Abstract
Heavy metal contaminated soils may pose risk and hazards to humans and the ecosystem. Physiochemical approaches have been widely used for remedying polluted soil; however, their large-scale application is difficult due to high cost and side effects. Current research interests are moving towards the application of in situ strategies to reduce costs and to resolve pollution dispersal problems. Phytoremediation is a plant-based, environment-friendly, and cost-effective technology that can potentially be used to remediate contaminated media. However, phytoremediation is with some critical shortcomings, such as phytotoxicity, slower than mechanical method and limited mechanical uptake. Some plant-associated bacteria can overcome these constraints by assisting plants to accumulate higher amount of metal without increasing phytotoxity. While rhizosphere microorganisms can enhance phytoremediation according to numerous studies, endophytes appear to do so more efficiently by interacting closely with host plants. Endophytes are all microorganisms that inhabit the interior of plant tissues, causing no harm to the host and developing no external structures. In the plant endophyte symbiosis, endophytes receive carbohydrates from plants and improve plant resistant to biotic and abiotic stresses in return. Moreover, recent studies showed that many endophytes are metal resistant, able to degrade organic contaminants and have been successfully used in phytoremediation. This paper focuses on the overview of studies on the ability of endophytes to support plant absorption of heavy metals as an object with potential in environmentally friendly and inexpensive bioremediation.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
13.1 Introduction
Heavy metals are distinguished by their high relative atomic weight (> 4 g/cm3). Some of them, such as zinc (Zn), iron (Fe), or manganese (Mn), are microelements that plants require and absorb through their roots since they are essential to plant survival. These metals, however, can be harmful in excessive doses. Other elements, such as lead (Pb), cobalt (Co), and arsenic (As), have no biological role and are harmful even at low doses; they are categorized as Toxic Heavy Metals (THM) (Rahman and Singh 2019).
Heavy metal pollution is the excessive deposition of hazardous heavy metals in soil as a result of human activity. Lead (Pb), cadmium (Cd), zinc (Zn), mercury (Hg), arsenic (As), silver (Ag), chromium (Cr), copper (Cu), iron (Fe), and platinum group elements are among the heavy metals found in soil. With the expansion of the global economy recently, both the type and concentration of heavy metals in the soil from anthropogenic sources (mining, electroplating and wielding, urbanization) (Mishra and Bharagava 2016; Wang and Chen 2009) and the use of metal-based fertilizers and pesticides have gradually increased, resulting in environmental degradation. Heavy metals are extremely dangerous to both the environment and organisms. It can be enhanced via the food chain and finally integrated into living creatures (Sim et al. 2019). Once the soil has been contaminated with heavy metals, it is difficult to be remediated (Bayata 2020).
The main purpose of heavy metal removal is to minimize their negative effects on ecosystems and human health. Numerous remediation techniques for heavy metal contaminated soils have been introduced to cope with this problematic issue, which can be divided into three main groups: physical, chemical and biological. Physical and chemical technologies are often expensive and time-consuming besides the negative environmental impacts due to generating a large quantity of hazardous waste (Sim et al. 2019). On the contrary, phytoremediation is attracting great interest because of its outstanding advantages of being a green technology, environmentally friendly and more economical than traditional methods. Phytoremediation of heavy metal contaminated soil is more effective due to the interaction between plants and plant-associated microorganisms. While rhizobacteria increase plant metal uptake and translocation through various processes, endophytes live inside plant tissues and form a mutualistic relationship with host plant (Sim et al. 2019).
Endophytes support the survivability of their host plants in unfavorable environments, especially in heavily polluted soils. Endophytes residing in such harsh environments are hypothesized to have adapted and evolved to acquire increased tolerance toward the adverse conditions.
In addition to heavy metal tolerance, plant-associated microbes can operate as biocontrol agents against a variety of pathogens and herbivores (Sim et al. 2019), ensure nitrogen fixation and growth regulator production, and contribute to heavy metal tolerance and treatment in plants. The nature of endophytes, their potential in heavy metal removal, and their function in heavy metal uptake by plants utilized in bioremediation will be examined in this paper.
13.2 Endophyte Characteristics
Endophytes were first defined as microorganisms that reside inside plant tissues by Barry in 1866. Then, in 1904, endophytic bacteria were identified in Darnel, Germany. Smith et al. developed the concept of endophytic actinomycetes in 1975 when they isolated Micromonospora sp. and discovered that it had a considerable inhibitory impact on the pathogenic fungus Fusarium oxysporum in uninfected tomato plant tissues (Shimizu 2011; Zhenhua et al. 2012). Endophytes are non-pathogenic microorganisms that reside inside plants, according to Carroll et al. (Araújo et al. 2008).
Endophytes are microorganisms that invade interior plant tissues without inflicting obvious harm to their hosts (Stępniewska and Kuźniar 2013). Endophytes, according to Wilson and Carroll, are bacteria and fungi that penetrate the tissues of live plants for all or part of their life cycle without causing damage or disease symptoms to the host plant. The most frequently recognized definition is that of Bacon and White, who agree with the aforementioned writers that “endophytes are microorganisms that live inside live tissues of plants and exert no obvious and direct harmful impact on host plants” (Bacon et al. 2000). This definition has a very important implication: because of its asymptomatic nature, the colonization of plant tissues by endophytes suggests a mutualistic relationship between endophytes and host plants.
Endophytes in plants are classified as either obligatory or facultative. The former require particular environmental conditions in the laboratory for successful culture (Waheeda and Shyam 2017). According to some authors, facultative endophytic bacteria may be isolated from surface sterilized plant parts or from plant internal tissues and are not harmful to the host plant. The preceding definitions do not take into account bacteria that are unculturable under laboratory circumstances and have just recently been detected using molecular techniques. As a result, endophytic bacteria might be culturable or unculturable (Araújo et al. 2008).
Each of the 300,000 higher plant species on Earth is a host plant for one or more endophytes, implying that Earth may have a huge endophytic community (Strobel et al. 2004). Endophytes are abundant, yet diverse, in the environment (soil, cultivated areas) (Seghers et al. 2004; Conrath et al. 2006; Hardoim et al. 2008; Singh et al. 2009). Their numbers vary depending on the plant and environmental factors. According to Xu et al., the number of endophytes and their species richness are greater in tropical and subtropical plants than in cold and dry locations, more in fast-growing and perennial plants than in slow-growing and short-term plants, and greater in healthy plants than in polluted plants (Zhenhua et al. 2012).
Endophytic microorganisms are not only asymptomatic, but they can also promote plant growth by producing growth-promoting substances and plant protection agents (Kandpal et al. 2012; Machavariani et al. 2014), protect host plants from biotic or abiotic stresses, and produce a diverse range of bioactive compounds (Waqas et al. 2012). Endophytes, in turn, obtain adequate nutrients from host plants for growth and survival (Sim et al. 2019). As a result, endophytic microbes are regarded as excellent genetic resources for the development of highly efficient, non-toxic, and environmentally friendly natural products for use in medicine, agriculture, industry, and environmental remediation.
13.3 Mechanisms Employed by Microorganisms to Gain Entry into the Host Plant and Become Endophytes
Endophytic bacteria are thought to originate from the rhizosphere when they have the chance to interact with the host plant (Ali et al. 2014a, b). Thus, endophytes use many mechanisms to gain entry into the host plant, particularly in roots (Santoyo et al. 2016). Typically, this process begins with their concentration in the rhizosphere. They begin to attach to the root surface after successfully colonizing the rhizosphere. This is a critical step in the penetration process. Microorganisms, according to some authors, can adhere to the root surface due to cell surface components such as pili, lipopolysaccharide, or exopolysaccharide etc. Binding sites are usually root tips, where there is a thin cell wall of root apex, or root hairs, or cracks at root emergence sites (Sørensen and Sessitsch 2006), except when endophytes were inoculated on the seeds from the beginning (Truyens et al. 2015).
