Abstract
Over the past decades, huge scientific efforts have been put into cardiac regeneration strategies. Although several strategies have been accepted for use in clinical practice, none has demonstrated great success in regenerating the cardiac tissue. Therefore, there are still significant challenges in repairing or regenerating cardiac tissue, which serves a predominantly biomechanical function. Furthermore, it is now evident that the mechanobiological interaction between cells and their mechanical environment is essential for tissue function and regeneration. Current cardiac regenerative strategies (i.e. cardiac cell therapy and tissue engineering) are based on administrating new healthy contractile cells within (or without) a healthy scaffold into the diseased cardiac environment. However, these strategies have widely omitted to restore the cellular mechanical environment present after cardiac injury. Therefore, the future cardiac regeneration strategies need to address the challenges of and questions on the role of biomechanics and mechanobiology in cardiac regeneration. This includes measurement and characterisation of multiscale mechanical properties of healthy and diseased cardiac tissue; development of in vitro and in silico models to understand the role of biomechanical factors in tissue regeneration; and translation of this information into the design of novel tissue-engineered strategies. In this chapter, we want to introduce the reader to the importance of mechanical considerations in the design of effective cardiac regeneration strategies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Current Regenerative Strategies Fail to Restore the Myocardial Mechanical Environment
According to the World Health Organization, one-third of worldwide deaths are caused by cardiovascular diseases, with an increasing trend in the recent years [1]. Specifically, heart failure (HF) following myocardial infarction (MI) is the most fatal cardiovascular disease with a 5-year survival rate of <50% [2]. At the tissue level, HF as a result of MI is characterised by a gradual loss of cardiomyocytes and, therefore, contractile tissue. The biological healing cascades following injury trigger the fibroblasts to change their phenotype and increase extracellular matrix (ECM) production, ultimately leading to the formation of hypo-contractile tissue scar [3]. At the same time, the remaining cardiomyocytes try to compensate for the loss of contractile tissue, initiating pathological cellular responses and further cardiac remodelling. Ultimately, tissue remodelling leads to an increased myocardial wall thickening, followed by dilation and eventually diminished cardiac function [4].
Nowadays, there is no doubt that cells actively respond to their mechanical environment and this interaction is essential in load-bearing and continuously contracting tissues such as the myocardium [5]. For instance, cellular contractility and electrical stability are highly dependent on mechanical environmental cues such as stiffness or cyclic strain [6,7,8,9]; and a more physiological, ‘healthy’ environment – or niche – will elicit beneficial cell behaviour in contrast to the environment present after injury. Still, this insight has been largely ignored in the design of strategies to repair or regenerate the myocardial tissue. For instance, cell-based therapies aim to restore tissue contractility by injecting new (contractile) cells in the injured area [10]. Despite promising results in in vitro settings, long-term preclinical and clinical studies have shown that these strategies fail to regenerate myocardial tissue and overall cardiac function [11,12,13,14]. One of the main reasons is that these studies mainly focus on cell phenotype and function before implantation, disregarding the effects of the highly injured ECM niche with altered mechanical properties that will be found upon transplantation. In an attempt to induce cell adaptation to the diseased environment prior to implantation, some studies exposed cells in vitro to mechanical or biological environmental cues before in vivo injection [15,16,17]. However, the toxic environment still caused low cell retention, high cell death and low mechanical stability upon injection in the diseased area.
Hence, it is difficult for the cells alone to overcome the highly destabilised mechanical tissue present after cardiac injury. Several studies have shown that cells upon implantation start synthesising and remodelling their own (healthy) ECM [18, 19]. This principle is copied by scientists in the context of in vitro cardiac tissue engineering (cTE) [20]. In vitro cTE seeks to create living myocardial tissue by combining cardiac cells with a temporary ‘healthy’ niche prior to transplantation, either as a patch or as an injectable gel replacing the damaged area [21]. Multiple biomaterials, from either natural or synthetic sources, have been developed in the form of hydrogels or scaffolds to provide for such niches [22,23,24,25,26]. The use of natural source biomaterials, such as collagen or other ECM components, seems attractive, as they can offer some of the biochemical cues present in the native tissue. Decellularised cardiac tissue – e.g. from animals – can provide scaffolds with appropriate mechanical, topological and biochemical properties [27,28,29]. On the other hand, synthetic polymers can provide scaffolds with better tuneable mechanical properties compared to the natural processed ECM but offer poor biochemical signals with fewer cell recognition motifs if applied in pristine form. Bioactive modification of such materials is pursued for the design of life-like materials that can mimic both mechanical and biochemical environmental cues [30,31,32,33]. Overall, these approaches fulfil the aim to provide new cardiac cells with a relevant micromechanical environment.
cTE constructs, based either on natural or on synthetic biomaterials, are usually implanted on the epicardium near the infarcted region of the myocardium and serve to constrain and mechanically reinforce the ventricular wall in order to prevent or halt pathological tissue remodelling [34]. However, mechanical consequences of patch transplantation or hydrogel injection are still largely overlooked. Proper mechanical integration of the delivered cTE constructs across length scales from local cell-cell and cell-ECM interactions to global tissue contraction is necessary for the success of these strategies. Mechanical mismatch will cause complications, including heart failure and diastolic dysfunction.
More recently, in situ cTE has emerged as a promising alternative to traditional in vitro cTE to achieve tissue regeneration directly at the functional site. In situ cTE is built on the notion that the injection of a biomimetic acellular scaffold into the injured myocardium stimulates endogenous repair processes [35]. However, in situ cTE approaches are still in their infancy, and the governing processes of regeneration are still largely unknown. These processes range from the activated immune response in response to the biomaterial inside the body to the mechanical factors governing de novo ECM formation and organisation. In situ cTE has major benefits over in vitro cTE in terms of costs, availability and regulatory complexity, as it uses an acellular scaffold to harness the regenerative potential of the native tissue. In addition, in situ cTE does not need to account for the mechanical integration of exogenous cells in the damaged tissue. Nevertheless, the mechanical and electrical coupling and stabilisation at the cellular, tissue and organ level upon biomaterial delivery need to be addressed.
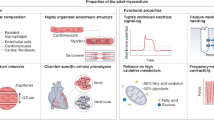
In summary, and with an eye to clinical translation, the design of successful cardiac regenerative strategies should address several aspects regarding the role of biomechanics in myocardial repair or regeneration (Fig. 1). These include questions such as: What are the mechanical properties of native and diseased tissues at the various length scales of the myocardium that will affect therapy outcomes? How will these properties influence cell and tissue behaviour in vitro and upon transplantation in vivo? How do biomechanical factors influence the remodelling of the regenerating myocardium in damaged and remote areas? In this chapter, we first describe the complex cardiac mechanical environment across length scales from macro to micro level and the techniques used for characterising mechanical behaviour at these length scales. Next, we review in vitro and in silico cardiac models to understand the impact of the cardiac mechanical environment on cellular behaviour and tissue regeneration. Finally, we provide an outlook on the requisites to design the next-generation engineering strategies for cardiac regeneration.
Cell-based therapies cannot restore the mechanical properties of the injured myocardium. In vitro and in situ tissue engineering strategies using hydrogels and scaffolds reinforce injured myocardium with moderate success. The main challenges to improve the mechanical stability of current cardiac regenerative strategies are highlighted. Partly created with BioRender.com
2 Understanding the Multiscale Biomechanical Properties of the Myocardium
Cardiac tissue, and especially the myocardium, is a complex tissue with multiple interconnected length scales (Fig. 2a–c) organised into a highly structural and functional hierarchy, ranging from whole heart biomechanics to the functional unit of contraction (the sarcomere) inside the cardiomyocytes [40].
At the macroscale, in particular in the left ventricular (LV) myocardial wall, tissue fibres form a right-handed helical structure close to the inner part of the wall (the endocardium), left-handed towards the outer part of the wall (the epicardium) and a circumferential structure in between [41, 42] (Fig. 2a). The changes in fibre orientation are smooth throughout the LV wall. During systole, due to this helical organisation, the myocardium rotates relative to the base to ensure complete blood ejection from the left ventricle [43]. Nonetheless, this description of LV wall organisation is a simplification because it omits other anatomical structures, including the extensive vascular network across the whole myocardium [44]. At the mesoscale, each of the ventricular fibres consists of an anisotropic array of cardiac cells (cardiomyocytes and fibroblasts) in parallel alignment with the ECM fibres (mainly collagen fibres) to ensure the coordinated contraction of the LV (Fig. 2b). At the microscale, cardiac cells, specifically the cardiomyocytes, form aligned, interconnected bundles that attach to ECM fibres to transduce the cellular contractile forces throughout all myocardium and generate coordinated contraction (Fig. 2c) [36].
The mechanical properties of the myocardium differ across length scales. However, basic mechanical concepts can be identified independently of these scales. These include the deformation and forces present in the tissue (strain, ε, and stress, σ), the elastic stiffness (Young’s modulus, E) and the complex G* modulus accounting for the viscoelastic properties (Table 1). Although they express the same physical concept, the interpretation of the values has to be correlated with tissue physiology and structure at each of the scales addressed.
In clinical practice, there are already established techniques to measure LV macroscopic strain based on ultrasound (echocardiography) and magnetic resonance imaging (MRI) [45,46,47]. One of the advantages of these techniques is that they are non-invasive, allowing the assessment of cardiac mechanical properties in patients. Echocardiography imaging is established as a gold standard technique, and it is based on analysing the LV wall motion by tracking speckles (natural acoustic markers) in the ultrasonic image. These acoustic markers appear physiologically in the myocardium and can be tracked frame to frame. By post-processing software, the geometric shift of each speckle can be calculated to assess tissue movement and, as such, tissue strain can be computed [48]. Similarly, MRI techniques are based on tracking features in the image over time to compute the displacement of the myocardium and, therefore, the strain present in the tissue (Fig. 2d, g) [49]. The information that can be extracted from these 4D images are the longitudinal, circumferential and radial strains. Usually, the strain values are represented by negative values as the reference deformation is set at the end of the diastole [50]. The strain present in the in vivo myocardium accounts for the tissue distensibility and gives an estimation of the overall function. After MI, strain at the affected area diminishes compared to the healthy area [51]. However, detailed knowledge of myocardial wall stress, particularly in humans, remains elusive. This lack of knowledge is primarily because forces or stresses cannot be directly measured in the intact myocardial wall [52]. To this end, several indirect methods, including analytical and finite-element modelling, have been used to estimate the stress present in the tissue [53,54,55,56]. Based on that, stress-strain relationships can be built, and the E value calculated.
Besides the active tissue contraction present in vivo, the passive mechanical properties of the myocardium significantly contribute to overall cardiac function [57, 58]. Throughout the literature, the E for soft biological tissues has physiological values in the range of 1–100 kPa [5]. The E gives an estimation of the elastic mechanical properties of the tissue. However, just an E value is not enough to fully understand the mechanical behaviour of soft biological tissues. Additionally, it is necessary to mention at which scale and with which technique the value has been measured as this includes the information of physiological structures that are being measured.
