Abstract
Introduction
In the current study, we aimed to compare the diagnostic efficacies of phase-contrast magnetic resonance imaging (PC-MRI) and three-dimensional constructive interference in steady-state (3D-CISS) sequence over detection of aqueductal stenosis (AS) on the basis of contrast-enhanced magnetic resonance cisternography (MRC).
Methods
Twenty-five patients with clinically and radiologically suspected AS were examined by PC-MRI, 3D-CISS, and MRC. Axial–sagittal PC-MRI and sagittal 3D-CISS were applied to view the cerebral aqueduct. Following injection of 0.5–1 ml intrathecal gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA) injection, postcontrast MRC images were obtained in three planes in early and late phases. Aqueductal patency was scored as follows: grade 0, normal; grade 1, partial narrowing; and grade 2, complete obstruction. Results of PC-MRI and 3D-CISS were compared with the findings of MRC.
Results
In PC-MRI, seven cases were assessed as grade 0, 16 cases grade 1, and two cases grade 2. As a result of 3D-CISS sequence, eight cases were evaluated as grade 0, 12 cases grade 1, and five cases grade 2. Based on MRC, nine cases were assessed as grade 0, whereas nine and seven cases were evaluated to be grades 1 and 2, respectively. Five cases that demonstrated partial patency in PC-MRI or 3D-CISS showed complete obstruction by MRC.
Conclusion
PC-MRI is helpful in confirming the AS. However, positive flow does not necessarily exclude the existence of AS. 3D-CISS sequence provides excellent cerebrospinal fluid-to-aqueduct contrast, allowing detailed study of the anatomic features of the aqueduct. MRC should be performed on patients who demonstrate suspected AS findings on PC-MRI and/or 3D-CISS sequences.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrocephalus types can be classified in three groups as purely obstructive (e.g., complete aqueductal stenosis), purely communicating (e.g., idiopathic normal-pressure hydrocephalus), or complex (a combination of pathologies, e.g., tubercular meningitis) [1, 2]. Aqueductal stenosis (AS) occurs due to many etiologies among which the most common one is known to be noncommunicating (obstructive) hydrocephalus [3]. Cause of AS is often idiopathic (primary). In some cases, X-linked recessive inheritance has been reported as well [4, 5]. Space-occupying lesions with periaqueductal localization (e.g., cyst and tumor) and trauma or certain infections (e.g., toxoplasmosis, EBV) can lead to AS [4]. Endoscopic third ventriculostomy (ETV) as an alternative to shunt procedures is an established treatment for complete AS [1, 6, 7]. However, patients who have complex hydrocephalus or incomplete AS could result in failure of ETV in spite of a patent stoma. Proper preoperative understanding of the basic pathology or the combination of pathologies leading to hydrocephalus in a given patient is essential to decide the best treatment option, improve postoperative outcome, and avoid an unnecessary operative procedure [1].
Diagnosis of AS and periaqueductal pathologies have been increased after the recent developments in magnetic resonance (MR) technology and availability of cranial MR. First, absence of flow void sign on conventional MR images has been used in AS diagnosis. However, absence of this sign is not specific for AS and can be affected by many other factors [8]. Moreover, routine magnetic resonance imaging (MRI) criteria have been described in AS diagnosis such as triventricular dilatation with a comparatively small fourth ventricle, narrowing of aqueduct in T2-weighted (W) images, and downwards and anterior bulging of third ventricle [6, 8]. Nevertheless, these criteria depend on a subjective neuroradiological evaluation, may be difficult to assess in some patients, and, thus, are hardly comparable in studies on postsurgical outcomes [6, 9].
