Abstract
Arachnoid cysts (ACs) are usually congenital, extraaxial, benign, cystic lesions filled with cerebrospinal fluid. The most common etiology is splitting of the arachnoid layer. They are usually asymptomatic and are discovered incidentally during prenatal fetal ultrasound and neuroimaging modalities. In this chapter, we present a pictorial review of ACs. We also discuss etiopathogenesis, imaging features, epidemiology, symptoms, natural history, and differential diagnosis of ACs.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
In 1831, English physician Bright first described arachnoid cysts (ACs) as serous cysts surrounded by a wall related to the arachnoid membrane [68]. ACs are the most common benign, non-neoplastic, intra-arachnoid abnormalities of the brain. ACs are encapsulated by the arachnoid membrane which is filled with a fluid like cerebrospinal fluid (CSF). They are usually asymptomatic. When they are symptomatic, symptoms are usually related to the mass effect on adjacent structures and locations. Prenatal ultrasound (US) screening and the widespread use of magnetic resonance imaging (MRI) and computed tomography (CT) for other head pathologies lead to incidentally diagnosed ACs [11, 41, 81].
The incidence of ACs is controversial because the asymptomatic course of ACs obscures the true incidence. In the literature, the incidence of ACs is reported as approximately 0.5–1.5% of the population [41, 82, 85]. Al-Holou et al. studied 11,738 patients aged 18 years or younger who had undergone brain MRI and found that the prevalence in childhood was about 2.6%. In another study in adults by Al-Holou et al., 661 of 48,417 patients had ACs, with a 1.4% prevalence [4, 5].
ACs can be seen throughout life, but nearly 75% of ACs are diagnosed in childhood. Males are twice more likely to be affected, and the left hemisphere is predominantly affected although the etiology is unknown [4, 5, 25, 30]. ACs are usually sporadic and present as a single malformation. Multiple ACs may occur in syndromes and metabolic disorders such as glutaric aciduria type 1, polycystic kidney disease, Down syndrome, neurofibromatosis, etc. in which the incidence of ACs increases [30, 48].
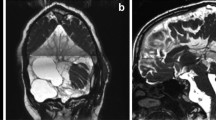
The location of the ACs in the cranial cavity varies; in adults, 60–70% of ACs are found in the middle cranial fossa and Sylvian fissure (Figs. 1 and 2). Infratentorial ACs are 10% of ACs and are located in the cerebellopontine angle or retrocerebellar area (Fig. 3). Infratentorial ACs are more common in children than in adults. ACs are also rarely found in the suprasellar, interhemispheric, intraventricular, and cerebellopontine angles and in the spinal region [4, 19]. Most ACs remain stable, but rarely progression or regression can be seen [5, 66, 73].
2 Pathology, Etiology, and Clinical Symptoms
ACs are fluid-filled arachnoid layers formed by cleavage of the arachnoid membrane due to congenital malformation or as a result of intracranial pathology. Primary ACs are a congenital developmental anomaly of the arachnoid mater due to cleavage of the arachnoid membrane at the edge of the cyst, which fills with CSF-like fluid, also called true ACs [8, 67]. Primary ACs are located in the arachnoid space and are surrounded by a vascularized collagen wall lined with multilayered hyperplastic arachnoid cells. ACs are distinguished from arachnoid granulations and membranes by a thick collagen wall lined with hyperplastic arachnoid cells and by the absence of trabecular processes. The inner and outer layers of the ACs are fused at the border, and they continue as normal arachnoid membrane. Choroid plexus and neuroglial cells can be seen in some cases of suprasellar or posterior fossa ACs. Primary ACs are the most common type [59, 67].
Secondary ACs are required ACs as a result of head trauma, infection, inflammation, etc., in which CSF-like fluid accumulates in the arachnoid space. Pericystic gliosis and hemosiderosis may accompany the cyst. The contents may be proteinaceous or xanthochromic, and an arachnoid cell layer is not present [59, 78].
ACs can be also classified as communicating and noncommunicating cysts depending on their relationship to the subarachnoid CSF space. In communicating ACs, there is a connection between the AC and the surrounding subarachnoid space, allowing CSF to enter the cyst space (Fig. 4). As for noncommunicating ACs, the AC has no communication, and CSF cannot enter the cyst. If surgical intervention is required for treatment of ACs, the difference between communicating and noncommunicating cysts should be established for treatment management [67].
ACs filled with CSF-like fluid are clear and contain no cellular debris, proteinaceous material, hemosiderin, or inflammatory cells with the same osmolarity and concentration of sodium, potassium, chloride, calcium, magnesium, and glucose. But some proteins and enzymes are minimal or absent, such as hemoglobin, carbonic anhydrate, lactate dehydrogenase, and alpha-1-antitrypsin, which distinguish CSF from ACs [9, 72].
ACs are often stable in size and behavior. Occasionally, they regress and disappear entirely [19, 69, 73, 80]. ACs rarely enlarge resulting in remodeling of the calvarium, mass effects on surrounding tissue, and other symptoms [19]. There are several theories that explain the enlargement of ACs. In the one-way valve mechanism theory, CSF enters the cyst space but cannot escape. The osmotic gradient theory explains the expansion by the hyperosmolar intracystic space and the influx of fluid into the cyst from the iso-osmolar environment. The other theory explains the expansion of the ACs by secretory arachnoid cells lining the inner wall of the ACs and secreting CSF-like fluid [14, 71]. It is believed that the combination of these three mechanisms contribute to the development of ACs, but this is still controversial [23].
Most ACs (80–90%) are located in the supratentorial space. The vast majority of ACs in the supratentorial space arise from the Sylvian fissure (50–60% of all intracranial ACs) and are located in the temporal fossa (Figs. 1 and 2). The quadrigeminal plate region and the chiasmatic cistern are other common sites for supratentorial ACs (Fig. 5). The interhemispheric fissure and convexity are less frequent sites of origin [4, 19] (Fig. 6). The lateral cerebellopontine (CP) angles (Fig. 7) and the midline retrocerebellar cisterns are fairly equivalent, whereas the interpeduncular and prepontine cisterns are a rare site of origin of the infratentorial ACs. Unlike in children, the retrocerebellar location of ACs in adults is as common as that of ACs in the middle cranial fossa [4, 5]. Some ACs located in the suprasellar and quadrigeminal plate may grow toward and into the cerebral ventricles (Fig. 5). Paraventricular cysts are usually primary ACs. The purely intraventricular cysts generally develop secondary to tumoral, inflammatory, posthemorrhagic, or postinfectious processes or are secondary to malformative lesions (cavum septi) or arise from the choroid plexus or ependyma [50, 63] (Fig. 4).
