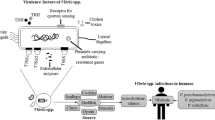
Abstract
The Vibrionaceae is a highly diverse family of aquatic bacteria. Some members of this ubiquitous group can cause a variety of diseases in humans ranging from cholera caused by Vibrio cholerae, severe septicemia caused by Vibrio vulnificus, to acute gastroenteritis by Vibrio parahaemolyticus. Planet Earth is experiencing unprecedented changes of planetary scale associated with climate change. These environmental perturbations paired with overpopulation and pollution are increasing the distribution of pathogenic Vibrios and exacerbating the risk of causing infections. In this chapter, we discuss various aspects of Vibrio infections within the context of the twenty-first century with a major emphasis on the aforementioned pathogenic species. Overall, we believe that the twenty-first century is posed to be both one full of challenges due to the rise of these pathogens, and also a catalyst for innovative and groundbreaking discoveries.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1.1 Vibrio Infections
The Vibrionaceae encompasses a group of ubiquitous aquatic bacteria that inhabit freshwater, estuarine, and marine environments (Reen et al. 2006; Baker-Austin et al. 2018; Austin et al. 2020). Some members of this family can be pathogenic to humans and cause the majority of human infections caused by bacteria of aquatic origin (Baker-Austin et al. 2018). V. cholerae represents the best known and most widely studied pathogenic species within the Vibrionaceae. A phylogenetically confined group of V. cholerae, the Pandemic Group (PG), causes the severe diarrheal disease cholera in humans (Chun et al. 2009; Boucher 2016; Shapiro et al. 2016). Toxigenic strains of V. cholerae belong to two serogroups, O1 and O139, the latter being close to extinction (Clemens et al. 2017; Kanungo et al. 2022). The O1 group can be further subdivided into Classical and El Tor strains, with the former having caused the first six pandemics of cholera, whereas El Tor being the source of the seventh and current pandemic (Balasubramanian et al. 2021). Other V. cholerae strains can cause sporadic disease in humans, known combined as non-O1 non-O139 strains. Even though infections by these strains are rare, they can cause severe gastrointestinal and extraintestinal infections (Deshayes et al. 2015). Cholera remains a major scourge in places with limited access to clean drinking water and poor sanitation infrastructure cholera outbreaks are increasing in frequency and intensity (Clemens et al. 2017; Kanungo et al. 2022). Nonetheless, as we discuss below, ambitious yet feasible frameworks are being proposed to eliminate the disease in the coming decades (Kanungo et al. 2022; Qadri et al. 2017; Editorial Lancet Gastroenterology Hepatology 2017; Francois 2020; Islam et al. 2022).
Infections by non-cholera Vibrios are commonly known as Vibriosis. The two most common and relevant ones are caused by V. vulnificus and V. parahaemolyticus. V. vulnificus is an emergent zoonotic pathogen that can cause a fulminant septicemia in susceptible hosts. The bacterium is typically contracted either through (a) the consumption of contaminated seafood, in particular oysters, resulting in gastroenteritis or primary septicemia or (b) exposure of wounds to sea water or products contaminated with the bacterium resulting in wound infections and secondary septicemia. V. vulnificus is the leading cause of seafood-associated deaths in the USA and is endemic to the Gulf and Southeastern coast (Phillips and Satchell 2017; López-Pérez et al. 2021). However, much uncertainty remains about the virulence of the organism (López-Pérez et al. 2019; Roig et al. 2018). For instance, recent genomic surveys determined that the known virulence factors of V. vulnificus strains are widespread within the species, with every strain analyzed encoding them (López-Pérez et al. 2019; Roig et al. 2018). Therefore, to date, the specific factors that allow only certain strains within the species to cause human disease remain to be elucidated. Furthermore, reliable markers that predict a high pathogenic potential of specific strains are still lacking, rendering this organism a unique threat to public health (Baker-Austin et al. 2018; Jones and Oliver 2009; Oliver 2012, 2015). V. vulnificus can also wreak havoc in aquaculture farms, a setting that allows the bacterium to quickly proliferate and be transmitted to humans. Besides the economic losses associated with this menace, novel hybrid strains can emerge in these settings as evidenced by a deadly Israeli outbreak in the 1990s (Paz et al. 2007; Amaro et al. 2015). Overall, efforts to understand and scrutinize the evolutionary and ecological trajectories of this pathogen are critical to prevent this zoonotic agent from expanding its narrow susceptible host range and habitat preference.
V. parahaemolyticus infections are associated with the consumption of raw or undercooked seafood and are characterized by a severe gastroenteritis that is distinct from cholera. Also, unlike V. cholerae, the bacterium cannot be transmitted from host to host or via the fecal-oral route. V. parahaemolyticus had been mostly restricted to Japan until the late 1960s, since then, infections associated with the bacterium started being reported worldwide turning this pathogen into a global public health concern (Baker-Austin et al. 2018; Letchumanan et al. 2014; Martinez-Urtaza and Baker-Austin 2020). In most cases, the disease resolves without the need for treatment, however, V. parahaemolyticus also can cause debilitating and dysenteric forms of gastroenteritis, necrotizing fasciitis, and septicemia in immunocompromised patients (Baker-Austin et al. 2018; Letchumanan et al. 2014; Martinez-Urtaza and Baker-Austin 2020; Zhang and Orth 2013). Since the date of its identification in 1953, V. parahaemolyticus infections have been reported in various parts of the world, causing outbreaks in Asia, Europe, and America. Pathogenic strains are mostly restricted to two serotypes, which are defined by somatic (O) and capsular (K) antigens (Baker-Austin et al. 2018; Letchumanan et al. 2014; Martinez-Urtaza and Baker-Austin 2020; Zhang and Orth 2013). Specifically, the O3:K6 (sequence type 3) and O4:K12 (sequence type 36) serotypes have been responsible for a large number of V. parahaemolyticus outbreaks and are associated with the pandemic expansion events of this pathogen (Baker-Austin et al. 2018; Letchumanan et al. 2014; Martinez-Urtaza and Baker-Austin 2020; Zhang and Orth 2013). The specific set of drivers that ultimately led to the expansion of these two serogroups remains unknown; however, molecular, and in vivo data indicate that these strains possess increased virulence capabilities compared with other serogroups (Martinez-Urtaza and Baker-Austin 2020; Zhang and Orth 2013). Strategies to reduce incidence of V. parahaemolyticus involve the identification and monitoring of the environmental abiotic conditions that significantly elevate its risk. Specifically, bivalve mollusks, such as oysters and mussels, can harbor large concentrations of this pathogen leading to increased risk of infection after ingestion (Martinez-Urtaza and Baker-Austin 2020). Therefore, monitoring virulent strains of V. parahaemolyticus in seafood products is a major health safety concern that must be prioritized to mitigate future outbreaks of this pathogen (Martinez-Urtaza and Baker-Austin 2020).
There are other Vibrio species that can be pathogenic to humans, however, their reduced incidence and severity are overshadowed by the three aforementioned pathogens. Those include Vibrio fluvialis, Vibrio mimicus, Vibrio hollisae, Vibrio metschnikovii, Vibrio cincinnatiensis, Vibrio furnissii, or more commonly Vibrio alginolyticus, which can cause gastroenteritis, wound, or ear infections (Baker-Austin et al. 2017, 2018). Nonetheless, the number of cases associated with some of these species such as V. alginolyticus or V. fluvialis continue increasing suggesting a potential source of concern over the coming years. Unfortunately, there are no global surveillance frameworks that systematically gather epidemiological data on pathogenic Vibrios, and very few countries have dedicated surveillance systems for them (Newton et al. 2012; Janda et al. 2015). Critically, it is imperative in order to prevent the unpredicted appearance of Vibrio outbreaks to prioritize the development of frameworks to assess the spread and distribution of these potential pathogens. Furthermore, monitoring is needed to reduce the impact that emergent strains or novel pathogenic species within the Vibrio group might have in human populations and aquaculture settings.
