Abstract
Zinc oxide (ZnO) is a very important material with diverse uses in solar cells, optoelectronic devices, and other applications. Optimizing the optical properties is required in such applications. In this work the influence of annealing in nitrogen atmosphere and aluminum doping on the optical properties was investigated. Undoped (ZnO) and aluminum doped (ZnO:Al) thin films were deposited using spray pyrolysis (SP) method on glass substrates, and some of these films were annealed in nitrogen atmosphere. The microstructure of the films was explored using X-ray diffraction, and it is found that the films are polycrystalline. Transmittance of the films was recorded as a function of wavelength in the range of 300–1100 nm. Transmittance was used to calculate the absorption coefficient, extinction coefficient, reflectance, refractive index, real and imaginary parts of the dielectric constant, and optical conductivity. All these parameters are found to be affected by Al-doping and annealing. It is found that optical parameters were influenced by Al-doping and annealing.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Characterization
- Electronic materials
- Energy conversion and storage
- Transparent conducting oxides
- Zinc oxide
- Spray pyrolysis
Introduction
Zinc oxide (ZnO) is a wide band gap, II-VI compound semiconductor that has attracted the attention of the scientific community. It has three phases; the cubic (zinc blend) phase of space group \(F\overline{4 }3m\) or \({T}_{d}^{2}\), which is a metastable phase that can be stabilized when it is grown on cubic substrates, rocksalt which can be obtained at relatively high pressures, and hexagonal (wurtzite) phase of space group P63mc or C6v4, which is the thermodynamically stable phase under ambient conditions. The lattice constants of the hexagonal phase are \( ~c = ~5.025~\;\mathop {\text{A}}\limits^{ \circ } \,{\text{and}}\,~a~ = ~3.249\;\mathop {\text{A}}\limits^{ \circ } \) [1, 2]. ZnO has several applications including solar cells, optoelectronic devices, gas sensors, piezo-electric transducers, and phosphors. It is a transparent conducting oxide (TCO) of transparency comparable to that of tin oxide (SnO2) and indium tin oxide (ITO), so it is an alternative to both of them. Naturally, ZnO is an n-type semiconductor due to intrinsic defects such as oxygen vacancies and/or zinc interstitials. The n-conductivity can be enhanced by intentional doping. Aluminum doping was found to be effective in improving the electrical and optical properties of ZnO, and aluminuim doped zinc oxide (ZnO:Al) is commonly used as a front contact in thin film solar cells such as CdTe-based and Cu(In,Ga)Se2 (CIGS)-based solar cells.
There are different physical and chemical methods to prepare ZnO thin films such as chemical solution deposition (CSD) [3], DC magnetron sputtering [4], spin coating [5], and spray pyrolysis technique (SP) [6,7,8,9,10]. The spray pyrolysis (SP) method was chosen because it is cost effective and simple method. The film’s microstructure was checked using X-ray diffraction, and the transmittance was recorded at room temperature. The transmittance was used to deduce the reflectance, absorption coefficient, refractive index, extinction coefficient real and imaginary parts of the dielectric function, and optical conductivity of the films. The results were analyzed and discussed in the light of using the films in solar cells’ applications.
Experimental Procedure
ZnO thin films were deposited on ultrasonically cleaned glass substrates using the spray pyrolysis technique. A precursor solution was prepared from 7.61 × 10–3 mol of zinc chloride (ZnCl2), and 1.33 × 10−5 mol of Al2O3, in 300 ml of distilled water, and about 1 ml of hydrochloric acid (HCl). The solution was intermittently sprayed on the hot substrates of temperature Ts = 450 °C. Nitrogen (N2) was used as the carrier gas, where the optimum carrier gas pressure was around 5 kg/cm3. The microstructure of the films was explored by X-ray diffraction (XRD) using XRD-7000 X-Ray diffractometer with Cu Kα radiation (λ = 1.54 A°). Transmittance was measured at room temperature using a double beam Shimadzu UV 1601 (PC) spectrophotometer in the wavelength range \(\lambda =290-1100 nm\) with respect to a piece of glass similar to the substrates. Annealing was performed in nitrogen atmosphere at 250 °C for 45 min.