Symptoms of ACs usually depend on the location and size. Headache, seizures, hydrocephalus, macrocrania, hormonal deficits, visual loss, hearing loss, and facial sensory loss can be observed. After head trauma, cyst rupture, hemorrhage, intracystic hemorrhage, or hygroma development leads the asymptomatic cysts to become symptomatic. Patients with ACs are prone to develop subdural hematomas after minor head trauma [4, 33, 34, 37, 53, 55].
3 Radiological Diagnosis of Arachnoid Cysts
3.1 Antenatal Ultrasonography
US has a role in diagnosing ACs, predominantly in prenatal, infantile, and early childhood periods. As the cranial bones thicken in the advanced ages, sonographic resolution decreases considerably and does not allow intracranial evaluation [83]. US is useful as a noninvasive, widely available, cost-effective, and radiation-free imaging technique with a high yield for detecting and characterizing cystic masses [10]. These features of US also allow serial examination in the follow-up of the AC and its complications [90].
The standard prenatal approach for evaluating the central nervous system (CNS) is US imaging [33]. Although rare, ACs of developmental origin (primary) can be detected in prenatal screening (0.2%) [31]. Prenatal US can reveal an AC as early as 20 weeks of gestation. However, there is a case in the literature diagnosed as a posterior fossa AC by transvaginal US at 13 weeks of gestation [15, 29, 90]. Most children with a prenatal diagnosis have a normal neurodevelopmental course. Large size (>2 cm), syndromic/genetic diagnosis, and/or presence of other intracranial abnormalities are reported as the predictors of pathological outcomes [17, 31]. Maternal obesity, oligohydramnios, fetal head engagement during late pregnancy, or acoustic shadowing by surrounding bony structures may impair sonographic imaging [10, 13]. In such restrictive situations, transvaginal neurosonography can be used. Convex probe (1–6 MHz), sector probe (3–5 MHz), and transvaginal probes (5–10 MHz) are used in the prenatal US [13, 90]. Fetal brain images are examined predominantly in the transverse axial plane, additionally in the coronal and parasagittal planes [13]. ACs are imaged as avascular extraaxial anechoic-hypoechoic lesions of variable dimensions with a thin regular outline, with or without septa on antenatal US [10, 16, 60, 90]. They do not communicate with the lateral ventricles and are not associated with loss of brain tissue. Larger cysts may cause compression in the adjacent cerebral parenchyma. The cysts are usually located in supratentorial (50–65% middle cranial fossa) and rarely infratentorial areas [15, 57, 89]. If the cyst blocks the foramen of Monro or the aqueduct, secondary hydrocephalus develops, and ventriculomegaly is observed on US [29, 90]. In addition, ACs and chromosomal abnormalities may co-exist, and accompanying structural abnormalities of the CNS such as frontal polymicrogyria, an abnormal corpus callosum, cerebellar dysplasia, and gray matter heterotopia can be visualized on the prenatal US [15, 17]. In the differential diagnosis of ACs in prenatal US, porencephalic cysts, choroid plexus cysts, aneurysms of the vein of Galen, schizencephaly, cystic neoplasms, and intracranial hemorrhage should be considered US [15, 17, 90].
AC consists of 1% of neonatal intracranial masses [15, 17, 29]. Transcranial US is a crucial diagnostic tool to evaluate the ACs during the first year of life. It is limited by the closure of the anterior fontanelle, which generally occurs in full-term infants 9–18 months of age. A sector probe (1–4 MHz) was used for the transcranial US. A high-frequency linear probe (5–10 MHz) is used for detailed visualization of superficial structures. Anterior-posterior-sphenoidal fontanelle, temporal area, high parietal convexity, supraorbital areas, and foramen magnum can be used as an acoustic window while performing US. Infratentorial structures are evaluated using acoustic windows, including posterior-lateral fontanelle, temporal approach, and foramen magnum. The sellar region is a challenging area for the transcranial US to evaluate small space-occupying lesions. Images should be symmetrical in the coronal and sagittal planes. ACs are imaged as extraaxial well-circumscribed smooth thin-walled anechoic-hypoechoic lesions. Color Doppler interrogation helps to assess the vascularization of lesions [32, 83]. ACs are avascular, which can be demonstrated by color Doppler imaging. Hydrocephalus and brain herniation, which may develop due to the mass effect caused by large cysts, can be determined in the US. The differential diagnosis of an AC is a subdural hygroma, dilatation of normal subarachnoid space secondary to underlying atrophy or encephalomalacia, and epidermoid cyst [58].
US can be used in the diagnosis of ACs as well as throughout surgical treatment. During the operation, intraoperative US can clearly define ACs, and treatment of these cysts, including drainage and fenestration of septations, is easily facilitated by intraoperative US guidance. Direct intraoperative real-time US of the brain and spinal cord is made with scan heads small enough for use after craniotomy/laminectomy or even through a burr hole. The sector probe (3–10 MHz) is generally used as the intraoperative probe [7, 40]. The cysts’ location and extent are evaluated before the neurosurgical approach for drainage or excision [40]. Thus, a safer and much more effective surgery becomes possible.
3.2 Computed Tomography
ACs are usually discovered incidentally during imaging studies (Fig. 3). On CT, ACs are sharply demarcated, unilocular, homogeneous, extraaxial lesions that have identical density with CSF. Hounsfield attenuation values range from 10 to 20 (hypodense). In the case of intracystic hemorrhage, they may be hyperintense. ACs do not usually enhance after intravenous injection of iodinated contrast medium, but ACs may enhance with delayed imaging. Bone remodeling such as calvarial bone thinning, bulging of the skull, and sphenoid wing displacements can be best distinguished on CT. ACs usually obliterate the subarachnoid space and compress surrounding tissue. ACs vary in size and may be unilocular or septated [30, 62, 87].
When CT is compared with MRI, soft tissue contrast is lower, and scanning CT requires an ionizing dose of radiation, which is a disadvantage in the pediatric population. But CT is a faster, cheaper, and more available neuroimaging test than MRI. CT is also helpful in detecting intracystic hemorrhage. CT imaging is often sufficient to establish the diagnosis of an AC. Thin-walled cysts that have CSF-like density at the usual locations generally help to determine the presence of ACs [30, 87, 88].
3.3 Magnetic Resonance Imaging
MRI is the gold standard imaging tool for ACs and distinguishes them from other intracranial cystic lesions. MRI is the most useful diagnostic tool for evaluating ACs to confirm their extraaxial location. On T2-weighted images, ACs exhibit hyperintense signal that attenuates on fluid-attenuated inversion recovery (FLAIR) images similar to that of CSF [24, 30, 87]. On T1-weighted images, the ACs have hypointense signal to the brain. Diffusion-weighted imaging is essential for distinguishing ACs from epidermoid cysts. Both ACs and epidermoid cysts generally have the same appearance on T1- and T2- weighted images (Figs. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, and 13). If ACs rupture or bleed into the cystic space, blooming can be seen on gradient echo (GRE) sequences. Uncomplicated ACs show no blooming on GRE [24, 75]. ACs do not enhance after gadolinium administration and do not have diffusion restriction, whereas epidermoid cysts usually restrict the diffusion on DWI [15] (Figs. 2, 6, 7, and 8).
