2020). A new era in forest restoration monitoring. Restoration Ecology, 28, 8–11.
de Souza, S. E. X. F., Vidal, E., Chagas, G. d. F., Elgar, A. T., & Brancalion, P. H. S. (2016). Ecological outcomes and livelihood benefits of community-managed agroforests and second growth forests in Southeast Brazil. Biotropica, 48, 868–881.
DellaSala, D. A., Martin, A., Spivak, R., Schulke, T., Bird, B., Criley, M., Van Daalen, C., Kreilick, J., Brown, R., & Aplet, G. (2003). A citizen’s call for ecological forest restoration: Forest restoration principles and criteria. Ecological Restoration, 21, 14–23.
Di Sacco, A., Hardwick, K. A., Blakesley, D., Brancalion, P. H. S., Breman, E., Cecilio Rebola, L., Chomba, S., Dixon, K., Elliott, S., Ruyonga, G., Shaw, K., Smith, P., Smith, R. J., & Antonelli, A. (2021). Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Global Change Biology, 27, 1328–1348.
Duchelle, A. E., Seymour, F., Brockhaus, M., Angelsen, A., Moeliono, M., Wong, G. Y., Pham, T. T., & Martius, C. (2018). REDD+: Lessons from national and subnational implementation. World Resources Institute.
Elliott, S. (2018). The interface between forest science and policy—A review of the IUFRO International and Multidisciplinary Scientific Conference 4–7 October 2016: Forestry-related policy and governance: Analyses in the environmental social sciences. Natural History Bulletin of the Siam Society, 63, 1–10.
Elliott, S., & Kuaraksa, C. (2008). Producing framework tree species for restoring forest ecosystems in northern Thailand. Small-Scale Forestry, 7, 403–415.
Elliott, S., Navakitbumrung, P., Zangkum, S., Kuarak, C., Kerby, J., Blakesley, D., & Anusarnsunthorn, V. (2000). Performance of six native tree species, planted to restore degraded forestland in northern Thailand and their response to fertiliser. In S. Elliott, J. Kerby, D. Blakesley, K. Hardwick, K. Woods, & V. Anusarnsunthorn (Eds.), Thailand: Restoration for wildlife conservation: International tropical timber organization and the forest restoration research unit (pp. 244–255). Chiang Mai University.
Elliott, S., Kuarak, C., Navakitbumrung, P., Zangkum, S., Anusarnsunthorn, V., & Blakesley, D. (2002). Propagating framework trees to restore seasonally dry tropical forest in northern Thailand. New Forest, 23, 63–70.
Elliott, S., Navakitbumrung, P., Kuarak, C., Zangkum, S., Anusarnsunthorn, V., & Blakesley, D. (2003). Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. Forest Ecology and Management, 184, 177–191.
Elliott, S., Kuarak, C., Tunjai, P., Polchoo, T., Thongtao, J., & Maxwell, J. (2008). A technical strategy for restoring Krabi’s lowland tropical forest. Forest Restoration Research Unit, Chiang Mai University.
Elliott, S., Blakesley, D., & Hardwick, K. (2013). Restoring tropical forests: A practical guide. Royal Botanical Garden.
Elliott, S., Chairuangsri, S. Shannon, D. Nippanon, P., & Amphon, R. (2018). Where science meets communities: Developing forest restoration approaches for Northern Thailand. Natural History Bulletin of the Siam Society, 63, 11–26.
Evans, K., Guariguata, M. R., & Brancalion, P. H. S. (2018). Participatory monitoring to connect local and global priorities for forest restoration. Conservation Biology, 32, 525–534.
FAO. (2014). Documenting ANR impacts on biodiversity, water quality and carbon sequestration (Internal Report, Project: TCP/RAS/3307).
FAO. (2019). Restoring forest landscapes through assisted natural regeneration (ANR) – A practical manual. Food and Agriculture Organization.