Results and Discussion
Figure 1 displays the X-ray diffractograms of two films of the set under study. The films are polycrystalline with a hexagonal (wurtzite) structure. The peaks were assigned to their corresponding Miller indices (hkl). The (100), (101), and (002) diffraction peaks are observed, with the highest intensity is that of the (002) peak. So, the preferential orientation is the one perpendicular to the surface (002), or in other words along the optical c-axis, which is perpendicular to the substrate [11]. This is because the c-axis has the lowest energy of the hexagonal crystalline structure [12].
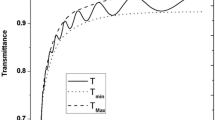
Figure 2 shows the transmittance \(T\%\) of as-deposited, annealed, undoped, and Al-doped ZnO thin films plotted against the wavelength \(\lambda \). . All the films showed high transmittance (60%-92%) in the visible and near infrared spectral regions. The figure shows that the maximum transmittance of the films reaches as high as more than 91% for the ZnO:Al as-deposited and annealed film in the red and near infrared regions, which means the films can be used as forecontacts, windows, or antireflection coatings in solar cells. It is observed that the transmittance increased when the films were doped with Al, and when the films were annealed. The highest transmittance was obtained for the Al-doped annealed film. The increase of transmittance after annealing is due to the increase of films crystallinity, and decrease of film defects especially surface defects, in addition to the decrease of surface roughness. The increase of transmittance with Al-doping is due to the decrease of the absorption coefficient in the visible region [13]. A slight shift of the band edge towards lower wavelengths was observed, where the annealed ZnO:Al has the shortest cut-off wavelength, while as-deposited ZnO has the longest one.
Figure 3 displays the reflectance \((R=1-T)\) of the same films deduced from the transmittance and plotted against wavelength \(\lambda \). As the figure shows, the reflectance decreases rapidly near the absorption edge of ZnO, then it decreases slowly and becomes approximately constant in the visible and near infrared region, where the films become highly transparent. The reflectance is smallest for the Al-doped and annealed film due to the increase in the density of free charge carriers, which are the electrons. The greatest reflectance is that for the as-deposited ZnO in most of the wavelength range.
ZnO has a direct band gap, and for a direct transition, the optical absorption edge is analyzed by the following equation [14],
where \(\alpha \) is the absorption coefficient, \(h\nu \) is the photon's energy, C is a constant, and \({E}_{g}\) is the direct band gap energy. The absorption coefficient \(\alpha \) was deduced from the transmittance T using the following formula
where \(T\) is the transmittance and \(t\) is the film thickness. To estimate the band gap energy of the films, a plot of \({(\alpha h\nu )}^{2}\) versus \((E = h\nu )\) was performed and displayed in Fig. 4. A linear fit was performed in the linear parts of the curves as shown in the figure, and the direct band gap energy was estimated and displayed in the figures. The obtained values of the annealed and doped films are all the same (3.28 eV), while that of the as-deposited undoped film is 3.27 eV. The reason why, the films have approximately the same value of band gap energy is that, they were deposited at high temperature (450 °C), which means the crystal growth was approximately complete, the grains have a large size as can be deduced from the sharp (002) peak, hence doping and annealing have unobservable influence on the band gap energy.
The extinction coefficient \(k\) was calculated from the following formula
Figure 5a shows the extinction coefficient, and Fig. 5b shows the refractive index for as-deposited, and annealed ZnO and ZnO:Al thin films, both as functions of wavelength in the range \(\lambda = 300 - 1100 \;{\text{nm}}\). As Fig. 5a shows the extinction coefficient \(k\) decreases abruptly at the absorption edge, then it becomes approximately constant. Its value for all films at λ = 550 nm is in the range 0.022–0.034, where the smallest value is for the annealed ZnO:Al films and the greatest value is for the as-deposited ZnO thin films. The small value of \(k\) is preferred for use of the thin film in solar cells.