For radiological evolution, the radiologist must first be informed with an accurate history of the patient by the clinician. During the radiological process, the radiologist must assess the mass effect on the adjacent tissues of the brain structures, such as nerves, vessels, and affected tissues. In addition, the radiologist must inform the physician about the cysts that may cause hydrocephalus or large cysts that may lead to cerebral herniation. CT imaging is often sufficient to establish the diagnosis of an AC. When additional information is needed about the anatomic location, size, and structural involvement of an AC or for follow-up, MRI is the best choice [88].
3.4 Cisternography
CT cisternography (CTC) and MR cisternography (MRC) can be performed to make a decision for surgery and to further evaluate an AC with adjacent tissue (small vessels, cranial nerves, etc.). Communication of ACs with the subarachnoid CSF space can be demonstrated by cisternography [26, 88]. In CTC, a safe, nonionic iodinated contrast agent is injected intrathecally, and the amount of filling and the time it takes to fill the cyst (dynamic images) can be obtained from CT imaging of the ACs. When cysts fill quickly and completely, they are considered as freely communicating ACs and treatment management changes [26, 84]. Communicating cysts are considered as subarachnoid space diverticulas. But slow-filling noncommunicating ACs are usually regarded as true ACs [88]. When ACs expand, it is because of an osmotic gradient, ball valve mechanism, or fluid-secreting arachnoid cells lining the cyst wall [14, 71].
MRC is another method to assess the connections of the ACs with the CSF space. MRC can be performed in two ways. Intrathecal injection of gadolinium and dynamic imaging of cyst filling can be demonstrated. 0.5–1-mL gadopentetate dimeglumine (Gd-DPTA) is injected intrathecally into the subarachnoid space, and serial fat-saturated T1-weighted images are acquired in three orthogonal planes. As the cyst fluid enhances, the connection between the cyst and the subarachnoid space becomes visible [1,2,3, 89]. In the other technique, lumbar puncture is not required, and it is more invasive than classical MRC (Fig. 8). High-resolution sequences will allow delineation of the cyst wall and neighboring structures. Constructive interference in steady state (CISS), fast imaging employing steady-state acquisition (FIESTA), and 3D T2-weighted sampling perfection with application-optimized contrast with different flip-angle evolutions (3D SPACE) can demonstrate the possible communications of the AC wall and CSF space and also the relationship between ACs and surrounding structures such as cranial nerves and vessels. Phase-contrast cine MRI combination with cardiac gating can be performed to detect CSF connection to an AC. It is usually preferred over CT cisternography because of its minimally invasive nature. If minimally invasive techniques demonstrate the connection, contrast-enhanced MRC should be performed to avoid false-positive results. MRC also provides the ability to distinguish ACs from enlarged subarachnoid spaces [35, 89].
An accurate history of the patient must be obtained from the clinician for radiological evolution. The radiologist must assess the mass effect on the adjacent tissues of the brain structures (nerves, vessels, affected tissues) and inform the clinician about the urgent situations such as hydrocephalus or large cysts that may lead to cerebral herniation.
3.5 Fetal Magnetic Resonance Imaging
Fetal MRI is an advanced diagnostic and evaluation modality. Approximately 80% of all fetal MRI examinations are performed to evaluate fetal CNS pathologies. Fetal MRI is a complementary tool for evaluating the various fetal pathologies diagnosed by ultrasound (US). Indications for fetal MRI include evaluation of an abnormality identified by US; even if the US examination is normal, there is a high risk of pathology due to familial, environmental, or laboratory findings. In cases such as maternal obesity, oligohydramnios, and fetal malposition, where US examination is suboptimal, fetal MRI is critical for diagnosis and management. In CNS pathologies, fetal MRI is usually used to check for ventriculomegaly, underlying pathologies such as hydrocephalus, dysgenesis of the corpus callosum, and posterior fossa anomalies, and to identify brain anomalies such as cortical dysgenesis that cannot be detected on US. MRI is more sensitive than US in fetuses with CNS anomalies [36, 51] (Fig. 9).
Currently, the effects of MRI exposure on the fetus are not known with certainty, regardless of the fetal week of gestation. However, due to the progressive development of the fetal brain, MRI of the CNS cannot be performed before 19 weeks of gestation. Fetal MRI is often performed with a phased array torso surface coil on 1.5-T MR devices, which has been shown to be sufficient to solve a standard clinical problem. In some centers, 3T fetal MRI is also used [21, 51]. Intravenous gadolinium-based contrast agents are not FDA-approved for use during pregnancy and are not recommended for fetal MRI. The development of fast MRI sequences has significantly reduced fetal motion artifacts and eliminates the need for fetal sedation. Single-shot fast spin-echo (SSFSE) T2-weighted sequences are used as the standard [70]. Ultrafast T2-weighted (W) sequences provide excellent visualization of fetal intracranial anatomy by optimizing the high tissue contrast between amniotic fluid and fetal tissue. T1W sequences are primarily used to visualize hemorrhage, fat, and calcium deposits. Balanced steady-state free precession (SSFP) sequences are useful for imaging fetal structures, particularly the vasculature. Diffusion-weighted imaging (DWI) plays an important role in assessing developmental and destructive processes in the brain [51, 70]. On fetal MRI, the AC is observed as a sharply demarcated and homogeneous mass with the same signal as the CSF in all sequences, resulting in displacement of the adjacent parenchyma. The location, size, and compression findings of the cyst are assessed on MRI images [20]. Prenatal MRI can detect CNS abnormalities (compression of the aqueduct, aberrant communication with the ventricles, dysgenesis of the corpus callosum, ventriculomegaly, other brain abnormalities) that often accompany ACs [20, 51].
4 Symptoms and Management of ACs
Clinical symptoms of ACs are often nonspecific and variable. Incidentally diagnosed ACs do not show any clinical symptoms [4]. When they become symptomatic, this is due to progressive enlargement or bleeding into the cyst. Clinical findings depend on the cyst’s size, its anatomic location, and its effect on CSF flow. Cysts may be small and clinically symptomatic because of their location, or they may enlarge and cause mass effect on the surrounding neuronal tissue, cranial nerves, and vessels, and then symptoms occur [4, 5, 30, 37, 41, 55, 58, 66, 87, 88].