Phase-contrast MRI (PC-MRI) and three-dimensional constructing imaging in the steady state (3D-CISS) sequence has been increasingly used during the last decade for evaluating cranial and spinal cerebrospinal fluid (CSF) flow [2, 6, 10, 11]. The PC-MRI is extremely sensitive, even to slow flow, and provides the potential for noninvasive flow quantification [11, 12]. The results of these measurements have yielded considerable information on the physiology of the CSF circulation [2, 11]. 3D-CISS is a gradient-echo imaging technique with high resolution, and it allows good visualization of the cerebral aqueduct and diagnosis of the underlying cause of obstruction (cyst, web, tumor), sometimes better than classical sequences [10]. PC-MRI and 3D-CISS sequence have been reported to be useful techniques in AS diagnosis and evaluation of the treatment response as well [2, 6, 11–13].
While computed tomography (CT) and MR ventriculography are recognized as gold standard for AS diagnosis, currently, they are not employed routinely in many clinics due to their highly invasive nature and potential for serious complications [9, 10, 14]. Radionuclide cisternography (RC) and CT cisternography (CTC) have been used as the gold standard in obstructive hydrocephalus; however, failure to show periaqueductal lesions, low soft tissue resolution, and radiation exposure can be mentioned among their disadvantages [4, 15]. On the other hand, MR cisternography (MRC) is a less invasive alternative which has a high soft tissue resolution and does not involve radiation [16, 17]. Recently, MRC has been applied in the investigation of CSF leaks and arachnoid cysts [16, 17]. As far as we know, there is no study on the use of MRC in AS diagnosis in the literature.
In the present prospective study, we aimed to compare the efficacies of PC-MRI and 3D-CISS in the detection of AS on basis of MRC.
Materials and methods
This prospective study was performed between March 2003 and September 2008, which included 34 cases (control and the patient group), referred to our department within this period. Informed consent was taken from all our patients. The approval of the ethical committee of our faculty was obtained for the study protocol.
The patient group
Patient group was consisted of 25 patients (11 women, 14 men; mean age 23 years; range 1–67) who were all diagnosed as probable AS both clinically and according to the routine MRI findings [4, 5, 15]. All of the cases were examined by an experienced neurosurgeon before the MRC examination, and no signs and symptoms of meningitis were detected. Three patients with clinical signs of meningitis were not included in the study. Symptoms and coexisting diseases of the patients were noted before MR examinations.
The control group
The control group consisted of nine cases (five women and four men; mean age 38; range 9–66) who underwent an equivalent MRI protocol with no symptoms except headache. Because of the invasiveness and the need for a contrast agent in the MRC technique, the cases with intracranial arachnoid cyst (AC) constituted the control cases that had no coexisting diseases other than AC. The patients with intraventricular or periventricular AC were excluded from the control group because the AC localization might have affected the CSF flow [17]. Therefore, only cases which had an AC localized in temporal fossa or convexity and those who had no AC compressing the ventricular system or aqueduct were enrolled in the study.
MRI protocol and statistical analysis
The examinations were performed with 1.5-T MRI machine (Magnetom Vision Plus, Siemens, Erlangen, Germany). The patients were in supine position during the examinations. T1W spin echo (SE) images (precontrast MRC) were obtained in axial, sagittal, and coronal planes (TR/TE 539/12 ms, flip angle 90°, matrix 192 × 256, NEX 2, FOV 25 × 25 cm, slice thickness 4 mm except sagittal plane) before contrast medium administration. Slice thickness of sagittal T1W SE images was 2 mm. Axial–sagittal planes T2W turbo gradient SE sequence (TR/TE 7,400/115 ms, FA 160°, NEX 1, FOV 230 mm, matrix 345 × 512, slice thickness 2 mm) and sagittal 3D-CISS sequence (supplemented by axial and coronal reformations in the cerebral aqueduct axis) were obtained. The reconstruction was performed using a built-in multiplanar reconstruction software program. Parameters of 3D-CISS sequence were as follows: TR/TE 12.3/5.90 ms, flip angle 70°, matrix 230 × 512, NEX 2, effective thickness 0.7 mm, FOV 26 × 26 cm, slab thickness 48 mm. The sections included the level of aqueductal canal and posterior third-superior fourth ventricles. T1W, T2W, and 3D-CISS images were evaluated by an experienced neuroradiologist blinded to PC-MRI and MRC results. Evans index was obtained as the ratio of maximal width of both anterior horns of the ventricles to the diameter of the inner table of skull along the line of measurement of the anterior horn transverse dimension [2]. Evans index >3 was recognized as hydrocephalus.
