Abstract
Nd-Fe-B magnets find wide range of applications due to its high magnetic properties. At its end-of-life, huge quantity of scrap Nd-Fe-B magnets are generated, which are promising alternative resource for rare earth elements (REEs). Recycling of scrap Nd-Fe-B magnets will mitigate the demand supply gap of REEs. Thus, the present paper reports development of feasible hydrometallurgical flow sheet to recover REEs from Nd-Fe-B magnets. The process consists of roasting of magnets with 20% NaCl at 750 °C for 2 h followed by water leaching of the roasted mass at 75 °C for 60 min to produce REEs containing leach liquor. About 99.9% REEs was recovered, and the left residue contained Fe, which was further calcined at 600 °C for 2 h to get red oxide pigment.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Neodymium-iron-boron (Nd-Fe-B) magnet are most important magnets which has essential range of applications such as electric vehicles due its high power and torque density [1], 1000–2000 kg in wind turbine generators [2], and computer hard disk drives [3]. Sintered Nd-Fe-B magnets have been widely used and continue to develop since they were invented because of their high performance and low cost. The average annual production growth in China and the world was 6310 tons and 6920 tons, respectively [4]. After the life circle of these devices is over, these rare earth magnets are being thrown away rapidly or dump in the land fill illegally. Present rare earth element in the permanent magnets gets dissolve and mixed in the water and soil, respectively. This causes severe diseases to the human life such as liver infection, difficulty in breathing, etc. The rapid industrialization and advancement of technology in EEE, electrical vehicle, and the turbines has increased demand of these permanent magnets rapidly [5]. The availability of rare earth Metals (REMs) in the earth is limited, and its mining process produce many essential difficulties especially with regard to the environment as REEs minerals containing some traces of radioactive elements such as uranium (U) and thorium (Th) [3]. The problem raising due to the unavailability of rare earth metals and decreasing primary resources by direct mining of rare earth metals has led to dependence on the secondary resources for the recovery of Rare earth metals. The spent Nd-Fe-B magnets after their end-of-life can be consider as the major source of rare earth elements.
A number of processes have been reported by many authors for the recovery of rare earth elements from spent Nd-Fe-B magnets by pyro-, hydro-, and electrometallurgy routes. Markarwa et al. [6] reported one step of REEs recovery with purity up to 93% from spent Nd-Fe-B magnets by electro-leaching with sulfuric acid and precipitation of REEs as oxalate with oxalic acid with same concentration 0.5 mol/L [6].
In addition, oxidative roasting carried out followed by organic acid leaching has been used to extract the REMs from Nd-Fe-B magnet. The Nd-Fe-B magnet was heated at 900 °C for 480 min to oxidize into acid susceptible species. The roasted materials were dissolved in a mix solution of malic and citric acids at 90 °C to leach more than 90% REEs [7]. Kumari et al. reported the extraction of neodymium by sulfuric acid leaching followed by precipitation using ammonia to recover the neodymium hydroxide. Almost ~ 99.99% recovery of REMs (Nd, Pr, Dy) [8]. Makarova et al. [9] was shown in their work, electrochemical leaching of rare earth elements of spent Nd-Fe-B magnets [9]. Ali et al. [10] reported nitration, calcinations, water leaching; ~ 95% REEs were recovered [10]. Lee et al. [11] proposed physical and chemical treatment of like demagnetization, grinding, screening, and leaching of Nd-Fe-B magnets [11]. In subsequent study, the precipitation and solvent extraction have also been studied to extract the rare earth metals from leach liquor of Nd-Fe-B magnet. Rabatho et al. [12] leached 81% of Dy and 98% of Nd in 1 M HNO3 at 25 °C by using of 0.3 M H2O2, without affecting Fe which was remained in the residue. Further, 91.5% of Nd and 81.8% of Dy were recovered by precipitation using oxalic acid (H2C2O2) in a range of pH 8 at room temperature.
Due to similarity of REEs, it hard to target to maximize the selectivity and feasibility of all the process used to recover REEs from Nd-Fe-B magnets, and they have many demerits such as high cost, high energy consumptions, and many more.