At the mesoscale, the mechanical properties of myocardial tissue are characterised from small ex vivo tissue strips of a few millimetres, subjected to uniaxial, biaxial or even triaxial tensile tests (Fig. 2e). The strips are attached to a lever arm controlled by an electromechanical or hydraulic servo-controlled displacement actuator that deforms the strip while it measures the force applied. Therefore, the σ-ε curve can be computed by calculating the relative deformation of the strip and the force applied per unit cross-sectional strip area (Table 1; Fig. 2h). This process is done to normalise deformation and force for the sample size and, hence, to allow a comparison of experimental results from strips with different sizes. The E value of the strip can easily be derived by computing the derivative of the σ-ε curve. However, the σ-ε curve exhibits a more complex mechanical behaviour than just elastic. The hysteresis of the curve (difference between loading and unloading curve) depicts energy loss behaviour, typical of viscoelastic materials. The energy loss results from frictional processes, such as tissue fluid movement, and is commonly observed in soft biological tissues [59]. Moreover, the σ-ε behaviour of living soft tissues is highly non-linear. This behaviour can be mainly explained by the state of the ECM fibres inside the tissue, including the structural organisation of the collagen fibres. At the relaxed state of the tissue (low strains), the collagen fibres are wavy. By increasing the strain present in the tissue, the fibres start to unfold, collagen fibre recruitment increases and the σ-ε curve becomes non-linear (Fig. 2h) [60]. This strain stiffening effect is advantageous for a tissue as it becomes increasingly resistant to extension in order to prevent excessive deformations and tissue damage [61].
The mammalian healthy and diseased mechanical properties of the myocardium at the mesoscale have been the subject of extensive investigation in the past two decades [62,63,64]. Healthy myocardium shows anisotropic behaviour (e.g. different mechanical properties depending on the measured direction). E of healthy myocardium ranges from 1 to 10 kPa at a physiological tissue strain [65]. On the other hand, the post-MI myocardium shows a stiffer and isotropic behaviour, correlating with the disorganised distribution of collagen fibres found in the post-MI fibrotic scar [66, 67]. These findings support the notion that the ECM fibre organisation dominates the mechanical properties of the scar. In order to measure only the contribution of the ECM in the fibrotic myocardial scar and exclude the cellular effect, some studies have decellularised the samples before testing [68, 69]. Knowledge of the mechanics of decellularised ECM will support the design of novel biomaterials for cardiac tissue engineering.
At the microscale, the mechanical properties are determined by individual ECM fibres and cells. The most suitable technique to measure mechanics at this scale is atomic force microscopy (AFM) [39]. AFM probes micromechanical properties of a thin tissue slice (or a single cell for that matter) by indenting its surface with a microfabricated cantilever ended with a pyramidal or spherical tip (Fig. 2f) [70]. This technique allows the measurement of tip displacement with nanometre resolution and simultaneous measurement of the applied force (Fig. 2i). AFM measurements provide essential mechanical information at the scale at which cells probe their own microenvironment. Micromechanical properties of heart tissue have been studied utilising AFM on fresh (ECM and cells) and decellularised cardiac slices of different species [38, 71,72,73,74]. Post-MI tissue mechanical properties showed a dramatic increased E compared to the healthy tissues (approximately three- to fourfold increase). Moreover, the loss tangent of G* (G”/G’, see Table 1) showed that the viscous tissue contribution of cardiac tissue at the microscale is around ten times lower than the elastic contribution, indicating that tissues show a solid-like behaviour [38].
Another major contributor to tissue mechanics are the cardiac cells. Characterisation of cardiomyocyte single-cell beating forces, frequency and cellular viscoelasticity has been studied using AFM [75]. A major advantage of AFM to measure cell mechanical parameters over other techniques, such as traction force microscopy or optical tweezers, is that AFM directly measures force and deformation without complex data processing. Different cell types largely used in cTE, such as human embryonic stem cells (hESC)- and induced pluripotent stem cells (iPSC)-derived cardiomyocytes, show different mechanical phenotype [76]. hESC-derived cardiomyocytes showed a beating force twofold lower than iPSC-derived cardiomyocytes, both showing single-cell forces in the range of nanonewton. Moreover, the most interesting fact is that cells showed an increased beating force when cultured in clusters showing that cell-cell connectivity plays a role in overall tissue force [76]. Additionally, cell viscoelasticity has been linked to several diseases [77, 78]. Laminopathies are a family of genetic diseases affecting the cardiomyocyte normal function and are caused by a mutation of the intracellular proteins called lamins. This mutation causes a loss of structural cell integrity, showed by a decreased E, with a lower cell-ECM adhesion affecting force generation and transmission towards surrounding cells and tissues [77]. Also, using primary cardiomyocytes from young and old rats, it was demonstrated that age correlates with a decreased cell shortening, increased relaxation time and increased E. These results indicate that cardiomyocytes from old animals are less deformable and contractile and suggest that cardiomyocyte mechanical changes per se can contribute to age-related diastolic LV dysfunction [79]. Overall, the data demonstrate that active and passive mechanical properties of cardiomyocytes also contribute to the overall tissue mechanics.
Obviously, mechanical data need to be handled with caution when designing novel strategies and biomaterials to mimic the healthy and diseased cardiac cellular niche. Additionally, there is still a lack of knowledge on the relation between cardiac mechanical properties and cardiac mechanical function. Fundamental insights into structure-function properties at all length scales from cell to organ are required and should be integrated to predict the consequences of mechanical changes at the microscale for cardiac function at the macroscale and vice versa. In the following sections, we will review the models used to understand the impact of cardiac mechanical properties on cell and tissue function.
Physiological structure, techniques to measure mechanical properties of cardiac tissue and mechanical properties at the three relevant scales. (a) Differential layer organisation of muscle fibres in the myocardium. (b) Cardiac cells (cardiomyocytes, red; fibroblasts, green) are anisotropically oriented along ECM fibres (blue). (c) Cardiomyocytes transmit the contraction force to the ECM by cell-ECM attachments (grey). (d) MR image of a human thorax. Red and green lines delineate the inner and outer parts of the myocardial wall necessary to compute myocardial strain. (e) Biaxial tensile tester setup. A porcine myocardial tissue strip is attached to four lever arms. (f) Microscopy phase-contrast images of 12 μm thick decellularised LV mouse heart probed with AFM. (g) Circumferential strain bull eye computed by MRI. Values are negative, depicting the contraction of the tissue. Regions closer to the centre correspond to myocardial regions near to the apex of the heart. (h) Representative strain-stress (σ-ε) curve measured by tensile testing. Non-linear viscoelastic behaviour is a characteristic of the passive mechanical properties of cardiac tissue. The hysteresis between mechanical loading (solid line) and unloading (dashed line) indicates energy loss. The progressive recruitment of collagen fibres explains the non-linear behaviour. (i) Representative force (stress) versus indentation (strain) curve acquired with AFM on a decellularised cardiac tissue slice. Mechanical properties at the microscale are also viscoelastic (the difference between approaching and retracting curves). The appropriate contact model depending on the AFM tip geometry is used to fit the experimental data. (a–c, h) Adapted from [36] and reprinted under Creative Commons (CC BY) license. (e) Adapted from [37] and reprinted under Creative Commons Attribution 4.0 International License. (f) Adapted from [38] and reprinted with permission from Elsevier. (i) Adapted from [39] and reprinted with permission from Elsevier
3 Mechanics-Based In Vitro Models to Understand Cardiac Behaviour at the Micro- and Mesoscale
cTE strategies are also designed to study the effect of the mechanical cardiac stimuli on cardiac cells. This section reviews in vitro cTE platforms mimicking the cardiac mechanical properties at the microscale (cells) and mesoscale (microtissues).
3.1 Cardiac ECM Organisation
The healthy myocardium at the micro- and mesoscale shows highly organised, anisotropic ECM fibres in contrast to the highly disorganised, isotropic ECM structure found after injury. Several in vitro methods have been described to mimic this organisation of healthy and diseased myocardium. First, a commonly used approach is microcontact printing that serves to pattern cell culture substrates with various ECM proteins, including fibronectin, laminin, collagen, Matrigel and gelatin [80,81,82,83,84,85,86,87]. Microcontact printing allows recreating the microscale two-dimensional environment by patterning the substrate with a high resolution of just a few micrometres. By changing pattern geometry, cells are forced to adopt specific (dis)organised alignment. A logical consequence of reproducing the highly organised healthy myocardium is that the cardiomyocytes become elongated with an increased contraction force compared to cells on a disorganised pattern [81, 84, 86].
At the mesoscale, the cTE gold standard technique to study cardiac in vitro conditions is the engineered heart tissue (EHT) pioneered at the end of the last century [88]. EHTs are cardiac microtissues composed of cell-laden and ECM mimicking biomaterial, moulded between constraints or stretching posts. Classical EHTs are composed of two stretching posts creating a unidirectional microtissue mimicking ECM anisotropy of the myocardium. More recently, to mimic the chaotic tissue organisation and force distribution after injury, EHTs were fabricated either with uniaxial and biaxial post distribution. To this end, the organisation and mechanical forces have been manipulated from organised to disorganised, respectively [89, 90] (Fig. 3a). This mesoscale model will enable the understanding of the principles of ECM remodelling after injury and how it can be restored towards an organised ECM.
3.2 ECM Stiffness
A myriad of biomaterials has been developed to simulate the stiffness of healthy and diseased myocardium. Hydrogels from natural (non-cardiac ECM) and synthetic sources such as gelatin methacryloyl, polyethylene glycol (PEG), alginate, polyacrylamide (PAA) and polydimethylsiloxane (PDMS) are regularly used due to tuneability of E [84, 94,95,96,97,98,99,100]. It has been demonstrated that culturing cardiomyocytes on substrates with a physiological E maximises their contractility and the number of intracellular actin stress fibres, whereas increased or decreased E disrupts their cytoskeletal structure and reduces their contractile force [84, 91] (Fig. 3b). On the other hand, hydrogels from reconstituted natural cardiac ECM, such as decellularised ECM, induce better biochemical activity and increased remodelling capacity of the encapsulated cells while being able to control their mechanical properties via chemical crosslinking of the gel [29, 101, 102].
Additionally, the viscoelastic properties of some of these materials can be controlled. A recent study developed a nanostructured alginate-based hydrogel allowing control over stress-relaxation properties without changing the E. Using this material, the authors showed that stress relaxation affects cardiomyocyte intracellular contraction [103]. Another study indicated that varying the monomer and crosslinker concentration of PAA hydrogels allows to control the viscoelastic properties [104].
Overall, the described hydrogels can mimic the mechanical properties of healthy and diseased cardiac tissue but cannot capture the dynamics of cardiac remodelling after injury due to the covalent crosslinking between structural polymeric fibres in the gels. A new class of hydrogels offers control over crosslinking dynamics and consequent manipulation of hydrogel dynamic mechanical properties. By incorporating reversible crosslink methods, the properties of such hydrogels can be changed instantly by applying an external trigger, such as temperature, light or a chemical agent [105, 106]. For example, PEG was modified with a photo-sensible and reversible crosslinker that allowed to dynamically tune the viscoelastic properties by the use of blue light. Importantly, these changes were even possible when culturing cells inside the hydrogel [107]. Another recent example addresses a pH-sensitive hydrogel that enables changing the crosslinking degree by dynamic pH changes [108].
