Abstract
In patients suffering from Coronavirus Disease 2019 (COVID-19), dyspnoea is less likely to occur despite hypoxemia. Even if the patient develops severe hypoxemia, it cannot be detected from subjective symptoms. In other words, it becomes more serious without the person or the surroundings noticing it. Initially less talked about, hypoxemia without dyspnoea (silent hypoxemia or happy hypoxia: hypoxemia that does not coincide with dyspnoea) is now experienced in many institutions. Dyspnoea is defined as “the unpleasant sensation that accompanies breathing.” Dyspnoea occurs when afferent information is transmitted to the sensory area. Receptors involved in the development of dyspnoea include central and peripheral chemoreceptors, chest wall receptors, lung receptors, upper respiratory tract receptors and corollary discharge receptors. In the present study, we considered mechanisms mediating the silent hypoxemia through three cases experienced at our hospital as a dedicated coronavirus treatment hospital. We have treated about 600 people infected with COVID-19, of which about 10% were severe cases. In the present study, the patients’ condition was retrospectively extracted and analysed. We investigated three typical cases of COVID-19 pneumonia admitted to our hospital (men and women between the ages of 58 and 86 with hypoxemia and tachypnoea). Silent hypoxemia is not entirely without dyspnoea, but hypoxemia does not cause dyspnoea commensurate with its severity. The virus may have specific effects on the respiratory control system. In our cases, respiratory rate significantly increased with hypoxemia, and hyperventilation occurred. Therefore, information about hypoxemia is transmitted from the carotid body. Since hyperventilation occurs, it is suggested that information is transmitted to effectors such as respiratory muscles. The fact that these patients did not feel the unpleasant sensation indicates that information is not accurately transmitted to the sensory area of the cerebral cortex. These cases suggest that there may be a problem somewhere in the path from the respiratory centre to the sensory area.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Currently, many people are infected due to the pandemic of the novel coronavirus infection which can lead to Coronavirus Disease 2019 (COVID-19). In Japan as well, the number is steadily increasing, and the number of home care recipients has exceeded 17,000 (as of 2021.8.11). Under such circumstances, there are many cases in which the symptoms are poor and there is a delay before aggravation is noticed. In COVID-19, dyspnoea is less likely to occur despite hypoxemia. Even if the patient develops severe hypoxemia, it cannot be detected from subjective symptoms. In other words, it becomes more serious without the person or the surroundings noticing it. Initially less talked about hypoxemia without dyspnoea (silent hypoxemia or happy hypoxia: hypoxemia that does not coincide with dyspnoea) is now experienced in many institutions. If we cannot understand the concept of silent hypoxemia, patients in home care may not receive hospital care unless they become seriously ill.
Dyspnoea is defined as “the unpleasant sensation that accompanies breathing.” Dyspnoea occurs when afferent information is transmitted to the sensory area. Receptors involved in the development of dyspnoea include central and peripheral chemoreceptors, chest wall receptors, lung receptors, upper respiratory tract receptors, and corollary discharge receptors [1,2,3,4,5,6,7,8]. In the present study, we considered mechanisms mediating the silent hypoxemia by reviewing the cases experienced at our hospital as a dedicated coronavirus treatment hospital.
2 Methods
We have treated about 600 people infected with COVID-19, of which about 10% were severe cases. Because patients with severe pneumonia were not in a good condition, informed consent and the following data were obtained from only three patients (men and women between the ages of 58 and 86 with hypoxemia and tachypnoea). We do not believe that these three cases reflect all COVID-19 patients, possible causes of silent hypoxemia could be retrospectively analysed. Clinical symptoms, vital signs, arterial blood gas data and the modified Borg scale values for dyspnoea (range 0–10) were analysed. As shown in Table 1, this is a measure to assess the difficulty of breathing. It starts at number 0, where the patient’s dyspnoea does not cause any difficulty, and progresses to number 10, where the patient’s dyspnoea is maximum [9]. The institutional review board approved this study.
3 Results
-
Case 1 (Fig. 1).
A 58-year-old man had a BMI of 28.2 (mildly obese). The underlying disease was dyslipidaemia. He had a temperature of 39.1 °C. At the time of admission, his SpO2 was 93% (room air) and the modified Borg scale was 2 (slight). As shown in Fig. 1, his chest CT images showed ground glass opacities in the bilateral lung fields. Arterial blood gas data on the third day of hospitalisation showed mild hypoxemia and A-aDO2 was increased.
-
Case 2 (Fig. 2).
A 58-year-old man had a BMI of 30.1 (obese). Underlying diseases included diabetes and hypertension. Body temperature was 38.7 °C. Although his SpO2 was 87% (room air) at the time of admission, which indicates his PaO2 was less than 60 mmHg, the modified Borg scale was only 4 (somewhat severe). As shown in Fig. 2, his chest CT images showed diffuse pneumonic shadows in the bilateral lung fields, indicating moderate to severe COVID-19 pneumonia. Arterial blood gas data on the second day of hospitalisation showed acute respiratory alkalosis. His PaO2 was 72.0 mmHg under high flow of oxygen 7 L/min. His respiratory rate increased to 34/min and hyperventilation was observed, but the modified Borg scale remained unchanged and no strong dyspnoea was observed.