There is a general consensus for treating patients with clear symptoms. Headache, epilepsy, psychomotor retardation, attention deficit, hyperactivity, maniac depression, schizophrenia-like symptoms, paranoia, psychoses, anosmia, Menière’s disease, and hearing loss are commonly encountered symptoms, but these clinical findings can rarely be entirely attributed to the cyst. Detailed history and examination must be performed for appropriate treatment. If the symptoms are attributable to the cyst, ACs need treatment [34, 57, 64].
Cyst fenestration or endoscopic fenestration (formation of a communication between the cyst and the subarachnoid space) may be suitable for small, nonobstructive, nonseptated cysts in the first instance. Ventriculocystostomy or ventriculocisternostomy is also a good choice for long-term treatment [20, 38, 44]. Placement of a cystoperitoneal shunt is also one of the surgical treatments for ACs [18, 39]. However, the rate of long-term complications is higher than with other surgical management. Appropriate surgery is almost always curative, but recurrences have been reported. Re-accumulation of the cyst requires shunt placement [65, 87]. In this manner, there is a higher risk of complications such as shunt blockage, infection, overdrainage, craniocerebral disproportion, and shunt dependency [6, 47, 79]. Craniotomy must be performed for complete resection of intracranial ACs, and temporal cysts are more often treated [57].
There is still controversy about treatment protocols for ACs. If the AC is asymptomatic and harmless, treatments with a high risk of unnecessary complications must be avoided. However, the results of the mentioned surgical methods are satisfactory for cysts that are demonstrably symptomatic.
ACs may require emergency treatment with the acute onset of symptoms such as obstructive hydrocephalus, acute myelopathy due to expansion or hemorrhage of a spinal AC, hemorrhage into a cyst, sudden onset of headache, and neurologic deficit. If symptoms are acute, appropriate surgical treatment must be performed immediately [37, 39, 57].
A symptomatic patient should be considered as asymptomatic if they are without symptoms related to the cyst. Most of the cases need no treatment. Most ACs in children are discovered incidentally, but in the case of expansion tendency, rupture, and subdural effusion, ACs can be treated conservatively [76]. Most pediatric neurosurgeons advise a “wait-and-see” approach when ACs are diagnosed incidentally and do not recommend further diagnostic examinations in asymptomatic patients. However, close clinical and radiological follow-up is recommended [57, 79]. MRI is the best choice for radiological follow-up. The timing of follow-up must be determined according to the size and location of the cyst and the age of the patient. Larger cysts in patients of younger age must be closely followed [52].
5 Intracranial Location of Arachnoid Cysts
5.1 Sylvian Fissure/Middle Cranial Fossa
In children, when ACs increase in volume, they may open the fissure and expose the middle cerebral artery. This exposure can lead to compression and underdevelopment of the anterior superior surface of the temporal lobe. In adults and children, nearly 50–60% of ACs are located in the middle cranial fossa—most commonly temporal lobe—or Sylvian fissure. The left hemisphere is predominantly affected, and headache is the most common presenting symptom [4, 30, 86] (Figs. 1 and 2).
The origin of middle cranial fossa ACs is controversial since either they may originate directly from the meninges adjacent to the temporal pole or partial agenesis of the temporal lobe may lead to a potential space for cyst formation [27]. ACs of the middle cranial fossa were classified into types 1–3 by Galassi in 1989 on CT [26]. Type 1 cysts are small and usually spindle-shaped. They are located below the sphenoid ridge and limited to the anterior portion of the middle cranial fossa. Type 2 cysts lie along the Sylvian fissure and displace the temporal lobe. Type 3 types of ACs fill the middle cranial fossa, are very large, and compress adjacent lobes such as the temporal, anterior, and parietal lobes. In type 3 ACs, proptosis, contralateral motor weakness and seizures, and mental impairment with developmental delay are more common in these patients. Asymmetric bulging of the skull and macrocrania may occur in infants [6, 25, 30, 34, 87]. Symptomatic patients are usually treated with surgery, but children with bitemporal ACs may have glutaric aciduria type 1 (GAT1), so even simple surgical procedures may be harmful for them. All pediatric patients with bitemporal ACs should be evaluated for GAT1 before surgical treatment, especially if there is also macrocephaly, acute encephalitis-like disease, or a dystonic cerebral palsy-like condition [48]. Bitemporal ACs are rare but may occur in other diseases such as neurofibromatosis [50]. Middle cranial fossa ACs most commonly present with headache, seizures, and motor deficits [6, 30, 87]. Symptoms are usually related to the size of the cyst and the mass effect on adjacent neuronal structures. However, the relationship between ACs and epilepsy, cognitive impairment, nausea, and dizziness is unclear [42].
5.2 Sellar Region
ACs in the sellar region are located in the suprasellar or intrasellar part of the sella turcica. They usually occur in pediatric patients and account for 10% of ACs [26]. Suprasellar cysts are located between the diaphragma sella and the optic chiasm. Because of their mass affect, they may cause obstruction of the third ventricle at the level of the foramen of Monro. Hydrocephalus and enlargement of the head may occur. If they compress the optic chiasm or intracerebral portions of the optic nerve, visual impairment such as unilateral or bilateral decrease in visual acuity and/or bitemporal hemianopsia may occur. Compression of the infundibulum, hypothalamus, or pituitary gland may lead to endocrine dysfunction. Growth retardation and developmental delay may occur [23, 55, 56, 91]. Due to compression of the third ventricle and dorsal thalamic nuclei by large suprasellar ACs, a rare condition called bobble-head doll syndrome may occur, with anteroposterior involuntary movements of the head, gait ataxia, and opisthotonus [38]. On MRI, suprasellar ACs are usually smooth, oval, or round masses that may push the pituitary gland down and the chiasm up, occasionally with erosion of the sella turcica [56, 91]. The differential diagnosis of ACs in the sellar region includes sellar diverticulum of the suprasellar cistern, also known as empty sella, Rathke’s cleft cyst and cystic craniopharyngioma, and epidermoid cysts [74]. Bypass shunt and resection are surgical options for ACs in the sella region [56] (Fig. 10).
5.3 Interhemispheric Region
ACs arise from the cistern of the corpus callosum. Although their formation is associated with compression of the corpus callosum and disruption of its development by the mechanical mass effect of the cyst, agenesis of the corpus callosum can be observed even in the presence of a small AC. When a normally formed corpus callosum is observed, these are referred to as “parasagittal” ACs [22]. These ACs have a relatively higher frequency of signs and symptoms of increased intracranial pressure (ICP) and are found in up to two-thirds of cases, more often than other sites of the ACs [46].
Asymmetric macrocrania is the most common clinical sign usually seen in infants. When the sutures are closed, headache and vomiting may be observed in older children and are more likely to be associated with hydrocephalus. Epilepsy, developmental delay, hemiparesis, hypotonia, and ocular changes are other symptoms observed [12]. MRI is usually the first choice for imaging when there is a large, giant parafalcine cyst compressing the adjacent brain or partial or complete agenesis of the corpus callosum and the absence of a normal falx cerebri [45] (Fig. 8).