3.3 Strain on Cells
Cardiac tissue is constantly subjected to static (pre-stress present in the tissue) and cyclic strain (beating), and these strains may change with disease progression. Therefore, it is of utmost importance to investigate the mechanoresponse of cardiac cells to the experienced strain under conditions of health and disease. Most studies investigating strain responses make use of two-dimensional systems to apply (cyclic) uniaxial or equibiaxial strain [109]. Commonly, the cells are seeded on top of a flexible membrane that is stretched, transmitting the deformation of the membrane to the cells [92] (Fig. 3c). Because study designs differ in strain magnitude and frequencies used, comparison of study outcomes is cumbersome [110,111,112]. However, a common phenomenon has been observed in cardiac fibroblasts in response to cyclic strain. This phenomenon is called strain avoidance and refers to the re-orientation of cells perpendicular to the direction of applied cyclic strains [113, 114]. The physiological interpretation of this behaviour is that cells turn away from the stretch direction to experience minimal deformation on their cell body and nucleus. In fibroblasts, strain avoidance has been observed in two-dimensional and three-dimensional in vitro models [115,116,117]. However, strain avoidance is less clear in cardiomyocytes with several studies indicating that cardiomyocyte strain avoidance depends on the differentiation state of the cell, the strain rate and strain duration [92]. More recently, it was demonstrated that cardiomyocytes derived from a pluripotent stem cell source do not show strain avoidance. However, when co-cultured with cardiac fibroblasts (with a strain avoidance response), the cardiomyocytes did show strain avoidance and rotated along with the fibroblasts [118]. Hence, the effect of strain on cardiac cell behaviour is also influenced by the interplay between different cell types present in the tissue.
Strain generated on cardiac cells also has a significant impact on their phenotype via mechanotransduction pathways. One of the main current challenges regarding cell models in cTE is to obtain highly mature cardiomyocytes from pluripotent stem cell sources resembling adult cardiomyocytes found in vivo. Mechanical factors and, in particular, tissue strain have been shown to play a critical role in maturation process [119]. Numerous studies have been conducted to understand how cyclic mechanical strain affects cardiomyocyte maturation at single-cell and tissue levels [120,121,122,123,124]. Most of these studies showed that cyclic strain increases sarcomere formation, cardiac ion channel expression and contraction force and frequency of cells and tissue. Importantly, the strain magnitude and frequency applied to the tissues are essential to achieve better maturation [125, 126]. Cyclic strains around 10% showed to induce increased cardiomyocyte maturation compared to lower strains of 5% [127]. On the other hand, large strains (mimicking increased afterload) have shown to cause pathological hypertrophy in vitro with larger cardiomyocytes but with decreased contractile function [128, 129].
3.4 Mechanoelectrical Feedback
The highly interconnected cardiomyocyte network controls the coordinated contraction of the myocardium and whole heart rhythm. Cardiomyocytes, at the microscale, depolarise their cellular membrane in the presence of an electrical stimulus. This depolarisation triggers the release of intracellular calcium ions responsible for activating the cell’s contractile machinery. In an in vitro setting, generally, the mesoscale tissues containing mature adult cardiomyocytes start beating due to their autorhythmic properties [89]. However, in pathophysiological conditions these tissues often beat non-synchronously due to a lack of proper cardiomyocyte and fibroblast organisation and cell-cell contact inside the hydrogel [130]. To overcome the appearance of arrythmia in cTE tissues, these are generally paced by applying an external electric field during long-term tissue culture. To this end, several approaches have been implemented with notable success [109, 131]. As an example, the Biowire and Biowire II platforms (Fig. 3d) have been demonstrated to improve intracellular calcium handling, contraction force and synchronicity of beating [93, 132].
3.5 Developing Integrated Models for Mechanical Consideration In Vitro
In the context of designing strategies for cardiac regeneration, it is not only necessary to understand how mechanical stimuli influence cell and tissue behaviour, but it is also fundamental to integrate such stimuli across length scales to better recapitulate the in vivo situation. For this purpose, the development and use of bioreactors are pursued. A bioreactor is typically defined as a device that provides tight control of the environmental conditions and external stimuli (biochemical and biomechanical) that influence cell and tissue culture processes [133].
Oxygen tension is of paramount importance in affecting cardiac cellular and tissue behaviour [134, 135]. After MI, there is a loss of perfusion in the scar region that leads to a decreased oxygen level or hypoxia. It has been previously shown that hypoxia enhances the migration and differentiation capacity of pluripotent stem cells derived to cardiomyocytes [136, 137]. Moreover, low oxygen tensions stimulate the ECM-producing phenotype of cardiac fibroblast, maintaining the presence of fibrotic tissue after injury, having a direct impact on cardiac tissue mechanics [138]. Therefore, the use of bioreactors capable to mimic (patho)physiological oxygen tensions is critical to further understand the mechanical implications on the behaviour of cTE strategies.
Biomechanically, the main goal for cTE is to synchronise contraction with appropriately timed mechanical or electrical stimulation to mimic ventricular filling. Independent control over individual input signals further allows for manipulating disease progression, e.g. via changes in the stretch (haemodynamics) and electrical signal patterns (e.g. arrythmias). Various bioreactors have been designed for applying mechanical and electrical stimuli simultaneously [139,140,141]. For example, an electromechanical bioreactor platform was able to provide static stress to microtissues using a pneumatically driven stretch device. It consisted of a tissue culture chamber where several tissue constructs (20 mm × 20 mm × 3 mm) were subjected to frequencies and amplitudes of cyclic stretches and electrical pulses matching the native tissue [142]. Moreover, it is also important to track over time the changes in mechanical properties of the tissues inside bioreactors as a parameter to understand tissue growth and remodelling. A recent study developed a bioreactor to test cardiac tissues under dynamic loading together with an ultrasound system to trace non-invasively the mechanical properties of the tissue over time [143].
Mechanical-based microscale and mesoscale in vitro models to understand cardiac cell behaviour. (a) Mesoscale microtissue with PDMS uniaxial or biaxial constraints to manipulate tissue organisation. The cells (red) seeded inside the collagen (green)-based hydrogels compacted around the posts. Collagen fibres’ orientation shows an (an)isotropic distribution depending on the uniaxially or biaxially constrained tissues. Adapted from [89] and reprinted with permission from Oxford University Press. (b) Neonatal rat ventricular cardiomyocytes cultured on top of 1, 10 and 50 kPa PAA gels (left to right). Actin fibres (green) and contraction forces are maximised at physiological ECM stiffness of 10 kPa. Adapted from [91] and reprinted with permission from Elsevier. (c) Device based on a flexible membrane with cells seeded on top. The vacuum applied underneath the membrane stretches the flexible membrane which is supported by a loading post. Cardiomyocyte progenitor cells show strain avoidance behaviour depending on their differentiation state. Adapted from [92] and reprinted with permission from Elsevier. (d) Schematic representation of the Biowire II platform. Cell-seeded collagen-based hydrogels are attached to uniaxially constrained wires. The system can be electrically paced with carbon electrodes and using an external electrical source. Adapted from [93] and reprinted under ACS AuthorChoice License
4 In Silico Models to Study the Mechanics of Myocardial Remodelling and Regeneration
The rapid development of digital technologies has enabled the development and application of computational models in many fields nowadays. Computational models in bioengineering, commonly referred to as in silico models, enhance the knowledge of various biological tissues’ behaviour at different scales. Moreover, a multiscale approach can integrate knowledge from different scales into an overall simulation of the tissue behaviour and thus become more relevant for analysis and predictions.
The use of in silico models for simulating cardiac tissue function has rapidly expanded in the last years, especially for simulating drug testing and considering chemical coupling within the tissue. The computational platforms for testing novel/existing drugs have achieved widespread approval on different aspects – ethical, toxicological and economic [144]. Besides the platforms for drug testing, the major interest of in silico models lies in capturing cardiac systolic/diastolic functioning and electrical coupling, from micro- to macroscale [145]. These models usually neglect the biomechanics of the heart and/or miss to include the mechanical environment for the cells [146]. Moreover, there is a gap in linking electro-mechanical coupling of the heart to the contraction of the tissue at different length scales. Improvement of in silico models in this area is suggested to result in models that can predict the change of tissue mechanical function in response to tissue remodelling under conditions of health, degeneration and regeneration. A such, these models also have to translate basic research findings on cardiac regeneration into tissue engineering or other regenerative strategies under pathological conditions, including their altered hemodynamic, electroconductive and tissue mechanical features [147].
Current regenerative strategies fail to properly restore the cellular microenvironment and aligned structural organisation after cardiac injury, relevant for coordinated contraction and tissue mechanical homeostasis, and this is where in silico models can be very useful in answering the questions about (changing) mechanical impact on cardiac tissue regeneration [148]. They can be developed to replicate certain experimental observations, e.g. at cell and tissue level, and be further extended to in vivo-like conditions at the organ scale. By employing a multiscale approach, they can be also used to investigate the effect of microscale modifications on macroscale function, much easier than experiments can do [149].
The starting point in the development of in silico models is the connection of simulated biological processes with data from in vitro or in vivo experiment(s), or those from the literature, since each computational model needs meaningful input data for robust predictions (Fig. 4). In this first phase of development, model outputs are compared with – or validated against – additional experimental findings so that initially applied boundary conditions of the proposed in silico model are in line with real-life data. As there are now various in silico models in cardiac research, there is a tendency to standardise in silico cardiac models in terms of verification, validation and uncertainty quantification of scientific software [150].
In the scheme depicted in Fig. 4, the input from an in vitro microtissue model is used to feed the computational model at the mesoscale (tissue level). The outputs from the in silico simulations can include, for instance, mechanical properties of the tissue, structural organisation and mechanical contraction in response to the experimental starting conditions. Once validated, the in silico model can be used to understand and predict outputs in response to new boundary/loading conditions, e.g. to mimic healthy/diseased states of the microtissue. The added value of such mesoscale in silico models can be found in the enhanced understanding and prediction of microtissue behaviour and the reduction and optimisation of further in vitro models. An additional benefit is the possibility to integrate and translate insights from the mesoscale level to the macroscale level using multiscale in silico models [149], which is particularly useful for predicting the outcome of cardiac regenerative strategies. The final advantage is the usage of in silico models as a platform for existing/novel drug testing – as has been mentioned before. This section aims to present current in silico models that mimic the myocardium at different length scales and with a special reference to mechanical consideration for regenerative strategies. Our opinion on future directions in this area will be discussed in the concluding section.
In the context of cardiac regeneration, in silico models have been employed to predict the mechanical consequences and optimise the design or placement of next-generation cardiac patches in terms of structural organisation and mechanical properties of the myocardium and to help define the mechanical constraints invoked by such patches that would lead to reversion or inhibition of adverse cardiac remodelling.