1.2 Vibrios and the Environment
In their natural environment, pathogenic Vibrios can be frequently found associated with other aquatic dwellers such as copepods and crustaceans (Huq et al. 1983; Tamplin et al. 1990; de Magny et al. 2011; Turner et al. 2014), arthropods and chironomid egg masses (Broza and Halpern 2001; Purdy and Watnick 2011), cyanobacteria (Epstein 1993; Greenfield et al. 2017; Reddi et al. 2018), shellfish (Phillips and Satchell 2017; Twedt et al. 1981; Hood et al. 1981; de Sousa et al. 2004), waterfowl (Halpern et al. 2008), or fish (Amaro et al. 2015; Senderovich et al. 2010; Novoslavskij et al. 2015; Messelhäusser et al. 2010). In addition, Vibrios generally face a wide range of abiotic and biotic stressors that pose a threat to their survivability such as nutrient limitation, pH changes, temperature, and salinity fluctuations, or protozoal grazing and phage predation (Almagro-Moreno and Taylor 2013; Lutz et al. 2013; Jayakumar et al. 2020). It appears that some of the mechanisms that allow these bacteria to colonize and persist in their natural environment provide preadaptations for virulence in the human host (Phillips and Satchell 2017; Zhang and Orth 2013; Broberg et al. 2011; Sakib et al. 2018; Cabanyero and Amaro 2020).
During adverse environmental conditions (e.g. antibiotic exposure, nutrient limitation) Vibrio cells enter a non-sporulating protective dormant state that enhances their survival and long-term persistence called viable but nonculturable (VBNC) (Almagro-Moreno and Taylor 2013; Lutz et al. 2013; Jayakumar et al. 2020). When external conditions become favourable (e.g. nutrient influx, reduction of antibiotics) dormant cells can recover from the VBNC state, a phenomenon also known as awakening or resuscitation. VBNC cells pose a major public health risk, as these pathogens can be found in this state during interepidemic periods, furthermore, they are a difficult to detect and eradicate source of food and water contamination (Almagro-Moreno and Taylor 2013; Lutz et al. 2013).
The growth and overall distribution of pathogenic Vibrios is severely affected by external environmental conditions. Vibrio infections naturally have very marked seasonal distribution as their abundance is primarily driven by increased temperature, salinity, and rainfall events (Huq et al. 1984, 2013; Lobitz et al. 2000). During warm summer months, Vibrios populations experience drastic blooms, which increase the likelihood of susceptible individuals to become in contact with them and contract the diseases associated with their pathogenic species. Furthermore, extended periods of warm weather, driven by climate change, have provided suitable conditions for the proliferation of pathogenic Vibrio spp. (Baker-Austin et al. 2018; Austin et al. 2020). As we discuss below, a multidecadal study recently demonstrated a steady increase in the abundance of pathogenic Vibrios, including V. cholerae, over the past half-century (Vezzulli et al. 2016). Furthermore, some water bodies are warming up faster than the global average such as the Baltic Sea, the White Sea, and those along the US east coast, posing a very high risk of Vibrio infections (Baker-Austin et al. 2013; Martinez-Urtaza et al. 2013; Rice and Jastram 2014). These patterns only exacerbate the problem of the emergence and reemergence of pathogenic Vibrios, the spread of virulence genes and their proliferation (Trinanes and Martinez-Urtaza 2021).
1.3 Life on a Warming Planet: Climate Change and the Global Vibrio Expansion
Human activity since the beginning of the industrial age has had an unprecedented impact on climate and on the future of life on the planet. The combustion of coal and other fossil fuels has generated levels of greenhouse gases that has caused a deep change on global climate patterns with impacts being perceptible at all ecological scales. The effects of climate change have a strong regional component, with geographical areas showing a faster rate of warming than others. In general terms, warming is having a greater effect on marine ecosystems because oceans capture more than 90% of all the heat (Zanna et al. 2019). In coastal areas, the most relevant impacts of climate change include the increase of temperatures, frequency of extreme weather events, and rise of sea level. Some areas are experiencing faster warming rates than others (Lima and Wethey 2012). For instance, the Baltic Sea, the Mediterranean Sea and the Northeastern USA are three marine regions with warming rate above the global average (Karmalkar and Horton 2021). Events of extreme weather, such as heat waves or torrential rains have a strong impact on coastal areas due to their shallow waters. Extreme weather rapidly influences temperature and salinity conditions in adjacent areas capturing the heat or assimilating the rainwater, causing a rise in temperature or sudden drops in salinity. The thawing of ice masses at the poles and large glaciers is mobilizing large masses of fresh water into the oceans with drastic consequences for oceanic currents, as well as generating a rise in sea water level that is causing the flooding of shorelines globally (Llovel et al. 2019).
Not every living organism is being affected negatively by climate change. For instance, some insects, such as mosquitoes, are being favoured by this new climatic situation with higher temperatures and higher humidity that facilitates the expansion towards the poles and they occupy new ecological niches at high latitudes that until recently were not suitable for these organisms. Interestingly, from the many examples of species benefitting from the conditions imposed by climate change, Vibrios have emerged as a barometer of climate change (Baker-Austin et al. 2017). Vibrio species have some of the fastest growth rates among bacteria (Joseph et al. 2008; Aiyar et al. 2002). This key characteristic shared by all members is critical to understand their adaptive ecological success and pathogenic potential (Baker-Austin et al. 2017). Under favourable conditions, Vibrios can double their populations in a matter of minutes. This facilitates their expansion and rapid occupation of new niches, which provides the ideal conditions to trigger infections (Baker-Austin et al. 2013). The shift in ecological conditions has two major potential effects on Vibrio populations: (a) increase the seasonal abundance (occurrence for longer periods) and (b) an expansion of their distribution range towards the poles.
Recent studies demonstrate the impacts of climate change on Vibrio populations showing the steady expansion of these species across coastal areas worldwide during the last 30 years (Baker-Austin et al. 2018). Around 71% of the world's coastal areas are warming at different rates. In the waters of enclosed or nearly enclosed seas (e.g. Mediterranean Sea or Gulf of Mexico), the rate of warming is even greater than the one in the oceans (Dutheil et al. 2022). As a result of these changes, the number of days with suitable conditions for the presence of Vibrio in shorelines across the planet has increased since the 1980s by about 10%. Vibrios have been identified in areas located at high latitudes as suitable ecological conditions have been amplified toward the poles reaching areas near the Arctic Circle (Baker-Austin et al. 2013, 2016). Recent progress in our understanding of the ecology of Vibrio has been a key element in the development of new frameworks for the construction of models to generate epidemiological and predictive tools (Semenza et al. 2017). For instance, these tools aid at remotely identifying areas with favourable ecological conditions for Vibrio growth and dispersal based on environmental data obtained from satellites and other remote sensing technologies (Semenza et al. 2017). The use of environmental data that dates back to the pre-industrial period together with the application of advanced climate models, has been combined to build a new generation of monitoring systems that enable to reconstruct the past, understand the present and predict the future of the environmental conditions for Vibrio on the planet (Trinanes and Martinez-Urtaza 2021). These studies show that the extent of coastal zones favourable for Vibrio remained relatively stable until 1980. Since then, the expansion of Vibrios has been increasing rapidly and in parallel to the rate of global warming, with an expansion towards the poles. Suitable periods for the occurrence of Vibrio have been amplifying at a rate of 1 month every 30 years. Furthermore, the distribution of these bacteria is reaching new areas that were considered adverse for the presence of Vibrio only a few years ago (Fig. 1.1). In fact, at the current rate of warming, their distribution is expected to extend about 38,000 km by the year 2100 (Fig. 1.1) (Trinanes and Martinez-Urtaza 2021).
