Abstract
Severe deficiency of either folate or vitamin B12 leads to megaloblastic anemia which clinically manifests as fatigue, weakness, and shortness of breath owing to a low red blood cell count. Hematologically, megaloblastic anemia is characterized by the presence of large, immature, nucleated cells (megaloblasts) in the bone marrow and macrocytes in the peripheral blood. Folate deficiency typically arises when folate requirement is increased (e.g., in pregnancy) and/or when folate availability is reduced as a result of low dietary intakes or malabsorption (e.g., in celiac disease). Pernicious anemia, the clinical condition of severe deficiency of vitamin B12, arises from an autoimmune gastritis characterized by B12 malabsorption owing to loss of intrinsic factor. A more subtle depletion of vitamin B12 status can however arise from mild atrophic gastritis leading to reduced gastric acid production, thereby diminishing B12 absorption from food. Apart from folate and B12, riboflavin deficiency can also lead to nutritional anemia. There are consequences of B-vitamin deficiency throughout the lifecycle, adversely affecting metabolic functioning and contributing substantially to the global burden of disease. This chapter will consider the causes, detection, and consequences of B-vitamin deficiency, ranging from the anemia of clinical deficiency to other manifestations associated with less severe deficiency, and the potential for prevention through effective public health measures.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Anemia
- B-vitamin deficiency
- Folate
- Folic acid
- Vitamin B12
- Cobalamin
- Riboflavin
- Food fortification
- Health outcomes
- Disease burden
Introduction
Reduction of anemia is one of the World Health Organization Global Nutrition Targets for 2025 [1]. Megaloblastic anemia is the major clinical manifestation of overt folate or B12 deficiency. Although the anemia arising from a deficiency of either vitamin is clinically indistinguishable, the causes are very different and differential diagnosis is essential in order to provide effective treatment. Furthermore, the absence of anemia does not imply that status of these vitamins is sufficient. Less obvious “nonanemic” manifestations can arise with low nutrient status through the lifecycle, with adverse health impacts from early life to older age. An understanding of the causes, detection, and consequences of insufficient B-vitamin status is necessary, so that appropriate public health strategies for prevention can be implemented.
Folate Deficiency
Metabolic Role of Folate
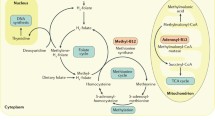
Folate is essential for one-carbon metabolism (Fig. 13.1). This involves the transfer and utilization of one-carbon units in a network of pathways required for DNA and RNA biosynthesis, amino acid metabolism, and methylation processes via the production of S-adenosylmethionine. The generation of S-adenosylmethionine in tissues is dependent on an adequate supply of folate and vitamin B12 and is used for a great number of methylation reactions by donating its methyl group to over 100 methyltransferases for a range of substrates such as DNA, hormones, proteins, neurotransmitters, and membrane phospholipids, all of which are regulators of important biological processes [2]. Of note, in order to function effectively within one-carbon metabolism, folate needs to interact closely with related B-vitamins, namely, vitamin B12, vitamin B6, and riboflavin. This means that deficient status of one or more of these B-vitamins, or polymorphisms in folate genes, can impair one-carbon metabolism, even if folate intakes are adequate.
Overview of B vitamins in one-carbon metabolism. Abbreviations: DHF dihydrofolate; DHFR dihydrofolate reductase; DMNT DNA methyltransferase; dTMP deoxythymidine monophosphate; dUMP deoxyuridine monophosphate; MTHFR methylenetetrahydrofolate reductase; SAH S-adenosylhomocysteine; SAM S-adenosylmethionine; THF tetrahydrofolate. (Adapted from Linus Pauling Institute at https://lpi.oregonstate.edu/mic/vitamins/folate)
Causes of Folate Deficiency
The discovery of folate as an essential nutrient dates back to the 1930s when a fatal anemia of pregnancy was first described in India which was subsequently proven to be responsive to treatment with food sources of the vitamin [3]. There are various causes of folate deficiency, arising from increased requirements and/or reduced availability [2]. Pregnancy is a time when folate requirement is greatly increased to sustain the demand for rapid cell replication and growth of fetal, placental, and maternal tissue. Certain gastrointestinal conditions, most notably celiac disease, can also lead to deficient folate status through chronic malabsorption. Heavy alcohol consumption and certain drugs, e.g., phenytoin and primidone (anticonvulsants) and sulfasalazine (used in inflammatory bowel disease), are also linked with folate deficiency.
Dietary folate intakes can be considered sub-optimal in the diets of many people worldwide in that, although adequate in preventing megaloblastic anemia, they are often found to be insufficient in achieving biomarker concentrations of folate associated with the lowest risk of neural tube defects or other disease linked with low folate status. This widespread under-provision of folate is generally attributed to the poor stability and incomplete bioavailability of natural food folates when compared with the synthetic vitamin, folic acid. Food folates occur naturally in richest supply in green leafy vegetables, beans, liver and yeast, whereas folic acid is found in the human diet only in fortified foods and supplements. Because of their chemical structure, natural food folates are inherently unstable outside living cells and tend to have poor bioavailability [4]. In addition to their limited bioavailability, food folates (particularly green vegetables) can undergo losses during cooking, and this will substantially reduce the folate content of the food before it is even ingested.
As a result of the poor stability and limited bioavailability of folate from natural food sources, achieving optimal folate status can be challenging in practice [3]. Folate intakes and recommendations in the US and certain other countries are expressed as Dietary Folate Equivalents (DFEs), a calculation that was devised to take into account the greater bioavailability of folic acid from fortified foods compared to naturally occurring dietary folates [4, 5].
