Abstract
Traumatic nerve injuries range from blunt force contusions causing temporary numbness to complete destruction of major nerves with permanent functional loss. The type and extent of injury, as well as the specific nerve(s) involved, determine the potential functional threat to the patient. A thorough clinical evaluation, along with judicious use of ancillary tests, guides treatment. Most closed nerve injuries are best initially observed to allow for spontaneous recovery. In some closed cases, neurolysis can facilitate distal axon progression. Other closed cases ultimately prove higher grade and require more complex reconstruction. Most lacerative nerve injuries should be taken emergently to the operating room for direct repair before gap formation. In the absence of excess tension, acute lacerations may simply be sutured back together under a high-magnification surgical microscope. Once separated over time, the two nerve ends retract, swell, and fibrose, creating a gap that must undergo reconstruction. Short nerve gaps can be bridged with cadaveric processed nerve allografts. Longer gaps targeting motor receptors may require autogenous cable nerve grafts harvested from the patient. Nerve and tendon transfers are final options.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara OverviewTraumatic peripheral nerve injuries threaten permanent loss of function. The risk of functional loss is proportionate to the type and extent of nerve injury and the distance from target receptors. Many closed nerve injuries will recover spontaneously without intervention. Lacerations should be repaired or reconstructed promptly to permit regenerating axons to reach their receptors as soon as possible. Sensory receptors are more tolerant of delayed reinnervation. Motor end plates become permanently depleted over time, precluding functional recovery if regenerating axons arrive later than 12–18 months after injury. The mechanism of injury, clinical examination, and ancillary tests guide treatment decisions that include various surgical techniques to reinnervate distal receptors.
1 Definition
Traumatic injury to a peripheral nerve includes any acute process that compromises nerve function. Closed mechanisms of injury include blunt force trauma involving sudden compression or traction forces. Open mechanisms of injury range from pure sharp lacerations to combinations of cutting, crushing, and tearing.
2 Epidemiology
Unlike some other medical conditions, traumatic nerve injury is not limited to any select population. From the very young to the very old, any patient can encounter a set of circumstances leading to nerve injury. The greatest incidence occurs during the working years of adult life when patients are most exposed to conditions likely for nerve injury.
3 Etiology/Pathogenesis
Sharp laceration accounts for the majority of nerve injuries. Common environments include the kitchen where knives and broken glass easily slip and cut nerves in the hand and wrist. Punching or falling through glass panes can lacerate nerves in the more proximal upper extremity. Laceration by sharp edges of metal occurs in manufacturing workplaces. Industrial machinery or vehicular trauma can impart heavy crush or avulsion injury to nerve. Even sleeping with the body weight on a nerve for many hours is enough to wake with a nerve palsy.
4 Classification
The cross-sectional architecture of a peripheral nerve consists of numerous individual nerve cells (axons) traveling together, longitudinally connecting the proximal cell bodies in the spinal column to the distal sensory and motor receptor. Each axon is enveloped in a conductive sheath of myelin protein manufactured by Schwann cells and housed in its own structural conduit, the endoneurial tube. A Sunderland grade I injury disrupts the myelin sheath or endoneurial environment enough to cease nerve function, but the axon itself survives. Sunderland grade II signifies injury to the nerve cell resulting in axon death, but the endoneurial tube is not disrupted. Multiple axons in their endoneurial tubes are grouped together into fascicles encased by the next structural layer called perineurium, an extension of the blood-brain barrier. Sunderland grade III injuries disrupt the axons and their endoneurial tubes, but the perineurium remains intact. Sunderland grade IV injuries further disrupt the protective perineurium, leaving intact only the most peripheral encasement, epineurium. With disruption of the epineurium, Sunderland grade V injuries render the nerve fully separated with an intervening gap. The original five-part Sunderland classification was subsequently modified to include grade VI, representing a pattern of mixed grades occurring in adjacent fascicles. Different types of nerve injury are reported in Fig. 77.1.
5 Diagnosis
Peripheral nerves may be pure motor, pure sensory, or mixed. Diagnosis begins with the history of injury, focusing on the mechanism and anatomic location. This establishes the likely type and grade of injury and the specific nerve(s) involved. Assessment continues with the physical examination, mapping the exact distribution of functional loss, sensory and motor. In most cases, this information is sufficient to establish the treatment plan. More complex scenarios may leave uncertainty regarding multilevel injuries that overlap to produce the observed deficits. With closed mechanisms of injury, the Sunderland grade cannot be known with certainty just based on the clinical deficit alone.