Suitable Vibrio habitats over time. Changes in the extent (in thousands of km) of coastal areas with suitable ecological conditions for Vibrio in the planet since the pre-industrial period (1840), and distribution of these areas in 1900, 2000 and projections for 2090 according to different climate scenarios. Adapted from J. Trinanes and J. Martinez-Urtaza, The Lancet Planetary Health, 5:e426–35
Global human populations living in coastal regions with suitable conditions for Vibrio grew over the past century and reached an estimated value of 610 million people by 1980 (Trinanes and Martinez-Urtaza 2021). The projection for 2020 duplicated the estimate for 1980, ranging from 1107 to 1133 million according to different scenarios (Trinanes and Martinez-Urtaza 2021). This trend is expected to continue to increase until 2050 and after this point simulations show a stabilization in the projections or even a slight decline (Trinanes and Martinez-Urtaza 2021). Population at risk for Vibrio infections in suitable regions almost doubled from 1980 to 2020 (from 610 million to 1100 million), although the increment will be more moderate in the future, and it is expected to reach stable conditions after 2050 at 1300 million (Trinanes and Martinez-Urtaza 2021). According to these estimates, the global estimate for non-cholera Vibrio infections would be around half a million of cases worldwide in 2020. Geographical areas with the largest population at risk are in coastal areas of the north of Europe, southeast Asia, the Gulf of Guinea, the Atlantic northeast, the Pacific northwest, and some specific hot spots in the Gulf of Venice, the south coast of the Black Sea, and coastal areas of Egypt (Trinanes and Martinez-Urtaza 2021). New regions for populations at risk identified in high latitudes in the northern hemisphere (Russia and Canada) are a clear indication of the poleward expansion of Vibrio infections (Fig. 1.1). However, projections indicate that the growth trend in the number of cases will be weakened for the next decades primarily due to (a) the stabilization of the world population in regions with Vibrio risk and (b) the low population in new areas at high latitudes reaching favourable conditions for Vibrio.
1.4 The Future of Cholera
Cholera is an ancient disease that remains a major scourge in places with limited access to clean drinking water, poor sanitation practices or social unrest (Kanungo et al. 2022; Lancet 2017; Grant et al. 2021). Estimates indicate that the disease continues to infect over 3 million people and kill over 100,000 per year (Kanungo et al. 2022; Islam et al. 2022). Nonetheless, the real disease burden is difficult to calculate due to the large number of cases that remain unreported. Currently, cholera remains endemic and continues to be reported from several countries in Asia (Bangladesh, India, Philippines, and Myanmar), Africa (Cameroon, Democratic Republic of Congo, Kenya, Somalia, Sudan, and Mozambique), the Caribbean (Haiti) and the Middle East (Yemen and Syria) (Kanungo et al. 2022; Islam et al. 2022). Recent outbreaks of epidemic cholera due to war and/or natural disasters have been reported in refugee camps in Bangladesh, Syria, Yemen, and Lebanon (Kanungo et al. 2022; Islam et al. 2022; Connolly et al. 2004). For instance, human displacement due to a civil war in Yemen led to the largest cholera outbreak recorded in human history (Qadri et al. 2017; Lancet 2017).
The Global Roadmap to 2030 proposes to end the disease within this decade and suggests a comprehensive approach based on (a) early detection of cholera cases and prompt responses to contain outbreaks, (b) a targeted multisectoral approach to prevent disease recurrence, and (c) an effective and coordinated mechanism for technical support, mobilization of resources and partnerships at local and global levels (Kanungo et al. 2022; Islam et al. 2022). This approach must be cheap and must require limited expertise to be widely implemented. Furthermore, it must be delivered and maintained on the ground by community health workers and should include rapid diagnostics, real-time reporting, and proper treatment for mild and severe cases (Islam et al. 2022).
There are two complementary approaches for the prevention and control of cholera: (1) Short term, Oral Cholera Vaccines (OCVs), as they provide faster but temporary protection, rapid diagnostics and real-time reporting, and (2) Long term, the WASH framework, which stands for improving water, sanitation, and hygiene. The latter lacks immediacy but can lead to sustained reductions in transmission of V. cholerae O1.
-
1.
Short term
-
(a)
OCVs. Three types of OCVs are available: killed whole-cell vaccines (Shanchol and Euvichol), killed whole-cell vaccines with a recombinant B subunit (Dukoral), and a live attenuated vaccine (Vaxchora) (Clemens et al. 2017; Bhattacharya et al. 2013; Clemens et al. 1988; Sur et al. 2009, 2011; Baik et al. 2015; Bi et al. 2017). The latter two are primarily used by people travelling to cholera-endemic areas, whereas Shanchol and Euvichol are the OCVs used during cholera outbreaks. OCVs stockpiles were created after the cholera outbreaks in Zimbabwe and Haiti to facilitate and ease the supply of vaccines during emergencies. The number of doses has increased from 2 million per year (2013), to 25 million (2021); however, given the large demand of OCVs, vaccine supply must increase over the coming years to lead to a lasting effect on the disease.
-
(b)
Rapid diagnostics and real-time reporting. Rapid diagnostic tests should be used in the home of patients with suspected cases of cholera using some of the tests that are currently available, such as Cholkit (Incepta Pharmaceuticals) and Crystal VC (Arkray Healthcare) (Chowdhury et al. 2021). Even though these tests do not always provide 100% accuracy, they are inexpensive and widely accessible. The WHO’s global task force has developed a cell phone-based app for cholera reporting: GTFCC cholera (Islam et al. 2022). The app acts as a real-time reporting method after a case is identified in the field and notifies health authorities helping map disease transmission and evaluate control strategies.
-
(c)
Treatment. Patients with mild to moderate signs of dehydration can be effectively treated at home with an oral rehydration solution plus zinc (Davies et al. 2017; Pietroni 2020; Sousa et al. 2020). If a patient is deemed to have severe dehydration, they must be referred to a local hospital and receive immediate intravenous fluid replacement over three hours for adults and six hours for children less than 1 year of age (Davies et al. 2017; Pietroni 2020; Sousa et al. 2020). Antibiotics should be used only in patients with severe dehydration, options including macrolides, fluoroquinolones, and tetracycline (Davies et al. 2017; Pietroni 2020; Sousa et al. 2020). Azithromycin can be used prophylactically for household contacts after cholera detection in a home as it is effective both for the treatment of cholera and in preventing colonization of V. cholerae in the gut.
-
(a)
-
2.
Water, sanitation, and hygiene framework (WASH). Numerous basic characteristics of cholera outbreaks are shared among settings (e.g. the pathophysiology of the disease, the waterborne nature of transmission, etc.). Nonetheless, recent findings suggest that transmission within households in endemic settings may play a larger role in cholera outbreaks than previously appreciated (D’Mello-Guyett et al. 2020; Sugimoto et al. 2014; Meszaros et al. 2020). Focused interventions around the households of medically attended patients with cholera represent an efficient way of interrupting transmission (Ratnayake et al. 2020). Specifically, approaches that include WASH interventions have been shown to reduce the duration of outbreaks at a community scale in Haiti (Michel et al. 2019). Furthermore, mathematical models of cholera that incorporate transmission within and between households show that variation in the magnitude of household transmission changes multiple features of disease dynamics, including the severity and duration of outbreaks (Meszaros et al. 2020). Importantly, integrating household transmission into cholera models influences the effectiveness of possible public health interventions (e.g. water treatment, antibiotics, OCVs) indicating vaccine interventions are more effective than water treatment or antibiotic administration when direct household transmission is present.
Approximately 1.6 billion people in the world live without safe water at home and 2.8 billion people without safe sanitation. Major infrastructure improvements, including piped water and sewage systems, are needed in order to achieve potential elimination of cholera as it was previously achieved in parts of Latin America and Europe (Balasubramanian et al. 2021). Nonetheless, while these are implemented, there are several smaller-scale WASH interventions that can be used to reduce cholera risk. For instance, safe storage of water systems and point-of-use water treatment, provision of sanitation facilities and campaigns targeted at increasing handwashing and other sanitary practices (Kanungo et al. 2022; Balasubramanian et al. 2021; Islam et al. 2022). These smaller-scale interventions can lead to sustainable reductions in cholera incidence and will ease the implementation of longer term ones that will lead to the control and eventual demise of this scourge.
