Abstract
Splenic injuries are among the most frequent trauma-related injuries. The optimal treatment strategy for splenic trauma patients has shifted towards a predominantly nonoperative approach. This has been made possible with close monitoring of the patients and the use of adjuncts such as angioembolization. There is however, still a role for splenectomy in penetrating injuries and in unstable patients. The final management pathway taken, is ultimately based on the physiology of the patient, the anatomy of the injury, and the associated lesions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Learning Goals
-
To understand the AAST and WSES classification of splenic injury.
-
To be able to recognize patients suitable for nonoperative management [NOM] and understand the nuances of NOM.
-
To have a good grasp on the latest evidence regarding angioembolization and its role in NOM.
-
To understand the indications for splenectomy.
2 Epidemiology
There is currently no consensus on the overall incidence of splenic injury. According to a European study, the incidence of blunt splenic injury is low, but it accounts for significant mortality [1]. Studies from the United States show that the overall mortality of blunt splenic injury varies from an average of 8.2 to 13% from 1981 to 2000 [2]. In a Taiwanese study, the incidence of blunt splenic injury was also not common (8.33 per million/year), with injured patient numbers being consistent every year [3].
3 Etiology
The spleen is typically injured when there is trauma involving the lower left chest or the upper left abdomen [4, 5], primarily because of its juxtaposition in the left upper abdomen to the 9th, 10th, and 11th ribs.
The three mechanisms of injury:
-
Penetrating trauma, e.g. abdominal gunshot wounds.
-
Blunt trauma, e.g. a punch or kick to the abdomen.
-
Indirect trauma, e.g. a tear in the splenic capsule during colonoscopy or traction on the splenocolic ligament.
Most mechanisms of injuries are similar between children and adults. These include motor vehicle crashes and pedestrian accidents. Conversely, certain mechanisms of injury such as motorcycle accidents, sports injuries, gunshots or stab-related injuries, and assaults are more frequent in adults [6].
4 Classification
The traditional classification system is the AAST system (Table 96.1) which takes the anatomical insult as the main consideration in injury grading. However, this does not take into account the overall clinical status of the patient.
The World Society of Emergency Surgery (WSES) has recently published an updated classification, that factors the clinical picture into the management algorithm [7]. The WSES classification is as follows (Table 96.2):
-
Minor (WSES class I) includes hemodynamically stable AAST grade I–II blunt and penetrating lesions.
-
Moderate (WSES classes II) includes hemodynamically stable AAST grade III blunt and penetrating lesions.
-
Moderate (WSES classes III) includes hemodynamically stable AAST grade IV–V blunt and penetrating lesions.
-
Severe (WSES class IV) includes hemodynamically unstable AAST grade I–V blunt and penetrating lesions.
5 Diagnosis
5.1 Clinical Presentation
Patients will present with a history of trauma, abdominal pain, and varying stages of shock.
5.2 Diagnostic Imaging
-
1.
Extended focused assessment sonography for trauma (E-FAST) has replaced diagnostic peritoneal lavage (DPL) in the management of abdominal trauma [8,9,10]. Studies have shown a sensitivity of up to 91% and a specificity of up to 96% for a small fluid amount [11, 12]. The E-FAST can detect the presence of free fluid and can also provide an ultrasonographic image of the spleen itself. Moreover, the E-FAST is readily available at the bedside, thus increasing its utility and applicability.
-
2.
Contrast tomography (CT) scan is considered the gold standard in trauma with a sensitivity and specificity for splenic injuries near to 96–100% [9, 13, 14]. However, hilar injuries may be underestimated [9]. The main considerations prior to using the CT scanner, are that the scanner must be rapidly available and must be performed only in hemodynamically stable patients or in those responding to fluid resuscitation [15, 16]. The CT scan is particularly useful as it can help delineate the anatomy of the injured spleen, which helps in the AAST grading of the injury. The delayed phase can also further differentiate patients with active bleeding from those with contained vascular injuries [17].
The identification of an active contrast extravasation is a classic sign of active hemorrhage [18]. Contrast blush occurs in about 17% of cases and has been demonstrated to be an important predictor of failure of NOM (>60% of patients with blush failed NOM). However, the absence of a blush on the CT scan in high-grade splenic injuries does not definitively exclude active bleeding and should not preclude angioembolization [13, 19, 20].
6 Management
6.1 Nonoperative Management (NOM) for Blunt Splenic Trauma
For hemodynamically stable patients, with the absence of other abdominal organ injuries that require surgery, these patients should undergo a trial of NOM regardless of injury grade [13, 21,22,23,24]. The caveat is that the hospital must have the capability for intensive monitoring, facilities and expertise for angioembolization, the ready access to available operating theatres, and immediate access to blood products. The presence of a CT scanner is paramount, as a baseline CT scan with intravenous contrast is necessary to define the anatomical splenic injury and to identify associated injuries.
The success rate of NOM in such circumstances is approximately 90% [25]. The advantages of NOM include reduced hospital costs, avoidance of nontherapeutic laparotomies, lower rates of blood transfusions, lower mortality, and the prevention of overwhelming post-splenectomy infection [OPSI] [23, 26, 27]. Routine laparotomy in hemodynamically stable patients with blunt splenic injury is not indicated [28, 29].
Risk factors for NOM failure include age > 55 years old, high ISS, and moderate to severe splenic injuries [15, 37, 40]. Other relative risk factors for NOM failure include age > 55 years old alone, large hemoperitoneum alone, hypotension before resuscitation, GCS < 12, low hematocrit level upon admission, associated abdominal injuries, blush at CT scan, anticoagulation drugs, HIV disease, drug addiction, cirrhosis, and need for blood transfusions [13, 17, 18, 25, 30,31,32,33,34,35,36,37,38,39,40].
An exception however exists for patients with WSES classes II–III spleen injuries with associated severe traumatic brain injury. In these patients, NOM could be considered only if absolutely efficient and rapid rescue therapy is available; otherwise, splenectomy should be performed.
6.2 Nonoperative Management (NOM) for Penetrating Trauma
Laparotomy has been the gold standard in penetrating abdominal trauma [e.g., gunshot and stab wounds]. Overall, the rate of negative laparotomy ranges between 9% and 14% [41, 42] in these cases. However, if the patient is found to have concomitant pancreatic, diaphragmatic, colic, and splenic injuries, they tend to have a significantly increased mortality rate [43]. The associated pancreatic injuries also frequently require spleno-pancreatectomy [43]. Although there is a trend toward adopting NOM for gunshot and stab injuries [44, 45], the decision for NOM in penetrating trauma should be still decided on a case-by-case basis.
6.3 Role of Angiography and Angioembolization [AG/AE] in NOM
The main indications for AG/AE are [46,47,48]:
-
1.
WSES I and II patients who have vascular injuries detected via CT scan (contrast blush, pseudo-aneurysms, and arteriovenous fistula). Hemodynamically stable patients with WSES class I and II lesions without blush should not undergo routine AG/AE but may be considered for prophylactic proximal embolization in presence of risk factors for NOM failure.
-
2.
WSES III patients who are hemodynamically stable regardless of the presence of CT blush.
-
3.
Patients who are stable with signs of persistent hemorrhage regardless of the absence of CT blush once extra-splenic source of bleeding has been excluded.
Some considerations during AG/AE:
-
1.
In presence of a single vascular abnormality (contrast blush, pseudo-aneurysms and arteriovenous fistula) in minor and moderate injuries, it is unclear whether proximal or distal embolization should be adopted [49]. Both methods were found to be similar with regard to the incidence of major infarctions, infections, and major rebleeding [50].