Detection of Deficient Folate Status
Biomarker status of folate is routinely assessed by measurement of folate concentrations in serum/plasma or in red blood cells as determined by either microbiological assay, or more typically in clinical laboratories, by protein binding assays. Serum folate is the earliest and most sensitive indicator of altered folate exposure and will reflect recent dietary intake [6]. Red blood cell folate parallels liver concentrations (accounting for about 50% of total body folate) and is thus considered to reflect tissue folate stores. It responds slowly to changes in dietary folate intake but is a good indicator of longer-term status in that it reflects folate intake/status over the previous 3–4 months when circulating folate is incorporated into the maturating red cells [6]. The measurement of plasma homocysteine concentration provides a functional indicator of folate status, on the basis that normal homocysteine metabolism requires an adequate supply of folate. When the status of folate is deficient or low, plasma homocysteine is invariably found to be elevated. Homocysteine is not specific for folate status, however, as it will also be elevated with B12 or other B-vitamin deficiency, certain lifestyle factors, and renal insufficiency [7].
Consequences of Deficient Folate Status
Severe Deficiency: Anemia
Clinical folate deficiency is manifested as megaloblastic anemia, a condition characterized by immature, enlarged red blood cells (reflecting impaired DNA synthesis), which is reversible with folic acid treatment. Folate-related anemia was recently reported to occur in >20% of women of reproductive age in many low- and middle-income countries, but was typically <5% in higher income economies [8]. Estimates of folate deficiency can however vary considerably depending on the method used and cut-points applied, but the microbiologic assay is recommended by the WHO and widely considered to be superior to other assays for measurement of folate status. Red blood cell and serum folate concentrations typically decrease throughout pregnancy; however, supplementation with folic acid prevents this decline [9] and can thus prevent the occurrence of megaloblastic anemia of pregnancy [10].
Other Manifestations of Insufficient Folate Status
Emerging scientific evidence supports a number of roles for folate through the lifecycle in maintaining health and preventing disease (Fig. 13.2). Because homocysteine is elevated with low folate status, it is possible that some of the reported manifestations of folate insufficiency are mediated via increased homocysteine concentrations, but it remains unclear whether homocysteine per se is a true risk factor for cardiovascular and other disease outcomes. In any case, homocysteine is a sensitive indicator of functional folate deficiency; in human studies, plasma homocysteine is inversely correlated with folate and shows marked lowering in response to folic acid intervention.
Neural tube defects (NTD): Maternal folate status has a major impact on early development of the embryo up to the first 4 weeks of pregnancy. Conclusive evidence has existed for over 25 years of the benefits at this time of folic acid in preventing both first occurrence [11] and recurrence [12] of NTD. These are major birth defects occurring as a result of failure of the neural tube to close properly in the first few weeks of pregnancy, leading to death of the fetus or newborn, or to various disabilities involving the spinal cord, the most common form of which is spina bifida. This conclusive evidence has led to clear folic acid recommendations for women of reproductive age which are in place worldwide.
The proposed mechanisms to explain the beneficial effects of periconceptional folic acid against NTD have focussed on the factors that could potentially impair normal folate metabolism, including polymorphisms in folate genes. Of these, an increased risk of NTD is strongly associated with the 677C → T variant in the gene coding for the folate metabolizing enzyme methylenetetrahydrofolate reductase (MTHFR) [13]. Autoantibodies against folate receptors have also been implicated in pregnancies affected by NTD [14].
Of note, although folate insufficiency is considered the major contributing factor, there is convincing evidence that low maternal vitamin B12 is an independent risk factor for an NTD-affected pregnancy [15]. Thus, future public health efforts to reduce NTD may need to target the status of not only folate but also B12 in women entering pregnancy. In addition, apart from preventing NTD, there is good evidence that periconceptional folic acid use may prevent congenital heart defects in infants [16], and possibly orofacial clefts, although the latter evidence is somewhat controversial.
Other adverse pregnancy outcomes: As pregnancy progresses, folate continues to play an important role in maternal, fetal, and neonatal health (Fig. 13.2). Deficient maternal folate status (and/or elevated homocysteine) is associated with an increased risk of a number of adverse pregnancy outcomes including gestational hypertension, preeclampsia, placental abruption, pregnancy loss, low birth weight, and intrauterine growth restriction [17]. Although there is some evidence that folic acid supplementation in pregnancy can reduce the risk of gestational hypertension and preeclampsia [18], this is conflicting. One recent RCT reported that high dose folic acid beyond the first trimester of pregnancy had no beneficial effect on preeclampsia in women at high risk for this condition [19].
In addition to protecting against the development of anemia in the mother and NTD in the offspring, there is emerging evidence linking maternal folate during pregnancy with neurodevelopment and cognitive function in the child. The biological mechanism linking maternal folate with the offspring brain is unclear, but likely involves folate-mediated epigenetic changes related to brain development and function [20]. Indeed, a wealth of literature supports the fetal origins of human disease throughout the lifecycle and emerging evidence implicates epigenetic modifications as the likely mechanism. DNA methylation, the most widely studied epigenetic mechanism for gene regulation, is dependent upon the supply of methyl donors provided by folate and related B-vitamins via S-adenosylmethionine [2]. Folate deficiency could thus lead to aberrant gene expression with consequential adverse health outcomes [21].
Cardiovascular disease (CVD): There is considerable evidence to link low status of folate and related B-vitamins (B12 and B6) with an increased risk of CVD and stroke in particular [22]. Although much of this evidence focuses specifically on plasma homocysteine (high concentrations of which are associated with endothelial dysfunction, atherosclerosis, and thrombosis), it is possible that the link with CVD is through mechanisms that are independent of homocysteine, including a role for folate and related one-carbon metabolism in blood pressure and prevention of hypertension, an important risk factor for CVD [3].