Urdeitx and Doweidar developed a mesoscale finite element model to simulate cardiac cellular behaviour such as proliferation, migration, maturation and cell-matrix adhesion in response to mechanical and electrical cues from the environment [151]. Cell behaviour was described as a function of cell deformation due to changes in ECM stiffness and/or electrical stimulation. The model predicted that on soft ECM, cell alignment and migration improved with increasing (directional) electrical stimulation. On stiffer ECM, cells showed enhanced maturation and proliferation. However, the mechanical impact considered in the model is limited as it only considers changes in ECM stiffness, without incorporating the cause of ECM stiffness changes or hemodynamic loading.
To better understand the potential of cardiac regeneration, tissue (ECM) production and organisation by resident or newly delivered cells have been studied in vitro. When cultured in 3D environments, such as collagen hydrogels enriched with Matrigel, cardiac cells (both cardiac fibroblasts and cardiomyocytes) contribute to the production and maintenance of the ECM by ECM synthesis, degradation and cell-matrix interactions, including cell traction forces [89]. They also respond to environmental strains by (re)orienting their cell body as well as the ECM fibres around them (see Sect. 3.3). Mesoscale in silico models of the cardiac tissues have been developed to successfully describe the underlying biological phenomena of these processes of healthy tissue remodelling, where fibroblasts align their internal cytoskeleton (stress fibres) and ECM to form an anisotropic tissue [152,153,154,155,156]. In response to cardiac injury, for instance, due to ischemia, the heart’s primary response is to create a scar-like tissue and protect the damaged tissue from rupturing by ongoing remodelling. Under these conditions the original cell and tissue anisotropy is lost and fibroblasts differentiate into myofibroblasts, which show a higher production of ECM proteins (mainly collagen), leading to reduced compliance and stiffening of the scar [157]. In silico models that can predict structural (re)organisation of the cells and collagen can thus predict disease development of the tissue but are also instrumental for testing the effects of mechanical conditions (e.g. constraints by tissue patches) that would allow the transition from an isotropic to an anisotropic organisation. To this end, the models should describe how cardiac fibroblasts remodel the collagenous matrix and incorporate cell-mediated traction forces [158] under both physiological and pathophysiological conditions [154,155,156]. When successfully validated, the output from an in silico model that describes tissue structural remodelling can also be used to design engineered tissues with optimised and load-bearing collagen organisations [159, 160].
When modelling the mechano-response of cardiomyocytes, in vitro and in silico models have focused on describing cardiomyocyte organisation and alignment at the mesoscale, either or not in the presence of fibroblasts. These models show that cardiomyocytes align with the stress direction and alongside collagen fibres in uniaxially constrained microtissues, but show no alignment in biaxially constrained microtissues [89, 161]. The aligned structure is essential for coordinated and synchronised contraction and proper propagation of electrical signals. Cross-sectional compaction of uniaxial constrained tissues further contributes to alignment and increases with the percentage of fibroblasts present in the tissue. Forceful contraction requires a high percentage of aligned cardiomyocytes and fibroblasts to establish conduction and synchronised beating. Hence, next to structural remodelling, cell ratios and alignment at the mesoscale should be incorporated when mimicking regenerative strategies to reverse or halt remodelling of cardiac scar tissue at the macroscale. Vice versa, the influence of hemodynamic loading conditions as well as active and passive mechanical behaviour at the macroscale [162] will influence cell alignment at the meso- and microscale.
Macroscopically, the myocardium is organised in differently orientated two-dimensional anisotropic sheets that follow a helicoidal shape from the epicardium to the endocardium. Hence, the local coordinate system is represented by three axes: fibre orientation, sheet orientation and normal to the sheet orientation to capture anisotropy of the tissue. There are two approaches to computationally include myocardial organisation within the cardiac geometry at the macroscale: patient-specific and numerically approximated. The inclusion of patient-specific organisation is the ultimate goal of all predictive in silico models, since it takes into account the real structure of the tissue, obtained by image reconstruction of patient-specific MR images. The fibre directions can be measured from MR images leading to the generation of patient-specific structure and geometry, which is a great improvement in numerical modelling despite their time-consuming feature [163]. Sometimes it can be more convenient to avoid image reconstruction for each patient separately. By mapping fibre orientations from the geometry of one patient to the cardiac geometry of another patient, time, money and effort can be reduced, but this approach is less accurate [164]. To simulate generic behaviour of the myocardium at the macroscale, including the influence of tissue organisation, numerical approximations can be used, where myofibril helix angle changes linearly from −60 ° ± 10° at the epicardium to 60 ° ± 10° at the endocardium side [165]. Which approach will be selected depends on the purpose of the in silico model.
Cardiac in silico models describing macroscopic mechanical behaviour commonly model left ventricle (LV) behaviour. The LV, representing the largest shape and volume of the four-chambered heart, experiences the highest stresses and strains, and its function is most often and most severely affected by disease. The most valuable output from these LV models is the quantification of myocardial mechanical parameters under in vivo-like hemodynamic loading, in particular LV wall stress, which cannot be measured experimentally but is important in disease prediction since it is indicative of cardiac wall (dis)function. By changing the mechanical properties of the LV wall at certain locations, different pathological conditions can be simulated. For instance, when incorporating differential mechanical properties for scarred tissue, border zone and healthy remote tissue based on patient-specific MR images, these models can be used to predict the severity and functional consequence of myocardial infarction [166]. Yet, the LV models generally do not account for tissue organisation and remodelling at lower length scales, so they cannot simulate the progression of fibrosis and functional consequences at later stages after infarction. A second disadvantage of these models is a simplified representation of electrical conduction and electro-mechanical coupling.
The final goal of in silico models is the integration of insights obtained across length scales (cell, tissue and organ) into multiscale models that can provide more detailed information on cardiac behaviour, predict disease progression and improve existing or establish novel regenerative strategies. So far, multiscale in silico models have mainly focused on describing the effects of pharmacological agents and electrical conduction from cell to organ, while multiscale mechanical interactions have remained largely unexplored. Future directions in the development of in silico myocardial models to support the improvement of existing or creation of novel regenerative strategies lie in predicting the effects of mechanical loading/unloading on tissue remodelling and mechanical functioning at different length scales.
Knowledge obtained from experiments and simulations at cell and tissue scale should be translated into the in vivo situation of the whole heart to ultimately understand how cardiac tissue will remodel under whole-organ hemodynamic loading conditions and depending on (changing/heterogeneous) cardiac wall mechanical properties. The SIMULIA Living Heart ProjectFootnote 1 could offer the next step in the development of more complex multiscale electromechanical cardiac models. This project gathers some of the most prominent researchers in cardiovascular area from different branches, with the purpose to create a multidisciplinary vision in the development of cardiac computational models for clinical use. The project integrates various features relevant for cardiac function, such as the detailed geometry of the whole heart, electrophysiology, active and passive mechanical properties of the cardiac wall, blood flow and the feedback of the circulatory system on cardiac function. Using the available computational methods to model myocardial infarction and tissue remodelling at the mesoscale, the macro model could, for instance, predict how tissue-engineered epicardial patches or biomaterial injection in the cardiac wall would influence tissue remodelling in the infarct area and subsequently how cardiac function as a whole could be improved.
5 Conclusion
Even though the progress in the development of cardiac regenerative strategies can be observed, long-term clinical benefits are still to be expected. Current strategies largely omit the mechanical consequences after implantation of cells or tissue-engineered constructs, suggesting that more attention should be paid to mechanical considerations in the processes of tissue formation and remodelling. The use of in vitro and in silico models, together with a thorough mechanical characterisation of myocardial tissue at different length scales, is required to understand and predict the effects of the mechanical cues (e.g. loading and ECM stiffness) on cells and tissues. In this context, it should be noted that mechanical properties of cardiac tissue are influenced by the scale at which they are measured, highlighting the need to use the appropriate technique at each scale. Microscale and mesoscale in vitro models need to integrate these mechanical properties to better understand the effect on cell and tissue behaviour. Moreover, in silico models can provide significant assistance in simulating a multiscale cardiac response upon improvement of existent or development of novel models. Regarding in silico models that describe mechanical behaviour, the literature mainly reports on models at the meso- and macroscales but does not provide multiscale models that can simulate cardiac tissue remodelling or regeneration based on such processes at cellular or tissue level. The development of multiscale predictive models could illuminate native-like mechanical conditions that can provoke remodelling of fibrotic tissue (e.g. change of mechanical loading and change of stiffness), but more importantly translation of these conditions into the effect of regenerative strategies, such as patches with regionally different stiffness.
Notes
- 1.
Living Heart Project | SIMULIA™ - Dassault Systèmes® (3ds.com)| SIMULIA™ - Dassault Systèmes® (3ds.com).