1.5 Emergence of Novel Pathogenic Variants: Vibrio vulnificus and Aquaculture
Aquaculture is one of the fastest-growing global food industries, accounting for more than 50% of the world’s fish supply. Most of this development has occurred in the past 50 years and is projected to rise significantly to meet the accelerating demand for seafood (Ahmad et al. 2021; Botta et al. 2020). However, the environmental implications of such a dramatic increase are far-reaching as the expansion of this industry has led to reduced land availability, nutrient over-enrichment, release of toxic chemicals into the ecosystem, and threats to the food chain (Ahmad et al. 2021; Botta et al. 2020). Moreover, the excessive use of antibiotics to control infections in fish farms has majorly influenced the occurrence and spread of antimicrobial resistance among many marine bacterial species (Elmahdi et al. 2016; Ibrahim et al. 2020). Heavy reliance on antibiotics, over-intensive exploitation of aquaculture, and unrestricted industrialized practices have ultimately contributed to the emergence of several aquaculture-associated diseases (Sanches-Fernandes et al. 2022; Sony et al. 2021; Deng et al. 2020).
Vibriosis is one of the most prevalent bacterial diseases affecting a diverse array of marine organisms (Sony et al. 2021; Chatterjee and Haldar 2012). The economic losses associated with diseases in aquaculture were estimated to have been over $3 billion per year by 1997 and have nearly tripled in the last two decades to over $9 billion per year (Sanches-Fernandes et al. 2022; Chatterjee and Haldar 2012; Novriadi 2016). Several members of the family Vibrionaceae, including V. vulnificus, V. parahaemolyticus, V. harveyi, V. alginolyticus, and V. anguillarum, have been linked to vibriosis in marine species (Deng et al. 2020; Chatterjee and Haldar 2012). For instance, over two-third of disease cases reported in the Epinephelus spp. of fish are due to V. parahaemolyticus and V. anguillarum infections (Deng et al. 2020). V. alginolyticus and V. harveyi infections in China, the largest aquaculture market in the world, exhibit mortality rates as high as 80% (Deng et al. 2020). V. vulnificus has been associated with drastic mortality rates in aquaculture-raised marine species including Anguilla spp., tilapia, and shrimp (Amaro et al. 2015; Rippey 1994; Fouz and Amaro 2003; Mahmud et al. 2010; Chen et al. 2006; Longyant et al. 2008). Overall, vibriosis has led to a significant decline in fish health and production globally, posing a significant threat to the aquaculture industry.
V. vulnificus, one of the most frequently isolated Vibrio spp. from diseased seafood, is also the leading cause of non-Cholera, Vibrio-associated infections in humans (Phillips and Satchell 2017; Jones and Oliver 2009; Cabanyero and Amaro 2020). The annual case counts of V. vulnificus infections in humans have steadily increased over the past 20 years in the USA (Phillips and Satchell 2017), over 75% of which occur during summer (Wright et al. 1996; Givens et al. 2014). This high incidence rate strongly coincides with increased prevalence of V. vulnificus in estuarine environments corresponding to the high sea surface temperatures (>20 °C) and low-to-moderate salinities (5–25 ppt) encountered during that season (Wright et al. 1996; Givens et al. 2014; Levine et al. 1993; Bisharat et al. 1999; Tilton and Ryan 1987). Recent reports further demonstrate an upsurge in the worldwide distribution and abundance of V. vulnificus in correlation with increasing sea surface temperature and climate change (Paz et al. 2007; Baker-Austin et al. 2017; Kaspar and Tamplin 1993). This has led to disease outbreaks in regions with no prior history of V. vulnificus infections (Paz et al. 2007; Baker-Austin et al. 2017; Kaspar and Tamplin 1993). Furthermore, recent studies underline a strikingly high diversity and recombination rates in V. vulnificus populations (Fig. 1.2) (López-Pérez et al. 2019, 2021). This is particularly worrisome as practices such as aquaculture can lead to the emergence of hybrid strains (Fig. 1.2). The most prominent example of this is the V. vulnificus outbreak in Israel stemming from a novel hybrid clade. Between the years 1996–1997, 62 cases of wound infection and bacteremia were recorded in Israel, the majority of which occurred during the summer months of Aug-Oct (Bisharat et al. 1999). Interestingly, all 62 patients reported contact with aquaculture-reared tilapia fish. Molecular typing and phenotypic characterizations showed that the causative agent was a new bio-group of V. vulnificus, Biotype 3 (BT3) (Bisharat et al. 1999; Zaidenstein et al. 2008). All cases reported in this period were caused by BT3 strains associated entirely with tilapia or carp aquaculture. Typing and molecular evolutionary analyses show that members of the new BT3 are hybrid organisms evolved through the acquisition of genes from two distinct and independent populations, BT1 and BT2 (Bisharat et al. 2005). Although BT3 strains exhibit a high degree of genetic homogeneity, they are distinct from BT1 and BT2. For the first time, it was evidenced that close contact between two distinct populations led to the emergence of an infectious outbreak caused by a new pathogenic variant.
Evolutionary model of cluster divergence in V. vulnificus. (a) VVCA. Clonal lineages start diverging from the V. vulnificus common ancestor (VVCA). (b) Divergence. The acquisition of different ecological determinants allowed the development of diverse lifestyles within the same environment, which has led to a higher divergence. This divergence led to a recombination and gene flow decrease, although frequent exchange of mobile genetic elements is found within the species and with other species. (c) Convergence. With the advent of aquaculture, we have created an artificial environment that has led to colocalization of strains from the two major clusters. Adapted from M. López-Pérez et al., mBio, 2019, 10:e02852-18
Prior to the 1996 outbreak, no cases of V. vulnificus human infections were reported in Israel. However, a single strain of halophilic bacteria that caused wound infection in a male patient after handling fish was reported in 1981, which proved to be genetically identical to the BT3 strains isolated after the 1996 outbreak (Paz et al. 2007). This suggested that the pathogen has been circulating within these water reservoirs long before the disease outbreak in 1996. Investigations assessing changing trends of V. vulnificus infections in Israel have reported patterns of increasing disease severity with rising sea surface temperatures, with more than 55% of cases occurring in patients with no known underlying diseases (Zaidenstein et al. 2008). Rising water temperatures fueled by climate change in the area could have increased prevalence of V. vulnificus populations over time, ultimately leading to the emergence of the disease outbreak in 1996 (Paz et al. 2007). Overall, given the distinctively high genome plasticity of this pathogen paired with the unexpected outcomes associated with manmade environmental changes and practices such as aquaculture, makes V. vulnificus a major threat to human health for which no effective therapeutic or surveillance strategies are available. The emergence of highly pathogenic hybrid variants of other Vibrio spp. could be a clamoring hazard in the coming decades that, as highlighted below, can only be exacerbated by the effects of climate change.
1.6 Vibrio Population Dynamics and Climate Change: The Vibrio parahaemolyticus Paradigm
To date, our understanding of the actual impacts that climate change has on Vibrios at the population and evolutionary level is still limited. For example it remains to be determined whether the colonization of new geographical areas is introducing any change on the effective population size of Vibrio populations. It is also possible that this expansion is the result of the dispersal of certain genetic variants that are adaptively successful in colonizing new areas, increasing the population census but with no effects on the effective population size. It remains to be addressed whether any recent event in the planet linked to human activity or initiated by natural causes facilitated the restructuring of Vibrio populations with the consequent effects on the demography and evolution of these populations. A major limiting factor to address these questions is our inadequate knowledge on the demographic oscillations and evolutionary histories of Vibrio populations. Vibrio species are characterized by a high genetic diversity, a highly variable genome rich in accessory genes, one of the highest recombination rates among all bacteria, and poorly structured populations as a consequence of their unique evolutionary dynamics (Roux and Blokesch 2018; Yang et al. 2019a). The high genetic diversity and large pangenomes (the entire set of genes from all strains) are partly defined by their complex lifestyle. Their presence in habitats with very different conditions (e.g. seawater, plankton microbiome, human gut) requires a very large and diverse genetic repertoire to adapt effectively to these diverse environments and survive under highly variable conditions (Vázquez-Rosas-Landa et al. 2020).
