Abstract
Low flow and microvascular shunts (MVS) is the final common pathway in cerebrovascular disease. Low flow in brain capillaries (diam. 3–8 μm) decreases endothelial wall shear rate sensed by the glycocalyx regulating endothelial function: water permeability; nitric oxide synthesis via nitric oxide synthase; leucocyte adhesion to the endothelial wall and penetration into the tissue; activation of cytokines and chemokines initiating inflammation in tissue. Tissue edema combined with pericyte and astrocyte capillary constriction increases capillary resistance. Increased capillary resistance diverts flow through MVS (diam. 10–25 μm) that are non-nutritive, without gas exchange, waste or metabolite clearance and cerebral blood flow (CBF) regulation. MVS predominate in subcortical and periventricular white matter. The shift in flow from capillaries to MVS is a pathological, maladaptive process. Low perfusion in the injured tissue exacerbates brain edema. Low blood flow and MVS alone can lead to all of the processes involved in tissue injury including inflammation and microglial activation.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
We recently showed the transition from brain capillary (CAP) to MVS flow with increased intracranial pressure (ICP), it’s role in the loss of cerebral blood flow (CBF) autoregulation and the circulatory isolation of injured brain tissue by perfusion through microvascular shunts (MVS) [5]. Our investigations into MVS were initiated by the study by Miller et al. in 1972 [1] reporting an unexplained decrease in the critical cerebral perfusion pressure (CPP) threshold of CBF autoregulation from 50 to 30 mmHg when CPP was decreased by increasing ICP instead of decreasing arterial pressure as conventionally done in studies [2, 3]. We hypothesized that the decrease in critical CPP when ICP rather than mean arterial pressure (MAP) was used to decrease CPP, was due to MVS flow resulting in a falsely elevated CBF and apparently maintained CBF at a lower critical CPP of 30 mmHg. We then showed brain MVS in our studies using two photon laser scanning microscopy (2PLSM) [4, 5].
2 Evidence on Microvascular Shunts
Shunt Flow in Traumatic Brain Injury
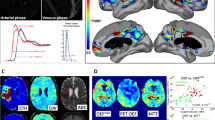
A dramatic example of non-nutritive shunt flow after traumatic brain injury was observed in a 7-year-old female who suffered severe left hemispheric infarction and at 7 days after injury was placed under barbiturate coma for treatment of intracranial hypertension (Fig. 1). CBF measured by stable xenon-CT showed that the infarcted left hemisphere was extremely hyperemic whereas the right hemisphere was hypoperfused due to barbiturate suppression of metabolism and depressed CBF. This remarkable demonstration of MVS triggered our search to prove the occurrence of brain MVS.
CT scan (top) and stable xenon CBF maps (bottom) a 7 year old female 7 days after severe brain trauma under barbiturate coma for high ICP. The left hemisphere is completely infarcted and the right hemisphere normal. CBF in the normal hemisphere is depressed by barbiturate indicating flow-metabolism coupling. The left hemisphere shows marked hyperemia and “nonnutritive flow” loss off CBF autoregulation
MVS in Human Cerebrum
Histological sections of human cerebrum suggest bridges of vessels without intervening capillary beds in the microvasculature [6]. These MVS 10–25 μm diameter are larger than capillaries which were measured at about 3–8 μm in outer diameter and arterio-venous and veno-venous anastomoses in the range of 25 μm.
Cerebral Capillary Flow Autoregulation
Hudetz et al. [7] reported two populations of microvessel flow velocities; one above and one below one mm/sec. High flow velocity microvessels showed no autoregulation whereas low flow velocity vessels showed constant flow velocity until the lower limit of autoregulation was reached.
Microvascular Shunts with Increased ICP
Our first definitive evidence of MVS in the healthy rat brain was by increasing ICP with a reservoir of mock CSF with a catheter into the cisterna magna using two photon laser scanning microscopy (2PLSM) [4, 5]. Increased ICP showed increased flow velocity in vessel diameters >10 μm which was not observed when CPP was decreased by decreasing mean arterial pressure (MAP) (Fig. 2).
Consistent with the effect of MVS as non-nutritive and without clearance of metabolic waste products or gas exchange, tissue hypoxia develops as indicated by the increase in NADH, i.e. a reflection of tissue hypoxia or low oxygen, with increasing ICP which was partially mitigated by increasing CPP. The effect of increasing ICP which also increased brain edema (water content) was partly mitigated by increasing CPP. Increasing ICP caused a progressive decrease in CAP/MVS ratio and shunting with increasing hypoxia and Doppler flux.
3 Low Blood Flow and Shear Rate on Endothelial Function
There are multiple shear stress related controls for blood vessels in the circulation in large arteries, small arteries to arterioles, capillaries, venules and veins [8]. For each of these vessels of different calibers, there are processes related to: blood vessel growth, angiogenesis, re thrombolysis and inflammatory processes such as sepsis all controlled by shear stresses in the blood vessels [9, 11].