References
Mendis S, Puska P, Norrving B, World Health Organization, World Heart Federation, World Stroke Organization (2011) Global atlas on cardiovascular disease prevention and control. World Health Organization, Geneva, p vi, 155
Stewart S, MacIntyre K, Hole DJ, Capewell S, McMurray JJV (2001) More “malignant” than cancer? Five-year survival following a first admission for heart failure. Eur J Heart Fail 3:315–322. https://doi.org/10.1016/S1388-9842(00)00141-0
Frangogiannis NG (2017) The extracellular matrix in myocardial injury, repair, and remodeling. J Clin Invest 127:1600–1612
Prabhu SD, Frangogiannis NG (2016) The biological basis for cardiac repair after myocardial infarction. Circ Res 119:91–112
Chaudhuri O, Cooper-White J, Janmey PA, Mooney DJ, Shenoy VB (2020) Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584:535–546. https://doi.org/10.1038/s41586-020-2612-2
Chin IL, Hool L, Choi YS (2019) A review of in vitro platforms for understanding cardiomyocyte mechanobiology. Front Bioeng Biotechnol 7:133. https://doi.org/10.3389/fbioe.2019.00133
Shapira-Schweitzer K, Seliktar D (2007) Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomater 3:33–41. https://doi.org/10.1016/j.actbio.2006.09.003
Boothe SD, Myers JD, Pok S, Sun J, Xi Y, Nieto RM, Cheng J, Jacot JG (2016) The effect of substrate stiffness on cardiomyocyte action potentials. Cell Biochem Biophys 74:527–535. https://doi.org/10.1007/s12013-016-0758-1
D’Urso M, Kurniawan NA (2020) Mechanical and physical regulation of fibroblast–myofibroblast transition: from cellular mechanoresponse to tissue pathology. Front Bioeng Biotechnol 8:609653
Nakamura K, Murry CE (2019) Function follows form—a review of cardiac cell therapy. Circ J 83:2399–2412
Karantalis V, Difede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM (2014) Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (PROMETHEUS) trial. Circ Res 114:1302–1310. https://doi.org/10.1161/CIRCRESAHA.114.303180
Aghajanian H, Kimura T, Rurik JG, Hancock AS, Leibowitz MS, Li L, Scholler J, Monslow J, Lo A, Han W, Wang T, Bedi K, Morley MP, Linares Saldana RA, Bolar NA, McDaid K, Assenmacher CA, Smith CL, Wirth D, June CH, Margulies KB, Jain R, Puré E, Albelda SM, Epstein JA (2019) Targeting cardiac fibrosis with engineered T cells. Nature 573:430–433. https://doi.org/10.1038/s41586-019-1546-z
Fisher SA, Brunskill SJ, Doree C, Mathur A, Taggart DP, Martin-Rendon E (2014) Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev 4:CD007888
Tenreiro MF, Louro AF, Alves PM, Serra M (2021) Next generation of heart regenerative therapies: progress and promise of cardiac tissue engineering. NPJ Regen Med 61(6):1–17. https://doi.org/10.1038/s41536-021-00140-4
Cassino TR, Drowley L, Okada M, Beckman SA, Keller B, Tobita K, Leduc PRHJ (2012) Mechanical loading of stem cells for improvement of transplantation outcome in a model of acute myocardial infarction: the role of loading history. Tissue Eng Part A 18:1101–1108. https://doi.org/10.1089/TEN.TEA.2011.0285
Wu KH, Mo XM, Han ZC, Zhou B (2012) Cardiac cell therapy: pre-conditioning effects in cell-delivery strategies. Cytotherapy 14:260–266. https://doi.org/10.3109/14653249.2011.643780
Lee J, Henderson K, Massidda MW, Armenta-Ochoa M, Im BG, Veith A, Lee B-K, Kim M, Maceda P, Yoon E, Samarneh L, Wong M, Dunn AK, Kim J, Baker AB (2021) Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat Biomed Eng 51(5):89–102. https://doi.org/10.1038/s41551-020-00674-w
Smits AM, van Laake LW, den Ouden K, Schreurs C, Szuhai K, van Echteld CJ, Mummery CL, Doevendans PA, Goumans M-J (2009) Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res 83:527–535. https://doi.org/10.1093/CVR/CVP146
Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, van der Schaft DW (2012) Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 53:497–508. https://doi.org/10.1016/J.YJMCC.2012.07.003
Montero P, Flandes-Iparraguirre M, Musquiz S, Pérez Araluce M, Plano D, Sanmartín C, Orive G, Gavira JJ, Prosper F, Mazo MM (2020) Cells, materials, and fabrication processes for cardiac tissue engineering. Front Bioeng Biotechnol 8:955
Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP, Radisic M (2010) Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 16:169. https://doi.org/10.1089/TEN.TEB.2009.0352
Radisic M (2015) Biomaterials for cardiac tissue engineering. Biomed Mater 10:030301. https://doi.org/10.1088/1748-6041/10/3/030301
Roshandel M, Dorkoosh F (2021) Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym Adv Technol 32:2290–2305. https://doi.org/10.1002/PAT.5273
Majid QA, Fricker ATR, Gregory DA, Davidenko N, Hernandez Cruz O, Jabbour RJ, Owen TJ, Basnett P, Lukasiewicz B, Stevens M, Best S, Cameron R, Sinha S, Harding SE, Roy I (2020) Natural biomaterials for cardiac tissue engineering: a highly biocompatible solution. Front Cardiovasc Med 0:192. https://doi.org/10.3389/FCVM.2020.554597
Efraim Y, Sarig H, Cohen Anavy N, Sarig U, de Berardinis E, Chaw SY, Krishnamoorthi M, Kalifa J, Bogireddi H, Duc TV, Kofidis T, Baruch L, Boey FYC, Venkatraman SS, Machluf M (2017) Biohybrid cardiac ECM-based hydrogels improve long term cardiac function post myocardial infarction. Acta Biomater 50:220–233. https://doi.org/10.1016/j.actbio.2016.12.015
Li Z, Guan J (2011) Hydrogels for cardiac tissue engineering. Polymers (Basel) 3:740–761
Perea-Gil I, Gálvez-Montón C, Prat-Vidal C, Jorba I, Segú-Vergés C, Roura S, Soler-Botija C, Iborra-Egea O, Revuelta-López E, Fernández MA, Farré R, Navajas D, Bayes-Genis A (2018) Head-to-head comparison of two engineered cardiac grafts for myocardial repair: from scaffold characterization to pre-clinical testing. Sci Rep 8. https://doi.org/10.1038/s41598-018-25115-2
Prat-Vidal C, Rodríguez-Gómez L, Aylagas M, Nieto-Nicolau N, Gastelurrutia P, Agustí E, Gálvez-Montón C, Jorba I, Teis A, Monguió-Tortajada M, Roura S, Vives J, Torrents-Zapata S, Coca MI, Reales L, Cámara-Rosell ML, Cediel G, Coll R, Farré R, Navajas D, Vilarrodona A, García-López J, Muñoz-Guijosa C, Querol S, Bayes-Genis A (2020) First-in-human PeriCord cardiac bioimplant: scalability and GMP manufacturing of an allogeneic engineered tissue graft. EBioMedicine 54. https://doi.org/10.1016/J.EBIOM.2020.102729
Bejleri D, Davis ME (2019) Decellularized extracellular matrix materials for cardiac repair and regeneration. Adv Healthc Mater 8:1801217. https://doi.org/10.1002/ADHM.201801217
Bouten CVC, Dankers PYW, Driessen-Mol A, Pedron S, Brizard AMA, Baaijens FPT (2011) Substrates for cardiovascular tissue engineering. Adv Drug Deliv Rev 63:221–241. https://doi.org/10.1016/J.ADDR.2011.01.007
Spaans S, Peter-Paul K, Fransen H, Schotman MJG, van der Wulp R, Lafleur RPM, Kluijtmans SGJM, Dankers PYW (2019) Supramolecular modification of a sequence-controlled collagen-mimicking polymer. Biomacromolecules 14:46. https://doi.org/10.1021/acs.biomac.9b00353
Mol EA, Lei Z, Roefs MT, Bakker MH, Goumans M, Doevendans PA, Dankers PYW, Vader P, Sluijter JPG (2019) Injectable supramolecular ureidopyrimidinone hydrogels provide sustained release of extracellular vesicle therapeutics. Adv Healthc Mater 8:1900847. https://doi.org/10.1002/adhm.201900847
Goor OJGM, Hendrikse SIS, Dankers PYW, Meijer EW (2017) From supramolecular polymers to multi-component biomaterials. Chem Soc Rev 46:6621–6637
Dwyer KD, Coulombe KLK (2021) Cardiac mechanostructure: Using mechanics and anisotropy as inspiration for developing epicardial therapies in treating myocardial infarction. Bioact Mater 6:2198–2220. https://doi.org/10.1016/J.BIOACTMAT.2020.12.015
Jarrell DK, Vanderslice EJ, VeDepo MC, Jacot JG (2020) Engineering myocardium for heart regeneration—advancements, considerations, and future directions. Front Cardiovasc Med 7:586261. https://doi.org/10.3389/fcvm.2020.586261
Jorba I, Mostert D, Hermans LHL, van der Pol A, Kurniawan NA, Bouten CVC (2021) In vitro methods to model cardiac mechanobiology in health and disease. Tissue Eng Part C Methods 27:139–151. https://doi.org/10.1089/TEN.TEC.2020.0342
Nemavhola F (2017) Biaxial quantification of passive porcine myocardium elastic properties by region. Eng Solid Mech 5:155–166. https://doi.org/10.5267/j.esm.2017.6.003
Andreu I, Luque T, Sancho A, Pelacho B, Iglesias-García O, Melo E, Farré R, Prósper F, Elizalde MR, Navajas D (2014) Heterogeneous micromechanical properties of the extracellular matrix in healthy and infarcted hearts. Acta Biomater 10:3235–3242. https://doi.org/10.1016/j.actbio.2014.03.034
Alcaraz J, Otero J, Jorba I, Navajas D (2018) Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. Semin Cell Dev Biol 73:71–81. https://doi.org/10.1016/j.semcdb.2017.07.020
Bers DM (2002) Cardiac excitation–contraction coupling. Nature 2002(415):198–205. https://doi.org/10.1038/415198a
Streeter D (1979) Gross morphology and fiber geometry of the heart
Arts T, Costa KD, Covell JW, McCulloch AD (2001) Relating myocardial laminar architecture to shear strain and muscle fiber orientation. Am J Physiol Heart Circ Physiol 280(5):H2222–H2229. https://doi.org/10.1152/AJPHEART.2001.280.5.H2222
Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH (1981) Left ventricular fibre architecture in man. Br Heart J 45:248–263. https://doi.org/10.1136/hrt.45.3.248
Burton RA, Plank G, Schneider JE, Grau V, Ahammer H, Keeling SL, Lee J, Smith NP, Gavaghan D, Trayanova N, Kohl P (2006) Three-dimensional models of individual cardiac histoanatomy: tools and challenges. Ann N Y Acad Sci 1080:301–319. https://doi.org/10.1196/ANNALS.1380.023
Phelan D, Collier P, Thavendiranathan P, Popović ZB, Hanna M, Plana JC, Marwick TH, Thomas JD (2012) Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 98:1442–1448. https://doi.org/10.1136/HEARTJNL-2012-302353
Saito M, Okayama H, Yoshii T, Higashi H, Morioka H, Hiasa G, Sumimoto T, Inaba S, Nishimura K, Inoue K, Ogimoto A, Shigematsu Y, Hamada M, Higaki J (2012) Clinical significance of global two-dimensional strain as a surrogate parameter of myocardial fibrosis and cardiac events in patients with hypertrophic cardiomyopathy. Eur Hear J Cardiovasc Imaging 13:617–623. https://doi.org/10.1093/EJECHOCARD/JER318