Florentine, S. K., Pohlman, C. L., & Westbrooke, M. E. (2016). The effectiveness of different planting frameworks for recruitment of tropical rainforest species on ex-rainforest land. Restoration Ecology, 24, 364–372.
Forest Restoration Research Unit. (2008). Research for restoring tropical forest ecosystems: A practical guide. Chiang Mai University.
FORRU-CMU. (2005). How to plant a forest: The principles and practice of restoring tropical forests. Chiang Mai University.
Fremout, T., Thomas, E., Taedoumg, H., Briers, S., Gutiérrez-Miranda, C. E., Alcázar-Caicedo, C., Lindau, A., Mounmemi Kpoumie, H., Vinceti, B., Kettle, C., Ekué, M., Atkinson, R., Jalonen, R., Gaisberger, H., Elliott, S., Brechbühler, E., Ceccarelli, V., Krishnan, S., Vacik, H., Wiederkehr-Guerra, G., Salgado-Negret, B., González, M. A., Ramírez, W., Moscoso-Higuita, L. G., Vásquez, Á., Cerrón, J., Maycock, C., & Muys, B. (2022). Diversity for Restoration (D4R): Guiding the selection of tree species and seed sources for climate-resilient restoration of tropical forest landscapes. Journal of Applied Ecology, 59, 664–679.
Galetti, M., Guevara, R., Côrtes, M. C., Fadini, R., Von Matter, S., Leite, A. B., Labecca, F., Ribeiro, T., Carvalho, C. S., Collevatti, R. G., Pires, M. M., Guimarães, P. R., Brancalion, P. H., Ribeiro, M. C., & Jordano, P. (2013). Functional extinction of birds drives rapid evolutionary changes in seed size. Science, 340, 1086–1090.
Gann, G. D., McDonald, T., Walder, B., Aronson, J., Nelson, C. R., Jonson, J., Hallett, J. G., Eisenberg, C., Guariguata, M. R., Liu, J., Hua, F., Echeverría, C., Gonzales, E., Shaw, N., Decleer, K., & Dixon, K. W. (2019). International principles and standards for the practice of ecological restoration (Restoration ecology) (Vol. 27, 2nd ed., pp. S1–S46).
Gilmour, D. (2007). Applying an adaptive management approach to FLR. In J. Reitbergen-McCraken, S. Maginnis, & A. Sarre (Eds.), The forest landscape restoration handbook (pp. 29–38). Earthscan.
González-Varo, J. P., Carvalho, C. S., Arroyo, J. M., & Jordano, P. (2017). Unravelling seed dispersal through fragmented landscapes: Frugivore species operate unevenly as mobile links. Molecular Ecology, 26, 4309–4321.
Goosem, S., & Tucker, N. I. G. (2013). Repairing the rainforest (2nd ed.). Wet Tropics Management Authority and Biotropica Australia.
Gregorio, N., Herbohn, J., Tripoli, R., & Pasa, A. (2020). A local initiative to achieve global forest and landscape restoration challenge—Lessons learned from a community-based forest restoration project in Biliran Province, Philippines. Forests, 11, 475.
Guariguata, M. R., & Brancalion, P. H. S. (2014). Current challenges and perspectives for governing forest restoration. Forests, 5, 3022–3030.
Guariguata, M. R., Garcia-Fernandez, C., Sheil, D., Nasi, R., Herrero-Jauregui, C., Cronkleton, P., & Ingram, V. (2009). Compatibility of timber and non-timber forest product management in natural tropical forests: Perspectives, challenges, and opportunities. Forest Ecology and Management, 259, 237–245.
Guevara, S., Purata, S. E., & Van der Maarel, E. (1986). The role of remnant forest trees in tropical secondary succession. Vegetatio, 66, 77–84.
Hagazi, N., Gebrekirstos, A., Birhane, E., Bongers, F., Kelly, R., & Brauning, A. (2020). Land restoration requires a shift from quantity to quality: Lessons from Tigray, Ethiopia. ETFRN News, p. 60.