5.4 Convexity
ACs of the convexity have no relationship to a subarachnoid cistern and are therefore distinct from other ACs. As mentioned earlier, clinical symptoms depend on the age of the patient and the size of the cyst. Thus, focal thinning and scalloping of the bone, asymmetric macrocrania, and sutural diastasis may occur in infants. Sometimes, giant convexity ACs can be asymptomatic in the first years of life and then show symptoms such as headache, neurologic deficits, and seizures, and these symptoms can also be observed in adults [46]. Subdural hygroma may be among the differential diagnoses, which is usually symptomatic and has a history of head injury.
Radiological studies may reveal local thinning/bulging of the overlying bone. On MRI, an extraaxial, CSF-like, fluid-filled mass can be seen that is hypointense on T1-weighted sequences and hyperintense on T2-weighted sequences, with varying degrees of compression of the underlying brain (Fig. 6).
5.5 Quadrigeminal Plate
The quadrigeminal plate is located between supratentorial and infratentorial spaces. Quadrigeminal cistern ACs present with different symptoms because adjacent structures such as the cisterna ambiens, superior cerebellum, interhemispheric fissure, posterior part of the third ventricle, mesencephalon, and the vein of Galen can be affected. Therefore, the clinical presentation may vary. Possible obstruction of the Sylvius aqueduct and resulting hydrocephalus may cause symptoms of increased ICP. Compression of the colliculus can lead to parinaud syndrome, with upward gaze paralysis and pupillary dysfunction, nystagmus, and hearing impairment. Compression of the brain stem can lead to paralysis of the trochlear nerve, respiratory failure, and limb weakness. Pineal gland suppression leads to premature puberty. Gait ataxia due to cerebellar compression and seizures due to temporal lobe compression are other symptoms. Like suprasellar ACs, quadrigeminal plate ACs are found mainly in children and rarely in adolescents and adults, probably because of the occurrence of hydrocephalus during their early natural development [28] (Fig. 9).
5.6 Intraventricular Region
Intraventricular ACs are very rare and appear to arise from ectopic remnants of the arachnoid or invagination of the arachnoid into the choroid or from the arachnoid layer in the choroidal fissure [49]. With the aid of high-resolution MRI, which provides a reliable differential diagnosis, intraventricular ACs are being identified with increasing frequency, especially in infants. Indeed, most intraventricular cystic lesions such as choroid plexus, ependymal tumor, or inflammatory cysts resemble ACs. Distortion and enlargement of the ventricles can be observed, and these ACs become symptomatic as soon as hydrocephalus occurs (Figs. 4 and 11).
5.7 Infratentorial Region
Infratentorial ACs account for 10% of all ACs. In the pediatric population, infratentorial ACs are more common than in adults. The retrocerebellar region or the CPA are affected. Retrocerebellar ACs must be distinguished from other cystic malformations of the posterior fossa, such as Dandy-Walker malformation or mega cisterna magna. The foramen of Magendie is present in ACs, but in Dandy-Walker malformation, the foramen of Magendie is not seen, and concomitant vermin hypoplasia or aplasia may be observed. Obstruction of the foramen of Magendie and enlargement of the fourth ventricle and rotation or upward displacement of the cerebellar vermis may be observed in Dandy-Walker malformation. In mega cisterna magna, cystic enlargement of the subarachnoid space, connected to the fourth ventricle by the foramen of Magendie, is observed [30, 63, 87] (Figs. 3, 7 and 12).
6 Differential Diagnosis
The cisterna magna is the largest subarachnoid cistern, located posteroinferior to the cerebellum, dorsal to the brain stem, and superior to the foramen magnum [77]. On sagittal plane imaging, the distance between the dorsal surface of the vermis and the internal table of the occipital bone in the posterior fossa is greater than 10 mm, called the mega cisterna magna (MCM). It is accepted as an asymptomatic normal variant in which the posterior fossa is slightly enlarged on imaging [54]. Because the foramen of Luschka and Magendie are open, they are in direct contact with the subarachnoid space. Therefore, CSF obstruction or hydrocephalus does not occur. Typically, the development of the cerebellar hemispheres and cerebellar vermis is normal, and atrophy is not expected in these locations. However, in large MCM, it may be difficult to distinguish from a retrocerebellar AC if there is a mild compressive effect on the cerebellar hemispheres and vermis. Free falx cerebelli and posterior dural folds are present in about half of MCM cases, and this finding is helpful in distinguishing them from retrocerebellar ACs.
In retrocerebellar ACs, the posterior fossa is usually normal in size, but scalloping may be seen in the internal table of the adjacent occipital bone. Although scalloping is a finding classically suggestive of a retrocerebellar AC, it can sometimes be seen in MCM cases. Hydrocephalus does not occur in the vast majority of retrocerebellar ACs and may cause a very mild, asymptomatic mass effect on the cerebellar hemispheres. However, when they become large, they can cause a mass effect on the cerebellum, resulting in compression of the fourth ventricle and obstruction of CSF flow [43] (Figs. 3 and 12).
This finding plays an important role in distinguishing it from MCM. Retrocerebellar ACs are separated from the subarachnoid space by a membrane, and this membrane can occasionally be visualized by thin section, gradient echo T2WI technique, or by cisternography. Because it is associated with the subarachnoid space in MCM, bright and black signals reflecting normal CSF flow are seen in the phase images of the CSF flow study, depending on the systole and diastole of the cardiac cycle. However, retrocerebellar ACs, which are separated from the subarachnoid space by a thin membrane, are often not expected to show a signal change reflecting CSF flow in the phase images. However, some retrocerebellar ACs are associated with CSF, and in such cases, cisternography is not helpful in distinguishing them from MCM. Therefore, the mass effect on the cerebellum and hydrocephalus seen in retrocerebellar ACs may be considered more useful findings in distinguishing MCM from retrocerebellar ACs.