Valente F, Gutierrez L, Rodríguez-Eyras L, Fernandez R, Montano M, Sao-Aviles A, Pineda V, Guala A, Cuéllar H, Evangelista A, Rodríguez-Palomares J (2020) Cardiac magnetic resonance longitudinal strain analysis in acute ST-segment elevation myocardial infarction: A comparison with speckle-tracking echocardiography. IJC Hear Vasc 29:100560. https://doi.org/10.1016/J.IJCHA.2020.100560
Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R (2009) Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Curr Cardiol Rev 5:133–148. https://doi.org/10.2174/157340309788166642
Pedrizzetti G, Claus P, Kilner PJ, Nagel E (2016) Principles of cardiovascular magnetic resonance feature tracking and echocardiographic speckle tracking for informed clinical use. J Cardiovasc Magn Reson 18:1–12. https://doi.org/10.1186/S12968-016-0269-7
Scatteia A, Baritussio A, Bucciarelli-Ducci C (2017) Strain imaging using cardiac magnetic resonance. Heart Fail Rev 224(22):465–476. https://doi.org/10.1007/S10741-017-9621-8
Mangion K, McComb C, Auger DA, Epstein FH, Berry C (2017) Magnetic resonance imaging of myocardial strain after acute ST-segment-elevation myocardial infarction: a systematic review. Circ Cardiovasc Imaging 10:e006498. https://doi.org/10.1161/CIRCIMAGING.117.006498
Huisman RM, Elzinga G, Westerhof N, Sipkema P (1980) Measurement of left ventricular wall stress. Cardiovasc Res 14:142–153. https://doi.org/10.1093/CVR/14.3.142
Guccione JM, McCulloch AD, Waldman LK (1991) Passive material properties of intact ventricular myocardium determined from a cylindrical model. J Biomech Eng 113:42–55. https://doi.org/10.1115/1.2894084
Feygin J, Hu Q, Swingen C, Zhang J (2008) Relationships between regional myocardial wall stress and bioenergetics in hearts with left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 294:H2313–H2321. https://doi.org/10.1152/AJPHEART.01288.2007
Walker JC, Ratcliffe MB, Zhang P, Wallace AW, Hsu EW, Saloner DA, Guccione JM (2008) Magnetic resonance imaging-based finite element stress analysis after linear repair of left ventricular aneurysm. J Thorac Cardiovasc Surg 135:1094–1102.e2. https://doi.org/10.1016/J.JTCVS.2007.11.038
Walker JC, Ratcliffe MB, Zhang P, Wallace AW, Fata B, Hsu EW, Saloner D, Guccione JM (2005) MRI-based finite-element analysis of left ventricular aneurysm. Am J Physiol Heart Circ Physiol 289:H692–H700. https://doi.org/10.1152/AJPHEART.01226.2004
Humphrey JD (2002) Cardiovascular solid mechanics, 1st edn. Springer, New York, pp 1–758. https://doi.org/10.1007/978-0-387-21576-1
Holmes JW, Borg TK, Covell JW (2005) Structure and mechanics of healing myocardial infarcts. Annu Rev Biomed Eng 7:223–253. https://doi.org/10.1146/ANNUREV.BIOENG.7.060804.100453
Fung Y-C (1993) Biomechanics, 2nd edn. Springer, New York, pp 1–568. https://doi.org/10.1007/978-1-4757-2257-4
Suki B (2014) Assessing the functional mechanical properties of bioengineered organs with emphasis on the lung. J Cell Physiol 229:1134–1140. https://doi.org/10.1002/jcp.24600
Storm C, Pastore JJ, MacKintosh FC, Lubensky TC, Janmey PA (2005) Nonlinear elasticity in biological gels. Nature 435:191–194. https://doi.org/10.1038/nature03521
Demer LL, Yin FC (1983) Passive biaxial mechanical properties of isolated canine myocardium. J Physiol 339:615. https://doi.org/10.1113/JPHYSIOL.1983.SP014738
Novak VP, Yin FCP, Humphrey JD (1994) Regional mechanical properties of passive myocardium. J Biomech 27:403–412. https://doi.org/10.1016/0021-9290(94)90016-7
Dokos S, Smaill BH, Young AA, LeGrice IJ (2002) Shear properties of passive ventricular myocardium. Am J Physiol Heart Circ Physiol 283:2650–2659. https://doi.org/10.1152/AJPHEART.00111.2002
Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE (2008) Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci 121:3794–3802. https://doi.org/10.1242/jcs.029678
Fomovsky GM, Holmes JW (2010) Evolution of scar structure, mechanics, and ventricular function after myocardial infarction in the rat. Am J Physiol Heart Circ Physiol 298. https://doi.org/10.1152/ajpheart.00495.2009
Sirry MS, Butler JR, Patnaik SS, Brazile B, Bertucci R, Claude A, Mclaughlin R, Davies NH, Liao J, Franz T (2016) Characterisation of the mechanical properties of infarcted myocardium in the rat under biaxial tension and uniaxial compression. J Mech Behav Biomed Mater 63:252–264. https://doi.org/10.1016/j.jmbbm.2016.06.029
Brazile BL, Butler JR, Patnaik SS, Claude A, Prabhu R, Williams LN, Perez KL, Nguyen KT, Zhang G, Bajona P, Peltz M, Yang Y, Hong Y, Liao J (2021) Biomechanical properties of acellular scar ECM during the acute to chronic stages of myocardial infarction. J Mech Behav Biomed Mater 116:104342. https://doi.org/10.1016/J.JMBBM.2021.104342
Farré N, Jorba I, Torres M, Falcones B, Martí-Almor J, Farré R, Almendros I, Navajas D (2018) Passive stiffness of left ventricular myocardial tissue is reduced by ovariectomy in a post-menopause mouse model. Front Physiol 9. https://doi.org/10.3389/fphys.2018.01545
Jorba I, Uriarte JJ, Campillo N, Farré R, Navajas D (2017) Probing micromechanical properties of the extracellular matrix of soft tissues by atomic force microscopy. J Cell Physiol 232. https://doi.org/10.1002/jcp.25420
Zhang S, Sun A, Ma H, Yao K, Zhou N, Shen L, Zhang C, Zou Y, Ge J (2011) Infarcted myocardium-like stiffness contributes to endothelial progenitor lineage commitment of bone marrow mononuclear cells. J Cell Mol Med 15:2245–2261. https://doi.org/10.1111/J.1582-4934.2010.01217.X
Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL (2006) Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 290:2196–2203. https://doi.org/10.1152/AJPHEART.01017.2005
Notari M, Ventura-Rubio A, Bedford-Guaus SJ, Jorba I, Mulero L, Navajas D, Martí M, Raya Á (2018) The local microenvironment limits the regenerative potential of the mouse neonatal heart. Sci Adv 4:eaao5553. https://doi.org/10.1126/sciadv.aao5553
Garcia-Puig A, Mosquera JL, Jiménez-Delgado S, García-Pastor C, Jorba I, Navajas D, Canals F, Raya A (2019) Proteomics analysis of extracellular matrix remodeling during zebrafish heart regeneration. Mol Cell Proteomics 18(9):1745–1755. https://doi.org/10.1074/mcp.RA118.001193
Borin D, Pecorari I, Pena B, Sbaizero O (2018) Novel insights into cardiomyocytes provided by atomic force microscopy. Semin Cell Dev Biol 73:4–12. https://doi.org/10.1016/J.SEMCDB.2017.07.003
Liu J, Sun N, Bruce MA, Wu JC, Butte MJ (2012) Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS One 7:e37559. https://doi.org/10.1371/JOURNAL.PONE.0037559
Lanzicher T, Martinelli V, Long CS, Del FG, Puzzi L, Borelli M, Mestroni L, Taylor MRG, Sbaizero O (2016) AFM single-cell force spectroscopy links altered nuclear and cytoskeletal mechanics to defective cell adhesion in cardiac myocytes with a nuclear lamin mutation. Nucleus 6:394–407. https://doi.org/10.1080/19491034.2015.1084453
Benech JC, Benech N, Zambrana AI, Rauschert I, Bervejillo V, Oddone N, Damián JP (2014) Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation. Am J Physiol 307:C910–C919. https://doi.org/10.1152/AJPCELL.00192.2013
Lieber SC, Aubry N, Pain J, Diaz G, Kim S-J, Vatner SF (2004) Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol Heart Circ Physiol 287:645–651. https://doi.org/10.1152/AJPHEART.00564.2003
Feinberg AW, Ripplinger CM, Van Der Meer P, Sheehy SP, Domian I, Chien KR, Parker KK (2013) Functional differences in engineered myocardium from embryonic stem cell-derived versus neonatal cardiomyocytes. Stem Cell Rep 1:387–396. https://doi.org/10.1016/j.stemcr.2013.10.004
Sheehy SP, Grosberg A, Qin P, Behm DJ, Ferrier JP, Eagleson MA, Nesmith AP, Krull D, Falls JG, Campbell PH, McCain ML, Willette RN, Hu E, Parker KK (2017) Toward improved myocardial maturity in an organ-on-chip platform with immature cardiac myocytes. Exp Biol Med (Maywood) 242:1643–1656. https://doi.org/10.1177/1535370217701006
Ariyasinghe NR, Reck CH, Viscio AA, Petersen AP, Lyra-Leite DM, Cho N, McCain ML (2017) Engineering micromyocardium to delineate cellular and extracellular regulation of myocardial tissue contractility. Integr Biol (United Kingdom) 9:730–741. https://doi.org/10.1039/c7ib00081b
Pasqualini FS, Agarwal A, O’Connor BB, Liu Q, Sheehy SP, Parker KK (2018) Traction force microscopy of engineered cardiac tissues. PLoS One 13:e0194706. https://doi.org/10.1371/journal.pone.0194706
McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK (2014) Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am J Physiol Circ Physiol 306:H1525–H1539. https://doi.org/10.1152/ajpheart.00799.2013
Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD (2003) Anisotropic stretch-induced hypertrophy in neonatal ventricular myocytes micropatterned on deformable elastomers. Biotechnol Bioeng 81:578–587. https://doi.org/10.1002/bit.10506
Ribeiro AJS, Ang Y-S, Fu J-D, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL (2015) Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci 112:12705–12710. https://doi.org/10.1073/pnas.1508073112
Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans M-J, Wang Y, Feinberg AW, Mummery CL, Passier R (2015) Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro – correlation between contraction force and electrophysiology. Biomaterials 51:138–150. https://doi.org/10.1016/j.biomaterials.2015.01.067
Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schäfer H, Bishopric N, Wakatsuki T, Elson EL (1997) Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB J 11:683–694. https://doi.org/10.1096/fasebj.11.8.9240969
Van Spreeuwel ACC, Bax NAM, Bastiaens AJ, Foolen J, Loerakker S, Borochin M, Van Der Schaft DWJ, Chen CS, Baaijens FPT, Bouten CVC (2014) The influence of matrix (an)isotropy on cardiomyocyte contraction in engineered cardiac microtissues. Integr Biol (United Kingdom) 6:422–429. https://doi.org/10.1039/c3ib40219c
van Spreeuwel ACC, Bax NAM, van Nierop BJ, Aartsma-Rus A, Goumans MJTH, Bouten CVC (2017) Mimicking cardiac fibrosis in a dish: fibroblast density rather than collagen density weakens cardiomyocyte function. J Cardiovasc Transl Res 10:116–127. https://doi.org/10.1007/s12265-017-9737-1