Similar to other free-living organisms, Vibrios are characterized by their large pangenomes and effective population size, which typically correlates with the efficacy of natural selection. In particular, V. parahaemolyticus has an effective population size greater than 108, which ranks it among the largest among all bacteria. This species also exhibits high recombination rates which progressively erase non-random associations between markers (linkage disequilibrium) and result in a less structured population in a near state of panmixia (in opposition to clonality characterized by very little or no genetic diversity among isolates) (Smith et al. 1993; Shapiro 2016; Yang et al. 2019b; Cui et al. 2020). The large availability of complete genomes of V. parahaemolyticus from global populations has enabled us to identify signals of the potential impacts of human activity on changes in demography or population structure of pathogenic Vibrios. The analysis of 1103 genomes revealed that the diversity patterns of V. parahaemolyticus populations are consistent with having arisen by progressive divergence through genetic drift during geographic isolation over most of its evolutionary history (Yang et al. 2019a). However, these analyses show that the genetic barriers keeping these populations isolated have been recently eroded by human-related activities or natural events that have enabled long-distance dispersals of local variants (Yang et al. 2019a). This dispersion has contributed to the introduction of new genetic variants in remote areas and the genetic exchange and overlap between different populations, consolidating a change in the biogeographical distribution of V. parahaemolyticus. Analyses based on time-calibrated divergence trees estimate that the processes of genetic mixing between the different populations occurred as recent as the past decades (Yang et al. 2019a).
Taken together these results indicate that human activity and/or recent profound ecological changes are responsible for the shift in the global distribution pattern of V. parahaemolyticus populations. Clearly certain human activities such as shipping, the global market of aquaculture products, or the increased migratory flows between continents may have contributed total or partially to the observed changes in these populations. All these activities have been intensified during the last decades and have originated a flow of water masses and living organisms from one continent to another. But natural causes, such as changes in plankton distribution patterns or ocean currents may also contribute to intensify long-distance migrations (Frémont et al. 2022). Climate change is restructuring the biogeography of plankton communities in the oceans at all scales, from viruses to mesozooplankton, and ocean currents are accelerating in response to warming (Richter et al. 2022). These complex and globally interconnected processes may be influencing a shift in the distribution of Vibrio populations given their planktonic nature and their connection to migratory process of other marine organisms. In the future, it is essential to introduce improvements into population analyses with the use of a more comprehensive collection of genomes and community structures (e.g. metagenomes) covering understudied areas of the world in the existing repositories. Furthermore, the development of novel sets of tools to analyze bacterial populations is essential to have a more robust inference of the basic parameters of these population genetics. Another key area of research will be the study of the biological dynamics of Vibrio in offshore waters, including oceans, to explore the possible existence of cross-oceanic migrations. Oceanic biological corridors, similar to those that exist for other species of plankton or fish, would break the genetic isolation and contribute to the dispersal of Vibrio populations, including those with pathogenic potential, with major consequences towards the global burden of Vibrio diseases.
References
Ahmad A, Abdullah SRS, Hasan HA, Othman AR, Ismail NI (2021) Aquaculture industry: supply and demand, best practices, effluent and its current issues and treatment technology. J Environ Manag 287:112271
Aiyar SE, Gaal T, Gourse RL (2002) rRNA promoter activity in the fast-growing bacterium Vibrio natriegens. J Bacteriol 184:1349–1358
Almagro-Moreno S, Taylor RK (2013) Cholera: environmental reservoirs and impact on disease transmission. Microbiol Spectr 1:149–165
Amaro C, Sanjuán E, Fouz B, Pajuelo D, Lee C-T, Hor L-I, Barrera R (2015) The fish pathogen Vibrio vulnificus biotype 2: epidemiology, phylogeny, and virulence factors involved in warm-water vibriosis. Microbiol Spectr 3
Austin CB, Pruzzo C, Oliver JD, Garzón DD (2020) Vibrios—from genes to ecosystems. Environ Microbiol 22:4093–4095
Baik YO, Choi SK, Olveda RM, Espos RA, Ligsay AD, Montellano MB, Yeam JS, Yang JS, Park JY, Kim DR, Desai SN, Singh AP, Kim IY, Kim CW, Park S (2015) A randomized, non-inferiority trial comparing two bivalent killed, whole cell, oral cholera vaccines (Euvichol vs Shanchol) in the Philippines. Vaccine 33:6360–6365
Baker-Austin C, Trinanes JA, Taylor NGH, Hartnell R, Siitonen A, Martinez-Urtaza J (2013) Emerging Vibrio risk at high latitudes in response to ocean warming. Nat Clim Change 3:73–77
Baker-Austin C, Trinanes JA, Salmenlinna S, Löfdahl M, Siitonen A, Taylor NGH, Martinez-Urtaza J (2016) Heat wave-associated vibriosis, Sweden and Finland, 2014. Emerg Infect Dis 22:1216–1220
Baker-Austin C, Trinanes J, Gonzalez-Escalona N, Martinez-Urtaza J (2017) Non-cholera Vibrios: the microbial barometer of climate change. Trends Microbiol 25:76–84
Baker-Austin C, Oliver JD, Alam M, Ali A, Waldor MK, Qadri F, Martinez-Urtaza J (2018) Vibrio spp. infections. Nat Rev Dis Primers 4:8
Balasubramanian D, Murcia S, Ogbunugafor CB, Gavilan R, Almagro-Moreno S (2021) Cholera dynamics: lessons from an epidemic. J Med Microbiol 20:106
Bhattacharya SK, Sur D, Ali M, Kanungo S, You YA, Manna B, Sah B, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Dhingra MS, Donner A, Nair GB, Lopez AL, Wierzba TF, Clemens JD (2013) 5 year efficacy of a bivalent killed whole-cell oral cholera vaccine in Kolkata, India: a cluster-randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 13:1050–1056
Bi Q, Ferreras E, Pezzoli L, Legros D, Ivers LC, Date K, Qadri F, Digilio L, Sack DA, Ali M, Lessler J, Luquero FJ, Azman AS, Oral Cholera Vaccine Working Group of The Global Task Force on Cholera Control, Cavailler P, Date K, Sreenivasan N, Matzger H, Luquero F, Grais R, Wiesner L, Ko M, Rouzier V, Peak C, Qadri F, Landegger J, Lynch J, Azman A, Sack D, Henkens M, Ciglenecki I, Ivers L, Diggle E, Weiss M, Hinman A, Maina K, Mirza I, Gimeno G, Levine M (2017) Protection against cholera from killed whole-cell oral cholera vaccines: a systematic review and meta-analysis. Lancet Infect Dis 17:1080–1088