Microorganisms form microcolonies of hundreds of cells at these sites. Endophytic bacteria have a cellulolytic enzyme system that can hydrolyze the exodermal cell wall, allowing for active penetration. Bacterial cell-wall degrading enzymes are also known to be involved in the elicitation of plant defense pathways, since many proteins involved in defense and repair are biosynthesized to prevent pathogen spread within the plant and are associated with plant cell walls. Endophytic microbes must be able to dodge or dramatically diminish the plant’s immune response, which they can achieve by entering from cracks at root emergence sites. This is the case that has been proposed for Azoacus and Burkholderia vietnamiensis (Malfanova et al. 2013).
Endophytes that pass the exodermal barrier can either remain at the site of entry or move deeper within to colonize the cortex’s intercellular space (apoplast). Endophytic microorganisms differ from legume root-nodule bacteria in that they do not generally enter plant cells and cause formation of specific morphological structures. Endophytic microorganisms, on the other hand, can trigger nodule development in some circumstances.
Only a few microbes are able to get through the endodermal barrier and invade the plant’s xylem vessels. They may feed on nutrients delivered by the xylem, such as water, ions, and low molecular weight organic compounds including sugars, amino acids, and organic acids. Although the concentration of these nutrients in the xylem is relatively low, it is adequate to support endophyte development. Endophytic microbes may metabolize carbon sources that rhizobacteria do not ordinarily use, such as D-sorbitol, D-galacturonic acid, and L-arabinose, which is one of the most readily accessible sugars in plant xylem fluid. Thus, the capacity to utilize certain plant metabolites may be required for successful endophytic colonization (Malfanova et al. 2013).
Normally, the concentration of accessible nutrients in the xylem declines along the plant axis. This explains why endophyte variety and population density diminish with distance from the base, and why only few numbers of them reach the plant’s upper parts, such as leaves and reproductive organs like flowers, fruits, and seeds. Some microorganisms can enter plants via different pathways, such as stomata on leaves, and become endophytic. They can also invade plants through flowers, fruits, and seeds, but this is more common with pathogens (Malfanova et al. 2013).
Endophytic microbes may have genetic differences from rhizosphere microorganisms in order to colonize interior plant tissues, however no specific group of genes responsible for the endophytic lifestyle has been identified so far (Santoyo et al. 2016). Many authors have recently conducted research on endophytic microbes on various host plants using 16S rDNA sequences to determine their relationships (Glick 2015; Romero et al. 2014; Shi et al. 2014). Ali et al. discovered an array of genes that may play a role in endophytic lifestyle by comparing the genomes of 9 endophytic Proteobacteria species, some of which have been experimentally confirmed to be involved in the colonization of internal plant tissues (Ali et al. 2012).
13.4 The Role of Endophytes in Host Plants
13.4.1 Production of Plant Growth Promoting Substance
Endophytic bacteria acquire nutrients from the host plant for survival in a mutualistic relationship (Shimizu 2011), and endophytes can be advantageous to plant development directly (by increasing growth) or indirectly (by inhibiting growth) through disease control. Endophytic microorganisms directly promote growth through supplying nutrients such as dissolved Fe or Mo in the soil (Tokala et al. 2002), nitrogen fixation (Shimizu 2011; Kandpal et al. 2012), either biosynthesis or regulation of growth-promoting substances (also known as growth hormones-phytohormones), especially indole-3-acetic acid (IAA) (Malfanova et al. 2013).
IAA’s physiological effects on plants include cell proliferation stimulation, induction of plant chemotaxis (photodynamic), inhibition of lateral shoot development to maintain apical dominance, promotion of fruit growth and seedless fruit production, and inhibition of leaf, flower, and fruit shedding. According to Mohamed Et-Otmeni et al., IAA increased the percentage of seed germination and the plant’s survival rate in following stages in citrus. IAA and naphthalene acetic acid (NAA) are efficient rooting aids when propagating citrus through cuttings. Bud-grafting success rates are increased by IAA and indolebutyric acid (IBA). To maintain grafted plants in vitro and promote lateral root initiation, 6-benzyladenine (BA) and IAA were added to the basal media (E1-Otmani et al. 2000).
Brick et al. identified and characterized the production of IAA by two types of bacteria, Pseudomonas and Bacillus, in 1991, and have since expanded their research by studying other strains (Bric et al. 1991). According to some authors, similar to other microorganisms, the production of plant hormones such as IAA in endophytic actinomycetes is one of the mechanisms to promote host plant growth and increase leaf and root dry weight and root length and are considered biological agents in agriculture (Ghodhbane-Gtari et al. 2010; Shutsrirung et al. 2013). Research has found that microorganisms synthesizes IAA from tryptophan (Trp) by deamination, decarboxylation and oxidation and the concentration of IAA biosynthesized by them is dependent on the amount of Trp in the culture medium (Yadav et al. 2010).
Research by P.T.H. Thao et al. showed that, out of 47 endophytic actinomycetes isolated from citrus, 42 strains (88%) were capable of biosynthesizing IAA, two of which (accounting for 4%) produced over 20 µg/ml IAA (Phan et al. 2016).
Endophytic microorganisms, like rhizosphere microorganisms, produce plant growth promoting substances but also have many more complex mechanisms to stimulate plant growth due to their close relationship. Endophytic actinomycetes improve root growth and development by absorbing nutrients, resulting in a larger root surface area and an increase in the number of lateral roots, and therefore increased nutrient uptake for the host plant. Streptomyces strains found in the roots have been proven to produce iron-sequestering siderophores and to solubilize inorganic and organic phosphates in order to promote plant health (Shimizu 2011).
Many endophytic microbial strains may produce IAA while also lowering ethylene concentration in plants via the (1-aminocyclopropane-1-carboxylate)-deaminase enzyme (Jaemsaeng et al. 2018; Tiwari et al. 2018). This is significant because high ethylene concentrations can hinder root growth in stressed plants. In addition to producing IAA, several endophytic actinobacterial strains isolated from Aloe vera, Mentha arvensis, and Ocimum sanctum have been shown to solubilize phosphate and produce hydroxamate and catechol (Gangwar et al. 2014) (Fig. 13.1).
The role of endophytes in host plants (Malfanova 2013)
Some endophytic bacteria have the ability to promote plant growth through increased availability of mineral elements or tolerance to heavy metals under living conditions. Therefore, when residing in plant tissue, endophytic microorganisms give plants many favorable conditions to help plants grow well (Malfanova et al. 2013).
The relationship between endophytic microorganisms, host plants and the production of bioactive natural products creates an opportunity to find specific preparations with potential applications in plant growth stimulation, fungal diseases control and increasing plant tolerance to adverse environmental conditions.
13.4.2 Disease Control
The primary mechanism of endophytic bacteria in plant disease control is resistance (antibiosis)-the synthesis of antibiotic compounds (Janso and Carter 2010; Kumar and SivaKumar 2011; Taechowisan et al. 2014) and fungal cell wall degrading enzymes (Shimizu 2011), competing for nutrients and habitat, and enhancing host plants’ systemic tolerance (Malfanova et al. 2013).
Endophytic microbes colonizing host plants can cause a variety of cell wall modifications, including the buildup of callose, pectin, cellulose, and phenolic compounds, resulting in the creation of a structural barrier at the site of pathogen infection (Mengistu 2020). Furthermore, when attacked by pathogens, plants colonized by endophytic microbes generally have increased biosynthesis of defense proteins such as peroxidase, chitinase, and −1,3-glucanase, which inhibit pathogen spread. Furthermore, because various antipathogenic compounds may activate the host plant’s immune response and even the immune system, many endophytic microbial species frequently adopt a combination of resistance strategies (Shimizu 2011; Priya 2012; Ongena et al. 2007).