Reflectance is related with the refractive index \(n\) and the extinction coefficient \(k\) by the following relationship [15]
and the complex refractive index of the film is given by
Solving Eq. (4) for the refractive index \(n\) gives
The refractive index decreases with wavelength \(\lambda\) as shown in Fig. 5b, where this decrease is strong in the region of the band edge, but becomes slower after that. Then the refractive index becomes constant in the visible and near infrared regions (1.35–3.5), and \(n_{\infty }\) can be estimated as 1.33 for annealed ZnO:Al, 1.41 for both annealed ZnO and as-deposited ZnO:Al, and 1.57 for as-deposited ZnO. These results are consistent with those obtained by Tüzemen et al. [16] for ZnO thin films prepared at room temperature using pulsed filtered cathodic vacuum arc deposition (PFCVAD), where the value of refractive index in the visible and near infrared spectral regions is in the range 1.5–2.4. Also from Fig. 5b it can be noticed that at λ = 550 nm, \(n\) is restricted in the range in the range 1.75- 2.17 for all films, where the smallest value is for the annealed ZnO:Al films, and the greatest value is for the as-deposited ZnO thin films. It is well known that the refractive index is mainly determined by the crystal quality, and it is correlated with crystallinity, surface roughness, and resistivity of the films.
The complex dielectric function is given as
where \(\varepsilon_{1}\) is the real part which is associated to the refractive index, and the imaginary part \(\varepsilon_{2}\) which is related to the absorption coefficient or extinction coefficient. The real and imaginary parts of the dielectric function are given by the following equations [15]
Figure 6 displays the real and imaginary parts of the dielectric function \(\varepsilon_{1}\), and \(\varepsilon_{2}\) respectively as functions of wavelength of light \(\lambda\). Both of \(\varepsilon_{1}\), and \(\varepsilon_{2}\) decrease with \(\lambda\), but the decrease of \(\varepsilon_{2}\) is faster, and in both cases the decrease is fast around the band edge, after which both of them become approximately constant, where \(\varepsilon_{1}\) for long wavelengths denoted as ε∞ is 1.68 for annealed ZnO:Al, 2.00 for both annealed ZnO and as-deposited ZnO:Al, and 2.43 for as-deposited ZnO. These values are consistent with the relationship \(\varepsilon_{\infty } = n_{\infty }^{2}\). Figure 6 shows that at \(\lambda = 550 nm\) the real dielectric function is in the range of 3.06–4.76, and \(\varepsilon_{2}\) is in the range of 0.083–0.161 for all films, where the smallest values are for annealed ZnO:Al and largest ones are for as-deposited ZnO. The values of \(\varepsilon_{1}\) are always larger than those of \(\varepsilon_{2}\). That is \(\varepsilon_{1}\) is related to the refractive index, while \(\varepsilon_{2}\) is related to the extinction coefficient.
The optical conductivity of the ZnO thin films can be determined by the formula [17];
where \(c\) is the speed of light. Figure 7 depicts the optical conductivity σ against wavelength of incident radiation \(\lambda\). It is observed that \(\sigma\) shows the same behavior as the other optical constants discussed before. That is it decreases with wavelength \(\lambda\), and the decrease is fast in the region of the absorption edge, then it becomes slow, and then constant in the visible and near infrared regions. The smallest \(\sigma\) is that of annealed ZnO:Al film and the largest one is for the as-deposited ZnO film.
Conclusions
Undoped and aluminum doped ZnO thin films were deposited on glass substrates by the spray pyrolysis method at substrate temperature Ts = 450 °C. X-ray diffraction was used to explore the microstructure of the films, where all films showed hexagonal (wurtzite) structure, with preferential orientation along the c-optical axis perpendicular to the substrate. UV–VIS spectroscopy was used to investigate the optical properties of the films. The transmittance was measured at room temperature and used to deduce reflectance, band gap energy, extinction coefficient, refractive index, real and imaginary parts of the dielectric function, and optical conductivity.
The maximum transmittance exceeds 91%, and transmittance for all films is in the range of 60–91% in the visible and infrared regions. The optical energy band gap \(E_{g}\) is 3.27 for as-deposited ZnO thin films, but 3.28 for annealed ZnO thin film, annealed, and as-deposited ZnO:Al. The extinction coefficient \(k\) and refractive index \(n\) decrease with wavelength, and the values of \(k\) are small which reflects the high quality of the films. Real and imaginary parts of the complex dielectric function decrease with radiation wavelength and go to a constant value. The behavior of \(\varepsilon_{1}\) follows the refractive index, while the behavior of \(\varepsilon_{2}\) follows that of the extinction coefficient. These results demonstrate that good quality of the prepared ZnO thin films and their potential use in solar cells and the optoelectronic industry.