Two-dimensional fast imaging with steady-state precession sequence with retrospective cardiac gating in axial–sagittal planes was applied for PC-MRI images. Sequence parameters were as follows: TR/TE/NEX 70/15.8/2, echo delay time 13 ms, flip angle 10°, matrix 192 × 256, FOV 25 cm, slice thickness 2 mm, venc 2 cm/s. Sagittal plane images were obtained as to encompass the cerebral aqueduct, whereas axial images were acquired from the narrowest portion of cerebral aqueduct on the sagittal plane. The axial plane was selected perpendicular to the presumed direction of the CSF flow. Twelve cine images were obtained involving the systolic and diastolic phases of cardiac pulse. CSF flow on reconstructed PC-MRI images and T1W–T2W images was evaluated by an experienced neuroradiologist blinded to the 3D-CISS and MRC results. Sequential cine images obtained from systolic and diastolic phases were investigated for presence of an AS. Presence of clearly visible black and white CSF flow shapes corresponding to systolic and diastolic flows within the aqueduct were recognized as a sign of patent aqueduct [11]. The acquisition time of T1W (precontrast MRC), T2W, 3D-CISS, and PC-MRI sequences was approximately 20 min.
For postcontrast MRC, 0.5–1 ml gadopentetate dimeglumine (Magnevist, Schering, Germany) was injected by a 24–26 gauge needle from intrathecal space at lower lumbar region (L4–L5), and the patient was allowed to rest in prone position in the observation room. Postcontrast images were obtained by repeating precontrast images with the same parameters in three planes during early (3–6 h) and delayed (12–24 h) phases. Detection of contrast medium flowing into the third ventricle on postcontrast images was interpreted as the sign of a patent aqueduct, and the examination was ended. Duration of each postcontrast MRC was approximately 10 min. The patients were then clinically monitored for 48 h after the MRC. MRC examinations were evaluated by an experienced radiologist unaware of the results of 3D-CISS sequence and PC-MRI.
While evaluating 3D-CISS, PC-MRI, and MRC regarding aqueductal patency, visual scoring was performed as explained below.
The grading of aqueductal patency by PC-MRI
-
Grade 0:
systolic and diastolic flows are clearly visible in the cerebral aqueduct
-
Grade 1:
aqueductal flow is narrowed and hardly visible (suspected patency)
-
Grade 2:
no aqueductal flow at systole or diastole
The grading of aqueductal patency by 3D-CISS
-
Grade 0:
aqueduct width is normal and aqueductal canal is patent. No web or contour irregularity is observed in the lumen
-
Grade 1:
Narrowed but patent aqueductal lumen
-
Grade 2:
Aqueductal lumen is occluded or there is a stenosis–prestenotic dilatation secondary to web
The grading of aqueductal patency by MRC
-
Grade 0:
aqueductal lumen width is normal. Contrast material flows into the third ventricle on early-phase images.
-
Grade 1:
narrowed lumen width and contrast material flows into the third ventricle on late-phase images.
-
Grade 2:
lumen demonstrates a marked narrowing. Contrast material migration is not observed into the third ventricle on early- and late-phase images
Statistical analysis was performed by SPSS 13.0 (SPSS Inc., Chicago, IL, USA). The comparison of scores between the three groups was performed with the Mann–Whitney U test. The statistically significant level was set at p < 0.05. Sensitivity, specificity, predictive values, and accuracy were calculated according to Nilsson [18]. While calculating those values, MRC was recognized as the gold standard in AS diagnosis. In MRC examinations, cases assessed as grades 0 and 1 had patent aqueduct, whereas cases with complete stenosis were evaluated as grade 2.