Hobbs, R. J., & Norton, D. A. (1996). Towards a conceptual framework for restoration ecology. Restoration Ecology, 4, 93–110.
Holl, K. D. (2007). Oldfield vegetation succession in the Neotropics. In R. J. Hobbs & V. A. Cramer (Eds.), Old fields (pp. 93–117). Island Press.
Holl, K. D. (2012). Tropical forest restoration. In J. Van Andel & J. Aronson (Eds.), Restoration ecology (pp. 103–114). Blackwell Publishing.
Holl, K. D. (2017). Research directions in tropical forest restoration. Annals of the Missouri Botanical Garden, 102, 237–250.
Holl, K. D. (2020). Primer of ecological restoration. Island Press.
Holl, K. D., & Aide, T. M. (2011). When and where to actively restore ecosystems? Forest Ecology and Management, 261, 1558–1563.
Holl, K. D., & Zahawi, R. A. (2014). Factors explaining variability in woody above-ground biomass accumulation in restored tropical forest. Forest Ecology and Management, 319, 36–43.
Holl, K. D., Loik, M. E., Lin, E. H. V., & Samuels, I. A. (2000). Tropical montane forest restoration in Costa Rica: Overcoming barriers to dispersal and establishment. Restoration Ecology, 8, 339–349
Holl, K. D., Zahawi, R. A., Cole, R. J., Ostertag, R., & Cordell, S. (2011). Planting seedlings in tree islands versus plantations as a large-scale tropical forest restoration strategy. Restoration Ecology, 19, 470–479.
Holl, K. D., Stout, V. M., Reid, J. L., & Zahawi, R. A. (2013). Testing heterogeneity-diversity relationships in tropical forest restoration. Oecologia, 173, 569–578.
Holl, K. D., Reid, J. L., Chaves-Fallas, J. M., Oviedo-Brenes, F., & Zahawi, R. A. (2017). Local tropical forest restoration strategies affect tree recruitment more strongly than does landscape forest cover. Journal of Applied Ecology, 54, 1091–1099.
Holl, K. D., Reid, J. L., Oviedo-Brenes, F., Kulikowski, A. J., & Zahawi, R. A. (2018). Rules of thumb for predicting tropical forest recovery. Applied Vegetation Science, 21, 669–677.
Holl, K. D., Reid, J. L., Cole, R. J., Oviedo-Brenes, F., Rosales, J. A., & Zahawi, R. A. (2020). Applied nucleation facilitates tropical forest recovery: Lessons learned from a 15-year study. Journal of Applied Ecology, 57, 2316–2328.
Howe, H. F. (2016). Making dispersal syndromes and networks useful in tropical conservation and restoration. Global Ecology and Conservation, 6, 152–178.
Howe, H. F., & Smallwood, J. (1982). Ecology of seed dispersal. Annual Review of Ecology and Systematics, 13, 201–228.
Jansen, P. A., Hirsch, B. T., Emsens, W.-J., Zamora-Gutierrez, V., Wikelski, M., & Kays, R. (2012). Thieving rodents as substitute dispersers of megafaunal seeds. Proceedings of the National Academy of Sciences, 109, 12610–12615.
Jantawong, K., Elliott, S., & Wangpakapattanawong, P. (2017). Above-ground carbon sequestration during restoration of upland evergreen forest in northern Thailand. Open Journal of Forestry, 7, 157–171.
Jantawong, K., Kavinchan, N., Wangpakapattanawong, P., & Elliott, S. (2022). Financial analysis of potential carbon value over 14 years of forest restoration by the framework species method. Forests, 13, 144.
Janzen, D. H. (2002). Tropical dry forest: Area de Conservación Guanacaste, northwestern Costa Rica. In M. R. Perrow & A. J. Davy (Eds.), Handbook of ecological restoration (pp. 559–583). Cambridge University Press.
Kavinchan, N., Wangpakapattanawong, P., Elliott, S., Chairuangsri, S., & Pinthong, J. (2015). Use of the framework species method to restore carbon flow via litterfall and decomposition in an evergreen tropical forest ecosystem, northern Thailand. Agriculture and Natural Resources, 49, 639–650.