Many endophytic actinomycetes have been proved to protect host plants from soil-borne pathogens such as Aspergillus niger, A. flavus, Alternaria brassicicola, Botrytis cinerea, Penicillium digitatum, Fusarium oxysporum, P. pinophilum, Phytophthora dresclea and Colletotrichum fulcatum (Gangwar et al. 2011). According to Conn et al., seed inoculation of Streptomyces sp EN27 and Micromonospora sp. EN43 increased Arabidopsis thaliana resistance to bacterial soft rot (caused by Erwinia carotovora) and root rot (caused by F. oxysporum); activated SA- or jasmanic acid/ethylene synthesis gene expression (Conn et al. 2008). They synthesize secondary metabolites that inhibit some pathogenic fungi such as A. porri, C. musae, C. gloeosporioides, Curvularia sp., Drechslera sp., Exserohilum sp., F. oxysporum, Verticillium sp. and Sclerotium rolfsii (Taechowisan et al. 2013), A. brassicicola (Phuakjaiphaeo et al. 2016).
Fungal stem end rot disease of pitaya caused by Alternaria alternata is one of the most destructive diseases in Binh Thuan province, Vietnam. The study by Luu The Anh et al. aimed to assess the antagonistic effects of some endophytic bacteria isolated from the weed plant (Echinochloa colonum) against A. alternata (Luu et al. 2021).
Pitaya confronts several obstacles in Vietnamese agriculture, including stem end rot disease caused by A. alternata. Nineteen endophytic bacteria were isolated from the weed (E. colonum). Five of them might suppress the mycelial development of A. alternata in distinct ways. Strain EC80 exhibited the strongest inhibition, while a weak inhibition was observed by EC79, EC83, EC90, and EC97. The results also showed that combining EC79 and EC80 significantly improved the reduction of biomass pathogenic fungi. The EC79 and EC80 enhanced seedling biomass in greenhouse studies. As a result, these bacteria have the potential to be highly valuable for agronomic applications and should be investigated further in the near future (Luu et al. 2021).
Streptomyces TQR12-4, isolated from the Ham Yen orange fruits of Northeast Vietnam, had high antibacterial activity against 2g-positive bacteria, and 4 phytopathogenic fungi. This strain’s crude antibacterial component was rather stable throughout a wide range of pH (3–9) and temperatures (40–100 °C). Separation on silica gel gave five fractions, two of which (X4 and X5) inhibited the growth of fungi at MIC (minimum inhibitory concentration) of 100 and 400 μg/mL, respectively (Hong-Thao et al. 2016).
In the research of Gangwar et al., three medicinal plants, Aloe vera, Mentha arvensis and Ocimum sanctum were explored for endophytic actinomycetes diversity, plant growth promoting and antimicrobial activity (Gangwar et al. 2014). Endophytic actinomycetes were most commonly recovered from roots (70% of all isolates) followed by stems (17.5%) and leaves (12.5%), respectively. Streptomyces was the most common genus (60% of all isolates) followed by Micromonospora (25%), Actinopolyspora (7.5%), and Saccharopolyspora (7.5%). The highest numbers of endophytic actinomycetes were isolated from Ocimum sanctum (45%). 12 of the 22 isolates tested were able to solubilize phosphate at concentrations ranging from 5.4 to 16.5 mg/100 ml, whereas 16 isolates generated indole-3 acetic acid (IAA) at concentrations ranging from 8.3 to 38.8 g/ml. Nine isolates produced the amount of hydroxamate-type of siderophore ranging between 5.9 and 64.9 µg/ml and only four isolates were able to produce catechol-type of siderophore in the range of 11.2–23.1 µg/ml. Of the nine, interestingly, eight endophytic actinomycetes (88.9%) showed a significant antagonistic activity against one or more phytopathogenic fungi indicating their potential role as plant biocontrol agents.
The roles of endophytes in host plants could be summarized as in Table 13.1:
13.5 Endophytes in Heavy Metal Bioremediation
Endophytes have recently been discovered to have an important role in pollution removal (Gai et al. 2009; Ho et al. 2012; Kim et al. 2012). Endophytes’ bioremediation potential was inspired by the use of plants in phytoremediation.
Phytoremediation is a type of biological treatment that uses plants to remove or degrade pollutants. It can be used to clean up soil, sediment, mud as well as surface and groundwater. To absorb nutrients for development, plants employ a variety of absorptive capacities, transport mechanisms, and metabolic reactions. As a result, phytoremediation entails growing plants in polluted environment for the duration of the growing period. The plant roots absorb pollutants from the soil and retain them in the rhizosphere, rendering them harmless by preventing leaching (Gupta et al. 2016).
The term “hyperaccumulator” refers to a group of plants that are distantly related but share the ability to grow on metalliferous soils and accumulate extraordinarily high levels of heavy metals in the aerial organs, far exceeding the levels found in the majority of species, without suffering phytotoxic effects. Hyperaccumulators are distinguished from comparable non-hyperaccumulating taxa by three basic characteristics: a significantly increased rate of heavy metal absorption, a quicker root-to-shoot translocation, and a greater ability to detoxify and sequester heavy metals in leaves. So far, around 450 angiosperm species have been discovered as heavy metal (As, Cd, Co, Cu, Mn, Ni, Pb, Sb, Se, Tl, Zn) hyperaccumulators, accounting for fewer than 0.2% of all known species. However, new reports of this type of plant continue to emerge, suggesting that numerous unidentified hyperaccumulators may exist in nature (Rascio and Navari-Izzo 2011).
Endophytes are naturally found in plants, and it has been proposed that endophytes in these plants have inherent tolerance and adaptability to contaminants (Fabiana et al. 2020). This “habitat-adapted symbiosis” is thought to provide adaptation and improved tolerance to contaminants on endophytes (Sim et al. 2019). Through overexpression of glutamylcysteine synthetase-glutathione synthetase derived from Streptococcus thermophiles, three transgenic lines of sugar beet (Beta vulgaris L.) with increased tolerance to different concentrations of cadmium, zinc, and copper were discovered (Chaudhary et al. 2016).
Thus, the development of plants and endophytic microorganisms carrying heavy metal assimilation or absorption genes can be a way to enhance heavy metal tolerance and absorption of hyperaccumulators.
Phytoremediation is seen as a potential strategy for the remediation of heavy metal polluted soils, and the harvested heavy metal-enriched hyperaccumulator biomass should be disposed of properly. Various thermal treatments of hyperaccumulators have recently gotten a lot of interest. Following thermal treatment, the hyperaccumulator was processed to bio-oil, biogas, charcoal, or ash according to the circumstances, and the heavy metals were separated, immobilized, or trapped. Metal ion migration and transformation during thermochemical conversion processes are crucial for the safe disposal and subsequent use of metal ion hyperaccumulators (Cui et al. 2021).
The use of endophytic microorganisms for heavy metals removal has been discovered from a variety plant species. They include cultivated plants such as Brassica napus (rapeseed), Nicotiana tabacum (cultivated tobacco), Solanum nigrum (black nightshade), ornamentals (Rosa longicuspis, Commelina communis), shrubs (Acacia decurrens), and hyperaccumulators (Phragmites sp.) (Sheng et al. 2008; Mastretta et al. 2009; Luo et al. 2011; Sim et al. 2016).
Endophytes isolated from these plants are similarly varied, including bacteria (Pseudomonas fluorescens, Stenotrophomonas sp., Clostridium aminovalericum, Microbacterium sp., Flavobacterium sp., Bacillus sp., Acinetobacter sp…), actinomycetes and fungi (Trichoderma asperellum, Phomopsis sp., Saccharicola bicolor, Phoma sp., Aspergillus sp., Mucor sp.) (Sim et al. 2019, 2016). Endophytic organisms from both hyperaccumulators and non-hyperaccumulators have been proven in studies to have metal removal capacity.