Results
Demographic–clinical data, existence of hydrocephalus, and symptoms of the case group are summarized in Table 1. Headache (15 cases, 60%) was the most common symptom. Eighteen cases (72%) had hydrocephalus.
MRC findings
MRC studies were well tolerated by all controls and patients. During the 48-h clinical follow-up period after MRC examination, no cases showed any neurological symptoms, epilepsy, or allergic reactions. Five patients and two controls (20%) had postural headache which responded well to conventional painkillers or spontaneously resolved in 48 h. Contrast material enhancement in third ventricle on early-phase images in all the controls was observed (grade 0).
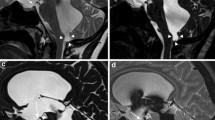
Based on the MRC studies performed on the patient group, nine cases were evaluated as grade 0, whereas nine and seven cases were assessed as grades 1 and 2, respectively (Table 2). All the cases that have been assessed to be grade 2 in MRC studies were operated on. Ventriculoperitoneal shunting (VPS) and ETV operations were performed on seven cases that have been evaluated as grade 0 or 1 in the MRC. One case, assessed as grade 1 in the MRC study (case 7), developed spontaneous ventriculostomy (Fig. 1). This case and other cases which were submitted to no surgery were clinically followed up. In brief, among the patient group, 11 cases were followed up and 14 cases were operated on. Following surgery, symptoms and Evans indexes of all these cases showed a reduction.
A 2-year-old male patient subjected to MRI by the prediagnosis of aqueductal obstruction (no. 7). Axial, sagittal T1W (a, b), axial T2W (c), and sagittal 3D-CISS images (d) showing AC in the posterior portion of third ventricle (septum pellucidum cyst, arrow). Narrowed but patent (grade 1) aqueduct on 3D-CISS images (d). Sagittal PC-MRI images (e, f) showing minimal, suspected aqueductal flow (arrow; grade 1). Sagittal–coronal early postcontrast images displaying no flow of contrast medium into the third ventricle (g, h). Axial delayed phase image demonstrating flow of contrast material into the third ventricle and lateral ventricles (grade 1; i). Moreover, signal changes consistent with spontaneous third ventriculostomy are observed at the suprasellar–prepontine cistern levels (d, e, f)
PC-MRI findings
All the cases in the control group exhibited black and white flow in the aqueduct (grade 0). Seven cases in the patient group were evaluated as grade 0, whereas 16 and two cases were classified as grades 1 and 2, respectively. PC-MRI and MRC results of 18 cases (72%) were totally consistent (Table 2). Two cases which have been assessed as grade 1 in the PC-MRI study were evaluated as normal by the MRC and 3D-CISS examinations (grade 0). Five cases showing partial stenosis in PC-MRI (grade 1) were evaluated as grade 2 by MRC (false negative; Fig. 2). There was no significant difference between PC-MRI scores and grades of MRC for AS (P > 0.05). The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of PC-MRI regarding demonstration of AS were 29%, 100%, 78%, 100%, and 80%, respectively.
A 20-year-old male patient examined due to headache (no. 17). T2W images showing hydrocephalus (Evans index 0.45; a, b). Sagittal PC-MRI images exhibiting minimal aqueductal narrowing with CSF flow (grade 1; c, d). Web is observed on sagittal 3D-CISS image (arrow). Prestenotic dilatation is present in the aqueduct (grade 2; e). Postcontrast MRC images showing no flow of contrast medium into the third ventricle (grade 2). f, g Coronal early-phase MRC images, h axial delayed phase MRC image, i, j sagittal early-phase MRC images, k, l sagittal delayed phase MRC images
3D-CISS sequence findings
Aqueductal width was normal in all the controls (grade 0). No lesion, narrowing the aqueduct, was found in all the controls. Eight cases in the patient group were classified as grade 0, whereas 12 and five cases were evaluated as grade 1s and 2, respectively (Table 2). 3D-CISS sequence and MRC examination results of 23 cases (92%) were totally consistent (Figs. 3 and 4). Two cases, assessed as grade 1 in the 3D-CISS examination, were evaluated as grade 2 by MRC (false negative). There was no significant difference between 3D-CISS sequence findings and results of MRC (P > 0.05). The sensitivity, specificity, negative predictive value, positive predictive value, and accuracy of 3D-CISS regarding demonstration of AS were 71%, 100%, 90%, 100%, and 92% respectively.