Both ACs and epidermoid cysts can have the same appearance on T1- and T2-weighted images, but epidermoid cysts have a heterogeneous signal, and the ACs do not [16, 58]. After head trauma, subdural hygromas may occur as a result of chronic leakage of CSF from the damaged meninges. They usually contain blood products that alter the fluid signal on T1-weighted and FLAIR sequences, so they typically do not follow CSF signal intensity on MRI. In contrast-enhanced sequences, they may have a peripherally enhanced membrane. Leptomeningeal cysts are also usually posttraumatic, and they are found near posttraumatic encephalomalacia and skull fractures. Cystic tumors include pilocytic astrocytomas and hemangioblastomas. They have brightly enhancing mural nodules and may have calcifications. Hemangioblastomas are associated with von Hippel Lindau disease. On CT, neurenteric cysts are slightly denser than CSF and do not show contrast enhancement. On MRI, they are isointense to hyperintense compared with CSF on T1-weighted images and hyperintense compared with CSF on T2-weighted images. On FLAIR sequences, the fluid content does not suppress. Neuroenteric cysts show facilitated diffusion on DWI/ADC imaging. Neuroglial cysts are located along the neuroaxis and are intraaxial cysts, whereas ACs are usually extraaxial. Porencephalic cysts communicate with the lateral ventricles, and gliosis is seen in the surrounding tissue. Ependymal cysts are located in the periventricular area. Choroidal fissure cysts are located in the choroidal fissure in the medial temporal lobe, where they are particularly common. Cerebral hydatid cysts are usually spherical and cannot be distinguished from ACs. Neurocysticercosis are usually small and may enhance peripherally [30, 58, 59, 61, 87].
For more information on differential diagnosis of intracranial ACs, please refer to chapter “Differential Diagnosis of Intracranial Arachnoid Cysts”.
7 Conclusion
ACs are benign intracranial cystic lesions, usually extraaxial, that are usually diagnosed incidentally during imaging procedures because they are usually asymptomatic [11, 41, 81]. They comprise 1% of all intracranial masses. With the widespread use of prenatal and cross-sectional imaging modalities, many ACs are discovered at a young age. Males are twice more likely to be affected compared to females. Symptoms of an AC depend on its size and/or location. When adjacent neuronal structures are affected, symptoms become more prominent. ACs are prone to rupture and hemorrhage after mild intracranial trauma, and complications such as rupture or hemorrhage require immediate treatment. ACs are usually stable in size, but they rarely regress or enlarge during follow-up. As a result of CSF flow obstruction or cyst enlargement, there may be an increase in intracranial pressure, resulting in headache, dizziness, and tinnitus [4, 85]. MRI is the gold standard and further imaging tool for ACs and distinguishes them from other intracranial cystic lesions [24, 30, 87]. Cisternography is performed for determining surgical indications [26, 88]. If there is a doubt about complication presence, CT is the best faster, cheaper tool for evoluation [30, 87].
References
Albayram S, Kilic F, Ozer H, Baghaki S, Kocer N, Islak C. Gadolinium-enhanced MR cisternography to evaluate dural leaks in intracranial hypotension syndrome. AJNR Am J Neuroradiol. 2008;29(1):116–21.
Algin O, Turkbey B. Intrathecal gadolinium-enhanced MR cisternography: a comprehensive review. AJNR Am J Neuroradiol. 2013;34(1):14–22.
Algin O, Hakyemez B, Parlak M. Phase-contrast MRI and 3D-CISS versus contrast-enhanced MR cisternography on the evaluation of the aqueductal stenosis. Neuroradiology. 2010;52(2):99–108.
Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in children. J Neurosurg Pediatr. 2010;5(6):578–85.
Al-Holou WN, Terman S, Kilburg C, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2013;118(2):222–31.
Arai H, Sato K, Wachi A, Okuda O, Takeda N. Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery. 1996;39(6):1108–12; discussion 12–3.
Auer LM, van Velthoven V. Intraoperative ultrasound (US) imaging. Comparison of pathomorphological findings in US and CT. Acta Neurochir (Wien). 1990;104(3–4):84–95.
Bannister CM, Russell SA, Rimmer S, Mowle DH. Fetal arachnoid cysts: their site, progress, prognosis and differential diagnosis. Eur J Pediatr Surg. 1999;9(Suppl 1):27–8.
Berle M, Wester KG, Ulvik RJ, Kroksveen AC, Haaland OA, Amiry-Moghaddam M, Berven FS, Helland CA. Arachnoid cysts do not contain cerebrospinal fluid: a comparative chemical analysis of arachnoid cyst fluid and cerebrospinal fluid in adults. Cerebrospinal Fluid Res. 2010;7:8.
Blaicher W, Prayer D, Bernaschek G. Magnetic resonance imaging and ultrasound in the assessment of the fetal central nervous system. J Perinat Med. 2003;31(6):459–68.
Bretelle F, Senat MV, Bernard JP, Hillion Y, Ville Y. First-trimester diagnosis of fetal arachnoid cyst: prenatal implication. Ultrasound Obstet Gynecol. 2002;20(4):400–2.
Caldarelli M, Di Rocco C. Surgical options in the treatment of interhemispheric arachnoid cysts. Surg Neurol. 1996;46(3):212–21.
Callen PW. The obstetric ultrasound examination. In: Callen PW, editor. Ultrasonography in obstetrics and gynecology. 5th ed. Philadelphia, PA: Saunders-Elseiver; 2008.
Catala M, Poirier J. [Arachnoid cysts: histologic, embryologic and physiopathologic review]. Rev Neurol (Paris). 1998;154(6–7):489–501.
Chen CP. Prenatal diagnosis of arachnoid cysts. Taiwan J Obstet Gynecol. 2007;46(3):187–98.
Chen CP, Chang TY, Wang W. Third-trimester ultrasound evaluation of arachnoid cysts. Taiwan J Obstet Gynecol. 2007;46(4):427–8.
Chen CY, Wong JS, Hsieh SC, Chu JS, Chan WP. Intracranial epidermoid cyst with hemorrhage: MR imaging findings. AJNR Am J Neuroradiol. 2006;27(2):427–9.
Cinalli G, Peretta P, Spennato P, Savarese L, Varone A, Vedova P, Grimaldi G, Ragazzi P, Ruggiero C, Cianciulli E, Maggi G. Neuroendoscopic management of interhemispheric cysts in children. J Neurosurg. 2006;105(3 Suppl):194–202.
Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109(10):837–43.
Cooper S, Bar-Yosef O, Berkenstadt M, Hoffmann C, Achiron R, Katorza E. Prenatal evaluation, imaging features, and neurodevelopmental outcome of prenatally diagnosed periventricular pseudocysts. AJNR Am J Neuroradiol. 2016;37(12):2382–8.
da Silva N, Adolfo JV, Sarian LO, Cognard C, Sévely A. Magnetic resonance imaging of the fetal brain at 3 Tesla. Medicine. 2018;97(40):e12602.
di Rocco C, Caldarelli M, di Trapani G. Infratentorial arachnoid cysts in children. Childs Brain. 1981;8(2):119–33.
Dubuisson AS, Stevenaert A, Martin DH, Flandroy PP. Intrasellar arachnoid cysts. Neurosurgery. 2007;61(3):505–13; discussion 13.
Dutt SN, Mirza S, Chavda SV, Irving RM. Radiologic differentiation of intracranial epidermoids from arachnoid cysts. Otol Neurotol. 2002;23(1):84–92.
