Abstract
In recent years, tantalum is being increasingly researched as a replacement for coatings for high-temperature applications. Tantalum is a refractory metal with low recycling rate of less than 1% because most tantalum secondary recovery techniques are primarily meant for recovery of other elements. In this study, the objective is to review various potential methods for recycling of tantalum coated steel composite, either at its end of life or for coating refurbishment purposes when coating is damaged. Tantalum can be recovered by both pyrometallurgical and hydrometallurgical methods, or a combination of the two, and it usually involves a whole process development with multiple steps for separation and purification from other elements. The main factor for selecting the best recovery method is dependent on the materials which are mechanically or chemically bonded with tantalum. This review summarizes various methods to recover tantalum from different secondary sources like tantalum capacitors, tantalum mill products, and tantalum in chemical processing industry. Lastly, we comment on the best method to recover tantalum from tantalum coated scrap.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Refractory metals like tantalum and tungsten are being extensively researched to replace traditional coatings in high-temperature applications. These metals offer properties of high melting point and superior resistance to extreme environments in high-temperature applications. Tantalum is a refractory metal with a high melting point of 3017 °C. It is a very inert and heat resistant metal which finds applications in many fields including semiconductor industry in electronic capacitors for automotive, mobile phones and computers; alloying element for steels in aerospace and oil industries; in superalloys; as carbides for use in cutting tools; and as coatings for medical implants [1, 2]. Tantalum is also highly resistant to most acids or aggressive mediums, including HNO3, HCl and aqua regia below 150 °C, because of which it also finds applications in chemical reaction vessels. Tantalum supply is majorly from primary mining (artisanal and small-scale mining accounting for 48% and conventional mining 25%), tin slags (7%), secondary recycling (20%) and inventories and stockpiles, as reported in 2020 [3].
Tantalum belongs to the critical metal category with recycling rate of less than 1% [4]. In 2021, tantalum consumption in USA from secondary sources was reported to be nearly 30% [2]. Tantalum coated composites are a potential source for secondary tantalum recoveries. Moreover, tantalum coatings and coatings in general must be removed from substrate for a variety of reasons—replacement of damaged coating, inspection of substrate, recycling of coated composite or for cleaning of substrate. Traditional ways to recycle tantalum include both hydrometallurgical and pyrometallurgical route, with advantages and disadvantages for both. In the present review, the objective is review of various potential methods to recover tantalum from tantalum coated steel composites, with or without damage to the substrate. There is a lack of research for recovering tantalum from this specific form of tantalum coated steel composite in scientific journals. Therefore, we will carry out a broad review of extracting tantalum from different secondary sources with hopes of shedding light on potential route for recovering tantalum from tantalum coated steel composite.
Pyrometallurgical Recovery
Pyrometallurgical processes are characterized by simple, high efficiency, energy intensive, high carbon footprint, and high-cost processing methods. There are various pyrometallurgy methods like high-temperature oxidation, chloride metallurgy, iodization, steam gasification, and pyrolysis, which have been reported to recover tantalum as its oxide or halogen compounds. For further separation and purification from other elements, hydrometallurgy like acid leaching, solvent extraction, or ion exchange may be used. To recover tantalum in metallic form, reduction using hydrogen gas or metallothermic reduction methods have been reported. The recovery rates are very high for tantalum using pyrometallurgy, but the purity rate solely depends on the secondary processes used to separate and purify the recovered tantalum. The various pyrometallurgical methods along with the complete process flow for recovering tantalum are summarized in Table 1.
Hydrometallurgical Recovery
Though pyrometallurgy is more commonly applied for tantalum recoveries from secondary sources, there are various alternate hydrometallurgical routes reported as well. Because of the inherent nature of tantalum to resist corrosion even in aggressive mediums, hydrometallurgical methods for recovering tantalum usually involve recovering elements attached to tantalum first. Hydrometallurgical processes are characterized by high efficiency, resource extensive, high waste or by-product generation and costly processes. Generally, for tantalum recovery, hydrometallurgical methods have to be used in conjunction with other unit processes for complete process flow. Some commonly used methods include acid leaching in leaching reagents like HF, NH4F, HNO3, HCl, or H2SO4 mediums; solvent extraction using solvents like methyl isobutyl ketone (MIBK), tributyl phosphate, cyclohexane, 2-octanol, and ionic liquids, and ion exchange method. Some methods to hydro-metallurgically recover tantalum from different sources are summarized in Table 2.
Conclusion
There are various processes to recover tantalum, broadly classified into high-temperature oxidation, pyrolysis, steam gasification, iodization, acid leaching, alkaline leaching, application of ionic liquid, and supercritical water treatment. Pyrometallurgy is energy intensive but produces tantalum of high purity, in short time and may generate less waste by-products. On the other hand, hydrometallurgy routes are less energy intensive but produce a lot of waste which needs to be further handled and may involve use of high quantities of toxic reagents. Both processes have their advantages and limitations. Table 3 lists some differences between pyrometallurgy and hydrometallurgy for tantalum recovery. The selection of recovery strategy for tantalum depends on the waste from which it needs to be extracted. As described in the sections above, tantalum recovery involves a whole process flow with multiple steps to purify and separate other components from the waste scrap.