An 11-year-old male patient subjected to MRI because of vomiting and headache (no. 8). Precontrast axial T1W image showing hydrocephalus (a). Sagittal 3D-CISS image displaying normal aqueduct patency (grade 0; b). Sagittal and axial PC-MRI images (c, d) showing normal aqueduct patency and presence of turbulent flow (arrows; grade 0). Axial–sagittal early-phase MRC images (e, f) demonstrating flow of contrast medium into the third ventricle at normal time (grade 0). Moreover, aqueductal flow void phenomenon is observed on sagittal 3D-CISS (b) and sagittal early-phase MRC images (e)
A 9-year-old male patient (no. 4). Sagittal and coronal images (a, b) showing marked narrowing of the aqueduct (grade 2). Compressed cerebral aqueduct by the arachnoid cyst extending from right lateral ventricle to right cerebellopontine angle (b). Sagittal PC-MRI image displaying no flow in the aqueduct (grade 2; c). MRC examination result (d precontrast sagittal image, e–g early-phase images, h, i delayed phase images) showing no flow of contrast medium into the third ventricle (grade 2)
Discussion
The cerebral aqueduct is one of the narrowest channels through which the CSF in the ventricles must flow [5]. AS may occur in utero as well as postnatally and may arise due to a variety of causes [15]. Tectal glioma, webs, ACs, neurofibromatosis type 1, and inflammatory disorders can cause AS [5, 10]. AS may be of partial or complete nature and is seen in approximately 10% of hydrocephalus cases [4]. Generally, the disease demonstrates a silent course. Clinical presentation may reveal itself at any time [9]. Clinically and radiologically, a critical distinction should be made between benign and neoplastic causes of obstruction or communicating and noncommunicating hydrocephalus [1, 15]. However, a patient with AS presents with various clinical and radiological features [6].
Diagnosis of AS and periaqueductal abnormalities have been increased after the recent developments in MR technology and availability of cranial MRI in routine studies. All patients with AS should undergo MR examination in order to allow more definitive evaluation of the cerebral aqueduct and tectum [15]. Routine MRI provides useful information in AS because it can show triventricular dilation, CSF pathway obstruction at the aqueductal level on sagittal T2W sequences, downward bulging of the floor of the third ventricle, anterior bulging of the third ventricle, etc. [6, 19]. All of these signs are operator dependent, difficult to reproduce, and lack validated cutoff values [6]. Moreover, these criteria prove to be inefficient in evaluation of the treatment response and patency of aqueduct following endoscopic aqueductoplasty [11, 12]. In brief, diagnosis of AS, determination of the pathology underlying AS, and assessment of the treatment response by routine cranial MRI are very difficult for radiologists.
Contrast-enhanced CT or MR ventriculography have been used to directly assess aqueductal patency, but these are invasive techniques that require a patent ventricular catheter for instillation of the contrast material and is currently considered only in selected cases [10, 14]. On the other hand, RC and CTC have been used in the diagnosis of obstructive hydrocephalus; however, they have been found to have some disadvantages such as failure to show periaqueductal lesions, low soft tissue resolution, and radiation exposure [4, 14, 15]. MRC is a less invasive alternative with a high soft tissue resolution which allows multiplanar images and involves no radiation [16, 17]. Moreover, MRC is a modality that can globally evaluate the CSF circulation and provide physiological information as well as morphological data. Recently, contrast-enhanced MRC is used in the investigation of CSF leaks and communicating ACs [16, 17]. Although intrathecal gadolinium application has not been recognized worldwide, it has been found to be safe in preliminary studies [14, 16, 17]. In the present study, except postural headache, no side effects or complications were observed during the 48-h clinical follow-up period after MRC examinations. Postural headache is secondary to intrathecal intervention. Headache observed following MRC is milder than the headache experienced after CTC [17]. More rapid closure of dural puncture due to usage of a thinner lumbar puncture needle (24–26 gauge) in MRC may be the reason behind this result. Also, the amount of iodinated contrast material for CTS is much more than the amount used for contrast-enhanced MRC. High viscosity and volume of the contrast material used for CTC pose practical difficulties, and larger needles (18 gauge) are used.