Fewel ME, Levy ML, McComb JG. Surgical treatment of 95 children with 102 intracranial arachnoid cysts. Pediatr Neurosurg. 1996;25(4):165–73.
Galassi E, Tognetti F, Gaist G, Fagioli L, Frank F, Frank G. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol. 1982;17(5):363–9.
García Santos JM, Martínez-Lage J, Gilabert Ubeda A, Capel Alemán A, Climent Oltrá V. Arachnoid cysts of the middle cranial fossa: a consideration of their origins based on imaging. Neuroradiology. 1993;35(5):355–8.
Garg K, Tandon V, Sharma S, Suri A, Chandra PS, Kumar R, Mahapatra AK, Sharma BS. Quadrigeminal cistern arachnoid cyst: a series of 18 patients and a review of literature. Br J Neurosurg. 2015;29(1):70–6.
Gedikbasi A, Palabiyik F, Oztarhan A, Yildirim G, Eren C, Ozyurt SS, Ceylan Y. Prenatal diagnosis of a suprasellar arachnoid cyst with 2- and 3-dimensional sonography and fetal magnetic resonance imaging: difficulties in management and review of the literature. J Ultrasound Med. 2010;29(10):1487–93.
Gosalakkal JA. Intracranial arachnoid cysts in children: a review of pathogenesis, clinical features, and management. Pediatr Neurol. 2002;26(2):93–8.
Grossman TB, Uribe-Cardenas R, Radwanski RE, Souweidane MM, Hoffman CE. Arachnoid cysts: using prenatal imaging and need for pediatric neurosurgical intervention to better understand their natural history and prognosis. J Matern Fetal Neonatal Med. 2022;35(24):4728–33.
Gupta N, Grover H, Bansal I, Hooda K, Sapire JM, Anand R, Kumar Y. Neonatal cranial sonography: ultrasound findings in neonatal meningitis—a pictorial review. Quant Imaging Med Surg. 2017;7(1):123–31.
Hanieh A, Simpson DA, North JB. Arachnoid cysts: a critical review of 41 cases. Childs Nerv Syst. 1988;4(2):92–6.
Helland CA, Wester K. A population-based study of intracranial arachnoid cysts: clinical and neuroimaging outcomes following surgical cyst decompression in children. J Neurosurg. 2006;105(5 Suppl):385–90.
Hoffmann KT, Hosten N, Meyer BU, Röricht S, Sprung C, Oellinger J, Gutberlet M, Felix R. Csf flow studies of intracranial cysts and cyst-like lesions achieved using reversed fast imaging with steady-state precession MR sequences. AJNR Am J Neuroradiol. 2000;21(3):493–502.
Hosny IA, Elghawabi HS. Ultrafast MRI of the fetus: an increasingly important tool in prenatal diagnosis of congenital anomalies. Magn Reson Imaging. 2010;28(10):1431–9.
Ildan F, Cetinalp E, Bağdatoğlu H, Boyar B, Uzuneyüoglu Z. Arachnoid cyst with traumatic intracystic hemorrhage unassociated with subdural hematoma. Neurosurg Rev. 1994;17(3):229–32.
Ishihara M, Nonaka M, Oshida N, Hamada Y, Nakajima S, Yamasaki M. “No-no” type bobble-head doll syndrome in an infant with an arachnoid cyst of the posterior fossa: a case report. Pediatr Neurol. 2013;49(6):474–6.
Jafrani R, Raskin JS, Kaufman A, Lam S. Intracranial arachnoid cysts: pediatric neurosurgery update. Surg Neurol Int. 2019;10:15.
Kane RA, Kruskal JB. Intraoperative ultrasonography of the brain and spine. Ultrasound Q. 2007;23(1):23–39.
Katzman GL, Dagher AP, Patronas NJ. Incidental findings on brain magnetic resonance imaging from 1000 asymptomatic volunteers. JAMA. 1999;282(1):36–9.
Koch CA, Voth D, Kraemer G, Schwarz M. Arachnoid cysts: does surgery improve epileptic seizures and headaches? Neurosurg Rev. 1995;18(3):173–81.
Kornienko VN, Pronin IN. Arachnoid cysts. In: Pronin IN, Kornienko VN, editors. Springer; 2008.
Kumar A, Sakia R, Singh K, Sharma V. Spinal arachnoid cyst. J Clin Neurosci. 2011;18(9):1189–92.
Lena G, van Calenberg F, Genitori L, Choux M. Supratentorial interhemispheric cysts associated with callosal agenesis: surgical treatment and outcome in 16 children. Childs Nerv Syst. 1995;11(10):568–73.
Li C, Yin L, Jiang T, Ma Z, Jia G. Shunt dependency syndrome after cystoperitoneal shunting of arachnoid cysts. Childs Nerv Syst. 2014;30(3):471–6.
Li L, Zhang Y, Li Y, Zhai X, Zhou Y, Liang P. The clinical classification and treatment of middle cranial fossa arachnoid cysts in children. Clin Neurol Neurosurg. 2013;115(4):411–8.
Lütcherath V, Waaler PE, Jellum E, Wester K. Children with bilateral temporal arachnoid cysts may have glutaric aciduria type 1 (GAT1); operation without knowing that may be harmful. Acta Neurochir (Wien). 2000;142(9):1025–30.
Maiuri F, Iaconetta G, Gangemi M. Arachnoid cyst of the lateral ventricle. Surg Neurol. 1997;48(4):401–4.
Martínez-Lage JF, Poza M, Sola J, Puche A. Congenital arachnoid cyst of the lateral ventricles in children. Childs Nerv Syst. 1992;8(4):203–6.
Masselli G, Vaccaro Notte MR, Zacharzewska-Gondek A, Laghi F, Manganaro L, Brunelli R. Fetal MRI of CNS abnormalities. Clin Radiol. 2020;75(8):640.e1–11.
Massimi L, Caldarelli M, Di Rocco C. Intracranial congenital arachnoid cysts. In: Di Rocco C, Pang D, Rutka JT, editors. Textbook of pediatric neurosurgery. Cham: Springer; 2020. p. 789–829.
Mastronardi L, Nardi M, Puzzilli F. Pupillary enlargement caused by an acute extradural haematoma associated with a non-symptomatic arachnoid cyst, from compression of the optic rather than the oculomotor nerve. Br J Neurosurg. 1999;13(3):341–2.
McKinney AM. Mega cisterna magna and retrocerebellar arachnoid cysts. In: Atlas of normal imaging variations of the brain, skull, and craniocervical vasculature. Springer; 2017.
Meyer FB, Carpenter SM, Laws ER Jr. Intrasellar arachnoid cysts. Surg Neurol. 1987;28(2):105–10.
Murakami M, Okumura H, Kakita K. Recurrent intrasellar arachnoid cyst. Neurol Med Chir (Tokyo). 2003;43(6):312–5.