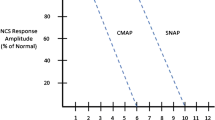
The most frequently employed ancillary study is electrical nerve testing. There are two components: nerve conduction study (NCS) and electromyography (EMG). During NCS, abnormalities of conduction are tested with a proximally applied electrical impulse recorded from a distal surface electrode. During EMG, thin needles inserted into specific muscles produce characteristic waveforms when stimulated, providing information about the condition of the nerve supplying that muscle. Similar techniques are also used during surgery with electrodes applied directly to the surface of the nerve to guide intraoperative treatment decisions. Less utilized are imaging studies: magnetic resonance imaging and ultrasound. Imaging studies have varying degrees of accuracy to reveal physical continuity of the nerve, constriction vs. enlargement, or signal intensity changes indicating response to injury.
6 Treatment
Optimal treatment is based on the contributing variables: mechanism of injury, age of the patient, location and time since injury, and specific motor/sensory deficits.
6.1 Nonoperative Treatment
The majority of closed mechanism of nerve injury traumas will ultimately recover spontaneously over the appropriate time period based on the grade of injury and distance from target receptors. For that reason, most closed injuries are initially observed for anticipated recovery. A baseline NCS/EMG is obtained at 3 weeks from injury. The relevant findings that distinguish a Sunderland I from higher grade injury will not be evident if obtained too early. In select rare cases, unique circumstances may indicate a higher grade injury and prompt early surgical evaluation including intraoperative electrical nerve testing. There are no current medical therapies that enhance nerve regeneration. Patients are taught therapy exercises while awaiting regeneration: desensitization, passive joint and tendon range of motion, and splinting to prevent contracture. If there are no clinical signs of functional recovery, a repeat NCS/EMG may demonstrate electrical signs of reinnervation that precede clinical signs. Distal motor receptors, motor end plates, are irreversibly lost over time. Potential to regain function declines at a rate of approximately 1% per week. Axons regenerating at 1 mm/day must reach the motor end plates by 12–18 months after initial injury for any meaningful recovery. The presence or absence of clinical/electrical signs of reinnervation guides appropriately timed next interventions.
6.2 Neurolysis
The decision to pursue neurolysis is typically made by 3–4 months following injury, certainly no later than 6 months. The surgical plan must include a treatment strategy to account for each possible variation of injury pattern. Neurolysis alone, as opposed to nerve graft or transfer, is indicated by demonstration of axon regeneration across the site of injury. Regenerating axons will have traveled a sufficient distance after 3–4 months to place the stimulating and recording electrodes far enough apart to accurately measure nerve action potentials (NAP). While there is no universally agreed-upon criterion, the amplitude of the NAP across the zone of injury should be at least 50% that of an NAP obtained proximal to the zone of injury. All dense investing scar tissue is removed from around the nerve at the site of injury along with decompression performed at known sites of anatomic narrowing such as the carpal or cubital tunnel passages.
6.3 Direct Repair
Unlike closed nerve injuries, direct nerve lacerations should be taken emergently to the operating room for direct repair. Within only a matter of days, tension-free approximation may become impossible because of retraction of the nerve stumps, local tissue edema, and fibrosis. Nerve suture is performed at high magnification under the operating microscope. Specialized microsurgical instruments permit delicate handling of the tissues and precise manipulation. Each nerve ending is trimmed perpendicular to its longitudinal axis, creating fresh margins of undamaged tissue without protruding fascicles. Using 9-0 or 10-0 nylon, the least number of sutures are placed only through epineurium to avoid damaging fascicles. Each fascicle should be aligned with itself across the junction. Multiple clues guide correct alignment including epineurial vessels and pattern of different-sized fascicle groups. The edges of the epineurium should just meet each other circumferentially and create a perfect cylinder without gaps. Fascicles should neither buckle nor escape. Forcing the two ends together too tightly distorts the repair, misaligns fascicles, and can hinder regeneration. The repair should remain intact without gaps when the adjacent joints are positioned in neutral extension or the range that permits limb function. If a reasonable limb position cannot be achieved without undue tension, consideration must be given to interposing a nerve graft. Axon regeneration is impaired across a junction under tension. The outcomes of nerve repair are influenced by multiple variables, the most powerful of which is patient age. Single-digit-aged children can achieve recovery indistinguishable from normal in many circumstances. The quality of regeneration and recovery then diminishes into the teens and adulthood. The second most important variable is distance to target receptors combined with time delay between injury and repair. Sensory receptors retain the capacity for reinnervation longer than the 1% per week attrition of motor recovery potential. Mixed nerves require proper alignment of motor and sensory fascicles; any misdirected axon regeneration will not result in functional recovery. Certain nerves with specific functional roles demonstrate relatively consistent outcomes such as the well-established hierarchy of recovery for radial nerve over median nerve over ulnar nerve [1]. Clean, sharp lacerations are more favorable as opposed to avulsion mechanisms that impart traction injury over substantial distance from the point of disruption.