Bisharat N, Agmon V, Finkelstein R, Raz R, Ben-Dror G, Lerner L, Soboh S, Colodner R, Cameron DN, Wykstra DL, Swerdlow DL, Farmer JJ (1999) Clinical, epidemiological, and microbiological features of Vibrio vulnificus biogroup 3 causing outbreaks of wound infection and bacteraemia in Israel. Lancet 354:1421–1424
Bisharat N, Cohen DI, Harding RM, Falush D, Crook DW, Peto T, Maiden MC (2005) Hybrid Vibrio vulnificus. Emerg Infect Dis 11(1):30–35
Botta R, Asche F, Borsum JS, Camp EV (2020) A review of global oyster aquaculture production and consumption. Mar Policy 117:103952
Boucher Y (2016) Sustained local diversity of Vibrio cholerae O1 biotypes in a previously cholera-Free country. MBio 7:e00570-16
Broberg CA, Calder TJ, Orth K (2011) Vibrio parahaemolyticus cell biology and pathogenicity determinants. Microbes Infect 13:992–1001
Broza M, Halpern M (2001) Pathogen reservoirs. Chironomid egg masses and Vibrio cholerae. Nature 412:40
Cabanyero CH, Amaro C (2020) Phylogeny and life cycle of the zoonotic pathogen Vibrio vulnificus. Environ Microbiol 22:4133–4148
Chatterjee S, Haldar S (2012) Vibrio related diseases in aquaculture and development of rapid and accurate identification methods. J Mar Sci Res Dev. https://doi.org/10.4172/2155-9910.S1-002
Chen C, Chao C, Bowser PR (2006) Infection of Tilapia oreochromis sp. by Vibrio vulnificus in freshwater and low-salinity environments. J World Aquacult Soc 37:82–88
Chowdhury G, Senapati T, Das B, Kamath A, Pal D, Bose P, Deb A, Paul S, Mukhopadhyay AK, Dutta S, Ramamurthy T (2021) Laboratory evaluation of the rapid diagnostic tests for the detection of Vibrio cholerae O1 using diarrheal samples. PLoS Neglect Trop D 15:e0009521
Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, Haley BJ, Taviani E, Jeon Y-S, Kim DW, Lee J-H, Brettin TS, Bruce DC, Challacombe JF, Detter JC, Han CS, Munk AC, Chertkov O, Meincke L, Saunders E, Walters RA, Huq A, Nair GB, Colwell RR (2009) Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc Natl Acad Sci USA 106:15442–15447
Clemens JD, Harris JR, Sack DA, Chakraborty J, Ahmed F, Stanton BF, Khan MU, Kay BA, Huda N, Khan MR, Yunus M, Rao MR, Svennerholm A-M, Holmgren J (1988) Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J Infect Dis 158:60–69
Clemens JD, Nair GB, Ahmed T, Qadri F, Holmgren J (2017) Cholera. Lancet 390:1539–1549
Connolly MA, Gayer M, Ryan MJ, Salama P, Spiegel P, Heymann DL (2004) Communicable diseases in complex emergencies: impact and challenges. Lancet 364:1974–1983
Cui Y, Yang C, Qiu H, Wang H, Yang R, Falush D (2020) The landscape of coadaptation in Vibrio parahaemolyticus. Elife 9:e54136
Davies HG, Bowman C, Luby SP (2017) Cholera—management and prevention. J Infect 74:S66–S73
de Magny GC, Mozumder PK, Grim CJ, Hasan NA, Naser MN, Alam M, Sack RB, Huq A, Colwell RR (2011) Role of zooplankton diversity in Vibrio cholerae population dynamics and in the incidence of cholera in the Bangladesh Sundarbans. Appl Environ Microbiol 77:6125–6132
Deng Y, Xu L, Chen H, Liu S, Guo Z, Cheng C, Ma H, Feng J (2020) Prevalence, virulence genes, and antimicrobial resistance of Vibrio species isolated from diseased marine fish in South China. Sci Rep-UK 10:14329
Deshayes S, Daurel C, Cattoir V, Parienti J-J, Quilici M-L, Blanchardière ADL (2015) Non-O1, non-O139 Vibrio cholerae bacteraemia: case report and literature review. Springerplus 4:575
de Sousa OV, RHSDF V, de Menezes FGR, dos Reis CMF, Hofer E (2004) Detection of Vibrio parahaemolyticus and Vibrio cholerae in oyster, Crassostrea rhizophorae, collected from a natural nursery in the Cocó river estuary, Fortaleza, Ceará, Brazil. Rev Inst Med Trop Sao Paulo 46:59–62
D’Mello-Guyett L, Gallandat K, den Bergh RV, Taylor D, Bulit G, Legros D, Maes P, Checchi F, Cumming O (2020) Prevention and control of cholera with household and community water, sanitation and hygiene (WASH) interventions: a scoping review of current international guidelines. PLoS One 15:e0226549
Dutheil C, Meier HEM, Gröger M, Börgel F (2022) Understanding past and future sea surface temperature trends in the Baltic Sea. Climate Dynam 58:3021–3039
Editorial (2017) Health catastrophe: the toll of cholera in Yemen. Lancet Gastroenterol Hepatol 2:619
Elmahdi S, DaSilva LV, Parveen S (2016) Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: a review. Food Microbiol 57:128–134
Epstein PR (1993) Algal blooms in the spread and persistence of cholera. Biosystems 31:209–221
Fouz B, Amaro C (2003) Isolation of a new serovar of Vibrio vulnificus pathogenic for eels cultured in freshwater farms. Aquaculture 217:677–682
Francois J (2020) Cholera remains a public health threat in Haiti. Lancet Glob Health 8:e984
Frémont P, Gehlen M, Vrac M, Leconte J, Delmont TO, Wincker P, Iudicone D, Jaillon O (2022) Restructuring of plankton genomic biogeography in the surface ocean under climate change. Nat Clim Change 12:393–401
Givens CE, Bowers JC, DePaola A, Hollibaugh JT, Jones JL (2014) Occurrence and distribution of Vibrio vulnificus and Vibrio parahaemolyticus—potential roles for fish, oyster, sediment and water. Lett Appl Microbiol 58:503–510
Grant T-A, Balasubramanian D, Almagro-Moreno S (2021) Vibrio cholerae: an opportunist of human crises. J Med Microbiol 70
Greenfield DI, Moore JG, Stewart JR, Hilborn ED, George BJ, Li Q, Dickerson J, Keppler CK, Sandifer PA (2017) Temporal and environmental factors driving Vibrio vulnificus and V. parahaemolyticus populations and their associations with harmful algal blooms in South Carolina detention ponds and receiving tidal creeks. GeoHealth 1:306–317
Halpern M, Senderovich Y, Izhaki I (2008) Waterfowl: the missing link in epidemic and pandemic cholera dissemination? PLoS Pathog 4:e1000173
Hood MA, Ness GE, Rodrick GE (1981) Isolation of Vibrio cholerae serotype O1 from the eastern oyster, Crassostrea virginica. Appl Environ Microbiol 41:559–560
Huq A, Small EB, West PA, Huq MI, Rahman R, Colwell RR (1983) Ecological relationships between Vibrio cholerae and planktonic crustacean copepods. Appl Environ Microbiol 45:275–283
Huq A, West PA, Small EB, Huq MI, Colwell RR (1984) Influence of water temperature, salinity, and pH on survival and growth of toxigenic Vibrio cholerae serovar 01 associated with live copepods in laboratory microcosms. Appl Environ Microbiol 48:420–424
Huq A, Hasan N, Akanda A, Whitcombe E, Colwell R, Haley B, Alam M, Jutla A, Sack RB (2013) Environmental factors influencing epidemic cholera. Am J Trop Med Hyg 89:597–607