References
Shakti N, Gupta PS (2010) Structural and optical properties of sol-gel prepared ZnO thin film. Appl Phys Res 2(1):19–28
Morkoç H, Özgür Ü (2008) Zinc Oxide: fundamentals, materials and device technology. Wiley-VCH. pp1–76. https://doi.org/10.1002/9783527623945
Rakhshani AE (2005) Thin ZnO films prepared by chemical solution deposition on glass and flexible conducting substrate. Appl Phys A 81:1497–1502
Abduev AK, Akhmedov AK, Asvarov AS, Abdullaev AA, Sulyanov SN (2010) Effect of growth temperature on properties of transparent conducting gallium_doped ZnO films. Semiconductors 44(1):32–36
Kumar SA, Jitendra K (2011) Effect of aluminum addition on the optical, morphology and electrical behavior of spin coated zinc oxide thin films. AIP Adv 1:032153
Studenikin SA, Golego N, Cocivera M (1998) Optical and electrical properties of undoped ZnO films grown by spray pyrolysis of zinc nitrate solution. J Appl Phys 83(4):2104–2111
Ikhmayies SJ, Abu El-Haija NM, Ahmad-Bitar RN (2010) Electrical and optical properties of ZnO:Al thin films prepared by the spray pyrolysis technique. Phys Scr 81:015703 (5pp)
Ikhmayies SJ, Abu El-Haija NM, Ahmad-Bitar RN (2010) Characterization of undoped spray-deposited ZnO thin films of photovoltaic applications. FDMP. 6(2):165–177
Ikhmayies SJ, Abu El-Haija NM, Ahmad-Bitar RN (2015) A comparison between different ohmic contacts for ZnO thin films. J Semicond 36(3):033005(5 pp)
Ikhmayies SJ, Abu El-Haija NM, Ahmad-Bitar RN (2010) The Influence of annealing in nitrogen atmosphere on the electrical, optical and structural properties of spray-deposited ZnO thin films. FDMP. 6(2):219–232
Kadi Allah F, Cattin L, Morsli M, Khelil A, Langlois N, Bernéde JC (2010) Microstructural properties of ZnO: Sn thin films deposited by intermittent spray pyrolysis process. J Mater Sci Mater Electron 21:179–184
Ekthammathat N, Phuruangrat A, Thongtem S, Thongtem T (2015) Hydrothermal-assisted synthesis and photoluminescence of ZnO microds. Dig J Nanomater Biostruct 10(1):149–153
Alrefaee M, Singh UP, Das SK (2021) Growth of aluminum doped zinc oxide nanostructure thin films by nonconventional sol-gel method. In: Macromolecular symposia 402(1) special issue: international conference on science and engineering of materials—ICSEM 2021 Part II. 2100350. https://doi.org/10.1002/masy.202100350
Benhaliliba M, Benouis CE, Aida MS, Yakuphanoglu F, Sanchez Juarez A (2010) Indium and aluminium-doped ZnO thin films deposited onto FTO substrates: nanostructure, optical, photoluminescence and electrical properties. J Sol-Gel Sci Technol 55:335–342. https://doi.org/10.1007/s10971-010-2258-x
Alnajjar AA, Al-Shaikley FY, Alias MFA (2012) Optical properties and photoconductivity of undoped and In-doped CdTe thin films. J Electron Devices 16:1306–1314
Tüzemen EŞ, Eker S, Kavak H, Esen R (2009) Dependence of film thickness on the structural and optical properties of ZnO thin films. Appl Surf Sci 255:6195–6200
Mishra RL, Mishra SK, Prakash SG (2009) Optical and gas sensing characteristics of tin oxide nano-crystalline thin film. J Ovonic Res 5(4):77–85
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Ikhmayies, S.J. (2023). Optical Parameters of ZnO Thin Films. In: Zhang, M., et al. Characterization of Minerals, Metals, and Materials 2023. TMS 2023. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-031-22576-5_4
Download citation
DOI: https://doi.org/10.1007/978-3-031-22576-5_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-22575-8
Online ISBN: 978-3-031-22576-5
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)