-
2.
In presence of multiple splenic vascular abnormalities or in presence of a severe lesion, proximal or combined AG/AE should be used, after confirming the presence of a permissive pancreatic vascular anatomy. Or in the absence of blush during angiography, if a blush was previously seen at CT scan, proximal angioembolization could be considered.
-
3.
In performing AG/AE, coils should be preferred to temporary agents.
-
4.
Conversely, when AG/AE is not rapidly available or in event of rapid hemodynamic deterioration, surgery should be considered.
The reported success rate of NOM with AG/AE ranges from 86 to 100% [46,47,48, 51,52,53,54,55,56,57,58]. AG/AE reduces the odds of splenectomy, with better results, the earlier the AG/AE was performed [58, 59]. Meta-analyses have shown a significant improvement in NOM success following introduction of AG/AE protocols (OR 0.26, 95% CI 0.13–0.53, p < 0.002) [37, 60,61,62].
Between 2.3 and 47% CT detected, contrast blushes could not be confirmed during angiography [63, 64]. Moreover an analysis on 143 patients with blush at CT scan suggested that an angiographic procedure without embolization increases twofold the risk of rebleeding and NOM failure [64].
The use of routine prophylactic AG/AE in high-grade splenic injuries is controversial [19, 46, 48, 54, 65,66,67,68]. NOM failure rates both with and without prophylactic AG/AE for high-grade injuries are 0–42% vs. 23–67%, respectively [19, 46, 48, 54, 65, 66]. Controversies exist regarding which kind of lesions should be considered as “high-grade” (AAST III–V or IV–V grade) and should undergo routine AG/AE [19, 46, 67, 68]. It has been reported that NOM could fail in up to 3% of grade III lesions without blush, with no AG/AE [19]. Considering the AG/AE-related morbidity of 47% (versus 10% related to NOM without AG/AE) [68], patients with grade III lesions without blush should not undergo routine AG/AE.
AG/AE major morbidity rates range from 3.7 to 28.5% including rebleeding, splenic infarction, splenic abscesses, acute renal insufficiency, pseudocysts, and puncture-related complications [19, 46, 48, 69,70,71,72,73,74,75,76]. The rates for minor morbidities range from 23 to 61% and include fever, pleural effusion, and coil migration [48, 69, 75, 76]. Comparatively, patients undergoing OM still reported significantly higher complication rates as compared to those who had AG/AE [68, 70, 71, 74].
AG/AE does not seem to totally compromise the splenic function, and, even in presence of an elevated leukocyte and platelet counts, no significant differences in immunoglobulin titers were found between splenic artery AG/AE patients and controls [66]. The spleen due to its intense vascularization, can maintain the necessary bloodflow to continue its immunological function.
6.4 Operative Management (OM)
The main indications of OM include:
-
1.
Patients with hemodynamic instability or with associated lesions like peritonitis or bowel evisceration requiring surgical exploration. The severity of splenic injury seems to be related to the incidence of hollow viscus injury (1.9, 2.4, 4.9, and 11.6% in minor, moderate, major, and massive injuries, respectively) [77].
-
2.
OM should be performed in moderate and severe lesions even in stable patients, in centers where intensive monitoring cannot be performed and/or when AG/AE is not rapidly available [78, 79].
-
3.
When NOM with AG/AE fails and patient remains hemodynamically unstable or shows a significant drop in hematocrit levels or continuous transfusion is required, OM is also indicated.
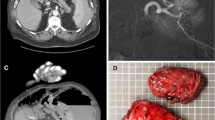
During OM, salvage of a part of the spleen is controversial [80, 81]. The use of splenic autologous transplantation—leaving pieces of spleen inside the abdomen—to avoid infective risk from splenectomy has not been shown to reduce morbidity or mortality [82]. Overall mortality of splenectomy in trauma is approximately 2%, and the incidence of postoperative bleeding after splenectomy ranges from 1.6 to 3%, but with mortality near to 20% [83]. Laparoscopic splenectomy in bleeding trauma patients is not recommended, and open splenectomy is mandatory [84, 85] (Fig. 96.1).
WSES spleen trauma management algorithm for adult patients is reported in Fig. 96.2.
6.5 Thromboprophylaxis in Splenic Trauma
Trauma patients are at high risk of venous thromboembolism (VTE). The transition to a hypercoagulation state occurs within 48 h from injury [86,87,88]. For patients who survive beyond the first 24 h, pulmonary embolism (PE) is the third leading cause of death. Even with chemical prophylaxis, deep venous thrombosis (DVT) can be detected in 15% of patients. If this progresses to PE, the mortality is about 50% [86, 87].
DVT prophylaxis is paramount in trauma patients. Mechanical prophylaxis is very safe and should be considered in all patients without absolute contraindication to its use.
Regarding chemical prophylaxis, it is best to consider using LMWH-based prophylactic anticoagulation. Splenic trauma without ongoing bleeding is not an absolute contraindication to this. If anything, prophylactic anticoagulation should be started as soon as possible from trauma [120]. Bellal et al. [89] found no difference in hemorrhagic complication and NOM failure rate in patients with early (< 48 h), intermediate (48–72 h), and late (> 72 h) VTE prophylaxis. Rostas et al. [86] show that VTE rates were over fourfold greater when LMWH was administered after 72 h from admission. Pertaining to oral anticoagulants, the risk-benefit balance of reversal should be individualized. Failing to resume anticoagulation in a timely fashion is associated with poor outcomes [90].
7 Prognosis: Short- and Long-Term Follow-up for NOM
Complete bed rest for the first 48-72hours, is the cornerstone of NOM treatment for patients with moderate and severe splenic traumatic lesions [16]. 19% of splenic-delayed ruptures happen within the first 48 h, with the majority of delayed ruptures occuring most frequently between 4 to 10 days after the trauma. Patients can present for a delayed splenectomy after discharge, anytime between 3 and 146 days after injury, and the rate of readmission for splenectomy was 1.4% [91] with approximately 2% of patients requiring late intervention [92].
Savage et al. [92] found an average of healing time of 12.5 days for patients with grades I–II splenic injury, with a complete healing after 50 days. Conversely patients with grades III–V injuries required 37.2 and 75 days respectively. In 2–2.5 months, regardless of the severity of the splenic injury, 84% of patients had complete healing [92]. Crawford et al. suggested that an early discharge is safe because late failure occurs infrequently [39, 93]. Nonetheless, the mortality of delayed rupturse range from 5 to 15% compared with 1% mortality in cases of acute rupture [31, 94]. In any case, patients who have undergone NOM should be counseled to not remain alone or in isolated places for the first weeks after the discharge, and they should be warned about the red flag symptoms to watch out for.
Repeated CT scans during the admission should be considered in patients with moderate or severe lesions, or those with decreasing hematocrit, or patients who were found to have vascular anomalies or underlying splenic pathologies or coagulopathies, and in neurologically impaired patients. However, there is no consensus regarding the timing and type of imaging (CT vs. US) [16, 31, 95,96,97,98]. More than 50% of patients demonstrated healing at an interval follow-up CT scan after 6 weeks, and subsequent further scans seemed to have no additional clinical utility [99]. However, routine post-discharge follow-up abdominal CT is not necessary in low-grade (AAST grade I or II) injuries [96].
Activity restriction may be suggested for 4–6 weeks in minor injuries and up to 2–4 months in moderate and severe injuries [92, 98, 100, 101]. Complete healing of almost all grades is observed 3 months after injury. The role of radiological follow-up before returning to normal activity remains controversial overall.