Randomized controlled trials (RCTs) show that folic acid intervention may decrease the risk of stroke by as much as 18%, and higher (over 25%) in trials with a treatment duration of >36 months, in participants with poorer baseline folate status or in those with no previous history of stroke [23]. Also, population data from the USA and Canada show an improvement in stroke mortality corresponding to the time that mandatory folic acid food fortification was introduced [24]. Thus, optimizing folate status through population-based folic acid intervention (i.e., food fortification) aimed at reducing NTD may also be beneficial in reducing CVD. Although a number of secondary prevention trials in at-risk patients failed to show a benefit of folic acid (typically in combination with vitamins B12 and B6) for CVD events generally [22], all such trials were aimed at preventing further cardiovascular events in patients with well-established pathology. A reasonable conclusion from the evidence therefore is that the administration of high dose B-vitamins to CVD patients is of no benefit in preventing another event. The same cannot be said for primary prevention, however, because the aforementioned trials did not investigate this question. Also, in one trial testing B-vitamin intervention in CVD risk, the HOPE-2 trial, a clear benefit in reducing the risk of stroke was detected but for some reason this result was overlooked in the original report [25], although subsequently reported separately [26].
Cognitive dysfunction and other neurological manifestations: Folate has a fundamental role in the nervous system throughout the lifecycle, from neural development in early life through to the maintenance of mental health and cognitive function in later life. A number of inherited disorders of folate transport and metabolism have been described [27]. These rare disorders are associated with variable neurological manifestations that can present any time from the neonatal period to adult life, including developmental delay, cognitive impairment, motor and gait abnormalities, behavioral or psychiatric symptoms, seizures, neuropathy, and vascular changes on magnetic resonance imaging (MRI).
A growing body of evidence indicates that folate and related B-vitamins may be important for maintaining cognitive health in aging, with lower B-vitamin status and/or elevated homocysteine concentrations associated with dysfunction [28]. Cognitive dysfunction refers to a spectrum ranging from mild memory loss to dementia, the latter referring to a state where the decline in memory and thinking are sufficient to impair functioning in daily living. Observational studies in this area are however complicated by the fact that poor diet can be both a cause and a consequence of impaired cognitive function and should be interpreted with caution. Only randomized trials can confirm whether low B-vitamin status is causatively linked with cognitive dysfunction. Research in this area has been very substantially underpinned by the VITACOG trial which showed that B-vitamin intervention not only improved cognitive performance in patients with mild cognitive impairment, but also slowed the rate of global and regional brain atrophy as determined using MRI [29, 30]. The totality of trial evidence suggests that any benefit of intervention with folic acid (alone or combined with vitamins B12 and B6) on cognitive function arises through correction of deficient/low status, whereas providing additional folic acid to those with optimal status likely has little effect on cognition.
Apart from memory deficits and cognitive dysfunction, depressive symptoms are well described in folate deficiency [31]. Furthermore, folate deficiency can affect the duration and clinical severity of depression and is associated with poorer response to antidepressant medication [31]. In a meta-analysis of observational studies, low folate was associated with a greater risk of depression [32]. A large cohort study of older Irish adults recently reported an incremental increase in the risk of depression as red blood cell folate declined, with an 80% higher rate of depression found among participants with the 20% lowest folate status [33]. The same study demonstrated that regular consumption of fortified foods increased dietary intakes of folate and related B-vitamins, substantially improved corresponding biomarker status, and was associated with a reduced risk of depression (by 50%) in those who consumed fortified foods on a daily basis compared to non-consumers.
Osteoporosis: Evidence implicating a role for folate and related B-vitamins in osteoporosis is typically focussed on hyperhomocysteinemia, arising from historical case reports on patients with homocystinuria, a rare autosomal recessive disease characterized by very high concentrations of plasma homocysteine (>100 μmol/L). Among the clinical features described in these patients were skeletal abnormalities, lower bone mineral density (BMD), and early onset osteoporosis.
The moderately elevated homocysteine concentrations found within the general population (and characteristic of low folate status) is also associated with an increased risk of osteoporosis, a crippling bone disease with major health and economic consequences. Potential mechanisms to explain this relationship link elevated homocysteine with increased bone resorption and bone fragility or a disturbance in collagen cross-linking. Thus, large cohort studies from various high-income countries report strong positive associations between homocysteine concentrations and risk of osteoporotic fracture or lower BMD [34, 35]. Correspondingly, a higher risk of hip fracture was reported in Norwegian women with lower (<2.9 nmol/L) compared to higher (>6.6 nmol/L) serum folate concentrations [35].
Cancer: Human and animal studies link deficient folate status with the development of various cancers, most notably of the colorectum [36]. The proposed mechanisms by which folate deficiency may promote malignant transformation are based on folate’s role in one-carbon metabolism, and specifically DNA methylation and the de novo synthesis of pyrimidines and purines, nucleotides required for DNA replication and repair. Low folate status may thus alter gene expression through defective cytosine methylation, or lead to catastrophic cycles of aberrant DNA repair [37]. Controversially, however, there is also evidence that folic acid at high doses may have adverse effects on carcinogenesis. Historical evidence of the potential for such effects lies in the fact that drugs such as methotrexate, whose mechanism of action is to block folate metabolism, are effective chemotherapeutic agents. Clinical studies have more recently raised the concern that high dose folic acid may promote colorectal tumorigenesis in patients with pre-existing lesions [38].
Thus, whereas higher folate status within the dietary range is considered to be protective against certain cancers, some remain concerned that exposure to excessively high folic acid intakes could increase the growth of pre-existing neoplasms. Such concerns in relation to cancer risk have impacted public health policy in countries worldwide, with many governments delaying decisions to implement population-wide folic acid fortification, despite the widespread recognition of the benefits of such policy in preventing NTD.
