Abstract
Plants are constantly challenged by an array of potential pathogens like fungi, bacteria, viruses, insects, nematodes, etc., which lead to a significant loss to plant yield. Plants commonly overcome these phytopathogens by showing resistance through plant defense mechanisms. Several general microbe elicitors allow plants to mitigate the harmful effects of pathogenic microbes by enhancing the capability of plants to identify anonymous pathogenic agents and act as surveillance systems for plants. Elicitors are small drug-like compounds released by pathogens that are composed of molecules like oligosaccharides, lipids, peptides, and proteins, and they activate various kinds of defense responses in plants. They deliver information to plants through perception and identification of signaling molecules by cell surface-localized receptors, which is followed by the triggering of signal transmission pathways that commonly induces the synthesis of active oxygen species (AOS), phytoalexin production, production of defense enzymes, and the aggregation of pathogenesis-related (PR) proteins. This article chiefly highlights the role of microbial elicitors in improving plant defense mechanisms as well as their modes of action that have been used to boost up the plant immune system.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
In the course of development, plants are systematically challenged by a broad range of biotic stresses in their natural habitat, such as fungi, bacteria, viruses, insects, nematodes, etc. There are numerous choices available for the plants to protect themselves from the disease (Abdul Malik et al. 2020). Plants usually overcome these biotic stresses by activating their separate defense pathways according to perceived signals from potential pathogens (Sarma et al. 2015; Barupal et al. 2020). There is an intricate type of defense mechanism employed by plants to detect microorganisms based on elicitor molecules produced during plant–pathogen interaction. Numerous elicitors of microbial origin belonging to distinct chemical groups have been identified, i.e., glycopeptides, carbohydrate polymers, glycoproteins, and lipids. This elicitor perception is followed by the stimulation of signal transmission pathways that commonly induces the synthesis of active oxygen species, production of phytoalexin, accumulation of pathogenesis-related proteins, deposition of callose, strengthening of the cell wall of plant cell related to phenyl propanoic compounds, and production of defense enzymes (Van Loon and Van Strien 1999; Patel et al. 2019). Active oxygen species (AOS) induce localized or fast death of limited cells at the site of infection, which induces a hypersensitive response in host plants to restrict the growth of invading pathogens. Activation of hypersensitive response (HR) results in the development of resistance in uninfected distal parts of the host plant to upcoming infection, which is called systemic acquired resistance (SAR) (Thakur and Sohal 2013). Systemic acquired resistance is mainly relying up on salicylic acid, where the first set of reactions brings on a complex modification in gene expression, enzymatic action, and metabolic changes (Garcia-Brugger et al. 2006; Barupal et al. 2019). Salicylic acid-dependent reaction is stimulated by biotrophic pathogens and distinct types of elicitors. Several microbial elicitors allow plants to mitigate the harmful effects of pathogenic microbes by enhancing the capability of plants to identify anonymous pathogenic agents and act as surveillance systems for plants (Newman et al. 2013). Elicitors are small drug-like compounds composed of molecules like oligosaccharides, lipids, peptides, and proteins, which activate various kinds of defense responses in plants. They are either secreted by pathogens or plants or pathogen cell walls by hydrolytic enzymes. Elicitor-activated signal transduction pathways bring on a hypersensitive response and systemic acquired resistance type of defense responses against a broad range of pathogens (Garcia-Brugger et al. 2006). Microbial biocontrol agents suppress the growth of phytopathogens through a wide array of distinct modes of actions. The most important advantage of using microbial biocontrol agents is that they display specificity for a particular pathogen and are expected to be harmless to nontarget species (Hussain et al. 2020a, b). In the last few decades, many studies have been done on the broad range of applications of microbial biocontrol agents in the plant disease management data given in Table 6.1 (Kokalis-burelle et al. 2002; Mavrodi et al. 2012; Singh et al. 2020). Environmentally friendly and sustainable attributes of biocontrol agents have driven profound investigation into the promising microbial candidates for the production of elicitors. In this chapter, we address the role of microbial elicitors in improving plant defense mechanisms as well as their modes of action that have been used to boost up the plant immune system.
6.2 Elicitors
Elicitors are small drug-like compounds composed of molecules like oligosaccharides, lipids, peptides, and proteins, which activate various kinds of defense responses in plants. Elicitors produced by pathogenic agents can be classified into two groups: general elicitors and specific elicitors (Montesano et al. 2003). General elicitors are engaged in the conventional resistance, which has the capacity to trigger defense reactions in both host and nonhost plants, whereas race-specific elicitors are released by specialized pathogens involved in R gene-mediated signal transduction (Gowthami 2018). General elicitors have the capacity to trigger defense in both nonhost and host plants through the realized incidence of potential pathogens (Onaga and Wydra 2016). Commonly, general elicitors are found in the cell walls of pathogens as structural constituents, for example, glucan, flagellin, chitin, and lipopolysaccharides (LPS) (Abdul Malik et al. 2020). Elicitor molecules act as ligands and generally bind to the specific receptor proteins located on the surface of plant cell membranes. According to the molecular pattern of elicitors recognized by receptors, an intracellular defense signaling has been triggered, which is echoed by the synthesis of secondary metabolites (Gowthami 2018; Zehra et al. 2021). It has long been recognized that microbial elicitors can induce many cellular defense responses in plants. Currently, elicitors of microbial origin have also been stated as microbe- or pathogen-associated molecular patterns (MAMPs or PAMPs). Following MAMP recognition, production of reactive nitrogen species, ion fluxes across the membrane, medium alkalinization, reactive oxygen species, and ethylene synthesis lead to activate plant pattern-triggered immunity (PTI) against broad range of microbial attack (Wu et al. 2014). Newman et al. (2013) stated that N-acetyl-chito-oligosaccharides, i.e., chitin oligomers, a fungal cell wall-derived elicitor molecule, can activate several defense responses in monocot as well as dicot plants. In recent years, numerous MAMPs and their corresponding PRRs have been recognized, such as flagellin, peptidoglycan, elongation factor (Tu), lipopolysaccharides, β-glucans from oomycetes and Ax21, fungal chitin, etc. (Newman et al. 2013).