Various studies have isolated and reported various species of strict aerobic As(III) and anaerobic As(V) detoxin-reducing bacteria from As contaminated sites (Suhadolnik et al. 2017). Bacteria such as Thermus thermophiles, Thermus aquaticus, P. arsenitoxidans, Crysiogenes arsenates, Bacillus arsenic oselenatis, Desulfutomaculum auripigmentu, Geospirillum barnesi and Geospirillum arsenophilus have the ability to synthesize arsenite oxidase and oxidize As(III) (Pongratz and Heumann 1999). Microorganisms can also decrease As(V) to As(III) via solubilization. Endophytes utilize As(V) as a final electron acceptor for anaerobic respiration in this process. Bacteria that can reduce As(V) include: Bacillus arsenic, Geospirillum arsenophilus, Geospirillum barnesi, Crysiogenes arsenatis, Sulfurospirillum barnesii, Sulfurospirillum arsenophilum, Oselenatis and Desulfutomaculum auripigmentu (Vaxevanidou et al. 2012).
Thus, it can be seen that endophytes which belong to groups of fungi, bacteria or actinomycetes are very diverse, present in both cultivated and wild plants, and can be either aerobic or anaerobic… In other words, these are potential objects which are readily available and easy to find in nature, so that they can be applied in combination with plants to clean up soil contaminated with heavy metal.
Endophytes remove heavy metals by two mechanisms: biosorption or bioaccumulation. Biosorption is a chemical and physical interaction that occurs between adsorbents (biosorbents) and pollutants (metal). Metal cations efficiently bind to functional groups of cell walls and membranes to promote efficient biosorption (Khalil et al. 2016). Some microbial strains have been reported to have potential for application in heavy metal absorption. For example, Trichoderma asperellum (removes 18 mg/g Zn2+, 17.26 mg/g Cu2+, 19.24 mg/g Pb2+, 19.78 mg/g Cd2+, and 16.75 mg/g Cr3+ (Sim et al. 2016), Microsphaeropsis sp. LSE10 adsorbed a maximum of 247.5 mg/g Cd2+ (Chen et al. 2010); and P. lilacinum removed Cu2+ and Cd2+ at 85.4% and 31.43% (El-Gendy et al. 2011).
Bioaccumulation (accumulation) is a process in which metal cations are transported through cell membranes. This is an energy-dependent process that occurs via precipitation, covalent bonding, redox reactions, and crystallization (Wilde and Benemann 1993; Malekzadeh et al. 2002). Metal cations are transported across the cell membrane and further compartmentalized or detoxified during bioaccumulation. Because surviving cells require a period of adaptation to metal stress, bioaccumulation in living cells is substantially slower than biosorption. The amount of metal removed by the former, however, was more than that of the latter (Sim et al. 2016). Deng et al. isolated the endophytic fungal strain Lasiodiplodia sp. MXSF31 from the hyperaccumulator Portulaca olercea capable of efficiently bioaccumulating Pb2+ up to 5.6 × 105 mg kg−1, Cd2+ (4.6 × 104 mg kg−1) and Zn2+ (7.0 × 104 mg kg−1) (Deng et al. 2014).
The functional groups in the cell walls are primarily responsible for metal removal by endophytes. The peptidoglycan of bacterial endophytes’ cell wall is rich in N-acetylglucosamine (NAG) and N-acetylmuramic acid (NAM), which assist in metal binding (Das et al. 2008). Furthermore, metal binding is supported by capsules or slime layers created by certain bacteria, since several functional groups such as sulfate and phosphate are present to chelate and bind metals (Sim et al. 2019). Metal complexation has also been found in fungal endophytes (Dursun et al. 2003).
Endophytes’ metal removal efficiency may be increased by providing optimal growth circumstances such as pH, temperature, and metal concentration. The most significant of them is pH, which influences precipitation, the existence of functional groups, and the competition between metal cations and hydroxyl groups (Bayramoglu et al. 2002). The optimal pH for metal removal is between 4 and 8. Because there are more hydrogen ions in acidic environments, hydrogen and metal cations are more competitive in low pH conditions. As a consequence, hydrogen ions attach to functional groups and repel metal ions, resulting in decreased metal bioabsorption. Other factors, such as starting metal concentration, adsorbent dose, and temperature, are also essential, although their importance is dependent on the kind of endophytic microbes and heavy metals (Sim et al. 2019).
The aforementioned experimental studies have clearly shown the mechanism, conditions and efficiency of removing heavy metals by endophytes, based on which we can grasp a deeper understanding of their optimal conditions for heavy metal remediation as well as opt for those suitable to the characteristics of each type of polluted environment for more efficient bioremediation.
Endophytic plant microorganisms’ biological features are also known to influence bioavailability and bio-characteristics that help or facilitate the metabolism of various kinds of inorganic and organic metals by the host plant environment in soil-plant systems (Ahmad et al. 2016; Pantsar-Kallio and Jørgensen 2002). In fact, not only soil microorganisms but endophytic microorganisms are involved in many important biochemical-biological processes that govern the behavior and fate of a metal in the soil-plant system objects (Abbas et al. 2018).
Ma et al. isolated a consortium of endophytes from a grass species growing in lead-contaminated soil at an abandoned mine site in Ballycorus, Co. Dublin, Ireland, and used it in a laboratory experiment with a plant to confirm their role in supporting and stimulating plant growth in heavy metal-contaminated soil (Ma et al. 2016). Overall, plants treated with endophytes germinated and tillered faster and produced more foliar biomass than control plants, demonstrating the importance of endophytes in boosting growth and minimizing the detrimental effects of heavy metals in plants. Furthermore, statistically significant improvements in germination and tillering rate were reported in plant endophytic treated at the highest lead concentrations (800 ppm). This shows that endophytes are especially critical for plant development in the most polluted soils. Although it is uncertain how endophytes enhance growth and toxin tolerance, past research suggests some probable mechanisms. Endophytes appear to limit lead bioavailability, preventing it from entering the plant. Indeed, identical findings were reported in the research by Bibi et al. (Bibi et al. 2018). They cultivated lettuce on cadmium-contaminated soil after inoculating it with a consortium of endophytes. The lettuce’s biomass output increased, which was connected with a decrease in accumulated metal in the edible section (the leaf). They claimed that the bacteria might absorb the metal in their membrane and then localize it at the root level.
Many endophytic fungi have been discovered to be resistant to heavy metals and/or capable of decomposing organic contaminants, and endophyte-assisted phytoremediation has been identified as a viable approach for in-situ remediation of polluted soils. Fungi have the biochemical and ecological potential to reduce the risk associated with metals, metalloids, and radionuclides, either by chemical alteration or by affecting chemical bioavailability. Furthermore, fungi with broad mycelial networks are particularly suited for bioremediation processes. The use of filamentous fungus can be an useful approach or a beneficial supplement in situations when bacterial cells fail to develop the mycelia network needed to react with pollutants (Venkateswarlu 2014). According to Deng et al., the Cd, Pb, Zn resistant endophytic fungi Lasiodiplodia sp. MXSF31 was isolated from metal accumulating Portulaca oleracea and re-isolated from the shoots and roots of inoculated rape (Deng et al. 2014). The endophytic fungus demonstrated great biosorption and bioaccumulation capabilities of Cd, Pb, and Zn from metal-contaminated solutions and improved rape metal extraction effectiveness in soils polluted with several metals. Endophytic fungi from plants accumulating various metals could be useful microorganism resources for bioremediation of multiple heavy metal polluted water and soils due to their vast host range, endophytic nature, and resistance to multiple metals.
Mesa et al. discovered the potential of indigenous arsenic-tolerant bacteria to enhance arsenic phytoremediation by the autochthonous pseudometallophyte Betula celtiberica, the results have showed that: a total of 54 cultivable rhizobacteria and 41 root endophytes, mainly affiliated with the phyla Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria, were isolated and characterized with respect to several potentially useful features for metal plant accumulation, such as the ability to promote plant growth, metal chelation, and/or mitigation of heavy-metal stress (Mesa et al. 2017).
In the contaminated field, the siderophore and indole-3-acetic acid (IAA) producers of the endophytic bacterial consortium enhanced arsenic accumulation in the leaves and roots of Betula celtiberica, whereas the rhizosphere isolate Ensifer adhaerens strain 91R mainly promoted plant growth.