Kim, S. (2012). Identifying framework tree species for restoring forest ecosystems in Siem Reap Province. Royal University of Agriculture.
Kulikowski, A. J., Zahawi, R. A., Werden, L. K., Zhu, K., Holl Karen, D. (2023). Restoration interventions mediate tropical tree recruitment dynamics over time. Philosophical Transactions of the Royal Society B-Biological Sciences, 378, 20210077.
Lamb, D., Erskine, P. D., & Parrotta, J. D. (2005). Restoration of degraded tropical forest landscapes. Science, 310, 1628–1632.
Lanuza, O., Casanoves, F., Zahawi, R. A., Celentano, D., Delgado, D., & Holl, K. D. (2018). Litterfall and nutrient dynamics shift in tropical forest restoration sites after a decade of recovery. Biotropica, 50, 491–498.
Lees, A. M., Sejian, V., Wallage, A. L., Steel, C. C., Mader, T. L., Lees, J. C., & Gaughan, J. B. (2019). The impact of heat load on cattle. Animals, 9, 322.
Letcher, S. G., & Chazdon, R. L. (2009). Rapid recovery of biomass, species richness, and species composition in a forest chronosequence in northeastern Costa Rica. Biotropica, 41, 608–617.
Mansourian, S. (2020). Enabling factors to scale up forest landscape restoration: The roles of governance and economics. WWF/IUFRO.
Mansourian, S., & Vallauri, D. (2014). Restoring forest landscapes: Important lessons learnt. Environmental Management, 53, 241–251.
Mappin, B., Ward, A., Hughes, L., Watson, J. E. M., Cosier, P., & Possingham, H. P. (2021). The costs and benefits of restoring a continent’s terrestrial ecosystems. Journal of Applied Ecology, 59, 408–419.
Marín-Spiotta, E., Cusack, D. F., Ostertag, R., & Silver, W. L. (2008). Trends in above and belowground carbon with forest regrowth after agricultural abandonment in the neotropics. In R. Myster (Ed.), Post-agricultural succession in the neotropics (pp. 22–72). Springer.
Maxwell, J. F. (2001). Vegetation and vascular flora of Doi Sutep-Pui National park, northern Thailand. Biodiversity Research and Training Program.
McDonald, T., Jonson, J., & Dixon, K. W. (2016). National standards for the practice of ecological restoration in Australia. Restoration Ecology, 24, S4–S32.
Meli, P., Martínez-Ramos, M., Rey-Benayas, J. M., & Carabias, J. (2014). Combining ecological, social and technical criteria to select species for forest restoration. Applied Vegetation Science, 17, 744–753.
Mendenhall, C. D., Sekercioglu, C. H., Brenes, F. O., Ehrlich, P. R., & Daily, G. C. (2011). Predictive model for sustaining biodiversity in tropical countryside. Proceedings of the National Academy of Sciences of the United States of America, 108, 16313–16316.
Moreno-Mateos, D., Barbier, E. B., Jones, P. C., Jones, H. P., Aronson, J., Lopez-Lopez, J. A., McCrackin, M. L., Meli, P., Montoya, D., & Benayas, J. M. R. (2017). Anthropogenic ecosystem disturbance and the recovery debt. Nature Communications, 8, 14163.
Nandakwang, P., Elliott, S., Youpensook, S., Dell, B., Teaumroon, N., & Lumyong, S. (2008). Arbuscular mycorrhizal status of indigenous tree species used to restore seasonally dry tropical forest in northern Thailand. Research Journal of Microbiology, 3, 51–61.
Nepstad, D. C., Stickler, C. M., Soares, B., & Merry, F. (2008). Interactions among Amazon land use, forests and climate: Prospects for a near-term forest tipping point. Philosophical Transactions of the Royal Society B-Biological Sciences, 363, 1737–1746.
