Abstract
Non-invasive brain stimulation (NIBS), encompassing Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS), is becoming more and more promising as a novel procedure to modulate cerebellar functions and as therapy for cerebellar patients. This chapter provides the elementary concept of this therapeutic approach, which is based on the modulation of cerebellar brain inhibition (CBI), and reports a brief overview of the studies focusing on the therapeutic potentials and effectiveness of these techniques. Evidences of the effectiveness of this appealing therapeutic approach are growing, but many issues remain to be clarified to include these treatments in the standard management of cerebellar patients.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Modulation of the cerebellar brain inhibition (CBI)
- Non-invasive brain stimulation (NIBS)
- Transcranial magnetic stimulation (TMS)
- Transcranial direct current stimulation (tDCS)
1 Introduction
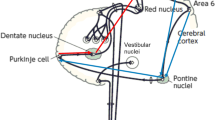
There is a consensus that both Transcranial Magnetic Stimulation (TMS) and Transcranial Direct Current Stimulation (tDCS) can effectively influence cerebellar functions, not only in the motor domain but also for the cognitive and affective operations handled by the cerebrocerebellar circuits (Grimaldi et al. 2014a). The cerebellum plays important roles in movement execution and motor control by modulation of the activity of the primary motor cortex (M1) through cerebello-thalamo-cortical connections (Fig. 106.1) (Ito 1984). The dentato-thalamo-cortical pathway itself is facilitatory. However, Purkinje cells of the cerebellar cortex inhibit cerebellar nuclei. Therefore, activation of Purkinje cells results in disfacilitation of the motor cortex (i.e., decreased excitability of the motor cortex: cerebellar brain inhibition—CBI). Both TMS and tDCS—non-invasive brain stimulation (NIBS) through electromagnetic induction and direct electrical current, respectively—modulate the electrical properties of the networks between the cerebellum and M1, tuning cerebellar excitability (Grimaldi et al. 2014a). This is the basic concept for therapeutic applications of neuromodulation techniques in cerebellar patients. Studies show that the normal effects of CBI are reduced or absent in patients with degeneration or lesions of the efferent system from the cerebellum, confirming the clinical potential of NIBS to manage motor deficits in cerebellar ataxias (Pope and Miall 2014).
Simplified scheme of the fronto-ponto-cerebello-thalamo-cortical loop. Solid lines indicate the cerebellar efferent pathways and dotted lines the cerebellar afferent pathways. The sign plus (+) indicates facilitatory effect. The sign minus (−) indicates inhibitory effect. Anodal tDCS increases cerebellar cortical exitability (arrow +), while cathodal decreases it (arrow −) (Adapted from Grimaldi et al. 2014a)
2 Transcranial Magnetic Stimulation (TMS)
TMS is based on electromagnetic induction by means of a magnetic field generator (the coil) which is placed over the scalp and produces small electric currents. This technique has been used in the last decades to investigate neural networks in human by stimulating/inhibiting neural structures non-invasively. Single-pulse TMS on the M1 and the recording of the motor evoked potential (MEP, generated in response to the TMS pulse: test stimulus) are used to measure motor cortical excitability. Applying a conditioning stimulus over the cerebellum before the test stimulus over the contralateral M1 allows to study the CBI (i.e., the cerebellar regulatory effects on M1) (Grimaldi et al. 2014a). Low-frequency repetitive TMS (rTMS) has an inhibitory effect on the cerebellar cortex thus decreasing CBI, high-frequency rTMS has an excitatory effect (Pope and Miall 2014).