Gadolinium is a paramagnetic contrast medium that shortens the T1 (lower concentrations) and T2 (higher concentrations) relaxation times of CSF [17]. In our study, 0.5–1 ml contrast medium diffused in the ventricular system and details of aqueductal canal and periaqueductal CSF spaces became clearly visible. In the current study, MRC examination successfully helped us to evaluate CSF flow and existence of physiological obstructions at the aqueduct level.
Recently, 3D-CISS sequence has been introduced to assess the normal and pathological features of the inner ear structures and cerebellopontine angle and proven to be superior to the conventional T2W sequence because of the heavy T2W effect and high spatial resolution [10, 14]. 3D-CISS is a gradient-echo imaging technique with high CSF-to-aqueduct contrast [17]. It enables locating the obstruction and determining the upstream impact. It provides anatomical information about morphology relationships of aqueduct before surgery [13]. Doll et al. conducted a study on 20 obstructive hydrocephalus patients and found 3D-CISS more effective in showing the aqueductal morphology and the cause of the obstruction compared to the classical sequences [10, 13]. The efficiency of this imaging technique for patency of endoscopic third ventriculostomy has also been reported [10, 13]. In the present study, 92% of the cases in the patient group showed consistent 3D-CISS and MRC results. In all the cases that showed complete obstruction (grade 2) in the aqueduct by 3D-CISS, no contrast material flow into third ventricle was observed by MRC examinations as well (grade 2). All the cases that were grade 0 in 3D-CISS were grade 0 in MRC results as well. Two cases (8%) demonstrated partial narrowing (grade 1) in the aqueductal lumen by 3D-CISS. MRC examinations of those two cases revealed no contrast material flow into the third ventricle (grade 2). In conclusion, we can say that MRC would be unnecessary if 3D-CISS sequences show complete obstruction or normal aqueduct. In case of suspected partial narrowing, presence of stenosis should be verified with MRC.
PC-MRI has been increasingly used during the last decade for evaluating CSF flow [2, 6, 11]. The flow void phenomenon was first defined on T2W sequences in the cerebral aqueduct [8]. The visualization of this effect on routine T1W or T2W MR images is not consistent. However, on sagittal T2W MR images, an aqueductal flow void sign can be detected in normal cases [11]. Also, the flow void phenomenon is a subjective parameter, being affected by technical factors like acquisition parameters, power of the gradient, flow compensation, and gradient moment nulling technique [6, 8].
PC-MRI enables reliable, noninvasive, and fast measurement of CSF flow and is sensitive even to slow CSF flows as seen in the aqueduct [6]. PC-MRI examination for CSF flow has been reported to be sensitive to the obstruction of the cerebral aqueduct [9]. CSF flow within the aqueduct is best described as a to-and-fro motion (black and white flow) with a very small net flow by cardiac pulsations [6, 11]. Studies indicate that if black and white flow in the aqueductal canal can be seen clearly by PC-MRI, then aqueduct can be evaluated as patent [11]. Fukuhara et al. reported that no flow at the cerebral aqueduct can be a supportive finding for AS and that AS may still exist even with some positive CSF flow [9]. The results of our study support the findings of the study of Fukuhara et al. In five cases that demonstrated grade 1 narrowing in the PC-MRI, we determined grade 2 stenosis by MRC (false negative). Turbulent flow in the narrowing site can be the underlying reason of this inconsistency [20]. Therefore, among cases displaying a flow in the aqueductal canal in the PC-MRI, if narrowing or obstruction is suspected in 3D-CISS, then aqueductal patency should be evaluated by MRC.