8 Pediatric Splenic Trauma (<15 Years Old)
The spleen is the most commonly injured solid organ in pediatric blunt trauma (25–30%) [6, 102]. The Eastern Association for the Surgery of Trauma (EAST) recommends NOM in blunt splenic trauma in all hemodynamically stable children irrespective of the AAST injury grade [103, 104].
NOM seems to be more effective in children [105] and is associated with reduced costs and lengths of hospital stay, less need for blood transfusions, vaccinations, and antibiotic therapy, as well as higher immunity and reduced rate of infections [106,107,108,109,110]. Even though it is not clear why NOM outcomes are superior in children compared with adults, this phenomenon may be related to certain unique pediatric characteristics (e.g., thicker splenic capsule, higher proportion of myoepithelial cells, more efficient contraction, and retraction of the splenic arterioles [111,112,113,114,115,116]).
WSES spleen trauma management algorithm for pediatric patients is reported in Fig. 96.3.
8.1 Diagnostic Procedures
Contrast-enhanced computer tomography (CT) is the gold standard for the evaluation of blunt abdominal trauma [6, 8]. However, patients should be hemodynamically stable as well as cooperative or sedated. Of note, surgeons should interpret CT findings cautiously before opting for OM because more than 50% of children present with grade III–IV lesions [6].
FAST (Focused Assessment with Sonography for Trauma): The role of FAST for the diagnosis of spleen injury in children is still unclear. The sensitivity of this imaging modality in children ranges from 50 to 92% [117,118,119]. The specificity of this exam is also quite low, and, therefore, in a hemodynamically stable patient, a positive FAST examination should be followed by an urgent CT. Bedside FAST may have utility in hemodynamically unstable patients to rapidly identify or rule out intraperitoneal hemorrhage when patients cannot undergo CT.
8.2 Nonoperative Management in Splenic Injury
NOM is recommended as first-line treatment for hemodynamically stable pediatric patients with blunt splenic trauma [105]. Patients with moderate-severe blunt and all penetrating splenic injuries should be considered for transfer to dedicated pediatric trauma centers after stabilization.
NOM of splenic injuries in children should be considered only in an environment that has the capability for patient continuous monitoring, angiography, trained surgeons, an immediately available OR, and immediate access to blood and blood products or alternatively in the presence of a rapid centralization system in those patients amenable to be transferred [120, 121]. NOM should be attempted even in the setting of concomitant head trauma, unless the patient is unstable due to intra-abdominal bleeding.
In particular, for blunt splenic injuries with hemodynamic stability and absence of other internal injuries requiring surgery, these patients should undergo an initial attempt of NOM irrespective of injury grade. The presence of contrast blush at CT scan is not an absolute indication for splenectomy or AG/AE in children. Intensive care unit admission in isolated splenic injury may be required only for moderate and severe lesions [122].
However, no sufficient data validating NOM for penetrating spleen injury in children exist. However, reports on successful nonoperative management of isolated penetrating spleen injuries in hemodynamically stable pediatric patients do exist [123,124,125].
NOM failure rates for pediatric splenic trauma have been shown to range from 2 to 5% [126, 127]. Of note, there is evidence suggesting that the rate of NOM failure peaks at 4 h and then declines over 36 h from admission [126]. Overall, the majority (72.5%) of NOM failures seem to occur during the first week after trauma, with 50% of them happening within the first 3–5 days [128].
8.3 The Role of Angiography/Angioembolization (AG/AE)
The vast majority of pediatric patients do not require AG/AE for CT blush or moderate to severe injuries [129,130,131].
However, there are several potential considerations:
-
1.
AG/AE may be considered if the patient has signs of persistent hemorrhage not amenable to NOM, once extra-splenic source of bleeding has been excluded.
-
2.
AG/AE may be considered for the treatment of post-traumatic splenic pseudo-aneurysms prior to patient discharge.
-
3.
Patients of more than 15 years old, or children of less than 13–15 years old that are more vulnerable to OPSI, should be managed according to adults AG/AE-protocols [132, 133].
The role of embolization in the management of pediatric splenic pseudo-aneurysms is also unclear. Of note, PSAs often undergo spontaneous thrombosis and could resolve without any interventions [97, 108, 131].
Mortality and major complications are rarely reported following AG/AE [131, 132, 134, 135]. Nevertheless, a post-embolization syndrome (PES), consisting of abdominal pain, nausea, ileus, and fever, seems to occur in 90% of children undergoing AG/AE. This syndrome is usually self-limited and tends to resolve spontaneously in 6–9 days [136]. In addition, pleural effusion (9%), pneumonia (9%), and coil migration (4.5%) can also be seen after splenic embolization [132]. Overall, AG/AE seems to preserve splenic function without lasting complications, but most children do not need this intervention [130].
8.4 Operative Management in Blunt and Penetrating Injuries
Patients should undergo OM in cases of hemodynamic instability, failure of conservative treatment, severe coexisting injuries necessitating intervention, and peritonitis, bowel evisceration, and impalement [122, 137,138,139,140].
Splenic preservation (at least partial) should be attempted whenever possible. Partial (subtotal) splenectomy or splenorrhaphy are safe and viable alternatives to total splenectomy and can be performed even in high-grade injuries [139, 141,142,143]. 1% of pediatric patients who undergo immediate OM are readmitted for intestinal obstruction within a year [140].
8.5 Splenic Trauma Associated with Head Injuries
Head injury is an important cause of morbidity and mortality in trauma patients of all ages (50–60%) and can also result in altered mental status, which can complicate the process of clinical evaluation [144]. Especially in the setting of concurrent head injury, blood pressure and heart rate are poor markers of hemorrhagic shock in pediatric patients [122]. Nevertheless, an analysis of the National Pediatric Trauma Registry suggested that the association of altered mental status from head injury with spleen injuries should not impact the decision for observational management in pediatric patients (< 19 years old) [144].
8.6 Short- and Long-Term Follow-Up in Splenic Trauma (Blunt and Penetrating)
In hemodynamic stable children without a drop in hemoglobin levels for 24 h, bed rest should be suggested. Initial APSA guidelines [106] recommended bed rest for a number of days equal to the grade of injury plus 1 day [106]. However, recent studies suggest a shorter bed rest of one night in solitary grade I–II splenic trauma and two nights for patients with more severe injuries (grade ≥ III) and stable hemoglobin level [145]. Longer admissions should be considered in patients with lower hemoglobin levels on admission, higher injury grade, suspected other abdominal injuries (as pancreatic or small bowel injuries), blush on the CT scan, bicycle handlebar injuries, and recurrent bleeding or patients at risk for missed injuries [122].
US (DUS, CEUS) follow-up seems reasonable to minimize the risk of life-threatening hemorrhage and associated complications in children [146].
After NOM in moderate and severe injuries, the reprise of normal activity could be considered safe after at least 6 weeks. The APSA guidelines [106] recommended 2–5 months of “light” activity before restart with normal activities and recommended 3 week–3 months of limited activity at home. In fact, the risks of delayed splenic rupture and post-traumatic pseudocysts seem to be increase within the first 3 weeks (incidence 0.2 and 0.3%, respectively) [106, 147]. Canadian guidelines suggested a discharge at home after reprise and good toleration of oral intake, able mobilization, and analgesia with oral medications; without a need for any imaging prior to discharge [148]. They reported a restriction of activities of no more than 6–8 weeks [148].