-
Case 3 (Fig. 3).
An 86-year-old female had a BMI of 23.5. The underlying diseases were diabetes, hypertension and angina. She had a temperature of 37.8 °C. Although her SpO2 was 88% (room air) at the time of admission, which indicates her PaO2 was less than 60 mmHg, the modified Borg scale was 4 (somewhat severe). As shown in Fig. 3, her chest CT images showed diffuse pneumonic shadows in the bilateral lung fields, indicating moderate to severe COVID-19 pneumonia. Arterial blood gas data on the second day of hospitalisation showed that her PaO2 was kept at 68.3 mmHg under high flow of oxygen 5 L/min. Her respiratory rate increased to 36/min and hyperventilation was observed, but the modified Borg scale remained unchanged and no strong dyspnoea was observed.
Despite hypoxemia, increased respiratory rate and hyperventilation in these cases, the modified Borg scale values were only 2–4. In particular, two cases (cases 2 and 3) developed severe respiratory failure, but the modified Borg scale values indicated “somewhat severe”.
4 Discussion
In the present study, only three cases were reviewed. Although these cases do not reflect clinical features of all COVID-19 patients, we think possible causes of silent hypoxemia could be analysed by reviewing these cases.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) mainly invades alveolar type-2 epithelial cells and spreads throughout the body through systemic circulation, neurotransmission and attachment to ACE2-expressing cells. The virus can spread directly to the central nervous system by the olfactory epithelium.
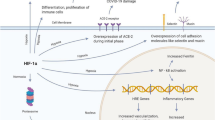
Silent hypoxemia would be defined as follows: Despite the intensity of hypoxemia, the intensity of dyspnoea is inappropriate to the degree of arterial hypoxemia. The clinical presentation of silent hypoxemia in patients with COVID-19 might be (1) impairment of peripheral oxygen-sensing and/or (2) impairment of central processing of hypoxia-stimulated afferent inputs within higher-order somatosensory brain regions that manifest perceptions of dyspnea [10]. The virus could bind to the ACE2 receptors on the glomus cells of the carotid body, resulting in damage of the mitochondrial electron transport chain in O2-sensing cells. This damage would reduce afferent information to the brain despite hypoxemia and may reduce dyspnoea sensation [11]. However, as shown in the results and Fig. 4, the fact that these patients developed tachypnoea and hyperventilation in the present study suggests that some afferent information, even if reduced, is processed centrally enough to produce an efferent ventilatory response. In addition to this, although it is possible that olfactory abnormalities may affect dyspnoea, olfactory dysfunction was not present in any cases throughout the course in the present study. From these data, it would be suggested that the virus may interfere with central processing of sensory afferent neural inputs in patients with silent hypoxemia. Since correlations between neurological symptoms and the brain pathology findings in COVID-19 patients remain largely unknown [12], future prospective studies with control and COVID-19 groups will be necessary to determine the mechanisms of silent hypoxemia.
Dysnonea and respiratory control system. (Modified from Ref. [10])
References
Martin JT, Franco L, Amal J (2020) Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med 202:356–360
Fung ML (2015) Expressions of angiotensin and cytokine receptors in the paracrine signaling of the carotid body in hypoxia and sleep apnea. Respir Physiol Neurobiol 209:6–12
Sedaghat AR, Gengler I, Speth MM (2020) Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg 5:194599820926464
Netland J, Meyerholz DK, Moore S et al (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82:7264–7275
Couzin-Frankel J (2020) The mystery of the pandemic’s ‘happy hypoxia’. Science 368:455–456
Wichmann D, Sperhake J-P, Lütgehetmann M et al (2020) Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med 173:268–277
Sanchez O, Caumont-Prim A, Riant E et al (2017) Pathophysiology of dyspnoea in acute pulmonary embolism: a cross-sectional evaluation. Respirology 22:771–777
Nakano T, Iwazaki M, Sasao G et al (2015) Hypobaric hypoxia is not a direct dyspnogenic factor in healthy individuals at rest. Respir Physiol Neurobiol 218:8–31
Borg AG (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Nishino T (2010) Respiratory center and respiratory sensation. J Jpn Soc Clin Aneseth 20:12
Jacquie B, Anthony VI, Richard JA et al (2021) Syncope and silent hypoxemia in COVID-19: implications for the autonomic field. Auton Neurosci 235:102842
Cosentino G, Todisco M, Hota N et al (2021) Neuropathological findings from COVID-19 patients with neurological symptoms argue against a direct brain invasion of SARS-CoV-2: a critical systematic review. Eur J Neurol 28(11):3856–3865. https://doi.org/10.1111/ene.15045
Acknowledgments
We would like to thank all the staff who treated the patients infected with the novel coronavirus.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this paper
Cite this paper
Ebihara, A., Kitahara, A., Iwamoto, T., Kuwahira, I. (2022). Silent Hypoxemia in COVID-19 Pneumonia. In: Scholkmann, F., LaManna, J., Wolf, U. (eds) Oxygen Transport to Tissue XLIII. Advances in Experimental Medicine and Biology, vol 1395. Springer, Cham. https://doi.org/10.1007/978-3-031-14190-4_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-14190-4_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-14189-8
Online ISBN: 978-3-031-14190-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)