Vitamin B12 Deficiency
Metabolic Role of Vitamin B12
Vitamin B12, also known as cobalamin, plays important roles in one-carbon metabolism and in mitochondrial metabolism.
In the form of methylcobalamin, vitamin B12 interacts closely with folate by acting as a cofactor for the folate-dependent enzyme, methionine synthase, which is required in the synthesis of methionine from homocysteine. Methionine, once formed, is activated by ATP to form S-adenosylmethionine, a methyl group donor used in many biological methylation reactions, including the methylation of a number of sites within DNA, RNA, and proteins [39].
In the form 5-deoxyadenosylcobalamin, vitamin B12 is required as a cofactor for the mitochondrial enzyme, methylmalonyl CoA mutase. This enzyme catalyzes the conversion of methylmalonyl CoA to succinyl-CoA, an intermediate step in the conversion of propionate to succinate, which in turn plays an important role in the metabolism of odd-chain fatty acids and ketogenic amino acids.
Causes of B12 Deficiency
Pernicious anemia is the clinical condition of severe deficiency of vitamin B12. This arises from an autoimmune gastritis characterized by B12 malabsorption owing to loss of intrinsic factor [40]. Much more commonly, however, a less severe depletion of vitamin B12 can arise from food-bound B12 malabsorption as a result of mild atrophic gastritis leading to reduced gastric acid production (hypochlorhydria), thereby diminishing B12 absorption from food because of the essential role of gastric acid in the release of B12 from food proteins during digestion. Food-bound B12 malabsorption commonly occurs in older adults and can lead to sub-clinical deficiency, where there is metabolic evidence of deficient status but without the classical hematological or neurological deficiency signs [41].
Vitamin B12 is present only in animal foods, including meat, poultry, fish and to a lesser extent eggs, milk, and dairy products. The deficient B12 status commonly found in older adults in high-income countries is rarely attributable to low dietary intakes, which are typically found to exceed dietary recommendations, but rather is the result of malabsorption related to atrophic gastritis as outlined above, and/or the use of proton pump inhibitors (e.g., omeprazole) and other gastric acid suppressant drugs, commonly prescribed for conditions such as simple heartburn or gastroesophageal reflux disease. Notably, one large community study in the US (of over 25,000 B12 deficient cases and nearly 200,000 controls) found that the long-term use of PPIs was associated with a 25–65% greater risk of vitamin B12 deficiency [42]. In addition, metformin usage in Type 2 Diabetes can also result in vitamin B12 deficiency and in turn a greater risk of cognitive dysfunction [43].
In low-income countries, however, B12 deficiency is largely the result of low dietary intakes, typically in regions where vegan diets or limited animal foods are consumed, but gastrointestinal infections and infestations, along with host–microbiota interactions, may also be contributory factors [44].
Detection of Deficient B12 Status
Vitamin B12 status can be assessed using up to four biomarkers, both direct and functional biomarkers. The direct measurement of serum total vitamin B12 (using microbiological assay or automated competitive protein binding assays) has been the standard clinical test for many years, with B12 deficiency generally identified at B12 concentrations <148 pmol/L, albeit this can vary between laboratories [44]. The sensitivity of total B12 assays has caused some concern, however, with false normal results reported in patients with pernicious anemia [41]. About 80% of total vitamin B12 is metabolically inert, therefore measurement of holotranscobalamin (holoTC), or “active B12,” is theoretically attractive because it represents only the metabolically active fraction (20%) of total B12 that is available for cellular processes. Although widely considered to be a robust biomarker of B12 status, serum HoloTC is not generally available in clinical settings.
The metabolites of B12-dependent reactions can be measured in blood to provide functional indicators of vitamin B12 status. With vitamin B12 depletion, the activity of the B-12 dependent enzyme methionine synthase is impaired, in turn leading to elevated homocysteine. Plasma homocysteine is however influenced by other vitamins (particularly folate) and non-nutrient factors (including renal function); therefore, it is not specific to vitamin B12 thus limiting its use as a biomarker of B12 status. Also, with B12 depletion, the activity of the second B-12 dependent enzyme in humans—methylmalonyl CoA mutase—is reduced, leading to an accumulation of the by-product methylmalonic acid (MMA). Measurement of MMA, unlike homocysteine, provides a specific and sensitive biomarker of B12 status, but limitations include the fact that it is greatly influenced by renal dysfunction and genetic variation, along with high running costs [44].
Given the limitations of each of the direct and functional B12 biomarkers, there is general agreement that two or more biomarkers should be used to more accurately diagnose B12 deficiency so that early intervention can be implemented, and any adverse health consequences prevented [45].
Consequences of Deficient B12 Status
Vitamin B12 deficiency in humans results in impaired activities of the two vitamin B12 dependent enzymes. The extent of functional deficiency and related adverse health outcomes that may arise will depend on the extent of B12 depletion.
Severe Deficiency: Anemia and Neuropathy
Severe vitamin B12 deficiency causes anemia which is reversible with treatment, and irreversible neurological disease leading to death if untreated.
Hematologically, a deficiency of B12 will be manifested as megaloblastic anemia, characterized by megaloblasts in the bone marrow and macrocytes in peripheral blood, and reflecting an underlying biochemical defect of impaired DNA synthesis. The explanation as to why the clinical sign of megaloblastic anemia is identical for a deficiency of either folate or vitamin B12 is because DNA synthesis will be impaired in either case. In B12 deficiency, folate recycling becomes impaired because of a decrease in the activity of the B12-dependent enzyme methionine synthase, therefore 5-methyltetrahydrofolate cannot be converted to tetrahydrofolate. Thus, folate cofactors becomes “trapped” in a form that cannot be used for DNA synthesis and, as a result, DNA synthesis becomes impaired—just like in folate deficiency. The mechanism to explain the occurrence of an identical anemia with folate or B12 deficiency is referred to as the “methyl trap hypothesis.”