9 Infection Prophylaxis in Asplenic and Hyposplenic Adult and Pediatric Patients
Patients should receive immunization against encapsulated bacteriae (Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis) [149, 150]. Vaccination programs should be started no sooner than 14 days after splenectomy or splenic total vascular exclusion. In fact, before 14 days, the antibody response is suboptimal [149, 151]; after that interval, the earlier the better. In asplenic/hyposplenic patients discharged before 15 days, where the risk to miss the vaccination is deemed high, the first vaccines should be given before discharge [151, 152]. The Center for Disease Control in 2016 proposed these last updated recommendations [153].
Most episodes of severe infections occur within the first 2 years after splenectomy, and for this reason, some authors recommended at least 2 years of prophylactic antibiotics after splenectomy. However, the duration of antibiotic prophylaxis is controversial.
Ideally, the vaccinations against S. pneumoniae, H. influenzae type B, and N. meningitidis should be given at least 2 weeks before splenectomy [6]. Patients should be informed that immunization can only reduce the incidence of OPSI (vaccines so far available do not allow an exhaustive coverage for S. pneumoniae—23 of 90 serotypes are included, nor for N. meningitidis—5 of 6 serotypes).
Annual immunization against seasonal flu is recommended for all patients over 6 months of age [154, 155]. Malaria prophylaxis is strongly recommended for travelers. Antibiotic therapy should be strongly considered in the event of any sudden onset of unexplained fever, malaise, chills, or other constitutional symptoms, especially when medical review is not readily accessible.
OPSI is a medical emergency. The risks of OPSI and associated death are highest in the first year after splenectomy, at least among young children, but remain elevated for more than 10 years and probably for life. The incidence of OPSI is 0.5–2%; the mortality rate is from 30 to 70%, and most death occurs within the first 24 h. Only prompt diagnosis and immediate treatment can reduce mortality [6, 150, 151, 154]. Asplenic/hyposplenic children younger than 5 years old have a greater overall risk of OPSI with an increased death compared with adults [149, 155]. The risk is more than 30% in neonates [6]. Evidence exists regarding the possible maintainence of function by the embolized spleen (hyposplenic patients); however, it is reasonable to consider it as less effective and proceed with vaccination as well [130, 156].
Asplenic/hyposplenic patients should be given an antibiotic supply in the event of any sudden onset of unexplained fever, malaise, chills, or other constitutional symptoms, especially when medical review is not readily accessible. The recommended options for emergency standby in adults include the following: (a) amoxicillin, 3 g starting dose followed by 1 g, every 8 h; (b) levofloxacin 500 mg every 24 h or moxifloxacin 400 mg every 24 h (for beta-lactam allergic patients). The recommended emergency standby treatment in children is amoxicillin 50 mg/Kg in three divided daily doses. For beta-lactam allergic patients, an alternative should be proposed by a specialist (fluoroquinolones are generally contraindicated in children, but due to the possible severity of OPSI, they might still be considered).
Dos and Dont’s
Dos
-
1.
All hemodynamically stable patients with blunt splenic trauma should be managed nonoperatively, with angioembolization applied when indicated.
-
2.
All unstable patients with splenic trauma should be managed operatively.
-
3.
Stable patients with severe injuries should be considered for operative management, if angioembolization and monitoring facilities are inadequate.
Don’ts
-
1.
Do not operate on pediatric patients with splenic trauma unless absolutely indicated, e.g., concomitant injuries requiring surgery.
Take-Home Messages
-
Nonoperative management is the first line of management for stable patients with blunt splenic trauma.
-
If unstable, or if monitoring facilities are inadequate, surgical management is the first line option.
-
For stable penetrating traumas, each case should be evaluated individually to decide if surgery or conservative management would be most ideal.
MCQ
-
1.
NOM for splenic trauma:
-
A.
Is always possible.
-
B.
Is possible only for stable patients.
-
C.
Is performed with splenectomy.
-
D.
Is not possible.
-
A.
-
2.
NOM for splenic trauma:
-
A.
Is performed in all hospitals (HUB and SPOKE).
-
B.
Is performed in HUB trauma centers.
-
C.
Is performed if surgical services arenot available.
-
D.
Is performed without interventional radiology.
-
A.
-
3.
NOM for splenic trauma:
-
A.
Is performed always with splenic artery embolization.
-
B.
Can be performed with bed rest and close observation alone.
-
C.
Is performed always with splenic artery distal embolization.
-
D.
Is performed always with embolization.
-
A.
References
Brady RRW, Bandari M, Kerssens JJ, Paterson-Brown S, Parks RW. Splenic trauma in Scotland: demographics and outcomes. World J Surg. 2007;31(11):2111–6.
Hartnett KL, Winchell RJ, Clark DE. Management of adult splenic injury: a 20-year perspective. Am Surg. 2003;69(7):608–11.
Soo KM, Lin TY, Chen CW, Lin YK, Kuo LC, Wang JY, Lee WC, Lin HL. More becomes less: management strategy has definitely changed over the past decade of splenic injury—a nationwide population-based study. Biomed Res Int. 2015;2015:124969. https://doi.org/10.1155/2015/124969. Epub 2015 Jan 5
Zarzaur BL, Rozycki GS. An update on nonoperative management of the spleen in adults. Trauma Surg Acute Care Open. 2017;2(1):e000075.
Yang K, Li Y, Wang C, Xiang B, Chen S, Ji Y. Clinical features and outcomes of blunt splenic injury in children: a retrospective study in a single institution in China. Medicine (Baltimore). 2017;96(51):e9419.
Lynn KN, Werder GM, Callaghan RM, Sullivan AN, Jafri ZH, Bloom DA. Pediatric blunt splenic trauma: a comprehensive review. Pediatr Radiol. 2009;39:904–16.
Coccolini F, Montori G, Catena F, et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg. 2017;12:40.
American College of Surgeon’s Commitee on Trauma. Advanced Trauma Life Support® (ATLS®) student manual. 9th ed. Chicago, IL: American College of Surgeon; 2012.
Carr JA, Roiter C, Alzuhaili A. Correlation of operative and pathological injury grade with computed tomographic grade in the failed nonoperative management of blunt splenic trauma. Eur J Trauma Emerg Surg. 2012;38:433–8.
Kirkpatrick AW, Sirois M, Laupland KB, Liu D, Rowan K, Ball CG, et al. Hand-held thoracic sonography for detecting post-traumatic pneumothoraces: the extended focused assessment with sonography for trauma (EFAST). J Trauma. 2004;57:288–95.
Doody O, Lyburn D, Geoghegan T, Govender P, Monk PM, Torreggiani WC. Blunt trauma to the spleen: ultrasonographic findings. Clin Radiol. 2005;60:968–76.
El-Matbouly M, Jabbour G, El-Menyar A, Peralta R, Abdelrahman H, Zarour A, et al. Blunt splenic trauma: assessment, management and outcomes. Surgeon. 2016;14:52–8.
Bee TK, Croce MA, Miller PR, Pritchard FE, Fabian TC. Failures of splenic nonoperative management: is the glass half empty or half full? J Trauma. 2001;50:230–6.
Clark R, Hird K, Misur P, Ramsay D, Mendelson R. CT grading scales for splenic injury: why can’t we agree? J Med Imaging Radiat Oncol. 2011;55:163–9.
Becker CD, Mentha G, Terrier F. Blunt abdominal trauma in adults: role of CT in the diagnosis and management of visceral injuries. Part 1: liver and spleen. Eur Radiol. 1998;8:553–62.
Shapiro MJ, Krausz C, Durham RM, Mazuski JE. Overuse of splenic scoring and computed tomographic scans. J Trauma. 1999;47:651–8.