In a view of the above aspect, the present study focused on the hydrometallurgical extraction of rare earth elements (Nd, Pr, and Dy) from discarded Nd-Fe-B magnet of hard disk. At first, magnet was demagnetized at elevated temperature; thereafter, roasting of crushed magnets was carried out with sodium chloride (NaCl) for the selective leaching of rare earth elements, and then, water leaching was carried out. To optimize the conditions for extraction of REEs, leaching studies were carried out at different parameters such as reaction time, temperature, and pulp density to recover the REEs from scrap Nd-Fe-B magnet. Further, the leached liquor contains REEs (Nd, Dy, and Pr) which undergoes evaporation to get the REEs salt.
Experimental
Materials
The waste computer CPU was collected from the local market and dismantled to separate hard disk drive and then magnet which treated for recovery of metals from it. The entire chemicals used in this study such as sulfuric acid, hydrogen peroxide, calcium hydroxide, nitric acid, and NaOH are analytical grade (AR) supplied by Rankem, India. Apart from concentrated chemical, all the dilute solutions were used in this study was prepared by using distilled water.
Pre-treatment and Characterization of Waste Hard Disk Drive
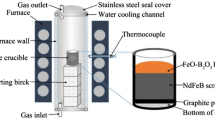
At first the waste hard disk is dismantled manually to separate magnet present inside it, the separated magnet from spent hard disk are collected into a crucible which subjected to demagnetized by heating at 310 °C for 2 h to sour their magnetic strength. The demagnetized magnets crushed and ground manually to reduce their size up to 100 meshes to convert the material into homogenous form, which helps to increases efficiencies of leaching of REEs. The flowchart showing pre-treatment of waste hard disk is shown in Fig. 1.
The demagnetized-crushed magnets are characterized by dissolving in a mixture of three parts HCl to one part HNO3 (Aqua regia) which is also summarized in Table 1 showing the total composition of metals present in Nd-Fe-B magnets determined by Induced Coupled Plasma-Optical Emission Spectrophotometer (ICP-OES). It is found that the excess of iron present in the magnet, some of them are associated with cobalt to improve corrosion properties of the magnets and the surface of magnets are coated by an electrochemical layer of nickel or some plastics [2].
Roasting Procedure
Roasting of Nd-Fe-B magnets was carried out for the effective liberation of rare earth metals (REMs) by using cylindrical alumina crucible. At first, the demagnetized-crushed magnets are mixed with 20% sodium chloride (NaCl) in the ratio of 1:4 by the help of spatula. Then, the homogenous mixture was roasted in a pre-heating muffle furnace at the range of temperature between 500 and 800 °C for 30–180 min. After the completion of roasting experiment, the crucible was taken out of the furnace and left the material in air for cooling. Since the material was not converted in paste, it was easily ground by the help of mortar pistol. Now, the material roasted undergoes leaching procedure with various leachants such as water, sulfuric acid, hydrogen peroxide, and nitric acid.
Leaching Procedure
Leaching process was carried out to recover rare earth metals from scrap magnets of hard disk. At first, the glass Pyrex leaching reactor (250 mL) was well setup with reflux condenser to neglect the loss of liquid in the form of vapor at elevated temperature and placed in temperature-controlled hot plate to maintain the optimized temperature throughout the experiments, and a constant stirring was provided to the whole setup by using magnetic stirrer. Sampling was done at regular time interval to optimize the suitable parameters. The leach liquor was separated from the residue by using vacuum filtration unit. The obtained leach liquor which contains REMs was further subjected to evaporation process at high temperature to get REMs salt, whereas the residue left which contains other non-ferrous metals such as Fe, Co, and Ni was washed and dried in a vacuum oven at 110 °C for 1 h, which can be further used to recover it by hydrometallurgical process.
Analytical Procedure
The demagnetized-crushed materials of spent permanent magnets of discarded hard disk drive are first analyzed in XRD for their phase detection, and then, the morphology of Nd-Fe-B magnets is studied by using SEM–EDS JXA-8230 Electron Probe Micro Analyzer (JEOL, Japan) with the same to know the accurate composition and morphology of metals present in the crushed materials of waste magnets (Figs. 2 and 3). The concentration of all metals (REEs, Fe, Co, Ni, B, etc.) present in the leach liquor was analyzed using Induced Coupled Plasma-Optical Emission Spectrophotometer (ICP-OES) (VISTA-PMX, CCD Simultaneous; Make: Australia) and Atomic Absorption Spectrophotometer (AAS) (Perkin Elmer model, Analyst 200; USA).