Jacot JG, McCulloch AD, Omens JH (2008) Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95:3479–3487. https://doi.org/10.1529/biophysj.107.124545
Mauretti A, Bax NAM, Van Marion MH, Goumans MJ, Sahlgren C, Bouten CVC (2016) Cardiomyocyte progenitor cell mechanoresponse unrevealed: Strain avoidance and mechanosome development. Integr Biol (United Kingdom) 8:991–1001. https://doi.org/10.1039/c6ib00117c
Wang EY, Rafatian N, Zhao Y, Lee A, Lai BFL, Lu RX, Jekic D, Davenport Huyer L, Knee-Walden EJ, Bhattacharya S, Backx PH, Radisic M (2019) Biowire model of interstitial and focal cardiac fibrosis. ACS Cent Sci 5:1146–1158. https://doi.org/10.1021/ACSCENTSCI.9B00052
Bracco Gartner TCL, Deddens JC, Mol EA, Magin Ferrer M, van Laake LW, Bouten CVC, Khademhosseini A, Doevendans PA, Suyker WJL, Sluijter JPG, Hjortnaes J (2019) Anti-fibrotic effects of cardiac progenitor cells in a 3D-model of human cardiac fibrosis. Front Cardiovasc Med 6:52. https://doi.org/10.3389/fcvm.2019.00052
Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, Prakash G, Antona S, Demarchi D, Buijsrogge MP, Sluijter JPG, Hjortnaes J, Khademhosseini A (2017) Engineered 3D cardiac fibrotic tissue to study fibrotic remodeling. Adv Healthc Mater 6:1–14. https://doi.org/10.1002/adhm.201601434
Crocini C, Walker CJ, Anseth KS, Leinwand LA (2020) Three-dimensional encapsulation of adult mouse cardiomyocytes in hydrogels with tunable stiffness. Prog Biophys Mol Biol 154:71–79. https://doi.org/10.1016/j.pbiomolbio.2019.04.008
Ma Y, Iyer RP, Jung M, Czubryt MP, Lindsey ML (2017) Cardiac fibroblast activation post-myocardial infarction: current knowledge gaps. Trends Pharmacol Sci 38:448–458
Lee S, Serpooshan V, Tong X, Venkatraman S, Lee M, Lee J, Chirikian O, Wu JC, Wu SM, Yang F (2017) Contractile force generation by 3D hiPSC-derived cardiac tissues is enhanced by rapid establishment of cellular interconnection in matrix with muscle-mimicking stiffness. Biomaterials 131:111–120. https://doi.org/10.1016/j.biomaterials.2017.03.039
Broughton KM, Russell B (2015) Cardiomyocyte subdomain contractility arising from microenvironmental stiffness and topography. Biomech Model Mechanobiol 14:589–602. https://doi.org/10.1007/s10237-014-0624-2
Corbin EA, Vite A, Peyster EG, Bhoopalam M, Brandimarto J, Wang X, Bennett AI, Clark AT, Cheng X, Turner KT, Musunuru K, Margulies KB (2019) Tunable and reversible substrate stiffness reveals a dynamic mechanosensitivity of cardiomyocytes. ACS Appl Mater Interfaces 11:20603–20614. https://doi.org/10.1021/acsami.9b02446
Spang MT, Christman KL (2018) Extracellular matrix hydrogel therapies: in vivo applications and development. Acta Biomater 68:1–14. https://doi.org/10.1016/J.ACTBIO.2017.12.019
Cramer MC, Badylak SF (2019) Extracellular matrix-based biomaterials and their influence upon cell behavior. Ann Biomed Eng 487(48):2132–2153. https://doi.org/10.1007/S10439-019-02408-9
Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee H-P, Lippens E, Duda GN, Mooney DJ (2016) Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater 15:326–334. https://doi.org/10.1038/NMAT4489
Charrier EE, Pogoda K, Wells RG, Janmey PA (2018) Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat Commun 9:449. https://doi.org/10.1038/s41467-018-02906-9
Zhao H, Xu K, Zhu P, Wang C, Chi Q (2019) Smart hydrogels with high tunability of stiffness as a biomimetic cell carrier. Cell Biol Int 43:84–97. https://doi.org/10.1002/cbin.11091
Accardo JV, Kalow JA (2018) Reversibly tuning hydrogel stiffness through photocontrolled dynamic covalent crosslinks. Chem Sci 9:5987–5993. https://doi.org/10.1039/c8sc02093k
Wu X, Huang W, Wu WH, Xue B, Xiang D, Li Y, Qin M, Sun F, Wang W, Bin ZW, Cao Y (2018) Reversible hydrogels with tunable mechanical properties for optically controlling cell migration. Nano Res 11:5556–5565. https://doi.org/10.1007/s12274-017-1890-y
Bastings MMC, Koudstaal S, Kieltyka RE, Nakano Y, Pape ACH, Feyen DAM, van Slochteren FJ, Doevendans PA, Sluijter JPG, Meijer EW, Chamuleau SAJ, Dankers PYW (2014) A fast pH-switchable and self-healing supramolecular hydrogel carrier for guided, local catheter injection in the infarcted myocardium. Adv Healthc Mater 3:70–78. https://doi.org/10.1002/adhm.201300076
Stoppel WL, Kaplan DL, Black LD (2016) Electrical and mechanical stimulation of cardiac cells and tissue constructs. Adv Drug Deliv Rev 96:135–155
Yamane M, Matsuda T, Ito T, Fujio Y, Takahashi KAJ (2007) Rac1 activity is required for cardiac myocyte alignment in response to mechanical stress. Biochem Biophys Res Commun 353:1023–1027. https://doi.org/10.1016/J.BBRC.2006.12.144
Salameh A, Wustmann A, Karl S, Blanke K, Apel D, Rojas-Gomez D, Franke H, Mohr FW, Janousek J, Dhein S (2010) Cyclic mechanical stretch induces cardiomyocyte orientation and polarization of the gap junction protein connexin43. Circ Res 106:1592–1602. https://doi.org/10.1161/CIRCRESAHA.109.214429
Debbi L, Drori S, Tzlil S (2018) The influence of the timing of cyclic load application on cardiac cell contraction. Front Physiol 0:917. https://doi.org/10.3389/FPHYS.2018.00917
Livne A, Bouchbinder E, Geiger B (2014) Cell reorientation under cyclic stretching. Nat Commun 51(5):1–8. https://doi.org/10.1038/ncomms4938
Tondon A, Hsu HJ, Kaunas R (2012) Dependence of cyclic stretch-induced stress fiber reorientation on stretch waveform. J Biomech 45:728–735. https://doi.org/10.1016/J.JBIOMECH.2011.11.012
Jungbauer S, Gao H, Spatz JP, Kemkemer R (2008) Two characteristic regimes in frequency-dependent dynamic reorientation of fibroblasts on cyclically stretched substrates. Biophys J 95:3470–3478. https://doi.org/10.1529/BIOPHYSJ.107.128611
Boccafoschi F, Bosetti M, Gatti S, Cannas M (2007) Dynamic fibroblast cultures: response to mechanical stretching. Cell Adhes Migr 1:124–128. https://doi.org/10.4161/CAM.1.3.5144
Foolen J, Deshpande VS, Kanters FMW, Baaijens FPT (2012) The influence of matrix integrity on stress-fiber remodeling in 3D. Biomaterials 33:7508–7518. https://doi.org/10.1016/j.biomaterials.2012.06.103
Mostert D, Klouda L, van Turnhout MC, Kurniawan NA, Bouten CVC (2021) Cardiac fibroblast mechanoresponse guides anisotropic organization of hiPSC-derived cardiomyocytes in vitro. bioRxiv. https://doi.org/10.1101/2021.02.16.431369
Carlos-Oliveira M, Lozano-Juan F, Occhetta P, Visone R, Rasponi M (2021) Current strategies of mechanical stimulation for maturation of cardiac microtissues. Biophys Rev 13:717–727. https://doi.org/10.1007/S12551-021-00841-6/FIGURES/4
Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK (2014) The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes. Biomaterials 35:2798–2808. https://doi.org/10.1016/J.BIOMATERIALS.2013.12.052
Marsano A, Conficconi C, Lemme M, Occhetta P, Gaudiello E, Votta E, Cerino G, Redaelli A, Rasponi M (2016) Beating heart on a chip: a novel microfluidic platform to generate functional 3D cardiac microtissues. Lab Chip 16:599–610. https://doi.org/10.1039/C5LC01356A
Llucià-Valldeperas A, Bragós R, Soler-Botija C, Roura S, Gálvez-Montón C, Prat-Vidal C, Perea-Gil I, Bayes-Genis A (2018) Unravelling the effects of mechanical physiological conditioning on cardiac adipose tissue-derived progenitor cells in vitro and in silico. Sci Rep 81(8):1–12. https://doi.org/10.1038/s41598-017-18799-5
LaBarge W, Mattappally S, Kannappan R, Fast VG, Pretorius D, Berry JL, Zhang J (2019) Maturation of three-dimensional, hiPSC-derived cardiomyocyte spheroids utilizing cyclic, uniaxial stretch and electrical stimulation. PLoS One 14:e0219442. https://doi.org/10.1371/JOURNAL.PONE.0219442
Kreutzer J, Viehrig M, Pölönen RP, Zhao F, Ojala M, Aalto-Setälä K, Kallio P (2020) Pneumatic unidirectional cell stretching device for mechanobiological studies of cardiomyocytes. Biomech Model Mechanobiol 19:291–303. https://doi.org/10.1007/S10237-019-01211-8/FIGURES/5
Dou W, Wang L, Malhi M, Liu H, Zhao Q, Plakhotnik J, Xu Z, Huang Z, Simmons CA, Maynes JT, Sun Y (2021) A microdevice platform for characterizing the effect of mechanical strain magnitudes on the maturation of iPSC-cardiomyocytes. Biosens Bioelectron 175:112875. https://doi.org/10.1016/J.BIOS.2020.112875
Lu K, Seidel T, Cao-Ehlker X, Dorn T, Batcha AMN, Schneider CM, Semmler M, Volk T, Moretti A, Dendorfer A, Tomasi R (2021) Progressive stretch enhances growth and maturation of 3D stem-cell-derived myocardium. Theranostics 11:6138–6153. https://doi.org/10.7150/THNO.54999
Kroll K, Chabria M, Wang K, Häusermann F, Schuler F, Polonchuk L (2017) Electro-mechanical conditioning of human iPSC-derived cardiomyocytes for translational research. Prog Biophys Mol Biol 130:212–222. https://doi.org/10.1016/J.PBIOMOLBIO.2017.07.003
Ovchinnikova E, Hoes M, Ustyantsev K, Bomer N, de Jong TV, van der Mei H, Berezikov E, van der Meer P (2018) Modeling human cardiac hypertrophy in stem cell-derived cardiomyocytes. Stem Cell Rep 10:794–807. https://doi.org/10.1016/J.STEMCR.2018.01.016
Viereck J, Bührke A, Foinquinos A, Chatterjee S, Kleeberger JA, Xiao K, Janssen-Peters H, Batkai S, Ramanujam D, Kraft T, Cebotari S, Gueler F, Beyer AM, Schmitz J, Bräsen JH, Schmitto JD, Gyöngyösi M, Löser A, Hirt MN, Eschenhagen T, Engelhardt S, Bär C, Thum T (2020) Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur Heart J 41:3462–3474. https://doi.org/10.1093/EURHEARTJ/EHAA519
Nagaraju CK, Dries E, Gilbert G, Abdesselem M, Wang N, Amoni M, Driesen RB, Sipido KR (2019) Myofibroblast modulation of cardiac myocyte structure and function. Sci Rep 9:1–11. https://doi.org/10.1038/s41598-019-45078-2
López-Canosa A, Perez-Amodio S, Yanac-Huertas E, Ordoño J, Rodriguez-Trujillo R, Samitier J, Castaño O, Engel E (2021) A microphysiological system combining electrospun fibers and electrical stimulation for the maturation of highly anisotropic cardiac tissue. Biofabrication 13:035047. https://doi.org/10.1088/1758-5090/ABFF12
Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M (2013) Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat Methods 10:781–787. https://doi.org/10.1038/nmeth.2524