6.4 Nerve Grafting
Nerve grafting is indicated for direct lacerations that cannot be repaired without excessive tension or Sunderland grade III–V crush/avulsion injuries that show no evidence of regeneration by intraoperative NAP. The key to successful grafting is elimination of damaged tissue on both the proximal and distal ends. Any fibrosis obstructs the endoneurial tubes and blocks regeneration. Standard teaching called for starting with the damaged end and trimming sequentially under the microscope until the cut end appeared normal. This often results in a repair margin that is still compromised. The better method is to begin the microscope examination in undamaged nerve tissue and progress towards the zone of injury. As soon as tissue change is encountered, that represents the correct margin for resection. After resecting damaged nerve tissue from both ends, the limb is positioned at full length to determine the tension-free size of the defect. A structural pathway for axon regeneration is then placed between the two ends in the form of nerve autograft or allograft [2]. The same surgical techniques employed in direct nerve repair apply to nerve grafting. Autograft refers to sections of nerve harvested from expendable sensory branches. The most common donor is the sural nerve that supplies sensation over the lateral ankle. Autograft provides a structural path for regeneration, nerve growth factors, and viable Schwann cells that are critical to promote regeneration over greater distances. To avoid the additional surgery and donor-site morbidity of autograft, hollow conduits were studied for decades. Conduits ultimately proved inferior to the other means of avoiding autograft, cadaveric nerve allograft. To avoid antigenicity, allograft must be laboratory processed to remove cellular material as well as tissue debris that obstructs the endoneurial tubes. Allografts still contain structural proteins and nerve growth factors but lack Schwann cells. Outcomes of nerve grafting are influenced by the same variables as direct repair, in addition to the length and type of graft matter. At shorter distances, the absence of Schwann cells has not created a discernible difference in outcomes. But with increasing defect size, especially for critical motor targets, autografts prove superior to allografts. The longest allografts available are 5–7 cm.
6.5 Nerve Transfer
When all of the contributing variables are considered and recovery potential from repair or graft is predicted to be minimal, nerve transfer may offer a better outcome. A nerve transfer refers to dividing an expendable donor nerve at a distal location and connecting it to the distal receiving end of a nerve that has been injured at a more proximal location [3]. The regenerating axons transferred from the donor nerve will arrive at the terminal receptors of the recipient nerve long before any regenerating axons from the proximal injury site. For example, outcome studies indicate no recovery of intrinsic muscle function in the hand following an ulnar nerve avulsion defect proximal to the elbow. Partial reinnervation of the hand intrinsic muscles can be accomplished by transferring the distal anterior interosseous nerve (AIN) to the ulnar motor branch. The junction of the nerve transfer is much closer to the progressively deteriorating motor end plates. Axons from the AIN can arrive within 12 months, whereas regeneration from proximal to the elbow would require more than 2 years. Nerve transfer is primarily used to restore motor function but can also be performed to restore critical sensory functions such as the ulnar digital nerve of the thumb and radial index finger. Principles of nerve transfer include tension-free coaptation, matching axon counts, and pure motor or pure sensory pairing. Nerve transfers continue to be studied with certain combinations demonstrating better outcomes than others. Some nerve transfers do not conflict with subsequent procedures, but for others, the donor nerve supplies the same muscle group that would otherwise be used in a tendon transfer to solve the same original deficit. If the nerve transfer fails, the corresponding tendon transfer has also been eliminated as an option.
6.6 Tendon Transfer
Before the advent of nerve transfers, motor deficits following nerve injury were managed by tendon transfers. Tendon transfer involves distal division of an expendable muscle-tendon unit with rerouting and connection to the recipient tendon associated with the lost motor function. Just like nerve transfers, some tendon transfers inherently work better than others. Principles of tendon transfer include matching excursion and power, a straight line of pull, one transfer for one function, and using muscles that naturally contract in the same phase of movement. One of the most reliable transfers, for a non-reconstructible radial nerve injury, restores digital extension using a wrist flexor muscle. Debate continues as to which is best in cases where either a nerve transfer or a tendon transfer could be used. Although a successful nerve transfer reinnervates the original muscle-tendon unit, nerve transfer has greater potential for complete failure. Also, nerve transfers must still be accomplished within the time window for motor recovery, but tendon transfers can be performed long after the original injury.