Microorganisms and plants have developed a variety of strategies for coping with arsenic, allowing them to resist and metabolize it. These features serve as the foundation for phytoremediation treatments, as well as the understanding that plant-soil bacterial interactions are critical for optimizing arsenic absorption. Endosphere and rhizosphere bacterial populations of the autochthonous Betula celtiberica plants growing in arsenic-contaminated soils were studied. Inoculating plants with indigenous bacteria that are As resistant, produce growth factors, and have the ability to convert As(V) to As(III), hence facilitating As detoxification, can increase the effectiveness of As phytoextraction by pseudometallophyte species like Betula. Furthermore, the usage of autochthonous plants and indigenous microbes relieved both partners’ autoecological requirements, guaranteeing effective plant growth (Xu et al. 2016) (Fig. 13.2).
Schematic diagram of arsenic uptake, translocation, detoxification, and sequestration in fern (Xie et al. 2009)
As(III) has been reported to be 60 times more toxic than As(V) and several hundred times more toxic than methylated arsenic to mammals (Neff 1997). As a result, the use of endophytes in arsenic metabolism is critical for organisms (Fig. 13.3).
Overview of bacterial As(III) oxidation and chemotaxis (Shi et al. 2020)
To date, different genera of As(III)-oxidizing bacteria and archaea have been reported, including α-, β-, γ-Proteobacteria, Thermus, green sulfur bacteria, filamentous green non-sulfur bacteria, Crenarchaeota and Euryarchaeota. Furthermore, some As(III)-oxidizing bacteria use As(III) oxidation as an energy source. Chemolithoautotrophic As(III)-oxidizing bacteria, such as Rhizobium sp. NT-26 maintain bacterial growth by using As(III) as an electron donor, oxygen as an electron acceptor, and carbon dioxide-bicarbonate as a carbon source. Moreover, heterotrophic As(III)-oxidizing bacteria, such as Hydrogenophaga sp. N14 and Agrobacterium tumefaciens GW4, employ As(III) oxidation as an energy resource (Shi et al. 2020).
While IAA promotes plant growth, siderophores solubilize Fe from soils, allowing plants to absorb more Fe. Many endophytic bacteria found in hyperaccumulators exhibit plant growth promotion (PGP) properties. For example, Luo et al. isolated 30 endophytes from Cd-hyperaccumulator Solanum nigrum from a mine tailing, with 70% strains producing IAA at 1.60–122 mg L−1 and 8 strains solubilizing phosphate at 22–400 mg L−1 (Luo et al. 2011). Visioli et al. isolated 5 endophytes from Ni-hyperaccumulator Noccaea caerulescens that were capable of producing siderophore (Visioli et al. 2014). Endophytes isolated from Prettis vitata (PV) cultivated in clean soils by Zhu et al., on the other hand, demonstrated minimal PGP features (Zhu et al. 2014). It is probable that a plant’s PGP ability is connected to its metal resistance. As a result, As-resistant endophytic bacteria from PV cultivated in As-contaminated soils must be isolated and characterized. Aside from PGP features, endophytes have strong tolerance to metal stress, which contributes in the reduction of metal phytotoxicity and the promotion of plant growth (Sessitsch et al. 2013). Endophytes have presumably developed mechanisms to adapt to heavy metal stress since hyperaccumulators have high metal concentrations in their biomass (Idris et al. 2004). For instance, Long et al. found endophyte from Sedum alfredii tolerated Zn and Cd while endophytes from hyperaccumulators Thlaspi goesingense, Alyssum bertolonii, and Solanum nigrum exhibited high tolerance to Ni and Cd (Xinxian et al. 2011). However, only one study focused on endophytes from PV growing in a clean soil (Zhu et al. 2014). Soil As pollution is known to influence microbial composition and diversity. Furthermore, endophytes from hyperaccumulators growing in polluted soils are likely to be more metal-tolerant than those from clean soils. Endophytic microbes have the ability to convert As to less toxic forms, allowing them to convert or immobilize As in soil-plant systems (Suhadolnik et al. 2017). Endophytes, or microbial bio-transformations in the host plant, play an essential part in arsenic biochemistry and are critical in risk assessment and remediation research (Anguita et al. 2018). Zhu et al. found that the ability of PV endophytes to reduce AsV and oxidize AsIII was related to their As tolerance (Zhu et al. 2014). Despite this, the study only included a small number of PV endophytes. Furthermore, PV rhizosphere bacteria were studied for As resistance and As transformation; certain rhizobacteria demonstrated a dual function of AsIII oxidation and AsV reduction (Rathinasabapathi et al. 2012). Endophytes linked with PV and their roles in As tolerance and transformation, on the other hand, are poorly understood. The study’s goals were to (1) isolate and characterize As-resistant endophytic bacteria from PV cultivated in soil containing 200 mg kg−1 AsV and (2) investigate the relationship between bacterial capability in As resistance and As transformation. The findings of these studies are critical for better understanding As tolerance and transformation mechanisms in As-resistant endophytes, as well as for improving phytoremediation of As-contaminated soils. According to Stazi et al., soil microbes and endophytes in hyperaccumulator plants enhance arsenic bioavailability by assisting in arsenic accumulation, release/conversion to other forms of As that are more mobile or soluble in water (As III) (Abbas et al. 2018). Microorganisms convert arsenic in host plants from one form to another via several processes/mechanisms such as methylation and demethylation (conversion of inorganic form to organic and vice versa) (Pantsar-Kallio and Jørgensen 2002). Microorganisms have been shown to transform inorganic arsenic to organic arsenic forms (Gadd 1993). Some (demethylated) microorganisms, on the other hand, may convert methylated arsenic to inorganic arsenic via biological methylation (Gao and Burau 1997).
In the study carried out by Xu et al., 43 As-resistant endophytic bacteria from the roots, stems, and leaflets of As hyperaccumulator PV were isolated and characterized (Xu et al. 2016). There was a huge variety, with four phyla and 17 genera, with Proteobacteria and Actinobacteria dominating. Among endophytes, Brevundimonas sp. was the main species in all three PV tissues. PGP characteristics were present in all endophytes. Endophytes from PV roots and stems were more capable of phosphate solubilization than leaflets. Furthermore, all endophytes were resistant to As. Six endophytes in particular grew faster in the presence of 10 mM AsV than the control, most likely due to As-stimulated growth. Endophytes from PV roots were also more resistant to AsV, whilst those from leaflets were more tolerant to AsIII. Bacterial resistance to AsV was shown to be positively associated to their capacity to reduce AsV but not to oxidize AsIII. According to the findings, As-resistant endophytes may promote plant growth in PV, increasing its effectiveness in phytoremediation of As-contaminated locations.
Because pollutants such as heavy metals, polycyclic aromatic hydrocarbons (PAHs), and halogenated hydrocarbons have phytotoxic effects on plants, phytoremediation may be hindered. However, in addition to their ability to promote plant growth, certain endophytes have been demonstrated to produce iron chelators, siderophores, organic acids, and other degrading enzymes, which can change the toxicity of pollutants. Plant-associated bacteria may improve phytoextraction by modifying the solubility, availability, and transport of heavy metals and nutrients via the formation of organic acids and redox changes. Furthermore, the release of low-molecular-mass organic acids by certain endophytes has been demonstrated to promote heavy-metal mobilization (Li et al. 2012).
The efficiency of phytoremediation of metal-contaminated soil is mainly dependent on metal uptake and accumulation in shoots. It has been demonstrated that some heavy-metalresistant/plant-growth-promoting endophytes can improve metal uptake and accumulation in plants. For example, Ma et al. discovered that inoculating Alyssum serpyllifolium with the plant-growth boosting endophytic bacteria Pseudomonas sp. A3R3 dramatically enhanced Ni concentration in plant (Ma et al. 2011). Chen et al. (2010) found that four heavy-metal-resistant endophytic bacteria improved Cd accumulation in root, stem, and leaf tissues of Solanum nigrum L. growing in three levels of Cd-contaminated soil; they also found that the accumulation capacity changed with the Cd concentration in the soil (Chen et al. 2010). Similarly, Mastretta et al. showed that the inoculation of Nicotiana tabacum with Cd-resistant endophyte Sanguibacter sp. increased the concentration of Cd in shoot tissues (Mastretta et al. 2009), while Sheng et al. (2008) discovered that the inoculation of Brassica napus with Pb-resistant endophytic bacteria increased Pb uptake into the shoot (Sheng et al. 2008).