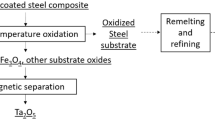
In case of tantalum coated steel composite, tantalum is attached to one other component which is steel and there may or may not be an interdiffusion layer which depends on the coating deposition method used. Thus, the main considerations here are the steel substrate and composite’s interface characteristics. If tantalum is to be recovered with minimum damage to the substrate, pyrometallurgy to extract tantalum as its oxides may be the most efficient method to protect the steel substrate. Tantalum and iron oxidize in oxygen containing environment to develop protective scales of Ta2O5 and oxides of the substrate, respectively. Thermodynamically, Ta2O5 is a very stable phase at high temperatures compared to most metals. Therefore, both tantalum coating and steel substrate will oxidize but at relatively different rates, which can be optimized. But if the whole tantalum coated steel composite is to be recycled, a combination of hydrometallurgy and pyrometallurgy may be the best method to selectively extract Fe (steel) and Ta.
References
“Fourth Annual Report on Carcinogens (1985) U.S. Department of Health and Human Services, NTP 85-002, p 85
USGS Report on Tantalum (2022) U.S. Geological Survey, Mineral Commodity Summaries, 2022.
Tantalum-Niobium International Study Center (2020) Beautiful metal. Oxide films on Tantalum and Niobium. TIC Bulletin No 181
Reck BK, Graedel TE (2012) Challenges in metal recycling. Science 337(6095):690–695
Mineta K, Okabe TH (2005) Development of a recycling process for tantalum from capacitor scraps. J Phys Chem Solids 66(2–4):318–321
Fujita T, Ono H, Dodbiba G, Yamaguchi K (2014) Evaluation of a recycling process for printed circuit board by physical separation and heat treatment. Waste Manage 34(7):1264–1273
Katano S, Wajima T, Nakagome H (2014) Recovery of tantalum sintered compact from used tantalum condenser using steam gasification with sodium hydroxide. APCBEE Proc 10:182–186
Lessard JD, Shekhter LN, Gribbin DG, Blagoveshchensky Y, McHugh LF (2015) A new technology platform for the production of electronic grade tantalum nanopowders from tantalum scrap sources. Int J Refract Metal Hard Mater 48:408–413
Vutova K, Vassileva V, Koleva E, Munirathnam N, Amalnerkar DP, Tanaka T (2016) Investigation of tantalum recycling by electron beam melting. Metals 6(11):287
Niu B, Chen Z, Xu Z (2017) Method for recycling tantalum from waste tantalum capacitors by chloride metallurgy. ACS Sustain Chem Eng 5(2):1376–1381
Niu B, Chen Z, Xu Z (2017) Application of pyrolysis to recycling organics from waste tantalum capacitors.“ J Hazard Mater 335:39–46
Niu B, Chen Z, Xu Z (2020) Recycling waste tantalum capacitors to synthesize high value-added Ta2O5 and polyaniline-decorated Ta2O5 photocatalyst by an integrated chlorination-sintering-chemisorption process. J Clean Prod 252:117206
Baba AA, Adekola FA, Faseki M (2005) A study of the kinetics of the dissolution of a Nigerian tantalite ore in hydrochloric acid. Ife J Sci 7(2):221–227
Baba, A. A., F. A. Adekola, O. I. Dele-Ige, and R. B. Bale (2007). “Investigation of dissolution kinetics of a Nigerian tantalite ore in nitric acid.“ Journal of Minerals & Materials Characterization & Engineering 7, no. 1:83–95
Wang X, Zheng S, Xu H, Zhang Y (2009) Leaching of niobium and tantalum from a low-grade ore using a KOH roast–water leach system. Hydrometallurgy 98(3–4):219–223
Nete M, Purcell W, Nel JT (2016) Hydrometallurgical separation of niobium and tantalum: a fundamental approach. Jom 68(2):556–566
Niu B, Chen Z, Xu Z (2017) Recovery of tantalum from waste tantalum capacitors by supercritical water treatment. ACS Sustain Chem Eng 5(5):4421–4428
Chen W-S, Ho H-J, Lin K-Y (2019) Hydrometallurgical process for tantalum recovery from epoxy-coated solid electrolyte tantalum capacitors. Materials 12(8):1220
Micheau C, Lejeune M, Arrachart G, Draye M, Turgis R, Michel S, Legeai S, Pellet-Rostaing S (2019) Recovery of tantalum from synthetic sulfuric leach solutions by solvent extraction with phosphonate functionalized ionic liquids. Hydrometallurgy 189:105107
Deblonde GJ-P, Bengio D, Beltrami D, Bélair S, Cote G, Chagnes A (2019) A fluoride-free liquid-liquid extraction process for the recovery and separation of niobium and tantalum from alkaline leach solutions. Sep Purif Technol 215:634–643
Legeai S, Pellet-Rostaing S (2020) Ionic liquids as extraction media in a two-step eco-friendly process for selective tantalum recovery. ACS Sustain Chem Eng 8(4):1954–1963
Dutta S, Mukhopadhyay S, Gaddam S, Shenoy KT, Mirji KV (2021) Process development for the separation of niobium and tantalum from fluoride medium using trioctyl amine and application of Taguchi's method to optimize solvent extraction parameters. Hydrometallurgy 199:105522
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Gupta, A., Mishra, B. (2023). Tantalum Recovery Technique for Recycling of Tantalum Coated Composite Materials. In: Ouchi, T., et al. Rare Metal Technology 2023. TMS 2023. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-22761-5_25
Download citation
DOI: https://doi.org/10.1007/978-3-031-22761-5_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22760-8
Online ISBN: 978-3-031-22761-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)