In our study, MRC and 3D-CISS examinations of two cases, evaluated as grade 1 by PC-MRI, assessed a normal aqueduct (grade 0). Therefore, cases that exhibit partial obstruction or suspected flow in the PC-MRI should be evaluated by 3D-CISS. In case aqueduct is found patent with 3D-CISS in those cases, then MRC can be deemed as unnecessary. MRC should be performed for AS diagnosis in cases where PC-MRI shows partial obstruction or suspected flow and 3D-CISS displays narrowed aqueduct or aqueductal web.
Original CTC studies outline the flow of contrast agent, delivered by intrathecal route, within CSF in detail [21, 22]. The finding in communicating hydrocephalus was the delay or absence of contrast reaching the vertex associated with a lack of parasagittal blushing [21]. Takahashi et al. reports no filling of the cortical sulci or parasagittal area with poor staining of the cortex, despite good staining in the sylvian fissures in cases with communicating hydrocephalus [22]. We determined communicating hydrocephalus coexisting with obstructive hydrocephalus (complex hydrocephalus) in eight cases (44%) of our study (Figs. 1i, 2f, g, and 4f, g). In such cases, VPS practice is mentioned to be more effective than ETV [1]. Lack of long-term postoperative clinical follow-up results is an important limitation of our study.
One of the limitations of our study was the failure of comparing 3D-CISS, PC-MRI, and MRC results with the findings of CT or MR ventriculography, recognized as the gold standard in AS diagnosis, due to ethical concerns. Another important limitation was the failure to evaluate aqueductal morphology intraoperatively due to ETV and VPS being the operation procedure, which prevented us from comparing the operation findings with the results of 3D-CISS, PC-MRI, and MRC. We believe that, in order to determine the roles of 3D-CISS, PC-MRI, and MRC, there is a need for further comprehensive studies encompassing comparison of these MRI techniques results with the findings of the endoscopic ventriculoplasty postoperative results.
Conclusion
3D-CISS sequence and PC-MRI are effective, practical, and noninvasive modalities in evaluation of the AS. Therefore, they should be applied first for detection of AS following routine cranial MRI. Because CSF hydrodynamics frequently alter and turbulent flow is often observed in aqueductal canal and periaqueductal spaces, PC-MRI may be insufficient in some cases. While 3D-CISS displays the aqueductal morphology well, it falls short for revealing the physiological properties in presence of partial stenosis. MRC may prevent these false-negative results. During evaluation of aqueductal patency where 3D-CISS or PC-MRI yields suspected results, MRC should be preferred instead of CTS because it is free from radiation/bony artifacts, can be applied more easily/tolerable, and provide us multiplanar images. In addition, intrathecal administration of Gd-DTPA was safe and thus consistent with the recent literature. MRC has a potential to become the preferred method of evaluating patients with abnormal CSF dynamics.