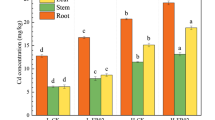
In Vietnam, soil contaminated with high concentrations of lead and cadmium was investigated and detected in the vicinity of the zinc/lead mining and processing factory in Tan Long commune, Dong Hy district, Thai Nguyen province. Lead and cadmium concentrations at Tan Long’s old solid waste dump from the zinc-lead factory ranged from 1100 to 13,000 mg kg−1 and 11.34 to 61.04 mg kg−1, respectively. Lead and cadmium Soil Pollution Indexes (SPI) were highest in the old solid waste dump region, followed by rice soils. The arsenic pollution of the soil in this area was caused by tin mining and sorting activities in Ha Thuong. Arsenic concentrations in soils were higher than 320 mg kg−1 (dry weight) and up to 3809 mg kg−1 in all evaluated areas (Chu 2011). In our study, 20 fern samples were collected at Nui Phao mine, Thai Nguyen province, Vietnam, where Cd and As concentrations at sampling points were about 0.648 mg kg−1 and ranged from 316 to 1606 mg kg−1, respectively (Tam et al. 2021). 205 strains of endophytic bacteria were isolated from the collected samples, with 73 growing on an LB agar plate containing 5 mM As(III) and 132 growing on an LB agar plate containing 5 mM As(V), distributed in all three parts: stem, root, and leaf. The percentage of endophytic bacterial colonies isolated from the roots was higher than those from branches and leaves (58%, 21% and 21%, respectively). Of the 205 endophytic bacterial isolates, 31 had minimal inhibitory concentrations in the range of 80–320 mM. Among them, strain Micrococcus luteus S3.4.1 can be tolerant to the concentration of As (V) up to 320 mM and synthesize high level of IAA (18.72 μg/ml) (Tam et al. 2021).
The experimental evidence presented above have been demonstrated that endophytes possess diverse biological characteristics such as the producing plant growth substances, heavy metal tolerance, reducing toxicity of heavy metals to plants, having wide spectrum of host plants, and degrading organic compounds to supply minerals for plants… This is an advantage of the host-endophyte mutualistic relationship, which can stimulate plant growth, development and tolerance, and thus contribute to promoting the efficiency of heavy metal treatment.
To generalize the content of this section, some of endophytes’ traits in heavy metal remediation have been summarized as shown in Table 13.2:
13.6 Conclusions and Future Prospects
The application of endophytic microorganisms in phytoremediation to enhance heavy metal removal efficiency is relatively new but is making rapid progress. Endophytes is clearly a novel group of microorganisms, with great potential in heavy metal removal, the use of which is an environmentally friendly and sustainable approach to bioremediation. Endophytes can be isolated from a variety of hosts, showing high heavy metal tolerance and removal capacity. In the future, introducing endophytic microorganisms with high heavy metal tolerance, absorption and assimilation capacity into polluted areas and host plants used in heavy metal bioremediation to clean the environment might well be possible.
With the presence of endophytic microorganisms, vitality and heavy metal hyperaccumulation capacity of host plants can be enhanced due to better growth, increased resistance to environmental stress, heavy metal transformation and absorption genes reducing heavy metal toxicity in cells. In Vietnam, studies on endophytes absorbing heavy metals and supporting host plants in heavy metal absorption are both few in number and small in scale. In the future, the application of endophytes into host plants (heavy metal hyperaccumulators) will be rapidly developed and the interaction mechanism between endophytes and host plants to enhance heavy metal absorption capacity will be further studied, clarified and effectively applied.
References
Abbas G, Murtaza B, Bibi I, Shahid M, Niazi NK, Khan MI et al. (2018) Arsenic uptake, toxicity, detoxification, and speciation in plants: physiological, biochemical, and molecular aspects. Int J Environ Res Public Health 15
Ahmad I, Akhtar MJ, Asghar HN, Ghafoor U, Shahid M (2016) differential effects of plant growth-promoting rhizobacteria on maize growth and cadmium uptake. J Plant Growth Regul 35:303–315
Ali S, Charles TC, Glick BR (2012) Delay of flower senescence by bacterial endophytes expressing 1-aminocyclopropane-1-carboxylate deaminase. J Appl Microbiol 113:1139–1144
Ali S, Charles TC, Glick BR (2014a) Amelioration of high salinity stress damage by plant growth-promoting bacterial endophytes that contain ACC deaminase. Plant Physiol Biochem PPB 80:160–167
Ali S, Duan J, Charles TC, Glick BR (2014b) A bioinformatics approach to the determination of genes involved in endophytic behavior in Burkholderia spp. J Theor Biol 343:193–198
Anguita JM, Rojas C, Pastén PA, Vargas IT (2018) A new aerobic chemolithoautotrophic arsenic oxidizing microorganism isolated from a high Andean watershed. Biodegradation 29:59–69
Araújo W, Lacava P, Andreote F, Azevedo J (2008) Interaction between endophytes and plant host: biotechnological aspects. pp 95–115
Bacon CW, White J, Stone J (2000) An overview of endophytic microbes: endophytism defined. Microb Endophytes 85–117
Bayata A (2020) Assessment, accumulation, toxicity and importance of heavy metals in agricultural soil and living system—review. Am J Environ Prot 9(6):116–119
Bayramoglu G, Denizli A, Bektas S, Yakup AM (2002) Entrapment of Lentinus sajor-caju into Ca-alginate gel beads for removal of Cd(II) ions from aqueous solution: preparation and biosorption kinetics analysis. Microchem J 72:63–76
Bibi S, Hussain A, Hamayun M, Rahman H, Iqbal A, Shah M et al (2018) Bioremediation of hexavalent chromium by endophytic fungi; safe and improved production of Lactuca sativa L. Chemosphere 211:653–663
Bric JM, Bostock RM, Silverstone SE (1991) Rapid in situ assay for indoleacetic acid production by bacteria immobilized on a nitrocellulose membrane. Appl Environ Microbiol 57:535–538
Chaudhary K, Jan S, Khan S (2016) Chapter 23-heavy metal ATPase (HMA2, HMA3, and HMA4) genes in hyperaccumulation mechanism of heavy metals. In: Ahmad P (ed) Plant metal interaction. Elsevier, pp 545–556
Chen L, Luo S, Xiao X, Guo H, Chen J, Wan Y et al (2010) Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl Soil Ecol 46:383–389
Chu H (2011) Survey on heavy metals contaminated soils in Thai Nguyen and Hung Yen provinces in Northern Vietnam. J Vietnamese Environ 1
Conn VM, Walker AR, Franco CM (2008) Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol Plant Microbe Interact MPMI 21:208–218
Conrath U, Beckers GJ, Flors V, García-Agustín P, Jakab G, Mauch F et al (2006) Priming: getting ready for battle. Mol Plant Microbe Interact MPMI 19:1062–1071
Cui X, Zhang J, Wang X, Pan M, Lin Q, Khan KY et al (2021) A review on the thermal treatment of heavy metal hyperaccumulator: fates of heavy metals and generation of products. J Hazard Mater 405:123832
Das N, Vimala R, Karthika P (2008) Biosorption of heavy metals—an overview. Indian J Biotechnol 7:159–169
Deng Z, Zhang R, Shi Y, Hu L, Tan H, Cao L (2014) Characterization of Cd-, Pb-, Zn-resistant endophytic Lasiodiplodia sp. MXSF31 from metal accumulating Portulaca oleracea and its potential in promoting the growth of rape in metal-contaminated soils. Environ Sci Pollut Res Int 21:2346–2357