Norden, N., Angarita, H. A., Bongers, F., Martinez-Ramos, M., Granzow-de la Cerda, I., Breugel, M., Lebrija-Trejos, E., Meave, J. A., Vandermeer, J., Williamson, G. B., Finegan, B., Mesquita, R., & Chazdon, R. L. (2015). Successional dynamics in Neotropical forests are as uncertain as they are predictable. Proceedings of the National Academy of Sciences of the United States of America, 112, 8013–8018.
Ong, R. (2011). Recent forest restoration initiatives in Sabah, Malaysia. In P. B. Durst, P. Sajise, & L. Leslie (Eds.), Forests beneath the grass (pp. 119–124). Food and Agriculture Organization, Regional Office for Asia and the Pacific.
Paetkau, D., Vazquez-Dominguez, E., Tucker, N. I. G., & Moritz, C. (2009). Monitoring movement into and through a newly planted rainforest corridor using genetic analysis of natal origin. Ecological Management & Restoration, 10, 201–216.
Parrotta, J. A., Knowles, O. H., & Wunderle, J. M. (1997). Development of floristic diversity in 10-year-old restoration forests on a bauxite mined site in Amazonia. Forest Ecology and Management, 99, 21–42.
Pedrini, S., & Dixon, K. W. (2020). International principles and standards for native seeds in ecological restoration. Restoration Ecology, 28, S286–S303.
Phongchiewboon, A. (2006). Recovery of lichen diversity during forest restoration in northern Thailand. Chiang Mai University, M.Sc. thesis.
Pirard, R., de Buren, G., & Lapeyre, R. (2014). Do PES improve the governance of forest restoration? Forests, 5, 404–424.
Porras, I., Grieg-Gran, M., & Neves, N. (2008). A review of payments for watershed services in developing countries (Natural resource issues no. 11). International Institute for Environment and Development.
Pothong, T., Elliott, S., Chairuangsri, S., Chanthorn, W., Shannon, D. P., & Wangpakapattanawong, P. (2021). New allometric equations for quantifying tree biomass and carbon sequestration in seasonally dry secondary forest in northern Thailand. New Forest, 53, 17.
Powers, J. S., & Marín-Spiotta, E. (2017). Ecosystem processes and biogeochemical cycles in secondary tropical forest succession. Annual Review of Ecology, Evolution, and Systematics, 48, 497–519.
Ramos, D. L., Pizo, M. A., Ribeiro, M. C., Cruz, R. S., Morales, J. M., & Ovaskainen, O. (2020). Forest and connectivity loss drive changes in movement behavior of bird species. Ecography, 43, 1203–1214.
Raupp, P. P., Ferreira, M. C., Alves, M., Campos-Filho, E. M., Sartorelli, P. A. R., Consolaro, H. N., & Vieira, D. L. M. (2020). Direct seeding reduces the costs of tree planting for forest and savanna restoration. Ecological Engineering, 148, 105788.
Reid, J. L., Mendenhall, C. D., Rosales, J. A., Zahawi, R. A., & Holl, K. D. (2014). Landscape context mediates avian habitat choice in tropical forest restoration. PLoS One, 9, e90573.
Reid, J. L., Holl, K. D., & Zahawi, R. A. (2015a). Seed dispersal limitations shift over time in tropical forest restoration. Ecological Applications, 25, 1072–1082.
Reid, J. L., Mendenhall, C. D., Zahawi, R. A., & Holl, K. D. (2015b). Scale-dependent effects of forest restoration on Neotropical fruit bats. Restoration Ecology, 23, 681–689.
Reid, J. L., Chaves-Fallas, J. M., Holl, K. D., & Zahawi, R. A. (2016). Tropical forest restoration enriches vascular epiphyte recovery. Applied Vegetation Science, 19, 508–517.
Reid, J. L., Fagan, M. E., Lucas, J., Slaughter, J., & Zahawi, R. A. (2019). The ephemerality of secondary forests in southern Costa Rica. Conservation Letters, 12, e12607.