Ibrahim M, Ahmad F, Yaqub B, Ramzan A, Imran A, Afzaal M, Mirza SA, Mazhar I, Younus M, Akram Q, Taseer MSA, Ahmad A, Ahmed S (2020) Antibiotics and antimicrobial resistance genes in the environment. Antibiotics Antimicrob Resist Genes Environ 39–69
Islam MT, Ross AG, Sleigh AC, Chowdhury F, Khan AI, McMillan NA, Qadri F (2022) A blueprint for eliminating cholera by 2030. Nat Med 1–3
Janda JM, Newton AE, Bopp CA (2015) Vibriosis. Clin Lab Med 35:273–288
Jayakumar JM, Balasubramanian D, Reddi G, Almagro-Moreno S (2020) Synergistic role of abiotic factors driving viable but non-culturable Vibrio cholerae. Environ Microbiol Rep 12:454–465
Jones MK, Oliver JD (2009) Vibrio vulnificus: disease and pathogenesis. Infect Immun 77:1723–1733
Joseph SW, Colwell RR, Kaper JB (2008) Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol 10:77–124
Kanungo S, Azman AS, Ramamurthy T, Deen J, Dutta S (2022) Cholera. Lancet 399:1429–1440
Karmalkar AV, Horton RM (2021) Drivers of exceptional coastal warming in the northeastern United States. Nat Clim Change 11:854–860
Kaspar CW, Tamplin ML (1993) Effects of temperature and salinity on the survival of Vibrio vulnificus in seawater and shellfish. Appl Environ Microbiol 59:2425–2429
Lancet T (2017) Yemen and cholera: a modern humanity test. Lancet 390:626
Letchumanan V, Chan K-G, Lee L-H (2014) Vibrio parahaemolyticus: a review on the pathogenesis, prevalence, and advance molecular identification techniques. Front Microbiol 5:376
Levine WC, Griffin PM, Gulf Coast Vibrio Working Group (1993) Vibrio infections on the Gulf Coast: results of first year of regional surveillance. J Infect Dis 167:479–483
Lima FP, Wethey DS (2012) Three decades of high-resolution coastal sea surface temperatures reveal more than warming. Nat Commun 3:704
Llovel W, Purkey S, Meyssignac B, Blazquez A, Kolodziejczyk N, Bamber J (2019) Global ocean freshening, ocean mass increase and global mean sea level rise over 2005–2015. Sci Rep-UK 9:17717
Lobitz B, Beck L, Huq A, Wood B, Fuchs G, Faruque AS, Colwell R (2000) Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc Natl Acad Sci U S A 97:1438–1443
Longyant S, Rukpratanporn S, Chaivisuthangkura P, Suksawad P, Srisuk C, Sithigorngul W, Piyatiratitivorakul S, Sithigorngul P (2008) Identification of Vibrio spp. in vibriosis Penaeus vannamei using developed monoclonal antibodies. J Invertebr Pathol 98:63–68
López-Pérez M, Jayakumar JM, Haro-Moreno JM, Zaragoza-Solas A, Reddi G, Rodriguez-Valera F, Shapiro OH, Alam M, Almagro-Moreno S (2019) Evolutionary model of cluster divergence of the emergent marine pathogen Vibrio vulnificus: from genotype to ecotype. MBio 10:697
López-Pérez M, Jayakumar JM, Grant T-A, Zaragoza-Solas A, Cabello-Yeves PJ, Almagro-Moreno S (2021) Ecological diversification reveals routes of pathogen emergence in endemic Vibrio vulnificus populations. Proc Natl Acad Sci USA 118:e2103470118
Lutz C, Erken M, Noorian P, Sun S, McDougald D (2013) Environmental reservoirs and mechanisms of persistence of Vibrio cholerae. Front Microbiol 4:375
Mahmud ZH, Wright AC, Mandal SC, Dai J, Jones MK, Hasan M, Rashid MH, Islam MS, Johnson JA, Gulig PA, Morris JG, Ali A (2010) Genetic characterization of Vibrio vulnificus strains from Tilapia aquaculture in Bangladesh. Appl Environ Microbiol 76:4890–4895
Martinez-Urtaza J, Baker-Austin C (2020) Vibrio parahaemolyticus. Trends Microbiol 28:867–868
Martinez-Urtaza J, Baker-Austin C, Jones JL, Newton AE, Gonzalez-Aviles GD, DePaola A (2013) Spread of Pacific Northwest Vibrio parahaemolyticus strain. N Engl J Med 369:1573–1574
Messelhäusser U, Colditz J, Thärigen D, Kleih W, Höller C, Busch U (2010) Detection and differentiation of Vibrio spp. in seafood and fish samples with cultural and molecular methods. Int J Food Microbiol 142:360–364
Meszaros VA, Miller-Dickson MD, Junior FB-A, Almagro-Moreno S, Ogbunugafor CB (2020) Direct transmission via households informs models of disease and intervention dynamics in cholera. PLoS One 15:e0229837
Michel E, Gaudart J, Beaulieu S, Bulit G, Piarroux M, Boncy J, Dely P, Piarroux R, Rebaudet S (2019) Estimating effectiveness of case-area targeted response interventions against cholera in Haiti. Elife 8:e50243
Newton A, Kendall M, Vugia DJ, Henao OL, Mahon BE (2012) Increasing rates of vibriosis in the United States, 1996–2010: review of surveillance data from 2 systems. Clin Infect Dis 54:S391–S395
Novoslavskij A, Terentjeva M, Eizenberga I, Valciņa O, Bartkevičs V, Bērziņš A (2015) Major foodborne pathogens in fish and fish products: a review. Ann Microbiol 66:1–15
Novriadi R (2016) Vibriosis in aquaculture. Omni-akuatika 12
Oliver JD (2012) Vibrio vulnificus: death on the half shell: a personal journey with the pathogen and its ecology. Microb Ecol 65:793–799
Oliver JD (2015) The biology of Vibrio vulnificus. Microbiol Spectr 3
Paz S, Bisharat N, Paz E, Kidar O, Cohen D (2007) Climate change and the emergence of Vibrio vulnificus disease in Israel. Environ Res 103:390–396
Phillips KE, Satchell KJF (2017) Vibrio vulnificus: from oyster colonist to human pathogen. PLoS Pathog 13:e1006053
Pietroni MAC (2020) Case management of cholera. Vaccine 38:A105–A109
Purdy AE, Watnick PI (2011) Spatially selective colonization of the arthropod intestine through activation of Vibrio cholerae biofilm formation. Proc Natl Acad Sci U S A 108:19737–19742
Qadri F, Islam T, Clemens JD (2017) Cholera in Yemen—an old foe rearing its ugly head. N Engl J Med 377:2005–2007
Ratnayake R, Finger F, Azman AS, Lantagne D, Funk S, Edmunds WJ, Checchi F (2020) Highly targeted spatiotemporal interventions against cholera epidemics, 2000–19: a scoping review. Lancet Infect Dis 21:e37–e48
Reddi G, Pruss K, Taylor RK, Cottingham KL, Almagro-Moreno S (2018) Catabolism of mucus components influences motility of Vibrio cholerae in the presence of environmental reservoirs. PLoS One 13:e0201383
Reen FJ, Almagro-Moreno S, Ussery D, Boyd EF (2006) The genomic code: inferring Vibrionaceae niche specialization. Nat Rev Microbiol 4:697–704
Rice KC, Jastram JD (2014) Rising air and stream-water temperatures in Chesapeake Bay region, USA. Clim Change 128:127–138
Richter DJ, Watteaux R, Vannier T, Leconte J, Frémont P, Reygondeau G, Maillet N, Henry N, Benoit G, Silva OD, Delmont TO, Fernàndez-Guerra A, Suweis S, Narci R, Berney C, Eveillard D, Gavory F, Guidi L, Labadie K, Mahieu E, Poulain J, Romac S, Roux S, Dimier C, Kandels S, Picheral M, Searson S, Acinas SG, Bork P, Boss E, Bowler C, Cochrane G, de Vargas C, Gorsky G, Grimsley N, Guidi L, Hingamp P, Iudicone D, Jaillon O, Kandels S, Karp-Boss L, Karsenti E, Not F, Ogata H, Pesant S, Raes J, Sardet C, Sieracki M, Speich S, Stemmann L, Sullivan MB, Sunagawa S, Wincker P, Pesant S, Aury J-M, Brum JR, Lemaitre C, Pelletier E, Bork P, Sunagawa S, Lombard F, Karp-Boss L, Bowler C, Sullivan MB, Karsenti E, Mariadassou M, Probert I, Peterlongo P, Wincker P, de Vargas C, d’Alcalà MR, Iudicone D, Jaillon O (2022) Genomic evidence for global ocean plankton biogeography shaped by large-scale current systems. Elife 11:e78129