Dursun A., Uslu G, Tepe Ö, Cuci Y, Eki̇z Hİ (2003) A comparative investigation on the bioaccumulation of heavy metal ions by growing Rhizopus arrhizus and Aspergillus niger. Biochem Eng J 15:87–92
E1-Otmani M, Coggins CW, Agustí M, Lovatt CJ (2000) Plant growth regulators in citriculture: world current uses. Crit Rev Plant Sci 19:395–447
El-Gendy MMA, Hassanein NM, Ibrahim HAEH, El-Baky DH (2011) Evaluation of some fungal endophytes of plant potentiality as low-cost adsorbents for heavy metals uptake from aqueous solution. Aust J Basic Appl Sci 5:466–473
Fabiana T, Francine Falcão de Macedo N, Ana Luisa G, Talita Bernardon M (2020) Endophytes potential use in crop production. Open access peer-reviewed chapter
Gadd GM (1993) Microbial formation and transformation of organometallic and organometalloid compounds. FEMS Microbiol Rev 11:297–316
Gai CS, Lacava PT, Quecine MC, Auriac MC, Lopes JR, Araújo WL et al (2009) Transmission of Methylobacterium mesophilicum by Bucephalogonia xanthophis for paratransgenic control strategy of citrus variegated chlorosis. J Microbiol (seoul, Korea) 47:448–454
Gangwar M, Dogra S, Sharma N (2011) Antagonistic bioactivity of endophytic actinomycetes isolated from medicinal plants. J Adv Lab Res Biol 2:154–157
Gangwar M, Phutela U, Dogra S, Kharwar R (2014) Diversity and biopotential of endophytic actinomycetes from three medicinal plants in India. Afr J Microbiol Res 8:184–191
Gao S, Burau RG (1997) Environmental factors affecting rates of Arsine evolution from and mineralization of Arsenicals in soil. J Environ Qual 26:753–763
Ghodhbane-Gtari F, Essoussi I, Chattaoui M, Chouaia B, Jaouani A, Daffonchio D et al (2010) Isolation and characterization of non-Frankia actinobacteria from root nodules of Alnus glutinosa, Casuarina glauca and Elaeagnus angustifolia. Symbiosis 50:51–57
Glick B (2015) Beneficial plant-bacterial interactions, pp 65–96
Gupta AK, Joia J, Sood AD, Sood R, Sidhu Y, Kaur G (2016) Microbes as potential tool for remediation of heavy metals: a review. J Microb Biochem Technol 8:364–372
Hardoim PR, van Overbeek LS, Elsas JD (2008) Properties of bacterial endophytes and their proposed role in plant growth. Trends Microbiol 16:463–471
Ho YN, Mathew DC, Hsiao SC, Shih CH, Chien MF, Chiang HM et al (2012) Selection and application of endophytic bacterium Achromobacter xylosoxidans strain F3B for improving phytoremediation of phenolic pollutants. J Hazard Mater 219–220:43–49
Hong-Thao PT, Mai-Linh NV, Hong-Lien NT, Van Hieu N (2016) Biological characteristics and antimicrobial activity of Endophytic Streptomyces sp. TQR12–4 isolated from Elite Citrus nobilis Cultivar Ham Yen of Vietnam. Int J Microbiol 2016, 7207818
Idris R, Trifonova R, Puschenreiter M, Wenzel WW, Sessitsch A (2004) Bacterial communities associated with flowering plants of the Ni hyperaccumulator Thlaspi goesingense. Appl Environ Microbiol 70:2667–2677
Jaemsaeng R, Jantasuriyarat C, Thamchaipenet A (2018) Molecular interaction of 1-aminocyclopropane-1-carboxylate deaminase (ACCD)-producing endophytic Streptomyces sp. GMKU 336 towards salt-stress resistance of Oryza sativa L. cv. KDML105. Sci Rep 8, 1950
Janso JE, Carter GT (2010) Biosynthetic potential of phylogenetically unique endophytic actinomycetes from tropical plants. Appl Environ Microbiol 76:4377–4386
Kandpal KC, Jain DA, Kumar U, Tripathi R, Kumar TS (2012) Isolation and screening of endophytic actinomycetes producing antibacterial compound from Citrus aurantifolia Fruit. Eur J Exp Biol 2:1733–1737
Khalil MMH, Abou-Shanab RAI, Salem AE-NM, Omer AM, Aboelazm TA (2016) Biosorption of trivalent chromium using Ca-alginate immobilized and alkali-treated biomass. J Comput Sci Technol 5:1–6
Kim TU, Cho SH, Han JH, Shin YM, Lee HB, Kim SB (2012) Diversity and physiological properties of root endophytic actinobacteria in native herbaceous plants of Korea. J Microbiol (seoul, Korea) 50:50–57
Kumar USA, SivaKumar T (2011) Isolation and screening of endophytic actinomycetes from different parts of Emblica officinalis. Ann Biol Res 2:423–434
Li H-Y, Wei D-Q, Shen M, Zhou Z-P (2012) Endophytes and their role in phytoremediation. Fungal Divers 54
Luo SL, Chen L, Chen JL, Xiao X, Xu TY, Wan Y et al (2011) Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 85:1130–1138
Luu TA, Phi QT, Nguyen TTH, Dinh MV, Pham BN, Do QT (2021) Antagonistic activity of endophytic bacteria isolated from weed plant against stem end rot pathogen of pitaya in Vietnam. Egypt J Biol Pest Control 31:14
Ma Y, Rajkumar M, Luo Y, Freitas H (2011) Inoculation of endophytic bacteria on host and non-host plants–effects on plant growth and Ni uptake. J Hazard Mater 195:230–237
Ma Y, Rajkumar M, Zhang C, Freitas H (2016) Beneficial role of bacterial endophytes in heavy metal phytoremediation. J Environ Manage 174:14–25
Machavariani NG, Ivankova TD, Sineva ON, Terekhova LP (2014) Isolation of endophytic actinomycetes from medicinal plants of the Moscow region, Russia. World Appl Sci J 30:1599–1604
Malekzadeh F, Latifi AM, Shahamat M, Levin M, Colwell RR (2002) Effects of selected physical and chemical parameters on uranium uptake by the bacterium Chryseomonas MGF-48. World J Microbiol Biotechnol 18:599–602
Malfanova NV (2013) Endophytic bacteria with plant growth promoting and biocontrol abilities. Doctoral thesis, Leiden University
Malfanova N, Lugtenberg BJJ, Berg G (2013) Bacterial endophytes: who and where, and what are they doing there? In: Molecular microbial ecology of the rhizosphere, pp 391–403
Mastretta C, Taghavi S, van der Lelie D, Mengoni A, Galardi F, Gonnelli C et al (2009) Endophytic bacteria from seeds of Nicotiana tabacum can reduce Cd phytotoxicity. Int J Phytorem 11:251–267
Mengistu AA (2020) Endophytes: colonization, behaviour, and their role in defense mechanism. Int J Microbiol 6927219–6927219
Mesa V, Navazas A, González-Gil R, González A, Weyens N, Lauga B et al. (2017) Use of Endophytic and Rhizosphere bacteria to improve phytoremediation of arsenic-contaminated industrial soils by Autochthonous Betula celtiberica. Appl Environ Microbiol 83
Mishra S, Bharagava RN (2016) Toxic and genotoxic effects of hexavalent chromium in environment and its bioremediation strategies. J Environ Sci Health C 34:1–32
Neff JM (1997) Ecotoxicology of arsenic in the marine environment. Environ Toxicol Chem 16:917–927
Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B et al (2007) Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol 9:1084–1090
Pantsar-Kallio M, Jørgensen DK (2002) Interactions between metals, microbes and plants: bioremediation of arsenic and lead contaminated soils. Ph.D. thesis, University of Helsinki, Helsinki
Phan T, ng H, Hong Lien N, Linh N (2016) Biological characteristics and production of plant growth regulator IAA by endophytic streptomyces hebeiensis TQR8–7. Vietnam J Sci Technol 54:31–39
Phuakjaiphaeo C, Chang CI, Ruangwong O, Kunasakdakul K (2016) Isolation and identification of an antifungal compound from endophytic Streptomyces sp. CEN26 active against Alternaria brassicicola. Lett Appl Microbiol 63:38–44
Pongratz R, Heumann KG (1999) Production of methylated mercury, lead, and cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere 39:89–102