Reid, J. L., Korrman, U., Zarate-Charry, D., Holl, K. D., & Zahawi, R. A. (2021). Multi-scale habitat selection of key frugivores predicts large-seeded tree recruitment in tropical forest restoration. Ecosphere, 12, e03868.
Rodrigues, R. R., Lima, R. A. F., Gandolfi, S., & Nave, A. G. (2009). On the restoration of high diversity forests: 30 years of experience in the Brazilian Atlantic Forest. Biological Conservation, 142, 1242–1251.
Sansupa, C., Purahong, W., Wubet, T., Tiansawat, P., Pathom-Aree, W., Teaumroong, N., Chantawannakul, P., Buscot, F., Elliott, S., & Disayathanoowat, T. (2021). Soil bacterial communities and their associated functions for forest restoration on a limestone mine in northern Thailand. PLoS One, 16, e0248806.
Sangsupan, H. A., Hibbs, D. E., Withrow-Robinson, B. A., & Elliott, S. (2018). Seed and microsite limitations of large-seeded, zoochorous trees in tropical forest restoration plantations in northern Thailand. Forest Ecology and Management, 419–420, 91–100.
Sapanthuphong, A., Thampituk, S., & SukIn, A. (2011). Restoration of degraded forests in dry areas: Concepts and practices for forest restoration in the western region. Elephant Conservation Network.
Schwartz, N. B., Aide, T. M., Graesser, J., Grau, H. R., & Uriarte, M. (2020). Reversals of reforestation across Latin America limit climate mitigation potential of tropical forests. Frontiers in Forests and Global Change, 3, 85.
Shaw, J. A., Roche, L. M., & Gornish, E. S. (2020). The use of spatially-patterned methods for vegetation restoration and management across systems. Restoration Ecology, 28, 766–775.
Silver, W. L., Ostertag, R., & Lugo, A. E. (2000). The potential for carbon sequestration through reforestation of abandoned tropical agricultural and pasture lands. Restoration Ecology, 8, 394–407.
Sinhaseni, K. (2008). Natural establishment of tree seedlings in forest restoration trials at Ban Mae Sa Mai, Chiang Mai Province. M.Sc. thesis, Chiang Mai University.
Sloan, S., Meyfroidt, P., Rudel, T. K., Bongers, F., & Chazdon, R. (2019). The forest transformation: Planted tree cover and regional dynamics of tree gains and losses. Global Environmental Change, 59, 101988.
Swinfield, T., Afriandi, R., Antoni, F., & Harrison, R. D. (2016). Accelerating tropical forest restoration through the selective removal of pioneer species. Forest Ecology and Management, 381, 209–216.
TEEB. (2009). TEEB climate issues update. http://www.teebweb.org/publication/climate-issues-update/
Toktang, T. (2005). The effects of forest restoration on the species diversity and composition of a bird community in Doi Suthep-Pui National Park Thailand from 2002–2003. M.Sc. thesis, Chiang Mai University.
Tucker, N. I. J. (2000). Linkage restoration: Interpreting fragmentation theory for the design of a restored habitat linkage in tropical north-eastern Queensland. Ecological Management & Restoration, 1, 35–42.
Tucker, N. I. J., & Murphy, T. M. (1997). The effects of ecological rehabilitation on vegetation recruitment: Some observations from the Wet Tropics of North Queensland. Forest Ecology and Management, 99, 133–152.
Tucker, N. I. G., & Simmons, T. (2009). Restoring a rainforest habitat linkage in north Queensland: Donaghy’s Corridor. Ecological Management & Restoration, 10, 98–112.
Vázquez-Yanes, C., & Orozco-Segovia, A. (1993). Patterns of seed longevity and germination in the tropical rainforest. Annual Review of Ecology and Systematics, 24, 69–87.