Anderson SW, Varghese JC, Lucey BC, P a B, Hirsch EF, J a S. Blunt splenic trauma: delayed-phase CT for differentiation of active hemorrhage from contained vascular injury in patients. Radiology. 2007;243:88–95.
Jeffrey RB, Olcott EW. Imaging of blunt hepatic trauma. Radiol Clin N Am. 1991;29:1299–310.
Bhullar IS, Frykberg ER, Tepas JJ, Siragusa D, Loper T, Kerwin AJ. At first blush: absence of computed tomography contrast extravasation in grade IV or V adult blunt splenic trauma should not preclude angioembolization. J Trauma Acute Care Surg. 2013;74:105–11; discussion 111-2
Hassan R, Aziz AA, Ralib ARM, Saat A. Computed tomography of blunt spleen injury: a pictorial review. Malaysian J Med Sci. 2011;18:60–7.
Juyia RF, Kerr HA. Return to play after liver and spleen trauma. Sports Health. 2014;6:239–45.
Fernandes TM, Dorigatti AE, Pereira BMT, Cruvinel Neto J, Zago TM, Fraga GP. Nonoperative management of splenic injury grade IV is safe using rigid protocol. Rev Col Bras Cir. 2013;40:323–9.
Stassen NA, Bhullar I, Cheng JD, Crandall ML, Friese RS, Guillamondegui OD, et al. Selective nonoperative management of blunt splenic injury: an eastern Association for the Surgery of trauma practice management guideline. J Trauma Acute Care Surg. 2012;73:S294–300.
Velmahos GC, Toutouzas KG, Radin R, Chan L, Demetriades D. Nonoperative treatment of blunt injury to solid abdominal organs: a prospective study. Arch Surg. 2003;138:844–51.
Smith J, Armen S, Cook CH, Martin LC. Blunt splenic injuries: have we watched long enough? J Trauma. 2008;64:656–63; discussion 663-5
Hafiz S, Desale S, Sava J. The impact of solid organ injury management on the US health care system. J Trauma Acute Care Surg. 2014;77:310–4.
Gaspar B, Negoi I, Paun S, Hostiuc S, Ganescu R, Beuran M. Selective nonoperative management of abdominal injuries in polytrauma patients: a protocol only for experienced trauma centers. Maedica. 2014;9:168–72.
Moore FA, Davis JW, Moore EE, Cocanour CS, West MA, McIntyre RC. Western trauma association (WTA) critical decisions in trauma: management of adult blunt splenic trauma. J Trauma. 2008;65:1007–11.
Rowell SE, Biffl WL, Brasel K, Moore EE, Albrecht RA, DeMoya M, et al. Western trauma association critical decisions in trauma: management of adult blunt splenic trauma-2016 updates. J Trauma Acute Care Surg. 2017;82:787–93.
Nix JA, Costanza M, Daley BJ, Powell MA, Enderson BL. Outcome of the current management of splenic injuries. J Trauma. 2001;50:835–42.
Peitzman AB, Heil B, Rivera L, Federle M, Harbrecht BG, Clancy K, et al. Blunt splenic injury in adults: multi-institutional study of the Eastern Association for the surgery of trauma. J Trauma Inj Infect Crit Care. 2000;49:177–89.
Malhotra AK, Latifi R, Fabian TC, Ivatury RR, Dhage S, Bee TK, et al. Multiplicity of solid organ injury: influence on management and outcomes after blunt abdominal trauma. J Trauma. 2003;54:925–9.
Velmahos GC, Zacharias N, Emhoff TA, Feeney JM, Hurst JM, Crookes BA, et al. Management of the most severely injured spleen: a multicenter study of the research consortium of New England centers for trauma (ReCONECT). Arch Surg. 2010;145:456–60.
Jeremitsky E, Kao A, Carlton C, Rodriguez A, Ong A. Does splenic embolization and grade of splenic injury impact nonoperative management in patients sustaining blunt splenic trauma? Am Surg. 2011;77:215–20.
Watson GA, Rosengart MR, Zenati MS, Tsung A, Forsythe RM, Peitzman AB, et al. Nonoperative management of severe blunt splenic injury: are we getting better? J Trauma. 2006;61(5):1113–8; discussion 1118-9
Schurr MJ, Fabian TC, Gavant M, Croce MA, Kudsk KA, Minard G, et al. Management of blunt splenic trauma: computed tomographic contrast blush predicts failure of nonoperative management. J Trauma. 1995;39(3):507–12; discussion 512-3
Bhangu A, Nepogodiev D, Lal N, Bowley DM. Meta-analysis of predictive factors and outcomes for failure of non-operative management of blunt splenic trauma. Injury. 2012;43:1337–46.
Aseervatham R, Muller M. Blunt trauma to the spleen. Aust N Z J Surg. 2000;70:333–7.
Crawford RS, Tabbara M, Sheridan R, Spaniolas K, Velmahos GC. Early discharge after nonoperative management for splenic injuries: increased patient risk caused by late failure? Surgery. 2007;142:337–42.
Jeremitsky E, Smith RS, Ong AW. Starting the clock: defining nonoperative management of blunt splenic injury by time. Am J Surg. 2013;205:298–301.
Demetriades D, Rabinowitz B. Indications for operation in abdominal stab wounds. A prospective study of 651 patients. Ann Surg. 1987;205:129–32.
Velmahos GC, Demetriades D, Toutouzas KG, Sarkisyan G, Chan LS, Ishak R, et al. Selective nonoperative management in 1,856 patients with abdominal gunshot wounds: should routine laparotomy still be the standard of care? Ann Surg. 2001;234(3):395–402; discussion 402-3
Carlin AM, Tyburski JG, Wilson RF, Steffes C. Factors affecting the outcome of patients with splenic trauma. Am Surg. 2002;68(3):232–9.
Renz BM, Feliciano DV. Gunshot wounds to the right thoracoabdomen: a prospective study of nonoperative management. J Trauma. 1994;37:737–44.
Inaba K, Barmparas G, Foster A, Talving P, David J-S, Green D, et al. Selective nonoperative management of torso gunshot wounds: when is it safe to discharge? J Trauma. 2010;68:1301–4.
Haan JM, Bochicchio GV, Kramer N, Scalea TM. Nonoperative management of blunt splenic injury: a 5-year experience. J Trauma. 2005;58:492–8.
Haan J, Scott J, Boyd-Kranis RL, Ho S, Kramer M, Scalea TM. Admission angiography for blunt splenic injury: advantages and pitfalls. J Trauma. 2001;51:1161–5.
Haan JM, Biffl W, Knudson MM, Davis KA, Oka T, Majercik S, et al. Splenic embolization revisited: a multicenter review. J Trauma Inj Infect Crit Care. 2004;56:542–7.
Frandon J, Rodière M, Arvieux C, Michoud M, Vendrell A, Broux C, et al. Blunt splenic injury: outcomes of proximal versus distal and combined splenic artery embolization. Diagn Interv Imaging. 2014;95:825–31.
Schnüriger B, Inaba K, Konstantinidis A, Lustenberger T, Chan LS, Demetriades D. Outcomes of proximal versus distal splenic artery embolization after trauma: a systematic review and meta-analysis. J Trauma. 2011;70:252–60.
Tugnoli G, Bianchi E, Biscardi A, Coniglio C, Isceri S, Simonetti L, et al. Nonoperative management of blunt splenic injury in adults: there is (still) a long way to go. The results of the Bologna-Maggiore Hospital trauma center experience and development of a clinical algorithm. Surg Today. 2015;45:1210–7.