Safety and feasibility of repetitive transcranial magnetic stimulation (rTMS, 1 Hz) over the cerebellum in ataxic patients with posterior circulation stroke have been demonstrated (Kim et al. 2014). rTMS, more specifically the iTBS protocol (intermittent theta burst stimulation, interstimulus interval of 15 ms), applied over the injured cerebellar hemisphere of stroke patients induces both neurophysiological changes (a decrease in CBI and an increase in intracortical facilitation) and a clinical improvement, thus suggesting that cerebellar iTBS could be a promising tool to promote recovery of cerebellar stroke patients (Bonnì et al. 2014). Improved cognition has been demonstrated using TMS (21 daily sessions of TMS over the cerebellum) in an ataxic patient (affected by idiopathic late-onset cerebellar atrophy) presenting speech and gait difficulties. The TMS-induced reduction in CBI resulted in the improvements in the patients’ functional mobility (postural control and walking) and dual-tasking (naming supermarket items while walking) after treatment (Farzan et al. 2013). Decreasing cortical excitability by administrating 1 Hz rTMS for 10 min over the right (unaffected) cerebellum in a patient with a left cerebellar lesion and a selective deficit in procedural learning induces recovery of the deficit and an improvement of the task performance (Torriero et al. 2007).
3 Transcranial Direct Current Stimulation (tDCS)
tDCS is based on the application of a steady current of small intensity (usually between 0.5 and 2 mAmp; anodal or cathodal) between two large electrodes fixed on the scalp (Tomlinson et al. 2013). The current causes a polarity-dependent modulation of brain activity which is site-specific (Nitsche et al. 2003). When applied over the cerebellum, tDCS can effectively modulate CBI by changing tonic Purkinje cell activity and thus resulting in modulation of cerebral cortical excitability (Fig. 106.1). Cathodal tDCS over the cerebellum reduces cortical excitability and leads to a lasting inhibition of CBI for up to 30 min after stimulation. On the other hand, anodal cerebellar tDCS, which increases cortical excitability, increases the magnitude of CBI (Galea et al. 2009). Anodal ctDCS causes walking adaptation to occur more rapidly, whereas cathodal ctDCS slowed it down relative to sham stimulation (Jayaram et al. 2012).
Recently, Pozzi et al. showed that anodal tDCS over the motor cortex in ataxic patients induced an improvement of the symmetry of step execution and reduction of base-width. This was associated with patients’ perception of improvement, lasting 30 days after stimulation (Pozzi et al. 2014). A study on the effect of anodal tDCS applied over the cerebellum in ataxic patients showed that tDCS exerts a favorable effect on upper limb stretch reflexes, reducing significantly the amplitudes of long-latency stretch reflexes (Grimaldi and Manto 2013). The effectiveness of tDCS on cerebellar patients improves when combining tDCS over the cerebellum and tDCS over the motor/premotor cerebral cortex (transcranial cerebello-cerebral DCS—tCCDCS). tCCDCS reduces both postural and action tremor, cancels hypermetria, and restores EMG activity associated with fast goal-directed movements (decrease of the onset latency of the antagonist EMG activity) (Grimaldi et al. 2014b). These findings suggest that tCCDCS impacts on the distorted timing of muscle discharges which is considered as a signature of a cerebellar lesion involving the cerebello-thalamo-cortical pathway.
tDCS is now considered a potential therapeutic tool as well as a valuable clinical tool for neurorehabilitation interventions. tDCS also raises researchers’ interest as a technique to provide novel information on cerebellar functions and to promote neuroplasticity (Grimaldi et al. 2014a). Moreover, this technique (similarly to TMS) is non-invasive, well-tolerated and safe, provided an appropriate current intensity is used, and interstimulation intervals are applied. However, important questions about therapeutic application of tDCS still remains open (Grimaldi et al. 2016): what is the optimal intensity? Are there any differences in responsiveness among cerebellar patients? How long the effects last? Is the effectiveness influenced by the extent of cerebellum volume loss in patients with cerebellar atrophy? Should tDCS be applied on a daily basis? Which are the optimal sites of stimulation and how to combine them? Could we consider a combination of stimulation of the cerebellum and the spinal cord (Priori et al. 2014)?