In addition to megaloblastic anemia, B12 deficiency affects the nervous system resulting in demyelination of peripheral and central neurons and neurological complications, including neuropathy and subacute combined degeneration of the spinal cord [40]. The progression of neurological complications is generally gradual and not reversible with treatment. The neurological manifestations of B12 deficiency can precede the appearance of hematological changes and may even occur in the absence of hematological complications [44].
Other Manifestations of Insufficient B12 Status
Emerging evidence indicates that low, though not necessarily deficient, vitamin B12 status is associated with increased risk of various diseases of aging including CVD (as discussed above), neuropsychiatric dysfunction, and osteoporosis.
Neuropsychiatric dysfunction: The reported neuropsychiatric effects of folate deficiency are remarkably similar to those described for vitamin B12 deficiency [31]. Both vitamins are required for the activity of methionine synthase, thus providing methyl groups for numerous central nervous system (CNS) reactions, and may therefore have overlapping roles in the prevention of disorders of CNS development and mood disorders, and in older people, cognitive dysfunction, and dementia (i.e., Alzheimer’s disease and vascular dementia). Vitamin B12 deficiency, like folate deficiency, leads to decreased synthesis of S-adenosylmethionine thereby adversely affecting methylation reactions essential for the metabolism of components of the myelin sheath of nerve cells as well as for synthesis of neurotransmitters [44]. Randomized trials show that intervention with vitamin B12 in combination with folic acid and vitamin B6 can help to prevent cognitive decline in aging, and slow the rate of brain atrophy in older patients with mild cognitive impairment [3, 29, 30]. As observed in folate deficiency, low B12 status is also associated with an increased risk of depression [31], and both vitamins may have roles in the long-term management of depression [46].
Osteoporosis: Low vitamin B12 status is linked with poor bone health, with evidence from large cohort studies showing lower than average BMD in middle-aged adults with low plasma B12 concentrations <148 pmol/L [47, 48]. One meta-analysis reported a modest association of lower B12 concentrations with a higher fracture risk [49]. As with folate, mechanisms to explain the relationship of B12 with bone health may be via homocysteine, higher concentrations of which are linked with increased bone resorption and interference with collagen cross-linking. Vitamin B12 may also have a direct effect on bone, with evidence that osteoblast activity is dependent on B12 and bone metabolism may be adversely affected by B12 deficiency [50].
Other B-Vitamins Implicated in Anemia
Apart from folate and B12, the B-vitamins most commonly implicated in anemia, riboflavin is another, often overlooked, B-vitamin that when deficient can lead to anemia. Riboflavin, in its cofactor forms flavin mononucleotide (FMN) and flavin dinucleotide (FAD), is essential for numerous oxidation-reduction reactions and plays a fundamental role in the metabolism of energy and in supporting cellular antioxidant potential. Also, riboflavin-dependent metabolism involves interaction with many other nutrients that are implicated in anemia, including iron, folate, and vitamin B6.
Interaction of Riboflavin with Iron
Riboflavin deficiency can alter iron metabolism though various mechanisms, such as impairing iron absorption, increasing intestinal loss of iron, and/or reducing the utilization of iron for the synthesis of hemoglobin. Riboflavin deficiency can thus contribute to iron deficiency anemia; for example, riboflavin supplementation of young women was shown to enhance circulating hemoglobin concentrations and improve the response of iron deficiency anemia to iron therapy [51]. Additionally, a randomized trial conducted in pregnant women with anemia in China showed that the inclusion of riboflavin (along with retinol) decreased the prevalence of anemia compared to supplementation with iron and folic acid only [52].
Interaction of Riboflavin with Folate
Riboflavin deficiency could contribute to megaloblastic anemia through its interaction with folate. Specifically, riboflavin acts as a cofactor for the key folate metabolizing enzyme methylenetetrahydrofolate reductase (MTHFR). The importance of riboflavin in folate metabolism is perhaps most evident in individuals homozygous for the C677T polymorphism in MTHFR, resulting in a thermolabile enzyme with reduced activity, thus impairing folate recycling. The homozygous variant MTHFR 677TT (“TT”) genotype affects about 10% of people globally, but this figure is much higher in some countries, including Mexico where a reported 32% of the population are affected by the TT genotype [53].
Although the health concerns in relation to this polymorphism have predominantly focussed on homocysteine as the well described phenotype, arguably of greater relevance to public health is the more recent emergence of a blood pressure phenotype, and a modulating role of riboflavin (as the MTHFR cofactor), in determining the risk of hypertension in affected individuals [53]. Three randomized trials to date in hypertensive patients showed that intervention with low-dose riboflavin (1.6 mg/day) results in lowering systolic blood pressure (by 6–14 mmHg), specifically in those with the variant TT genotype in MTHFR [53]. Moreover, very recent evidence from a large cohort of over 6000 Irish adults showed that the variant TT genotype in MTHFR was associated with higher blood pressure and an increased risk of hypertension from 18 years, whilst better biomarker status of riboflavin reduced this genetic risk [54]. The impact of this gene-nutrient effect may be particularly relevant in preventing adverse pregnancy outcomes, including gestational hypertension and preeclampsia, linked with this common folate polymorphism [17], but this remains to be demonstrated.
Riboflavin deficiency is a significant problem in low-middle income countries. Across the developed world also, deficient riboflavin status may be widespread, but this is largely undocumented as biomarker status is rarely measured in population-based studies [3]. The UK is in fact one of the very few countries worldwide to include a riboflavin biomarker as part of its rolling national nutrition survey. Riboflavin deficiency in pregnancy may be a particular concern across countries globally and, given its importance in both iron and folate metabolism, further research is needed investigating riboflavin-related anemia in pregnancy and the potential role of riboflavin in hypertensive disorders of pregnancy.