The shear rate on the endothelium of capillaries is highest as it is inversely proportional to the third power of the vessel diameter [8, 11]. Endothelial shear rate plays an important role in endothelial function through the glycocalyx, a carbohydrate-rich layer 0.2–0.5 μm thick gel-like layer lining the luminal membrane of the endothelium (8, 10, 12). It connects to the endothelium by core molecules containing proteoglycans and glycoproteins [12] triggered by the shear rate and torque applied to endothelial cells (Fig. 3). It consists of proteoglycans 50–90% of which is heparin sulfate and glycoproteins anchored to the endothelium by glycosaminoglycans. It maintains the colloid osmotic gradient of the vascular barrier; regulates vascular exchange of water and solutes and leucocyte adhesion; provides binding sites for several molecules antithrombin III, lipoprotein lipase vascular endothelial growth factor; and acts as a shear stress sensor and regulator [12]. The glycocalyx controls capillary endothelial function as the interface between the tissue and the circulation.
Schematic representation of the endothelial glycocalyx, showing its main components. Left: The endothelial glycocalyx observed in vivo as a red blood cell exclusion zone, located on the luminal side of the vascular endothelium. It consists of membrane bound and soluble molecules. Right: Components of the endothelial glycocalyx [10]. (Reproduced Springer Verlag)
4 Low Blood Flow and MVS in the Final Common Pathway in Cerebrovascular Disease
The role of low blood flow in cerebrovascular disease is increasingly recognized in the pathogenesis of all cerebrovascular diseases including vascular dementia, Alzheimer’s Disease [13]. MVS occur primarily in the deep cortical white matter and in the periventricular white matter where white matter hyperintensities (WMH) are most frequently observed. The role of MVS in the pathophysiology of cerebrovascular disease may be a means of isolating injured tissue from further perfusion shunting blood around dead and edematous tissue inducing further edema in injured tissue. MVS do not conduct nutrient nor gas exchange with tissue which would reduce further brain edema development in injured tissue.
References
Miller JD, Stanek A, Langfitt TW (1972) Concepts of cerebral perfusion pressure and vascular compression during intracranial hypertension. Prog Brain Res 35:411–432
Artru AA, Katz RA, Colley PS (1989) Autoregulation of cerebral blood flow during normocapnia and hypocapnia in dogs. Anesthesiology 70:288–292
Rapela CE, Green HD (1964) Autoregulation of canine cerebral blood flow. Circ Res 15(SUPPL):205–212
Bragin DE, Bush RC, Müller WS, Nemoto EM (2011) High intracranial pressure effects on cerebral cortical microvascular flow in rats. J Neurotrauma 28:775–785
Bragin DE, Bush RC, Nemoto EM (2013) Effect of cerebral perfusion pressure on cerebral cortical microvascular shunting at high intracranial pressure in rats. Stroke 44:177–181
Ravens JR (1974) Anastomoses in the vascular bed of the human cerebrum. In: Cervos-Navaro J (ed) Pathology of cerebral microcirculation. Walter de Bruter, Berlin, pp 16–38
Hudetz AG, Feher G, Kampine JP (1996) Heterogeneous autoregulation of cerebrocortical capillary flow: evidence for functional thoroughfare channels? Microvasc Res 51(1):131–136
Resnick N, Yahav H, Shay-Salit A, Shushy M, Schubert S, Zilberman LC, Wofovitz E (2003) Fluid shear stress and the vascular endothelium: for better and for worse. Prog Biophys Mol Biol 81(3):177–199
Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospinatascon GA, Hernandez G, Murray P, DeBacker D, ADQI XIV Workgroup (2016) The endothelium in sepsis. Shock 45:259–270
Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MGA (2007) The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch 454:345–359
Papaioannou TG, Stefanadis C (2005) Vascular wall shear stress: basic principles and methods. Hell J Cardiol 46:9–15
Schött U, Solomon C, Fries D, Bentzer P (2016) The endothelial glycocalyx and its disruption, protection and regeneration: a narrative review. Scand J Trauma Resusc Emerg Med 12(24):48
de la Torre JC (2016) Cerebral perfusion enhancing interventions: a new strategy for the prevention of Alzheimer Dementia. Brain Pathol 26:618–631
Acknowledgements
Supported by NIH and American Heart Association grants to Drs Nemoto and Bragin. NIH-NIGMS Sub-award from P20 GM109089-01A1, NIH-NINDS 1R21NS091600-01A1, NIH-NINDS RO1-NS051639-05, NIH-NINDS R03 NS061216-01A1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Nemoto, E.M., Bragin, D. (2022). Low Flow and Microvascular Shunts: A Final Common Pathway to Cerebrovascular Disease: A Working Hypothesis. In: Scholkmann, F., LaManna, J., Wolf, U. (eds) Oxygen Transport to Tissue XLIII. Advances in Experimental Medicine and Biology, vol 1395. Springer, Cham. https://doi.org/10.1007/978-3-031-14190-4_21
Download citation
DOI: https://doi.org/10.1007/978-3-031-14190-4_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-14189-8
Online ISBN: 978-3-031-14190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)