Priya M (2012) Endophytic actinomycetes from indian medicinal plants as antagonists to some phytopathogenic fungi. Open Access Sci Rep 1:1–5
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191(7):419
Rascio N, Navari-Izzo F (2011) Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci 180:169–181
Rathinasabapathi B, De Oliveira L, Guilherme L (2012) Bacteria-mediated Arsenic oxidation and reduction in the growth media of Arsenic hyperaccumulator Pteris vittata. Environ Sci Technol 46:11259–11266
Romero FM, Marina M, Pieckenstain FL (2014) The communities of tomato (Solanum lycopersicum L.) leaf endophytic bacteria, analyzed by 16S-ribosomal RNA gene pyrosequencing. FEMS Microbiol Lett 351:187–194
Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR (2016) Plant growth-promoting bacterial endophytes. Microbiol Res 183:92–99
Seghers D, Wittebolle L, Top EM, Verstraete W, Siciliano SD (2004) Impact of agricultural practices on the Zea mays L. endophytic community. Appl Environ Microbiol 70:1475–1482
Sessitsch A, Kuffner M, Kidd P, Vangronsveld J, Wenzel WW, Fallmann K et al (2013) The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol Biochem 60:182–194
Sheng XF, Xia JJ, Jiang CY, He LY, Qian M (2008) Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ Pollut 156:1164–1170
Shi Y, Yang H, Zhang T, Sun J, Lou K (2014) Illumina-based analysis of endophytic bacterial diversity and space-time dynamics in sugar beet on the north slope of Tianshan mountain. Appl Microbiol Biotechnol 98:6375–6385
Shi K, Wang Q, Wang G (2020) Microbial oxidation of arsenite: regulation, chemotaxis, phosphate metabolism and energy generation. Front Microbiol 11
Shimizu M (2011) Endophytic actinomycetes: biocontrol agents and growth promoters. pp 201–220
Shutsrirung A, Chromkaew Y, Pathom-Aree W, Choonluchanon S, Boonkerd N (2013) Diversity of endophytic actinomycetes in mandarin grown in northern Thailand, their phytohormone production potential and plant growth promoting activity. Soil Sci Plant Nutr 59:322–330
Sim CSF, Tan WS, Ting ASY (2016) Endophytes from Phragmites for metal removal: evaluating their metal tolerance, adaptive tolerance behaviour and biosorption efficacy. Desalin Water Treat 57:6959–6966
Sim C, Chen SH, Ting A (2019) Endophytes: emerging tools for the bioremediation of pollutants
Singh G, Singh N, Marwaha TS (2009) Crop genotype and a novel symbiotic fungus influences the root endophytic colonization potential of plant growth promoting rhizobacteria. Physiol Mol Biol Plants Int J Funct Plant Biol 15:87–92
Sørensen J, Sessitsch A (2006) Plant-associated bacteria lifestyle and molecular interactions. Mod Soil Microbiol 211–236
Stępniewska Z, Kuźniar A (2013) Endophytic microorganisms-promising applications in bioremediation of greenhouse gases. Appl Microbiol Biotechnol 97:9589–9596
Strobel G, Daisy B, Castillo U, Harper J (2004) Natural products from endophytic microorganisms. J Nat Prod 67:257–268
Suhadolnik MLS, Salgado APC, Scholte LLS, Bleicher L, Costa PS, Reis MP et al (2017) Novel arsenic-transforming bacteria and the diversity of their arsenic-related genes and enzymes arising from arsenic-polluted freshwater sediment. Sci Rep 7:11231
Taechowisan T, Chanaphat S, Ruensamran W, Phutdhawong WS (2013) Antibacterial activity of 1-methyl ester-nigericin from Streptomyces hygroscopicus BRM10; an endophyte in Alpinia galanga. J Appl Pharm Sci 3:104–109
Taechowisan T, Chanaphat S, Ruensamran W, Phutdhawong WS (2014) Antibacterial activity of new flavonoids from Streptomyces sp. BT01; an endophyte in Boesenbergia rotunda (L.) mansf. J Appl Pharm Sci 4:8–13
Tam NKB, Linh NT, Truc TT, Linh NVM, Nhung ĐT, Tra LT et al. (2021) Research on some biological characteristics of arsenate resistant endophytic bacteria from ferns. Vietnam Environ Adm Mag 51–55
Tiwari G, Duraivadivel P, Sharma S, Hariprasad P (2018) 1-Aminocyclopropane-1-carboxylic acid deaminase producing beneficial rhizobacteria ameliorate the biomass characters of Panicum maximum Jacq. by mitigating drought and salt stress. Sci Rep 8, 17513
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA et al (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68:2161–2171
Truyens S, Weyens N, Cuypers A, Vangronsveld J (2015) Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environ Microbiol Rep 7:40–50
Vaxevanidou K, Giannikou S, Papassiopi N (2012) Microbial arsenic reduction in polluted and unpolluted soils from Attica, Greece. J Hazard Mater 241–242:307–315
Venkateswarlu D (2014) Role of endophytic fungi in restoration of heavy metal contaminated soils. Indo Am J Pharm Res 4:5427–5436
Visioli G, D’Egidio S, Vamerali T, Mattarozzi M, Sanangelantoni AM (2014) Culturable endophytic bacteria enhance Ni translocation in the hyperaccumulator Noccaea caerulescens. Chemosphere 117:538–544
Waheeda K, Shyam KV (2017) Formulation of novel surface sterilization method and culture media for the isolation of endophytic actinomycetes from medicinal plants and its antibacterial activity. J Plant Pathol Microbiol 8:399
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH et al (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17:10754–10773
Wilde EW, Benemann JR (1993) Bioremoval of heavy metals by the use of microalgae. Biotechnol Adv 11:781–812
Xie Q-E, Yan X-L, Liao X, Li X (2009) The arsenic hyperaccumulator fern Pteris vittata L. Environ Sci Technol 43(22):8488–8495
Xinxian L, Xuemei C, Yagang C, Wong J, Zebin W, Qitang W (2011) Isolation and characterization endophytic bacteria from hyperaccumulator Sedum alfredii Hance and their potential to promote phytoextraction of zinc polluted soil. World J Microbiol Biotechnol 27:1197–1207
Xu J-Y, Han Y-H, Chen Y, Zhu L-J, Ma LQ (2016) Arsenic transformation and plant growth promotion characteristics of As-resistant endophytic bacteria from As-hyperaccumulator Pteris vittata. Chemosphere 144:1233–1240
Yadav J, Verma J, Tiwari K (2010) Effect of plant growth promoting Rhizobacteria on seed germination and plant growth Chickpea (Cicer arietinum L.) under in vitro conditions. Biol Forum Int J 2:15–18
Zhenhua X, Dongmei G, Xiuli S, Ying X (2012) A review of endophyte and its use and function. In: International conference on environmental engineering and technology advances in biomedical engineering, pp 124–130
Zhu LJ, Guan DX, Luo J, Rathinasabapathi B, Ma LQ (2014) Characterization of arsenic-resistant endophytic bacteria from hyperaccumulators Pteris vittata and Pteris multifida. Chemosphere 113:9–16
Acknowledgements
This research is funded by the Vietnam National University, Hanoi (VNU) under Project number QG.20.09
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Nguyen, K.B.T., Phan, T.H.T. (2023). Application of Plant Endophytic Microorganisms (Endophytes) in the Treatment of Heavy Metal Pollution in Soils. In: Vo, P.L., Tran, D.A., Pham, T.L., Le Thi Thu, H., Nguyen Viet, N. (eds) Advances in Research on Water Resources and Environmental Systems. GTER 2022. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-031-17808-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-17808-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-17807-8
Online ISBN: 978-3-031-17808-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)