Interaction of Riboflavin with Vitamin B6
The conversion of vitamin B6 to the metabolically active form in tissues, pyridoxal 5′-phosphate (PLP), is dependent on riboflavin in the cofactor form FMN. Riboflavin is thus an important determinant of vitamin B6 status throughout the lifecycle, with recent evidence indicating that riboflavin may be the limiting nutrient for maintaining PLP in older people [55]. PLP, in turn, functions as a coenzyme of 5-aminolevulinic acid synthase, which is involved in the synthesis of heme, the iron-containing component of hemoglobin. Vitamin B6 deficiency may thus impair hemoglobin synthesis and lead to microcytic anemia.
Public Health Measures to Address B-Vitamin Insufficiency and Related Policy
Reduction of anemia is one of the World Health Assembly Global Nutrition Targets for 2025 [1]. There are particular concerns regarding tackling anemia in women of reproductive age and pregnant women, and a recent WHO report (2020) has reiterated the critical importance of addressing anemia in these target groups and particularly in low-middle income countries.
Addressing Folate Insufficiency
Advice for individuals: For the prevention of NTD, women globally are recommended to take 400 μg/day folic acid as a supplement from preconception until the end of the first trimester of pregnancy. Some women are considered to be at higher risk (e.g., those with a previous pregnancy affected by NTD; those taking certain anticonvulsant drugs) and thus are recommended to take higher folic acid doses (4–5 mg/day).
Folic acid supplements provide a highly effective means to optimize folate status in individual women who take their supplements as recommended. However, supplementation is not an effective public health strategy for populations because in practice very few women correctly follow the recommendations, as discussed below.
Folic acid-fortified foods, like folic acid supplements, are highly effective as a means of optimizing folate status in women who are regular consumers of fortified foods. In countries with voluntary fortification in place, folic acid-fortified foods such as breakfast cereals are found to have very significant impacts on dietary intakes and folate biomarkers [56].
Public health challenges: Implementation of current folic acid policy to prevent NTD is problematic. For over 25 years, policy in many countries has been based on recommending women to take a supplement containing folic acid (0.4 mg/day) from before conceiving until the 12th week of pregnancy. As a sole public health measure (as is the case in European countries), however, supplementation has had little or no impact in preventing NTD, despite active health promotion campaigns over many years. The lack of success of this measure is primarily because women typically start taking folic acid after the period of neural tube closure (i.e., the 3rd to 4th week of pregnancy) [3]. This has resulted in unacceptably high rates of NTD in Europeans countries, estimated to be 1.6 times higher than in regions of the world with mandatory folic acid fortification policies in place [57].
Food Fortification
Food fortification is the process of adding essential micronutrients to foods. Food fortification can be conducted on a mandatory (i.e., regulated) or a voluntary (i.e., at the discretion of individual food manufacturers) basis. When folic acid fortification is undertaken via mandatory fortification of staple foods, it has resulted in a population-wide increase in folate status and decreased prevalence of anemia [2]. Over 85 countries worldwide to date (including North America, most of South America and Australia) have implemented mandatory folic acid fortification. In such countries, folate status is found to be optimal and this is reflected in, not only a much lower prevalence of anemia, but also a lower risk of NTD, with evidence that rates of NTD have declined by between 27 and 50% in the US, Canada and Chile in response to mandatory folic acid fortification [57].
Although the UK and Ireland have led the way in Europe in terms of considering folic acid fortification on a mandatory basis, for more than a decade both governments have delayed decisions to introduce mandatory fortification due to concerns relating to possible health risks. However, two extensive government-commissioned reports from Ireland and UK recently provided the basis for reforming folic acid policy [58, 59], with the balance of scientific evidence in both reports indicating that there are no health risks associated with the low levels of folic acid being proposed. Of note, rates of NTD in Ireland are among the highest in the world and there is particular concern that NTD trends have been increasing in recent years [58]. Although voluntary folic acid fortification is permitted in Ireland and appears to be beneficial in terms of reducing NTD to some extent, the benefit will only be achieved by consumers who choose to eat fortified food products. Mandatory folic acid fortification, in contrast, would reach all women, including those who have not planned their pregnancy, will not be taking FA supplements in early pregnancy and therefore are not protecting against NTDs in their babies. Moreover, an expert international panel tasked with reviewing all aspects of folate biology, recently concluded that the proven benefits of folic acid fortification would outweigh any potential risks [2].
Summary and Recommendations
Severe deficiency of either folate or vitamin B12 leads to megaloblastic anemia. Insufficiency of either of these vitamins, even if not severe enough to cause anemia, can be a major cause of ill health globally, especially for women of reproductive age (folate) and older adults (B12). Much less well recognized is that riboflavin deficiency can also contribute to anemia through adversely affecting the metabolism of iron and folate.
Folate is essential in one-carbon metabolism and is thus required for critical biological processes. A large body of evidence links folate insufficiency with adverse health outcomes from early to late life, however, the driver of public health policy worldwide relates to NTD where folic acid supplementation of mothers before and in early pregnancy has a proven preventative effect. Despite this, preventable NTDs are not being prevented in many countries. Implementation of a policy of mandatory folic acid fortification of staple foods would be highly effective in preventing NTD in regions without such policy in place.
Metabolically, vitamin B12 interacts closely with folate, and deficiency manifests in an identical anemia, along with irreversible neuropathy. In addition to these clinical signs of deficiency, B12 insufficiency is associated with increased risk of diseases of aging including CVD, neuropsychiatric dysfunction, and osteoporosis.