Bessoud B, Denys A, Calmes JM, Madoff D, Qanadli S, Schnyder P, et al. Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? Am J Roentgenol. 2006;186:779–85.
Brillantino A, Iacobellis F, Robustelli U, Villamaina E, Maglione F, Colletti O, et al. Non operative management of blunt splenic trauma: a prospective evaluation of a standardized treatment protocol. Eur J Trauma Emerg Surg. 2016;42:593–8.
Smith HE, Biffl WL, Majercik SD, Jednacz J, Lambiase R, Cioffi WG. Splenic artery embolization: have we gone too far? J Trauma. 2006;61:541–4; discussion 545-6
Capecci LM, Jeremitsky E, Smith RS, Philp F. Trauma centers with higher rates of angiography have a lesser incidence of splenectomy in the management of blunt splenic injury. Surgery. 2015;158:1020–4; discussion 1024-6
Zarzaur BL, Savage SA, Croce MA, Fabian TC. Trauma center angiography use in high-grade blunt splenic injuries: timing is everything. J Trauma Acute Care Surg. 2014;77:666–71.
Raikhlin A, Baerlocher MO, Asch MR, Myers A. Imaging and transcatheter arterial embolization for traumatic splenic injuries: review of the literature. Can J Surg. 2008;51:464–72.
Banerjee A, Duane TM, Wilson SP, Haney S, O’Neill PJ, Evans HL, et al. Trauma center variation in splenic artery embolization and spleen salvage: a multicenter analysis. J Trauma Acute Care Surg. 2013;75:69–74; discussion 74-5
Rosati C, Ata A, Siskin GP, Megna D, Bonville DJ, Stain SC. Management of splenic trauma: a single institution’s 8-year experience. Am J Surg. 2015;209:308–14.
Requarth JA, D’Agostino RB Jr, Miller PR. Nonoperative management of adult blunt splenic injury with and without splenic artery embolotherapy: a meta-analysis. J Trauma Inj Infect Crit Care. 2011;71:898–903.
Davis KA, Fabian TC, Croce MA, Gavant ML, Flick PA, Minard G, et al. Improved success in nonoperative management of blunt splenic injuries: embolization of splenic artery pseudoaneurysms. J Trauma. 1998;44:1008–13; discussion 1013-5
Dehli T, Bagenholm A, Trasti NC, Monsen SA, Bartnes K, Bågenholm A, et al. The treatment of spleen injuries: a retrospective study. Scand J Trauma Resusc Emerg Med. 2015;23:85.
Yuan K-C, Wong Y-C, Lin B-C, Kang S-C, Liu E-H, Hsu Y-P. Negative catheter angiography after vascular contrast extravasations on computed tomography in blunt torso trauma: an experience review of a clinical dilemma. Scand J Trauma Resusc Emerg Med. 2012;20:46.
Alarhayem AQ, Myers JG, Dent D, Lamus D, Lopera J, Liao L, et al. “Blush at first sight”: significance of computed tomographic and angiographic discrepancy in patients with blunt abdominal trauma. Am J Surg. 2015;210:1104. s
Gavant ML, Schurr M, Flick PA, Croce MA, Fabian TC, Gold RE. Predicting clinical outcome of nonsurgical management of blunt splenic injury: using CT to reveal abnormalities of splenic vasculature. Am J Roentgenol. 1997;168:207–12.
Skattum J, Naess PA, Eken T, Gaarder C. Refining the role of splenic angiographic embolization in high-grade splenic injuries. J Trauma Acute Care Surg. 2013;74:100–3; discussion 103-4
Miller PR, Chang MC, Hoth JJ, Mowery NT, Hildreth AN, Martin RS, et al. Prospective trial of angiography and embolization for all grade III to V blunt splenic injuries: nonoperative management success rate is significantly improved. J Am Coll Surg. 2014;218:644–8.
Chastang L, Bège T, Prudhomme M, Simonnet AC, Herrero A, Guillon F, et al. Is non-operative management of severe blunt splenic injury safer than embolization or surgery? Results from a French prospective multicenter study. J Visc Surg. 2015;152:85–91.
Ekeh AP, McCarthy MC, Woods RJ, Haley E. Complications arising from splenic embolization after blunt splenic trauma. Am J Surg. 2005;189:335–9.
Frandon J, Rodiere M, Arvieux C, Vendrell A, Boussat B, Sengel C, et al. Blunt splenic injury: are early adverse events related to trauma, nonoperative management, or surgery? Diagnostic Interv Radiol. 2015;21:327–33.
Demetriades D, Scalea TM, Degiannis E, Barmparas G, Konstantinidis A, Massahis J, et al. Blunt splenic trauma: splenectomy increases early infectious complications: a prospective multicenter study. J Trauma Acute Care Surg. 2012;72:229–34.
Kaseje N, Agarwal S, Burch M, Glantz A, Emhoff T, Burke P, et al. Short-term outcomes of splenectomy avoidance in trauma patients. Am J Surg. 2008;196:213–7.
Freitas G, Olufajo OA, Hammouda K, Lin E, Cooper Z, Havens JM, et al. Postdischarge complications following nonoperative management of blunt splenic injury. Am J Surg. 2016;211:744–9.
Wei B, Hemmila MR, Arbabi S, Taheri PA, Wahl WL, et al. Angioembolization reduces operative intervention for blunt splenic injury. J Trauma Inj Infect Crit Care. 2008;64:1472–7.
Ekeh AP, Khalaf S, Ilyas S, Kauffman S, Walusimbi M, McCarthy MC. Complications arising from splenic artery embolization: a review of an 11-year experience. Am J Surg. 2013;205:250–4.
Wu SC, Chen RJ, Yang AD, Tung CC, Lee KH. Complications associated with embolization in the treatment of blunt splenic injury. World J Surg. 2008;32:476–82.
Swaid F, Peleg K, Alfici R, Matter I, Olsha O, Ashkenazi I, et al. Concomitant hollow viscus injuries in patients with blunt hepatic and splenic injuries: an analysis of a National Trauma Registry database. Injury. 2014;45:1409–12.
Morrell DG, Chang FC, Helmer SD. Changing trends in the management of splenic injury. Am J Surg. 1995;170:686–9; discussion 690
Carter JW, Falco MH, Chopko MS, Flynn WJ, Wiles Iii CE, Guo WA. Do we really rely on fast for decision-making in the management of blunt abdominal trauma? Injury. 2015;46:817–21.
Garber BG, Yelle JD, Fairfull-Smith R, Lorimer JW, Carson C. Management of splenic injuries in a Canadian trauma centre. Can J Surg. 1996;39:474–80.
Garber BG, Mmath BP, Fairfull-Smith RJ, Yelle JD. Management of adult splenic injuries in Ontario: a population-based study. Can J Surg. 2000;43:283–8.
Pisters PW, Pachter HL. Autologous splenic transplantation for splenic trauma. Ann Surg. 1994;219:225–35.
Qu Y, Ren S, Li C, Qian S, Liu P. Management of postoperative complications following splenectomy. Int Surg. 2013;98:55–60.
Nasr WI, Collins CL, Kelly JJ. Feasibility of laparoscopic splenectomy in stable blunt trauma: a case series. J Trauma. 2004;57:887–9.
Hallfeldt KK, Trupka AW, Erhard J, Waldner H, Schweiberer L. Emergency laparoscopy for abdominal stab wounds. Surg Endosc. 1998;12:907–10.