Results and Discussion
Pre-treatment of Nd-Fe-B Magnets
Initially, spent hard disk drives of personal computers were dismantled manually and separated the required magnet for the recovery of REMs and the other parts, viz. steel frames, PCBs, platter, actuator, etc., are also separated. The magnets are demagnetized to diminish their magnetic strength at elevated temperature 310 °C for 2 h. Further, the demagnetized magnets are pulverized up to 100 mesh size. Figure 1 shows the systematic flowchart for pre-treatment of spent Nd-Fe-B magnets of hard disk drives. The pulverized materials of magnets were chemically digested and analyzed. About 24.74% of Nd, 2.5% of Pr, 1.25% of Dy, 55.8% of Fe, along with other traces metals, was present in the demagnetized-crushed magnets shown in Table 1.
Roasting Studies of Nd-Fe-B Magnets
Roasting study was carried out for selective recovery of rare earth metals from the Nd-Fe-B magnets by mixing with calcium hydroxide at optimal temperature. The roasting process was optimized for effectiveness at varying parameters as selection of additive, effect of concentration of sodium chloride, temperature, time, etc.
Selection of Additive
The roasting experiments were conducted with various additives, viz. calcium hydroxide, sodium hydroxide, sodium chloride, calcium carbonate, etc.; the concentration of the additive for each experiments remains the same which is 20%, and the pulverized materials of permanent magnets are mixed with the additive in the ratio of 1:4 for 1 h. The result obtained is shown in Table 2.
Effect of Temperature
To demonstrate the effect of temperature on roasting of Nd-Fe-B magnets, various set of roasting experiments were carried out viz. 500, 600, 700, 750, and 800 °C for 1 h. At first, the crushed materials are well mixed with paste of sodium chloride and then placed into muffle furnace by putting it in an alumina crucible. At 500 °C, no weight loss has been found after roasting; it is because the sample needs more temperature to fracture the internal caging of samples. On moving toward 600 °C, at this the REEs are leached in water with low leaching efficiency which is 43%. But, in case of 700 °C roasting temperature, leaching efficiency of REEs increased from 43 to 78%, and at 750 °C, complete recovery of rare earth elements has been found which is shown in Fig. 3.
Leaching Studies
Effect of Pulp Density
For more dissolution of metals, one factor pulp density plays significant role; as pulp density increases, it helps to increase the solution’s surface area per unit volume, which increases the reaction rate; pulp density also affects the leaching rate of REEs from Nd-Fe-B of hard disk. For knowing the better optimized condition, various sets were practiced with different pulp densities such as 25, 50, 75, 100, and 150 g/L. Results indicates that the leaching percentage at 100 g/L is maximum, and it became constant at 150 g/L and so on; it is because the pulp density is inversely proportional to the leaching rate, and it may be due to the sample requiring more amount of acid solution as we increase the amount of samples [13]. 100 g/L pulp density was considered as best optimized condition for the further experiments after practicing various sets of leaching with different pulp density with same condition (Fig. 5).
Effect of Temperature
As the temperature of the reaction increases, the rate of reaction increases and vice versa [14]. Therefore, to study the effect of temperature on leaching of roasted Nd-Fe-B magnets, many sets leaching experiment were practiced at different temperature such as 25, 60, 75, and 90 °C with pulp density 100 g/L and time 30 min. After analysis, results indicate that increase in temperature increases leaching efficiency of REMs contained in the solid materials, but at high temperature, it becomes constant, and at 75 °C, the leaching percentage of REMs was found maximum, while at 90 °C it seems almost constant. Therefore, further leaching experiments were carried out at 60 °C as optimized leaching temperature at this temperature leaching of REMs was found 99.9% (Fig. 6).
Effect of Time
To investigate the effect of time in the dissolution of REMs from Nd-Fe-B magnets of computers, the roasted products were leached in water at 75 °C by maintaining 100 g/L pulp density; various leaching sets were practiced such as 10, 15, 20, 30, 45, 60, and 75 min. Results show that the leaching efficiency of REEs reached ~ 57% in 30 min and the maximum recovery (~ 92%) was obtained in 60 min. which is shown in Fig. 4. The residue obtained after filtration contains iron was further calcined at 600 for 2 h.
After 10 min of the experiment, the leach liquor was taken in a test tube bottle {bottle (a) in Fig. 2} and analyzed which indicates 19.6% recovery. Further, after 30 min, it was increased by ~ 57.3% {bottle (b) in Fig. 2}. In addition, after 45 min and 60 min, the recovery rate of REEs was continuously increased by 69.8% and 93.4% {bottle (c) and (d)}. But, after 60 min of the reaction, the results show that the dissolution of iron started along with the REEs {bottle (e) in Fig. 2}; therefore, 60 min mixing time was considered as suitable parameter for further experiments (Fig. 7). The complete process flow sheet for extraction of REMs is presented in Fig. 8.