Take-Home Message
-
Nerve lacerations should be taken emergently to the operating room for direct repair.
-
Most closed nerve injuries should be initially followed nonsurgically and methodically evaluated with a timed plan for intervention in the absence of recovery.
-
If a tension-free direct repair of undamaged nerve tissue cannot be achieved, nerve graft or nerve transfer is indicated.
-
Tendon transfer remains the ultimate salvage for late-presenting cases or other circumstances precluding useful reinnervation.
Summary
Traumatic nerve injury immediately deprives the patient of the motor or sensory functions served by that nerve. Lesser degrees of closed mechanism injury typically recover with patience and time. Open lacerations are repaired immediately, but ultimate recovery is influenced by multiple variables. Delayed nerve reconstructions using more complex techniques of nerve graft and transfer demonstrate even further diminished outcomes.
Questions
Multiple correct answers are possible. Answers available in the book back matter.
-
1.
What is the difference between a Sunderland I and II injury?
-
(a)
The nerve cell survives in a Sunderland I injury and clinical recovery occurs with re-myelination, but Sunderland II injuries require axon regeneration
-
(b)
The nerve cell survives in a Sunderland II injury and clinical recovery occurs with re-myelination, but Sunderland I injuries require axon regeneration
-
(c)
Sunderland I injuries result in complete nerve cell death, leading to no clinical recovery
-
(d)
Sunderland II injuries cause immediate clinical recovery without the need for re-myelination
-
(a)
-
2.
How long after closed nerve injury should the initial NCS/EMG be performed?
-
(a)
After 3 weeks, the EMG changes appear that distinguish a Sunderland I from higher grade injury
-
(b)
After 1 week, the EMG changes appear that distinguish a Sunderland I from higher grade injury
-
(c)
The EMG must be performed immediately after injury to accurately diagnose nerve damage
-
(d)
NCS/EMG is not a reliable method for diagnosing closed nerve injuries
-
(a)
-
3.
When repairing a nerve laceration, what is the main indication to use a graft?
-
(a)
Excess tension or gap at the repair site
-
(b)
Signs of infection or association with exposed fractures or gap at the repair site
-
(c)
Grafts are mainly used for cosmetic purposes and are not essential for nerve repair
-
(d)
Grafts are never recommended for nerve repair due to the increased risk of complications
-
(a)
-
4.
In nerve repair or reconstruction, which variable has the greatest impact on outcome?
-
(a)
Young patient age has the greatest impact on clinical recovery
-
(b)
Sex has the greatest impact on clinical recovery
-
(c)
The severity of the initial nerve injury has the most significant influence on outcome
-
(d)
Genetic factors are the primary determinant of clinical recovery following nerve repair
-
(a)
-
5.
What is the main advantage of tendon transfer over nerve transfer?
-
(a)
There is no time limit following injury to perform a tendon transfer
-
(b)
It requires less costs and days of hospitalization
-
(c)
Tendon transfers have a higher risk of complications compared to nerve transfers
-
(d)
Tendon transfers can only be performed on upper limbs, limiting their applicability
-
(a)
References
Murovic JA. Upper-extremity peripheral nerve injuries: a Louisiana State University Health Sciences Center literature review with comparison of the operative outcomes of 1837 Louisiana State University Health Sciences Center median, radial, and ulnar nerve lesions. Neurosurgery. 2009;65(4 suppl):A11–7.
Brooks DN, Weber RV, Chao JD, et al. Processed nerve allografts for peripheral nerve reconstruction: a multicenter study of utilization and outcomes in sensory, mixed, and motor nerve reconstructions. Microsurgery. 2012;32(1):1–14.
Gordon T. Nerve regeneration: understanding biology and its influence on return of function after nerve transfers. Hand Clin. 2016;32(2):103–17.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Henry, M. (2023). Traumatic Nerve Injuries. In: Longo, U.G., Denaro, V. (eds) Textbook of Musculoskeletal Disorders. Springer, Cham. https://doi.org/10.1007/978-3-031-20987-1_77
Download citation
DOI: https://doi.org/10.1007/978-3-031-20987-1_77
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-20986-4
Online ISBN: 978-3-031-20987-1
eBook Packages: MedicineMedicine (R0)