References
Yadav YR, Mukerji G, Parihar V, Sinha M, Pandey S (2009) Complex hydrocephalus (combination of communicating and obstructive type): an important cause of failed endoscopic third ventriculostomy. BMC Res Notes. doi:10.1186/1756-0500/2/137
Bateman GA (2007) Magnetic resonance imaging quantification of compliance and collateral flow in late-onset idiopathic aqueductal stenosis: venous pathophysiology revisited. J Neurosurg 107:951–958
Allan R, Chaseling R, Graf N, Dexter M (2005) Aqueduct stenosis—benign? J Clin Neurosci 12:178–182
Tisell M (2005) How should primary aqueductal stenosis in adults be treated?—a review. Acta Neuro Scand 111:143–153
Grossman RI, Yousem DM (2003) Neuroradiology the requisites, 2nd edn. Mosby Elsevier, Philadelphia, pp 372–373
El Sankari SS, Lehmann P, Gondry-Jouet C, Fichten A, Godefroy O, Meyer ME, Baledent O (2009) Phase-contrast MR imaging support for the diagnosis of aqueductal stenosis. AJNR Am J Neuroradiol 30:209–214
Da Silva LRF, Cavalheiro S (2007) Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst 23:1263–1268
Atlas SW, Mark AS, Fram EK (1998) Aqueductal stenosis: evaluation with gradient-echo rapid MR imaging. Radiology 169:449–453
Fukuhara T, Luciano MG (2001) Clinical features of late-onset idiopathic aqueductal stenosis. Surg Neurol 55:132–136
Aleman J, Jokura H, Higano S, Akabane A, Shirane R, Yoshimoto T (2001) Value of constructive interference in steady-state, three-dimensional, Fourier transformation magnetic resonance imaging for the neuroendoscopic treatment of hydrocephalus and intracranial cysts. Neurosurgery 48:1291–1295
Schroeder HWS, Schweim C, Schweim KH, Gaab MR (2000) Analysis of aqueductal cerebrospinal fluid flow after endoscopic aqueductoplasty by using cine phase-contrast magnetic resonance imaging. Neurosurg Focus 9:1–8
Schroeder HWS, Oertel J, Gaab MR (2004) Endoscopic aqueductoplasty in the treatment of aqueductal stenosis. Childs Nerv Syst 20:821–827
Doll A, Christmann D, Kerhli P, Abu Eid M, Gillis C, Bogorin A, Thiebaut A, Dietemann JL (2000) Contribution of 3D-CISS MRI for the post therapeutic monitoring of obstructive hydrocephalus. J Neuroradiol 27:218–225
Joseph VB, Raghuram L, Korah IP, Chacko AG (2003) MR ventriculography for the study of CSF Flow. AJNR Am J Neuroradiol 24:373–381
Lee HS, Rao KCVG, Zimmerman RA (1999) Cranial MRI and CT, 4th edn. McGraw-Hill, New York, pp 173–241
Aydin K, Terzibasioglu E, Sencer S, Sencer A, Suoglu Y, Karasu A, Kiris T, Turantan MI (2008) Localization of cerebrospinal fluid leaks by gadolinium-enhanced magnetic resonance cisternography: a 5-year single-center experience. Neurosurgery 62:584–589
Algin O, Hakyemez B, Gokalp KE, Parlak M (2009) Phase-contrast cine MRI versus MR cisternography on the evaluation of the communication between intraventricular arachnoid cysts and neighbouring cerebrospinal fluid spaces. Neuroradiology 51:305–312
Nilsson S, Ortoft K, Mölstad S (2008) The accuracy of general practitioners' clinical assessment of chest pain patients. Eur J Gen Pract 14:50–55
Kehler U, Regelsberger J, Gliemroth J, Westphal M (2006) Outcome prediction of third ventriculostomy: a proposed hydrocephalus grading system. Minim Invasive Neurosurg 49:238–243
Kadowaki C, Hara M, Numoto M, Takeuchi K, Saito I (1995) Cine magnetic resonance imaging of aqueductal stenosis. Childs Nerv Syst 11:107–111
Drayer BP, Rosenbaum AE, Higman HB (1977) Cerebrospinal fluid imaging using serial metrizamide CT cisternography. Neuroradiology 28:7–17
Takahashi M, Arii H, Tamakawa Y (1978) Comparison of metrizamide CT cisternography with radionuclide cisternography in abnormal cerebrospinal fluid dynamics. Neuroradiology 16:199–202
Acknowledgement
We gratefully acknowledge Gokhan Ocakoglu (biostatistician), Gokhan Gokalp (radiologist), and Ender Korfalı (neurosurgeon) for their suggestions and review of the manuscript.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Algin, O., Hakyemez, B. & Parlak, M. Phase-contrast MRI and 3D-CISS versus contrast-enhanced MR cisternography on the evaluation of the aqueductal stenosis. Neuroradiology 52, 99–108 (2010). https://doi.org/10.1007/s00234-009-0592-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0592-x