Conclusions
Based on the above experimental results, the following conclusions have been made.
-
The Nd-Fe-B magnets can be demagnetized by heat treatment at 310 °C in 2 h.
-
The complete conversion of REEs in water-soluble species occurred at 750 °C by mixing with 20% of NaCl paste placed in muffle furnace.
-
Roasted magnets was leached in water at 75 °C by 60 min mixing time, maintaining 100 g/L. Results indicates complete dissolution of REMs by water leaching.
-
The leach liquor of discarded magnet can be evaporated at high temperature to get REEs salts.
-
Finally, the residue left which contains Fe was calcined 600 °C for 2 h to convert it as Fe-pigment red oxide.
References
Luk PCK, Abdulrahem HA, Xia B (2020) Low-cost high-performance ferrite permanent magnet machines in EV applications: a comprehensive review. Transportation 6:100080
Gruber V, Carsky M (2020) New technology for lanthanide recovery from spent Nd-Fe-B magnet. S Afr J Chem Eng 33:35–38
Nababan DC, Mukhlis R, Durandet Y, Pownceby MI, Prentice L, Rhamdhani MA (2021) Mechanism and microstructure evolution of high temperature oxidation of end-of-life NdFeB rare earth permanent magnets. Corros Sci 182:109290
https://www.statista.com/statistics/270277/mining-of-rare-earths-by-country/
Lu X, Wu Y, Lian J, Zhang Y, Chen C, Wang P, Meng L (2020) Energy management of hybrid electric vehicles: a review of energy optimization of fuel cell hybrid power system based on genetic algorithm. Energy Convers Manage 205:112474
Makarovaa I, Rylb J, Sunc Z, Kurilod I, Górnickae K, Laatikainena M, Repoa E (2020) One-step recovery of REE oxalates in electro-leaching of spent NdFeB magnets. Sep Purif Technol 251:117362
Kumari MK, Jha DD, Pathak (2020) An innovative environmental process for the treatment of scrap Nd-Fe-B magnets. J Environ Manage 273:111063
Makarova I, Soboleva E, Osipenko M, Kurilo I, Laatikainen M (2020) Electrochemical leaching of rare-earth elements from spent NdFeB magnets. Hydrometallurgy 192:105264
Onal MAR, Aktan E, Borra CR, Blanpain B, Gerven TV, Guo M (2017) Recycling of NdFeB magnets using nitration, calcination and water leaching for REE recovery. Hydrometallurgy 167:115–123
Reisdorfer G, Bertuol D, Tanabe EH (2019) Recovery of neodymium the magnets of hard disk drives using organic acids. Miner Eng 143:105938
Lee CH, Chen YJ, Liao CH, Popuri RS, Tsai SL, Hung CE (2013) Selective leaching process for Neodymium recovery from scrap Nd-Fe-B magnet. Metall Mater from Trans a Phys Metall Mater Sci. 44:5825–5833
Rabatho JP, Tongamp W, Takasaki Y, Haga K, Shibayama A (2013) Recovery of Nd and Dy from rare earth magnetic waste sludge by hydrometallurgical process. J Mater Cycles Waste Manag 15:171–178
Jha MK, Kumari A, Choubey PK, Lee JC, Kumar V, Jeong J (2012) Leaching of lead from solder material of waste printed circuit boards (PCBs). Hydrometallurgy 121–124:28–34
Choubey PK, Singh N, Panda R, Jyothi RK, Yoo K, Park I, Manis Kumar Jha (2021) Development of hydrometallurgical process for recovery of rare earth metals (Nd, Pr, and Dy) from Nd-Fe-B Magnets. Metals 11:1987
Acknowledgements
The authors are thankful to Director, CSIR-National Metallurgical Laboratory, Jamshedpur, India, for kind permission to publish the paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Singh, N., Choubey, P.K., Panda, R., Jyothi, R.K., Jha, M.K. (2023). Recycling of Rare Earth Elements (REEs) from Scrap Nd-Fe-B Magnets. In: Ouchi, T., et al. Rare Metal Technology 2023. TMS 2023. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-22761-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-22761-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22760-8
Online ISBN: 978-3-031-22761-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)