References
Bonnì S, Ponzo V, Caltagirone C, Koch G (2014) Cerebellar theta burst stimulation in stroke patients with ataxia. Funct Neurol 7:1–5
Farzan F, Wu Y, Manor B, Anastasio EM, Lough M, Novak V et al (2013) Cerebellar TMS in treatment of a patient with cerebellar ataxia: evidence from clinical, biomechanics and neurophysiological assessments. Cerebellum 12:707–712. CrossRef PubMed PubMedCentral
Galea JM, Jayaram G, Ajagbe L, Celnik P (2009) Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci 29(28):9115–9122. CrossRef PubMed PubMedCentral
Grimaldi G, Manto M (2013) Anodal Transcranial Direct Current Stimulation (tDCS) decreases the amplitudes of long-latency stretch reflexes in cerebellar ataxia. Ann Biomed Eng 41:2437–2447. CrossRef PubMed
Grimaldi G, Argyropoulos GP, Boehringer A, Celnik P, Edwards MJ, Ferrucci R, Galea JM, Groiss SJ, Hiraoka K, Kassavetis P, Lesage E, Manto M, Miall RC, Priori A, Sadnicka A, Ugawa Y, Ziemann U (2014a) Non-invasive cerebellar stimulation—a consensus paper. Cerebellum 13(1):121–138. CrossRef PubMed
Grimaldi G, Oulad Ben Taib N, Manto M, Bodranghien F (2014b) Marked reduction of cerebellar deficits in upper limbs following transcranial cerebello-cerebral DC stimulation: tremor reduction and re-programming of the timing of antagonist commands. Front Syst Neurosci 8:9. CrossRef PubMed PubMedCentral
Grimaldi G, Argyropoulos GP, Bastian A, Cortes M, Davis NJ, Edwards DJ, Ferrucci R, Fregni F, Galea JM, Hamada M, Manto M, Miall RC, Morales-Quezada L, Pope PA, Priori A, Rothwell J, Tomlinson SP, Celnik P (2016) Cerebellar Transcranial Direct Current Stimulation (ctDCS): a novel approach to understanding cerebellar function in health and disease. Neuroscientist 22(1):83–97. CrossRef PubMed PubMedCentral
Ito M (1984) Cerebellum and neural control. Raven, New York
Jayaram G, Tang B, Pallegadda R, Vasudevan EV, Celnik P, Bastian A (2012) Modulating locomotor adaptation with cerebellar stimulation. J Neurophysiol 107(11):2950–2957. CrossRef PubMed PubMedCentral
Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ (2014) Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med 46(5):418–423. CrossRef PubMed
Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F (2003) Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci 15:619–626. CrossRef PubMed
Pope PA, Miall RC (2014) Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Front Psychiatry 5:33. CrossRef PubMed PubMedCentral
Pozzi NG, Minafra B, Zangaglia R, De Marzi R, Sandrini G, Priori A, Pacchetti C (2014) Transcranial direct current stimulation (tDCS) of the cortical motor areas in three cases of cerebellar ataxia. Cerebellum 13(1):109–112. CrossRef PubMed
Priori A, Ciocca M, Parazzini M, Vergari M, Ferrucci R (2014) Transcranial cerebellar direct current stimulation and transcutaneous spinal cord direct current stimulation as innovative tools for neuroscientists. J Physiol 592(Pt 16):3345–3369. CrossRef PubMed PubMedCentral
Tomlinson S, Davis N, Bracewell R (2013) Brain stimulation studies of non-motor cerebellar function: a systematic review. Neurosci Biobehav Rev 37:766–789. CrossRef PubMed
Torriero S, Oliveri M, Koch G, Lo Gerfo E, Salerno S, Petrosini L et al (2007) Cortical networks of procedural learning: evidence from cerebellar damage. Neuropsychologia 45:1208–1214. CrossRef PubMed
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Grimaldi, G. (2023). Cerebellar Stimulation. In: Gruol, D.L., Koibuchi, N., Manto, M., Molinari, M., Schmahmann, J.D., Shen, Y. (eds) Essentials of Cerebellum and Cerebellar Disorders. Springer, Cham. https://doi.org/10.1007/978-3-031-15070-8_106
Download citation
DOI: https://doi.org/10.1007/978-3-031-15070-8_106
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-15069-2
Online ISBN: 978-3-031-15070-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)