Viani, R. A. G., Holl, K. D., Padovezi, A., Strassburg, B. B. N., Farah, F. T., Garcia, L. C., Chaves, R. B., Rodrigues, R. R., & Brancalion, P. H. S. (2017). Protocol for monitoring tropical forest restoration: Perspectives from the Atlantic Forest Restoration Pact in Brazil. Tropical Conservation Science, 10, 1940082917697265.
Viani, R. A. G., Barreto, T. E., Farah, F. T., Rodrigues, R. R., & Brancalion, P. H. S. (2018). Monitoring young tropical forest restoration sites: How much to measure? Tropical Conservation Science, 11, 1940082918780916.
Vieira, D. L. M., & Scariot, A. (2006). Principles of natural regeneration of tropical dry forests for restoration. Restoration Ecology, 14, 11–20.
Wangpakapattanawong, P., Kavinchan, N., Vaidhayakarn, C., Schmidt-Vogt, D., & Elliott, S. (2010). Fallow to forest: Applying indigenous and scientific knowledge of swidden cultivation to tropical forest restoration. Forest Ecology and Management, 260, 1399–1406.
Werden, L. K., Holl, K. D., Rosales, J. A., Sylvester, J. M., & Zahawi, R. A. (2020). Effects of dispersal- and niche-based factors on tree recruitment in tropical wet forest restoration. Ecological Applications, 30, e02139.
Werden, L. K., Holl, K. D., Chaves-Fallas, J. M., Oviedo-Brenes, F., Rosales, J. A., & Zahawi, R. A. (2021). Degree of intervention affects interannual and within-plot heterogeneity of seed arrival in tropical forest restoration. Journal of Applied Ecology, 58, 1693–1704.
Weyerhaeuser, H., & Kahrl, F. (2006). Planting trees on farms in southwest China. Mountain Research and Development, 26, 205–208.
Wilson, S. J., Alexandre, N. S., Holl, K. D., Reid, J. L., Zahawi, R. A., Celentano, D., Sprenkle-Hyppolite, S. D., & Werden, L. K. (2021). Applied nucleation guide for tropical forests. Conservation International.
Zahawi, R. A., & Reid, J. L. (2018). Tropical secondary forest enrichment using giant stakes of keystone figs. Perspectives in Ecology and Conservation, 16, 133–138.
Zahawi, R. A., Reid, J. L., & Holl, K. D. (2014). Hidden costs of passive restoration. Restoration Ecology, 22, 284–287.
Zahawi, R. A., Werden, L. K., San-José, M., Rosales, J. A., Flores, J., & Holl, K. D. (2021). Proximity and abundance of mother trees affects recruitment patterns in a long-term tropical forest restoration study. Ecography, 44, 1826–1837.
Zarin, D. J., Ducey, M. J., Tucker, J. M., & Salas, W. A. (2001). Potential biomass accumulation in Amazonian regrowth forests. Ecosystems, 4, 658–668.
Zimmerman, J. K., Aide, T. M., & Lugo, A. E. (2007). Implications of land use history for natural forest regeneration and restoration strategies in Puerto Rico. In R. J. Hobbs & V. A. Cramer (Eds.), Old fields (pp. 51–74). Island Press.
Acknowledgements
Thanks are due to NSF for financial backing connected to the Islas Project (DEB 05-15577; DEB 09-18112; DEB 14-56520, DEB 20-16623), which provided support for the work of K.D.H. and R.A.Z. Also, thanks are recorded to Biotropica Australia Pty Ltd. for supporting NT’s study and to Chiang Mai University for supporting S.E.’s work on this chapter. We are grateful to the many field assistants and landowners who made the results reported here possible.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tucker, N.I.J., Elliott, S., Holl, K.D., Zahawi, R.A. (2023). Restoring Tropical Forests: Lessons Learned from Case Studies on Three Continents. In: Florentine, S., Gibson-Roy, P., Dixon, K.W., Broadhurst, L. (eds) Ecological Restoration. Springer, Cham. https://doi.org/10.1007/978-3-031-25412-3_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-25412-3_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-25411-6
Online ISBN: 978-3-031-25412-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)