Riboflavin has several essential roles, including in folate metabolism. Deficiency is widespread but goes undetected in almost all countries. The recent emergence of a novel interaction between a common folate polymorphism and riboflavin status with impacts for blood pressure is potentially important in preventing hypertension in sub-populations globally, and may offer new insights into mechanisms linking impaired folate with disease outcomes throughout life.
In summary, folate, vitamin B12, and riboflavin are important throughout the lifecycle. More rigorous assessment and prevention of insufficient status of these nutrients should be prioritized and based on measurement of biomarkers rather than relying on dietary data only. In older populations, routine monitoring of vitamin B12 status will identify those with deficiency and enable early intervention. Fortified foods provide a bioavailable source of folate and other B-vitamins and offer a practical and highly effective means of improving status.
References
WHO Global Nutrition Targets 2025. Anaemia policy brief. Geneva: World Health Organization; 2014 (WHO/NMH/NHD/14.4). https://www.who.int/nutrition/publications/globaltargets2025_policybrief_anaemia/en/.
Bailey LB, Stover PJ, McNulty H, et al. Biomarkers of nutrition for development—folate review. J Nutr. 2015;145:1636S–80S.
McNulty H, Ward M, Hoey L, Hughes CF, Pentieva K. Addressing optimal folate and related B vitamin status through the lifecycle: health impacts and challenges. Proc Nutr Soc. 2019;78(3):449–62.
Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998.
European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific opinion on dietary reference values for folate. EFSA J. 2014;12(11):3893.
Gibson RS. Assessment of folate and vitamin B12 status. In: Principles of nutritional assessment. 2nd ed. New York, NY: Oxford University Press; 2005. p. 595–640.
Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32.
Rogers LM, Cordero AM, Pfeiffer CM, et al. Global folate status in women of reproductive age: a systematic review with emphasis on methodological issues. Ann N Y Acad Sci. 2018;1431:35–57.
McNulty B, McNulty H, Marshall B, et al. Impact of continuing folic acid after the first trimester of pregnancy: findings of a randomized trial of folic acid supplementation in the second and third trimesters. Am J Clin Nutr. 2013;98:92–8.
Blot I, Papiernik E, Kaltwasser JP, et al. Influence of routine administration of folic acid and iron during pregnancy. Gynecol Obstet Investig. 1981;12:294–304.
MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the Medical Council Vitamin Study. Lancet. 1991;338:131–7.
Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5.
Vollset E, Botto LD. Neural tube defects, other congenital malformations and single nucleotide polymorphisms in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene. In: Ueland P, Rozen R, editors. MTHFR polymorphisms and disease. Georgetown, TX: Landes Bioscience; 2004. p. 127–45.
Rothenberg SP, da Costa MP, Sequeira JM, et al. Autoantibodies against folate receptors in women with a pregnancy complicated by a neural-tube defect. N Engl J Med. 2004;350:134–42.
Molloy AM, Kirke PN, Troendle JF, Burke H, Brody LC, Scott JM, Mills JL. Maternal vitamin B12 status and risk of neural tube defects in a population with high neural tube defects and no folic acid supplementation. Pediatrics. 2009;123:917–23.
Van Beynum IM, Kapusta L, Bakker MK, et al. Protective effect of periconceptional folic acid supplements on the risk of congenital heart defects: a registry-based case-control study in the northern Netherlands. Eur Heart J. 2010;31:464–71.
Psara E, Pentieva K, Ward M, McNulty H. Critical review of nutrition, blood pressure and risk of hypertension through the lifecycle: do B vitamins play a role? Biochimie. 2020;173:76–90.
De Ocampo MPG, Araneta MRG, Macera CA, Alcaraz JE, Moore TRCC. Folic acid supplement use and the risk of gestational hypertension and preeclampsia. Women Birth. 2018;31:e77–83.
Wen SW, White RR, Rybak N, Gaudet LM, et al. Effect of high dose folic acid supplementation in pregnancy on pre-eclampsia (FACT): double blind, phase III, randomised controlled, international, multicentre trial. BMJ. 2018;362:k3478.
Caffrey A, McNulty H, Irwin RE, et al. Maternal folate nutrition and offspring health: evidence and current controversies. Proc Nutr Soc. 2019;78:208–20.
James P, Sajjadi S, Tomar AS, et al. Candidate genes linking maternal nutrient exposure to offspring health via DNA methylation: a review of existing evidence in humans with specific focus on one-carbon metabolism. Int J Epidemiol. 2018;47(6):1910–37.
McNulty H, Strain JJ, Pentieva K, Ward M. One-carbon metabolism and CVD outcomes in older adults. Proc Nutr Soc. 2012;71:213–21.
Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–82.
Yang Q, Botto LD, Erickson JD, et al. Improvement in stroke mortality in Canada and the United States, 1990 to 2002. Circulation. 2006;113:1335–43.
Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77.
Saposnik G, Ray JG, Sheridan P, et al. the HOPE 2 Investigators. Homcysteine-lowering therapy and stroke risk, severity, and disability: additional findings from the HOPE 2 trial. Stroke. 2009;40:1365–72.
Rosenblatt DS. Inherited disorders of folate transport and metabolism. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The metabolic basis of inherited disease. New York: Mc Graw; 1995. p. 3111–28.
Porter K, Hoey L, Hughes CF, et al. Causes, consequences and public health implications of low B-vitamin status in ageing. Nutrients. 2016;8:1–29.
Smith AD, Smith SM, de Jager CA, et al. Homocysteine-lowering by B-vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized controlled trial. PLoS One. 2010;5:e12244.
Douaud G, Refsum H, de Jager CA, et al. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc Natl Acad Sci. 2013;110:9523–8.
Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol. 2006;5:949–60.
Gilbody S, Lightfoot T, Sheldon T. Is low folate a risk factor for depression? A meta-analysis and exploration of heterogeneity. J Epidemiol Comm Hlth. 2007;61:631–7.
Moore K, Hughes CF, Hoey L, et al. B-vitamins in relation to depression in older adults over 60 years of age: the TUDA Cohort study. JAMDA. 2019;20(2):551–7.
Gjesdal CG, Vollset SE, Ueland PM, et al. Plasma homocysteine, folate, and vitamin B 12 and the risk of hip fracture: the Hordaland Homocysteine Study. J Bone Miner Res. 2007;22:747–56.
van Meurs JB, Dhonukshe-Rutten RA, Pluijm SM, et al. Homocysteine levels and the risk of osteoporotic fracture. N Engl J Med. 2004;350:2033–41.
Kim Y-I. Folate and carcinogenesis: evidence, mechanisms, and implications. J Nutr Biochem. 1999;10:66–88.
Blount BC, Mack MM, Wehr CM, et al. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: implications for cancer and neuronal damage. Proc Natl Acad Sci U S A. 1997;94:3290–5.
Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9.
Allen LH, Miller JW, de Groot L, Rosenberg IH, Smith AD, Refsum H, Raiten DJ. Biomarkers of nutrition for development (BOND): vitamin B-12 review. J Nutr. 2018;148:1995S–2027S.
Stabler SP. Vitamin B12 deficiency. New Engl J Med. 2013;368:2041–2.
Carmel R. Diagnosis and management of clinical and subclinical cobalamin deficiencies: why controversies persist in the age of sensitive metabolic testing. Biochimie. 2013;95:1047–55.
Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–42.
Porter KM, Ward M, Hughes CF. Hyperglycemia and metformin use are associated with B vitamin deficiency and cognitive dysfunction in older adults. J Clin Endocrinol Metab. 2019;104:4837–47.
Green R, Allen LH, Bjorke-Monsen AL, et al. Vitamin B12 deficiency. Nat Rev Dis Primers. 2017;3:17040.
Hughes CF, McNulty. Assessing biomarker status of vitamin B12 in the laboratory: no simple solution. Ann Clin Biochem. 2018;55:188–9.
Almeida OP, Ford AH, Flicker L. Systematic review and meta-analysis of randomized placebo-controlled trials of folate and vitamin B12 for depression. Int Psychogeriatr. 2015;27:727–37.
Tucker KL, Hannan MT, Qiao N, et al. Low plasma vitamin B12 is associated with lower BMD: the Framingham Osteoporosis study. J Bone Miner Res. 2005;20:152–8.
Morris M, Jacques P. Selhub J Relation between homocysteine and B-vitamin status indicators and bone mineral density in older Americans. Bone. 2005;37:234–42.
van Wijngaarden JP, Doets EL, Szczecinska A, et al. Vitamin B12, folate, homocysteine, and bone health in adults and elderly people: a systematic review with meta-analyses. J Nutr Metab. 2013;2013:486186.
Carmel R, Lau K, Baylink D, et al. Cobalamin and osteoblast-specific proteins. N Engl J Med. 1988;319:70–5.
Powers HJ, Hill MH, Mushtaq S, et al. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am J Clin Nutr. 2011;93:1274–84.
Ma AG, Schouten EG, Zhang FZ, et al. Retinol and riboflavin supplementation decreases the prevalence of anemia in Chinese pregnant women taking iron and folic Acid supplements. J Nutr. 2008;138:1946–50.
McNulty H, Strain JJ, Hughes CF, Pentieva K, Ward M. Evidence of a role for one-carbon metabolism in blood pressure: can B vitamin intervention address the genetic risk of hypertension owing to a common folate polymorphism. Curr Dev Nutr. 2020;4(1):nzz102.
Ward M, Hughes C, Strain JJ, aI. Impact of the common MTHFR 677C→T polymorphism on blood pressure in adulthood and role of riboflavin in modifying the genetic risk of hypertension: evidence from the JINGO project. BMC Med. 2020;18:318.
Jungert A, McNulty H, Hoey L, et al. Riboflavin is an important determinant of vitamin B6 status in healthy adults. J Nutr. 2020;150:2699–706.
Hopkins SM, Gibney MJ, Nugent AP, et al. Impact of voluntary fortification and supplement use on dietary intakes and biomarker status of folate and vitamin B12 in Irish adults. Am J Clin Nutr. 2015;101:1163–72.
Khoshnood B, Loane M, Dolk H, et al. Long term trends in prevalence of neural tube defects in Europe: population based study. BMJ (Online). 2015;351:1–5.
Food Safety Authority of Ireland FSAI. Update report on folic acid and the prevention of birth defects in Ireland. 2016. https://www.fsai.ie/news_centre/press_releases/folic_acid_report_04052016.html.
Scientific Advisory Committee on Nutrition (SACN) Folate and Disease Prevention Report (2006) and Folic acid updated SACN recommendations (2017). Available at https://www.gov.uk/government/publications/sacn-folate-and-disease-prevention-report https://www.gov.uk/government/publications/folic-acid-updated-sacn-recommendations.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this chapter
Cite this chapter
McNulty, H. (2022). The Role of B-Vitamins in Nutritional Anemia. In: Karakochuk, C.D., Zimmermann, M.B., Moretti, D., Kraemer, K. (eds) Nutritional Anemia. Nutrition and Health. Springer, Cham. https://doi.org/10.1007/978-3-031-14521-6_13
Download citation
DOI: https://doi.org/10.1007/978-3-031-14521-6_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-14520-9
Online ISBN: 978-3-031-14521-6
eBook Packages: MedicineMedicine (R0)