Rostas JW, Manley J, Gonzalez RP, Brevard SB, Ahmed N, Frotan MA, et al. The safety of low molecular-weight heparin after blunt liver and spleen injuries. Am J Surg. 2015;210:31–4.
Murphy PB, Sothilingam N, Charyk Stewart T, Batey B, Moffat B, Gray DK, et al. Very early initiation of chemical venous thromboembolism prophylaxis after blunt solid organ injury is safe. Can J Surg. 2016;59:118–22.
Alejandro KV, Acosta JA, Rodríguez PA. Bleeding manifestations after early use of low-molecular-weight heparins in blunt splenic injuries. Am Surg. 2003;69:1006–9.
Joseph B, Pandit V, Harrison C, Lubin D, Kulvatunyou N, Zangbar B, et al. Early thromboembolic prophylaxis in patients with blunt solid abdominal organ injuries undergoing nonoperative management: is it safe? Am J Surg. 2015;209:194–8.
Weinberger J, Cipolle M. Optimal reversal of novel anticoagulants in trauma. Crit Care Clin. 2017;33:135–52.
Zarzaur BL, Vashi S, Magnotti LJ, Croce MA, Fabian TC. The real risk of splenectomy after discharge home following nonoperative management of blunt splenic injury. J Trauma. 2009;66:1531–8.
Savage SA, Zarzaur BL, Magnotti LJ, Weinberg JA, Maish GO, Bee TK, et al. The evolution of blunt splenic injury: resolution and progression. J Trauma. 2008;64:1085–91; discussion 1091-2
Meguid AA, Bair HA, Howells GA, Bendick PJ, Kerr HH, Villalba MR. Prospective evaluation of criteria for the nonoperative management of blunt splenic trauma. Am Surg. 2003;69:238–42; discussion 242-3
Riezzo I, Di Battista B, De Salvia A, Cantatore S, Neri M, Pomara C, et al. Delayed splenic rupture: dating the sub-capsular hemorrhage as a useful task to evaluate causal relationships with trauma. Forensic Sci Int. 2014;234:64–71.
Clancy AA, Tiruta C, Ashman D, Ball CG, Kirkpatrick AW. The song remains the same although the instruments are changing: complications following selective non-operative management of blunt spleen trauma: a retrospective review of patients at a level I trauma Centre from 1996 to 2007. J Trauma Manag Outcomes. 2012;6:4.
Haan JM, Boswell S, Stein D, Scalea TM. Follow-up abdominal CT is not necessary in low-grade splenic injury. Am Surg. 2007;73:13–8.
Muroya T, Ogura H, Shimizu K, Tasaki O, Kuwagata Y, Fuse T, et al. Delayed formation of splenic pseudoaneurysm following nonoperative management in blunt splenic injury: multi-institutional study in Osaka, Japan. J Trauma Acute Care Surg. 2013;75:417–20.
Uecker J, Pickett C, Dunn E. The role of follow-up radiographic studies in nonoperative management of spleen trauma. Am Surg. 2001;67:22–5.
Lyass S, Sela T, Lebensart PD, Muggia-Sullam M. Follow-up imaging studies of blunt splenic injury: do they influence management? Isr Med Assoc J. 2001;3:731–3.
Lynch JM, Meza MP, Newman B, Gardner MJ, Albanese CT. Computed tomography grade of splenic injury is predictive of the time required for radiographic healing. J Pediatr Surg. 1997;32:1093–6.
Unal E, Onur MR, Akpinar E, Ahmadov J, Karcaaltincaba M, Ozmen MN, et al. Imaging findings of splenic emergencies: a pictorial review. Insights Imaging. 2016;7:215–22.
Linet MS, Nyrén O, Gridley G, Adami HO, Buckland JD, McLaughlin JK, et al. Causes of death among patients surviving at least one year following splenectomy. Am J Surg. 1996;172:320–3.
Alonso M, Brathwaite C, García V, Patterson L, Scherer T, Stafford P, et al. Practice management guidelines for the nonoperative management of blunt injury to the liver and spleen. Chicago, IL: Eastern Association for the Surgery of Trauma; 2003.
Moore EE, Cogbill TH, Jurkovich GJ, Shackford SR, Malangoni MA, Champion HR. Organ injury scaling: spleen and liver (1994 revision). J Trauma. 1995;38:323–4.
Bairdain S, Litman HJ, Troy M, McMahon M, Almodovar H, Zurakowski D, et al. Twenty-years of splenic preservation at a level 1 pediatric trauma center. J Pediatr Surg. 2015;50:864–8.
Stylianos S. Evidence-based guidelines for resource utilization in children with isolated spleen or liver injury. The APSA trauma committee. J Pediatr Surg. 2000;35:164–9.
McVay MR, Kokoska ER, Jackson RJ, Smith SD. Throwing out the “grade” book: management of isolated spleen and liver injury based on hemodynamic status. J Pediatr Surg. 2008;43:1072–6.
Martin K, Vanhouwelingen L, Bütter A. The significance of pseudoaneurysms in the nonoperative management of pediatric blunt splenic trauma. J Pediatr Surg. 2011;46:933–7.
Li D, Yanchar N. Management of pediatric blunt splenic injuries in Canada-practices and opinions. J Pediatr Surg. 2009;44:997–1004.
Bond SJ, Eichelberger MR, Gotschall CS, Sivit CJ, Randolph JG. Nonoperative management of blunt hepatic and splenic injury in children. Ann Surg. 1996;223:286–9.
Muehrcke DD, Kim SH, McCabe CJ. Pediatric splenic trauma: predicting the success of nonoperative therapy. Am J Emerg Med. 1987;5:109–12.
Delius RE, Frankel W, Coran AG. A comparison between operative and nonoperative management of blunt injuries to the liver and spleen in adult and pediatric patients. Surgery. 1989;106:788–92; discussion 792-3
Lynch JM, Ford H, Gardner MJ, Weiner ES. Is early discharge following isolated splenic injury in the hemodynamically stable child possible? J Pediatr Surg. 1993;28:1403–7.
Konstantakos AK, Barnoski AL, Plaisier BR, Yowler CJ, Fallon WF, Malangoni MA. Optimizing the management of blunt splenic injury in adults and children. Surgery. 1999;126:805–13.
Upadhyaya P. Conservative management of splenic trauma: history and current trends. Pediatr Surg Int. 2003;19:617–27.
Rodrigues CJ, Sacchetti JC, Rodrigues AJ. Age-related changes in the elastic fiber network of the human splenic capsule. Lymphology. 1999;32:64–9.
Murphy R, Ghosh A. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. The accuracy of abdominal ultrasound in paediatric trauma. Emerg Med J. 2001;18:208–9.
Scaife ER, Rollins MD, Barnhart DC, Downey EC, Black RE, Meyers RL, et al. The role of focused abdominal sonography for trauma (FAST) in pediatric trauma evaluation. J Pediatr Surg. 2013;48:1377–83.
Holmes JF, Gladman A, Chang CH. Performance of abdominal ultrasonography in pediatric blunt trauma patients: a meta-analysis. J Pediatr Surg. 2007;42:1588–94.
Mooney DP, Rothstein DH, Forbes PW. Variation in the management of pediatric splenic injuries in the United States. J Trauma Inj Infect Crit Care. 2006;61:330–3.
Todd SR, Arthur M, Newgard C, Hedges JR, Mullins RJ. Hospital factors associated with splenectomy for splenic injury: a national perspective. J Trauma. 2004;57:1065–71.