Rippey SR (1994) Infectious diseases associated with molluscan shellfish consumption. Clin Microbiol Rev 7:419–425
Roig FJ, González-Candelas F, Sanjuán E, Fouz B, Feil EJ, Llorens C, Baker-Austin C, Oliver JD, Danin-Poleg Y, Gibas CJ, Kashi Y, Gulig PA, Morrison SS, Amaro C (2018) Phylogeny of Vibrio vulnificus from the analysis of the core-genome: implications for intra-species taxonomy. Front Microbiol 8:716
Roux FL, Blokesch M (2018) Eco-evolutionary dynamics linked to horizontal gene transfer in Vibrios. Annu Rev Microbiol 72:1–22
Sakib SN, Reddi G, Almagro-Moreno S (2018) Environmental role of pathogenic traits in Vibrio cholerae. J Bacteriol 200:2123
Sanches-Fernandes GMM, Sá-Correia I, Costa R (2022) Vibriosis outbreaks in aquaculture: addressing environmental and public health concerns and preventive therapies using gilthead seabream farming as a model system. Front Microbiol 13:904815
Semenza JC, Trinanes J, Lohr W, Sudre B, Löfdahl M, Martinez-Urtaza J, Nichols GL, Rocklöv J (2017) Environmental suitability of Vibrio infections in a warming climate: an early warning system. Environ Health Perspect 125:107004
Senderovich Y, Izhaki I, Halpern M (2010) Fish as reservoirs and vectors of Vibrio cholerae. PLoS One 5:e8607
Shapiro BJ (2016) How clonal are bacteria over time? Curr Opin Microbiol 31:116–123
Shapiro BJ, Levade I, Kovacikova G, Taylor RK, Almagro-Moreno S (2016) Origins of pandemic Vibrio cholerae from environmental gene pools. Nat Microbiol 2:16240
Smith JM, Smith NH, O’Rourke M, Spratt BG (1993) How clonal are bacteria? Proc Natl Acad Sci USA 90:4384–4388
Sony M, Sumithra TG, Anusree VN, Amala PV, Reshma KJ, Alex S, Sanil NK (2021) Antimicrobial resistance and virulence characteristics of Vibrio vulnificus, Vibrio parahaemolyticus and Vibrio harveyi from natural disease outbreaks of marine/estuarine fishes. Aquaculture 539:736608
Sousa FBM, Nolêto IRSG, Chaves LS, Pacheco G, Oliveira AP, Fonseca MMV, Medeiros JVR (2020) A comprehensive review of therapeutic approaches available for the treatment of cholera. J Pharm Pharmacol 72:1715–1731
Sugimoto JD, Koepke AA, Kenah EE, Halloran ME, Chowdhury F, Khan AI, LaRocque RC, Yang Y, Ryan ET, Qadri F, Calderwood SB, Harris JB Jr, IML. (2014) Household Transmission of Vibrio cholerae in Bangladesh. PLoS Negl Trop Dis 8:e3314
Sur D, Lopez AL, Kanungo S, Paisley A, Manna B, Ali M, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Van NT, Donner A, Ganguly NK, Nair GB, Bhattacharya SK, Clemens JD (2009) Efficacy and safety of a modified killed-whole-cell oral cholera vaccine in India: an interim analysis of a cluster-randomised, double-blind, placebo-controlled trial. Lancet 374:1694–1702
Sur D, Kanungo S, Sah B, Manna B, Ali M, Paisley AM, Niyogi SK, Park JK, Sarkar B, Puri MK, Kim DR, Deen JL, Holmgren J, Carbis R, Rao R, Van NT, Han SH, Attridge S, Donner A, Ganguly NK, Bhattacharya SK, Nair GB, Clemens JD, Lopez AL (2011) Efficacy of a low-cost, inactivated whole-cell oral cholera vaccine: results from 3 years of follow-up of a randomized, controlled trial. PLoS Neglect Trop D 5:e1289
Tamplin ML, Gauzens AL, Huq A, Sack DA, Colwell RR (1990) Attachment of Vibrio cholerae serogroup O1 to zooplankton and phytoplankton of Bangladesh waters. Appl Environ Microbiol 56:1977–1980
Tilton RC, Ryan RW (1987) Clinical and ecological characteristics of Vibrio vulnificus in the Northeastern United States. Diagn Microbiol Infect Dis 6:109–117
Trinanes J, Martinez-Urtaza J (2021) Future scenarios of risk of Vibrio infections in a warming planet: a global mapping study. Lancet Planet Heal 5:e426–e435
Turner JW, Malayil L, Guadagnoli D, Cole D, Lipp EK (2014) Detection of Vibrio parahaemolyticus, Vibrio vulnificus and Vibrio cholerae with respect to seasonal fluctuations in temperature and plankton abundance. Environ Microbiol 16:1019–1028
Twedt RM, Madden JM, Hunt JM, Francis DW, Peeler JT, Duran AP, Hebert WO, McCay SG, Roderick CN, Spite GT, Wazenski TJ (1981) Characterization of Vibrio cholerae isolated from oysters. Appl Environ Microbiol 41:1475–1478
Vázquez-Rosas-Landa M, Ponce-Soto GY, Aguirre-Liguori JA, Thakur S, Scheinvar E, Barrera-Redondo J, Ibarra-Laclette E, Guttman DS, Eguiarte LE, Souza V (2020) Population genomics of Vibrionaceae isolated from an endangered oasis reveals local adaptation after an environmental perturbation. BMC Genomics 21:418
Vezzulli L, Grande C, Reid PC, Hélaouët P, Edwards M, Höfle MG, Brettar I, Colwell RR, Pruzzo C (2016) Climate influence on Vibrio and associated human diseases during the past half-century in the coastal North Atlantic. Proc Natl Acad Sci USA 113:E5062-71
Wright AC, Hill RT, Johnson JA, Roghman MC, Colwell RR, Morris JG (1996) Distribution of Vibrio vulnificus in the Chesapeake Bay. Appl Environ Microbiol 62:717–724
Yang C, Pei X, Wu Y, Yan L, Yan Y, Song Y, Coyle NM, Martinez-Urtaza J, Quince C, Hu Q, Jiang M, Feil E, Yang D, Song Y, Zhou D, Yang R, Falush D, Cui Y (2019a) Recent mixing of Vibrio parahaemolyticus populations. ISME J 13:2578–2588
Yang C, Cui Y, Didelot X, Yang R, Falush D (2019b) Why panmictic bacteria are rare. BioRxiv. https://doi.org/10.1101/385336
Zaidenstein R, Sadik C, Lerner L, Valinsky L, Kopelowitz J, Yishai R, Agmon V, Parsons M, Bopp C, Weinberger M (2008) Clinical characteristics and molecular subtyping of Vibrio vulnificus illnesses, Israel. Emerg Infect Dis 14:1875–1882
Zanna L, Khatiwala S, Gregory JM, Ison J, Heimbach P (2019) Global reconstruction of historical ocean heat storage and transport. Proc Natl Acad Sci USA 116:1126–1131
Zhang L, Orth K (2013) Virulence determinants for Vibrio parahaemolyticus infection. Curr Opin Microbiol 16:70–77
Acknowledgements
This work was supported by a National Science Foundation CAREER award (#2045671) and a Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease award (#1021977) to S.A.M.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Almagro-Moreno, S., Martinez-Urtaza, J., Pukatzki, S. (2023). Vibrio Infections and the Twenty-First Century. In: Almagro-Moreno, S., Pukatzki, S. (eds) Vibrio spp. Infections. Advances in Experimental Medicine and Biology, vol 1404. Springer, Cham. https://doi.org/10.1007/978-3-031-22997-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-031-22997-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22996-1
Online ISBN: 978-3-031-22997-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)