Mustansir F, Bashir S, Darbar A. Management of arachnoid cysts: a comprehensive review. Cureus. 2018;10(4):e2458.
Nadgir R, Yousem D. Congenital anomalies of the central nervous system. In: Yousem D, Nadgir R, editors. Neuroradiology: the requisites the core requisites. 4th ed. Philadelphia, PA: Elseiver; 2016.
Oberbauer RW, Haase J, Pucher R. Arachnoid cysts in children: a European co-operative study. Childs Nerv Syst. 1992;8(5):281–6.
Paladini D, Volpe P. Central and peripheral nervous system anomalies. In: Paladini D, editor. Ultrasound of congenital fetal anomalies. 2nd ed. CRC Press; 2014.
Pascual-Castroviejo I, Roche MC, Martínez Bermejo A, Arcas J, García Blázquez M. Primary intracranial arachnoidal cysts. A study of 67 childhood cases. Childs Nerv Syst. 1991;7(5):257–63.
Patel AP, Oliverio PJ, Kurtom KH, Roberti F. Spontaneous subdural hematoma and intracystic hemorrhage in an arachnoid cyst. Radiol Case Rep. 2009;4(3):298.
Pierre-Kahn A, Hanlo P, Sonigo P, Parisot D, McConnell RS. The contribution of prenatal diagnosis to the understanding of malformative intracranial cysts: state of the art. Childs Nerv Syst. 2000;16(10–11):619–26.
Rabiei K, Jaraj D, Marlow T, Jensen C, Skoog I, Wikkelsø C. Prevalence and symptoms of intracranial arachnoid cysts: a population-based study. J Neurol. 2016;263(4):689–94.
Raffel C, McComb JG. To shunt or to fenestrate: which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery. 1988;23(3):338–42.
Rao G, Anderson RC, Feldstein NA, Brockmeyer DL. Expansion of arachnoid cysts in children: report of two cases and review of the literature. J Neurosurg. 2005;102(3 Suppl):314–7.
Rengachary SS, Watanabe I, Brackett CE. Pathogenesis of intracranial arachnoid cysts. Surg Neurol. 1978;9(2):139–44.
Reports of medical cases, selected with a view of illustrating the symptoms and cure of diseases, by a reference to morbid anatomy. Med Chir Rev. 1831;15(30):289–330.
Russo N, Domenicucci M, Beccaglia MR, Santoro A. Spontaneous reduction of intracranial arachnoid cysts: a complete review. Br J Neurosurg. 2008;22(5):626–9.
Saleem SN. Fetal MRI: an approach to practice: a review. J Adv Res. 2014;5(5):507–23.
Santamarta D, Aguas J, Ferrer E. The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg. 1995;38(4):133–7.
Schachenmayr W, Friede RL. Fine structure of arachnoid cysts. J Neuropathol Exp Neurol. 1979;38(4):434–46.
Seizeur R, Forlodou P, Coustans M, Dam-Hieu P. Spontaneous resolution of arachnoid cysts: review and features of an unusual case. Acta Neurochir (Wien). 2007;149(1):75–8; discussion 78.
Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke’s cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999;84(11):3972–82.
Silbergleit R, Brunberg JA, Patel SC, Mehta BA, Aravapalli SR. Imaging of spinal intradural arachnoid cysts: MRI, myelography and CT. Neuroradiology. 1998;40(10):664–8.
Sprung C, Armbruster B, Koeppen D, Cabraja M. Arachnoid cysts of the middle cranial fossa accompanied by subdural effusions—experience with 60 consecutive cases. Acta Neurochir (Wien). 2011;153(1):75–84; discussion 84.
Standring S. Gray’s anatomy. 39th ed. Elsevier under the Churchill Livingstone imprint; 2011.
Talamonti G, D’Aliberti G, Picano M, Debernardi A, Collice M. Intracranial cysts containing cerebrospinal fluid-like fluid: results of endoscopic neurosurgery in a series of 64 consecutive cases. Neurosurgery. 2011;68(3):788–803; discussion 03.
Tamburrini G, Dal Fabbro M, Di Rocco C. Sylvian fissure arachnoid cysts: a survey on their diagnostic workout and practical management. Childs Nerv Syst. 2008;24(5):593–604.
Thomas BP, Pearson MM, Wushensky CA. Active spontaneous decompression of a suprasellar-prepontine arachnoid cyst detected with routine magnetic resonance imaging. Case report. J Neurosurg Pediatr. 2009;3(1):70–2.
Timor-Tritsch IE, Monteagudo AR, Cohen HL. Ultrasonography of the prenatal and neonatal brain. Appleton & Lange; 1996.
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–8.
Wang HS, Kuo MF, Huang SC, Chou ML, Hung PC, Lin KL. Transcranial ultrasound diagnosis of intracranial lesions in children with headaches. Pediatr Neurol. 2002;26(1):43–6.
Wang X, Chen JX, You C, Jiang S. CT cisternography in intracranial symptomatic arachnoid cysts: classification and treatment. J Neurol Sci. 2012;318(1–2):125–30.
Weber F, Knopf H. Incidental findings in magnetic resonance imaging of the brains of healthy young men. J Neurol Sci. 2006;240(1-2):81–4.
Wester K. Peculiarities of intracranial arachnoid cysts: location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999;45(4):775–9.
Westermaier T, Schweitzer T, Ernestus RI. Arachnoid cysts. Adv Exp Med Biol. 2012;724:37–50.
White ML, Das JM. Arachnoid cysts. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2022, StatPearls Publishing LLC; 2022.
Yildiz H, Erdogan C, Yalcin R, Yazici Z, Hakyemez B, Parlak M, Tuncel E. Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. AJNR Am J Neuroradiol. 2005;26(1):145–51.
Yin L, Yang Z, Pan Q, Zhang J, Li X, Wang F, Ye Y, Deng X, Hu C. Sonographic diagnosis and prognosis of fetal arachnoid cysts. J Clin Ultrasound. 2018;46(2):96–102.
Zada G, Lopes MBS, Mukundan S, Laws E. Sellar region arachnoid cysts. In: Lopes M, Zada G, Mukundan Jr S, Laws Jr E, editors. Atlas of sellar and parasellar lesions. Springer; 2016.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Demir, P.İ., Bilge, A.C., Turgut, A.T. (2023). Radiological Evaluation of Arachnoid Cysts. In: Turgut, M., Akhaddar, A., Turgut, A.T., Hall, W.A. (eds) Arachnoid Cysts. Springer, Cham. https://doi.org/10.1007/978-3-031-22701-1_14
Download citation
DOI: https://doi.org/10.1007/978-3-031-22701-1_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22700-4
Online ISBN: 978-3-031-22701-1
eBook Packages: MedicineMedicine (R0)