Notrica DM, Eubanks JW, Tuggle DW, Maxson RT, Letton RW, Garcia NM, et al. Nonoperative management of blunt liver and spleen injury in children: evaluation of the ATOMAC guideline using GRADE. J Trauma Acute Care Surg. 2015;79:683–93.
Narci A, Solak O, Turhan-Haktanir N, Ayçiçek A, Demir Y, Ela Y, et al. The prognostic importance of trauma scoring systems in pediatric patients. Pediatr Surg Int. 2009;25:25–30.
Richards JR, McGahan JP, Jones CD, Zhan S, Gerscovich EO. Ultrasound detection of blunt splenic injury. Injury. 2001;32:95–103.
Tataria M, Nance ML, Holmes JH 4th, Miller CC 3rd, Mattix KD, Brown RL, et al. Pediatric blunt abdominal injury: age is irrelevant and delayed operation is not detrimental. J Trauma. 2007;63:608–14.
Holmes JH 4th, Wiebe DJ, Tataria M, Mattix KD, Mooney DP, Scaife ER, et al. The failure of nonoperative management in pediatric solid organ injury: a multi-institutional experience. J Trauma. 2005;59:1309–13.
Sharma OP, Oswanski MF, Singer D, Raj SS, Daoud YAH. Assessment of nonoperative management of blunt spleen and liver trauma. Am Surg. 2005;71:379–86.
McIntyre LK, Schiff M, Jurkovich GJ. Failure of nonoperative management of splenic injuries: causes and consequences. Arch Surg. 2005;140:563–8; discussion 568
Cloutier DR, Baird TB, Gormley P, McCarten KM, Bussey JG, Luks FI. Pediatric splenic injuries with a contrast blush: successful nonoperative management without angiography and embolization. J Pediatr Surg. 2004;39:969–71.
Gross JL, Woll NL, Hanson CA, Pohl C, Scorpio RJ, Kennedy AP Jr, et al. Embolization for pediatric blunt splenic injury is an alternative to splenectomy when observation fails. J Trauma Acute Care Surg. 2013;75:421–5.
Kiankhooy A, Sartorelli KH, Vane DW, Bhave AD. Angiographic embolization is safe and effective therapy for blunt abdominal solid organ injury in children. J Trauma. 2010;68:526–31.
Skattum J, Gaarder C, Naess PA. Splenic artery embolisation in children and adolescents—an 8 year experience. Injury. 2014;45:160–3.
Mayglothling JA, Haan JM, Scalea TM. Blunt splenic injuries in the adolescent trauma population: the role of angiography and embolization. J Emerg Med. 2011;41:21–8.
Schuster T, Leissner G. Selective angioembolization in blunt solid organ injury in children and adolescents: review of recent literature and own experiences. Eur J Pediatr Surg. 2013;23:454–63.
van der Vlies CH, Saltzherr TP, Wilde JCH, van Delden OM, de Haan RJ, Goslings JC. The failure rate of nonoperative management in children with splenic or liver injury with contrast blush on computed tomography: a systematic review. J Pediatr Surg. 2010;45:1044–9.
Ben-Ishay O, Gutierrez IM, Pennington EC, Mooney DP. Transarterial embolization in children with blunt splenic injury results in postembolization syndrome: a matched case-control study. J Trauma Acute Care Surg. 2012;73:1558–63.
Akinkuolie AA, Lawal OO, Arowolo OA, Agbakwuru EA, Adesunkanmi ARK. Determinants of splenectomy in splenic injuries following blunt abdominal trauma. S Afr J Surg. 2010;48:15–9.
Polites SF, Zielinski MD, Zarroug AE, Wagie AE, Stylianos S, Habermann EB. Benchmarks for splenectomy in pediatric trauma: how are we doing? J Pediatr Surg. 2015;50:339–42.
Nwomeh BC, Nadler EP, Meza MP, Bron K, Gaines BA, Ford HR. Contrast extravasation predicts the need for operative intervention in children with blunt splenic trauma. J Trauma. 2004;56:537–41.
Jen HC, Tillou A, Cryer HG, Shew SB. Disparity in management and long-term outcomes of pediatric splenic injury in California. Ann Surg. 2010;251:1162–6.
Mohamed AA, Mahran KM, Zaazou MM. Blunt abdominal trauma requiring laparotomy in polytraumatized patients. Saudi Med J. 2010;31:43–8.
Lo A, Matheson A-M, Adams D. Impact of concomitant trauma in the management of blunt splenic injuries. N Z Med J. 2004;117:U1052.
Resende V, Petroianu A. Functions of the splenic remnant after subtotal splenectomy for treatment of severe splenic injuries. Am J Surg. 2003;185:311–5.
Keller MS, Sartorelli KH, Vane DW. Associated head injury should not prevent nonoperative management of spleen or liver injury in children. J Trauma. 1996;41:471–5.
St Peter SD, Aguayo P, Juang D, Sharp SW, Snyder CL, Holcomb GW, et al. Follow up of prospective validation of an abbreviated bedrest protocol in the management of blunt spleen and liver injury in children. J Pediatr Surg. 2013;48:2437–41.
Minarik L, Slim M, Rachlin S, Brudnicki A. Diagnostic imaging in the follow-up of nonoperative management of splenic trauma in children. Pediatr Surg Int. 2002;18:429–31.
Pachter HL, Guth AA, Hofstetter SR, Spencer FC. Changing patterns in the management of splenic trauma: the impact of nonoperative management. Ann Surg. 1998;227:708–9.
Zabolotny B, Hancock BJ, Postuma R, Wiseman N. Blunt splenic injuries in a Canadian pediatric population: the need for a management guideline. Can J Surg. 2002;45:358–62.
Leone G, Pizzigallo E. Bacterial infections following splenectomy for malignant and nonmalignant hematologic diseases. Mediterr J Hematol Infect Dis. 2015:7.
Skattum J, P a N, Gaarder C. Non-operative management and immune function after splenic injury. Br J Surg. 2012;99(Suppl 1):59–65.
Shatz DV. Vaccination practices among North American trauma surgeons in splenectomy for trauma. J Trauma. 2002;53:950–6.
Shatz DV, Romero-Steiner S, Elie CM, Holder PF, Carlone GM. Antibody responses in postsplenectomy trauma patients receiving the 23-valent pneumococcal polysaccharide vaccine at 14 versus 28 days postoperatively. J Trauma. 2002;53:1037–42.
ACIP Vaccine Recommendations. Centers for disease control and prevention, recommended immunization schedules, 2016.
Spelman D, Buttery J, Daley A, Isaacs D, Jennens I, Kakakios A, et al. Guidelines for the prevention of sepsis in asplenic and hyposplenic patients. Intern Med J. 2008;38:349–56.
Salvadori MI, Price VE. Preventing and treating infections in children with asplenia or hyposplenia. Paediatr Child Heal. 2014;19:271–4.
Schimmer JAG, Van Der Steeg AFW, Zuidema WP. Splenic function after angioembolization for splenic trauma in children and adults: a systematic review. Injury. 2016;47:525–30.
Further Reading
Coccolini, F., Montori, G., Catena, F. et al. Splenic trauma: WSES classification and guidelines for adult and pediatric patients. World J Emerg Surg 12, 40 Thursday (2017).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Anthony, T.W.C.B., Vallicelli, C., Catena, F. (2023). Splenic Trauma. In: Coccolini, F., Catena, F. (eds) Textbook of Emergency General Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-22599-4_96
Download citation
DOI: https://doi.org/10.1007/978-3-031-22599-4_96
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22598-7
Online ISBN: 978-3-031-22599-4
eBook Packages: MedicineMedicine (R0)