Paez-Mayorga J, Hernández-Vargas G, Ruiz-Esparza GU, Iqbal HMN, Wang X, Zhang YS, Parra-Saldivar R, Khademhosseini A (2019) Bioreactors for cardiac tissue engineering. Adv Healthc Mater 8:1701504. https://doi.org/10.1002/ADHM.201701504
Abe H, Semba H, Takeda N (2017) The roles of hypoxia signaling in the pathogenesis of cardiovascular diseases. J Atheroscler Thromb 24:884–894. https://doi.org/10.5551/JAT.RV17009
D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F (2019) Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol 10:1347. https://doi.org/10.3389/FPHYS.2019.01347/BIBTEX
Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, Nanthakumar K, Woodhouse K, Husain M, Kumacheva E, Zandstra PW (2009) Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng 102:493–507. https://doi.org/10.1002/BIT.22065
Correia C, Serra M, Espinha N, Sousa M, Brito C, Burkert K, Zheng Y, Hescheler J, Carrondo MJT, Šarić T, Alves PM (2014) Combining hypoxia and bioreactor hydrodynamics boosts induced pluripotent stem cell differentiation towards cardiomyocytes. Stem Cell Rev Rep 10:786–801. https://doi.org/10.1007/S12015-014-9533-0/FIGURES/7
Wang JH, Zhao L, Pan X, Chen NN, Chen J, Gong QL, Su F, Yan J, Zhang Y, Zhang SH (2016) Hypoxia-stimulated cardiac fibroblast production of IL-6 promotes myocardial fibrosis via the TGF-β1 signaling pathway. Lab Investig 968(96):839–852. https://doi.org/10.1038/labinvest.2016.65
Isenberg BC, Tranquillo RT (2003) Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Ann Biomed Eng 31:937–949. https://doi.org/10.1114/1.1590662
Lu L, Mende M, Yang X, Körber HF, Schnittler HJ, Weinert S, Heubach J, Werner C, Ravens U, Lu L, Mende M, Yang X, Körber HF, Schnittler HJ, Weinert S, Heubach J, Werner C, Ravens U (2013) Design and validation of a bioreactor for simulating the cardiac niche: a system incorporating cyclic stretch, electrical stimulation, and constant perfusion. Tissue Eng Part A 19:403–414. https://doi.org/10.1089/TEN.TEA.2012.0135
Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, Radisic M (2014) Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation. Biofabrication 6. https://doi.org/10.1088/1758-5082/6/2/024113
Wang B, Wang G, To F, Butler JR, Claude A, McLaughlin RM, Williams LN, de Jongh Curry AL, Liao J (2013) Myocardial scaffold-based cardiac tissue engineering: application of coordinated mechanical and electrical stimulations. Langmuir 29:11109–11117. https://doi.org/10.1021/LA401702W
van Kelle MAJ, Oomen PJA, Bulsink JA, Janssen-van den Broek MWJT, Lopata RGP, Rutten MCM, Loerakker S, Bouten CVC (2017) A bioreactor to identify the driving mechanical stimuli of tissue growth and remodeling. Tissue Eng Part C Methods 23:377–387. https://doi.org/10.1089/TEN.TEC.2017.0141
Savoji H, Mohammadi MH, Rafatian N, Toroghi MK, Wang EY, Zhao Y, Korolj A, Ahadian S, Radisic M (2019) Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 198:3–26. https://doi.org/10.1016/j.biomaterials.2018.09.036
Mijailovich SM, Prodanovic M, Poggesi C, Geeves MA, Regnier M (2021) Multiscale modeling of twitch contractions in cardiac trabeculae. J Gen Physiol 153. https://doi.org/10.1085/JGP.202012604
Campbell SG, McCulloch AD (2011) Multi-scale computational models of familial hypertrophic cardiomyopathy: genotype to phenotype. J R Soc Interface 8:1550–1561. https://doi.org/10.1098/RSIF.2011.0184
Günter J, Wolint P, Bopp A, Steiger J, Cambria E, Hoerstrup SP, Emmert MY (2016) Microtissues in cardiovascular medicine: regenerative potential based on a 3d microenvironment. Stem Cells Int 2016. https://doi.org/10.1155/2016/9098523
Butler DL, Goldstein SA, Guldberg RE, Guo XE, Kamm R, Laurencin CT, McIntire LV, Mow VC, Nerem RM, Sah RL, Soslowsky LJ, Spilker RL, Tranquillo RT (2009) The impact of biomechanics in tissue engineering and regenerative medicine. Tissue Eng Part B Rev 15:477–484
Mihalef V, Passerini T, Mansi T (2020) Multi-scale models of the heart for patient-specific simulations. Artif Intell Comput Model Hear 2020:3–42. https://doi.org/10.1016/B978-0-12-817594-1.00011-5
Land S, Gurev V, Arens S, Augustin CM, Baron L, Blake R, Bradley C, Castro S, Crozier A, Favino M, Fastl TE, Fritz T, Gao H, Gizzi A, Griffith BE, Hurtado DE, Krause R, Luo X, Nash MP, Pezzuto S, Plank G, Rossi S, Ruprecht D, Seemann G, Smith NP, Sundnes J, Rice JJ, Trayanova N, Wang D, Wang ZJ, Niederer SA (2015) Verification of cardiac mechanics software: benchmark problems and solutions for testing active and passive material behaviour. Proc R Soc A Math Phys Eng Sci 471:20150641. https://doi.org/10.1098/RSPA.2015.0641
Urdeitx P, Doweidar MH (2020) A computational model for cardiomyocytes mechano-electric stimulation to enhance cardiac tissue regeneration. Mathematics. 8:1875. https://doi.org/10.3390/MATH8111875
Obbink-Huizer C, Foolen J, Oomens CWJ, Borochin M, Chen CS, Bouten CVC, FPT B (2014) Computational and experimental investigation of local stress fiber orientation in uniaxially and biaxially constrained microtissues. Biomech Model Mechanobiol 135(13):1053–1063. https://doi.org/10.1007/S10237-014-0554-Z
Obbink-Huizer C, Oomens CW, Loerakker S, Foolen J, Bouten CV, Baaijens FP (2014) Computational model predicts cell orientation in response to a range of mechanical stimuli. Biomech Model Mechanobiol 13:227–236. https://doi.org/10.1007/S10237-013-0501-4
Loerakker S, Obbink-Huizer C, Baaijens FPT (2013) A physically motivated constitutive model for cell-mediated compaction and collagen remodeling in soft tissues. Biomech Model Mechanobiol 135(13):985–1001. https://doi.org/10.1007/S10237-013-0549-1
Ristori T, Obbink-Huizer C, Oomens CW, Baaijens FP, Loerakker S (2016) Efficient computational simulation of actin stress fiber remodeling. Comput Methods Biomech Biomed Engin 19:1347–1358. https://doi.org/10.1080/10255842.2016.1140748
Ristori T, Notermans TMW, Foolen J, Kurniawan NA, Bouten CVC, Baaijens FPT, Loerakker S (2018) Modelling the combined effects of collagen and cyclic strain on cellular orientation in collagenous tissues. Sci Rep 81(8):1–14. https://doi.org/10.1038/s41598-018-26989-y
Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70:9–18. https://doi.org/10.1016/J.YJMCC.2013.10.019
Meshel AS, Wei Q, Adelstein RS, Sheetz MP (2005) Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol 72(7):157–164. https://doi.org/10.1038/ncb1216
Ristori T, Bouten CVC, Baaijens FPT, Loerakker S (2018) Predicting and understanding collagen remodeling in human native heart valves during early development. Acta Biomater 80:203–216. https://doi.org/10.1016/J.ACTBIO.2018.08.040
Emmert MY, Schmitt BA, Loerakker S, Sanders B, Spriestersbach H, Fioretta ES, Bruder L, Brakmann K, Motta SE, Lintas V, Dijkman PE, Frese L, Berger F, Baaijens FPT, Hoerstrup SP (2018) Computational modeling guides tissue-engineered heart valve design for long-term in vivo performance in a translational sheep model. Sci Transl Med 10. https://doi.org/10.1126/SCITRANSLMED.AAN4587
Thavandiran N, Dubois N, Mikryukov A, Massé S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, Keller GM, Radisic M, Zandstra PW (2013) Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci U S A 110:E4698–E4707. https://doi.org/10.1073/PNAS.1311120110
Shim J, Grosberg A, Nawroth JC, Kit Parker K, Bertoldi K (2012) Modeling of cardiac muscle thin films: pre-stretch, passive and active behavior. J Biomech 45:832–841. https://doi.org/10.1016/J.JBIOMECH.2011.11.024
Toussaint N, Stoeck CT, Schaeffter T, Kozerke S, Sermesant M, Batchelor PG (2013) In vivo human cardiac fibre architecture estimation using shape-based diffusion tensor processing. Med Image Anal 17:1243–1255. https://doi.org/10.1016/J.MEDIA.2013.02.008
Cao Y, Miller MI, Winslow RL, Younes L (2005) Large deformation diffeomorphic metric mapping of vector fields. IEEE Trans Med Imaging 24:1216–1230. https://doi.org/10.1109/TMI.2005.853923
Genet M, Lee LC, Kuhl E, Guccione J (2014) Abaqus/Standard-based quantification of human cardiac mechanical properties. In: 2014 SIMULIA Community Conference (SCC2014), May 2014, Providence, Rhode Island, United States
Haddad SMH (2016) A novel composite material-based computational model for left ventricle biomechanics simulation. Electronic Thesis and Dissertation Repository
Acknowledgements
The authors would like to thank Gemma Burcet, MD from Hospital Universitari Vall d’Hebron and Institut de Diagnòstic per la Imatge (Barcelona, Spain), for the supply of magnetic resonance images and strain processing. This work is supported by the partners of ‘Regenerative Medicine Crossing Borders’ (RegMed XB) and by Health~Holland, Top Sector Life Sciences & Health. We also gratefully acknowledge funding from the Ministry of Education, Culture and Science for the Gravitation Program 024.003.103 ‘Materials-Driven Regeneration’ and Nederlandse Organisatie voor Wetenschappelijk Onderzoek for the NWO Open Competition Domain Science grant, OCENW.XS21.4.146.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Ethics declarations
Funding: This work was funded by 1) the partners of ‘Regenerative Medicine Crossing Borders’ and Health~Holland, Top Sector Life Sciences & Health (project Cardiac Moonshot), 2) the Ministry of Education, Culture and Science for the Gravitation Program ‘Materials-Driven Regeneration’ (grant number 024.003.103), and 3) by the Dutch Research Council (OCENW.XS21.4.146).
Conflict of Interest: All authors declare they have no conflict of interest.
Ethical Approval: This chapter does not contain any studies with animals performed by any of the authors. For Fig. 2d, g informed consent was obtained from the patients.
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jorba, I., Nikolic, M., Bouten, C.V.C. (2023). Mechanical Considerations of Myocardial Tissue and Cardiac Regeneration. In: Hecker, M., Duncker, D.J. (eds) Cardiac Mechanobiology in Physiology and Disease. Cardiac and Vascular Biology, vol 9. Springer, Cham. https://doi.org/10.1007/978-3-031-23965-6_8
Download citation
DOI: https://doi.org/10.1007/978-3-031-23965-6_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23964-9
Online ISBN: 978-3-031-23965-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)