6.2.1 Microbial Agents as a Source of Elicitors
Induction of plant defense response is a crucial step during plant–pathogen interaction via several factors. The first step of inducible response is carried out by the plant by the perception of molecules derived by microbes known as elicitors. While the plant percept these molecules, it results in a plant response that provides effective resistance toward pathogens; hence, they can be described as “defense elicitors” (Wiesel et al. 2014). These elicitors may be of proteinaceous, polysaccharide, laminarin, and other chemical nature. Apart from the pathogenic role of microbes, there are some beneficial microbes that live in plant tissue as endophytes, plant growth promoting rhizobacteria (PGPR), arbuscular mycorrhizal fungi (AMF), and some algae, oomycetes, and viruses also play a significant role in inducing resistance in plants (Siah et al. 2018; Yadav and Meena 2021). The primary work of these elicitors is to induce production of reactive oxygen species (ROS) or oxidative burst, which ultimately evokes plant defense responses like cross-linking of plant cell wall proteins, upregulation of defense-related genes, stimulation of synthesis of phenolic compounds (phytoalexins), and induction of hypersensitive response (Low and Merida 1996). The biological agents evoke plant defense via several modes like production of siderophores, antibiotic secretion, lytic enzyme production, hyperparasitism, and inducing systemic response (ISR); all mechanisms are induced by secretion of elicitor molecules (Pieterse et al. 2014; Navarro et al. 2019; Singh et al. 2020).
6.2.2 Some Potent Elicitor-Producing Microbial Agents
6.2.2.1 Fungi
Fungal groups possess some cell wall breakdown products like chitin, β-glucans, and mannoproteins that act as potent elicitors and can evoke defense response; for example, yeast extract can be used widely for the study of defense response in plants via closing of stomata and peroxidase-mediated ROS production (Khokon et al. 2010). Sclerotinia culture filtrate elicitor1 (SCFE1) is a proteinaceous elicitor secreted by Sclerotinia sclerotiorum that induces BAK1-dependent PTI responses in A. thaliana (Zhang et al. 2013). Among fungal-derived elicitors, chitin and chitosan (a deacetylated derivative of chitin) are potent elicitors that increase resistance in plants toward several fungal and bacterial pathogens (Hadrami et al. 2010). Fungal cell wall polysaccharides, especially chitin and carboxymethyl cellulose, are active elicitors that stimulate synthesis and accumulation of a secondary metabolite tolytoxin (phytoalexin) in a cyanobacterium Scytonema ocellatum, which provides chemical defense against fungal pathogens (Patterson and Bolis 1997; Meena and Samal 2019). Transcription of retrotransposons is also carried out by some fungal genera to increase host defense; for example, application of crude extracts of Trichoderma viride induces transcription of Tnt1 gene, which accumulates capsidiol (a phytoalexin) in tobacco plants (Pouteau et al. 1994; Meena and Swapnil 2019). Some other examples also suggest transcription activation by fungal elicitors as it has been seen in Phaseolus vulgaris where plant cells show upregulation of genes related to phytoalexin metabolism such as phenylalanine ammonium lyase (PAL) and chalcone synthase (CHS) (Lawton and Lamb 1987). Some other species of Trichoderma like T. virens induce plant defense response by producing an elicitor named Sm1 (small protein 1), which triggers an increased production of reactive oxygen species in seedlings of monocot and dicot plants and proves as a potent elicitor in defense against foliar pathogen Colletotrichum sp. (Djonović et al. 2006). Trichoderma harzianum is also reported as an inducer of antioxidant defense system in tomatoes against Fusarium wilt disease (Zehra et al. 2017a, b). It is reported that oxidative burst during plant defense is dependent on external calcium (Ca+2) and protein kinase activity (Schwacke and Hager 1992). Hypersensitive response is also stimulated by the same fungus in Vitis vinifera by increasing the level of endogenous H2O2, which ultimately activates oxidative phenolic metabolism in respective plants (Calderón et al. 1993). A proteinaceous elicitor PeaT1 produced by Alternaria tenuissima enhances plant defense response against tomato aphid (Myzus persicae), which is evidenced by accumulation of defense-related substances such as jasmonic acid (JA), salicylic acid (SA), and ethylene (ET) (Meena et al. 2017a, b; Basit et al. 2021). PeaT1 is also responsible for systemic acquired resistance (SAR) in tobacco plants (Mao et al. 2010). Other than the above described fungal genera, there are several fungi that are sources of potent elicitors and regulate plant defense responses as given in Table 6.2.
6.2.2.2 Bacteria
In addition to fungal-derived elicitors, bacteria-derived elicitors have also been shown to regulate plant defense mechanisms and reduce pathogen infections in plants. There are several pieces of evidence that justify this statement, for example, Ralstonia solanacearum produce extracellular polysaccharides (EPS), which trigger a defense response in tomato plants in the case of bacterial wilt (Milling et al. 2011). Gram-negative bacteria-derived lipopolysaccharides (LPS)-mediated induction resistance is also shown in many crop plants (Erbs and Newman 2012). At concentrations of 1 g/ml, lipopolysaccharides from Xanthomonas campestris induce transcription of genes of β-1,3-glucanase, which ultimately shows defense responses in turnip (Newman et al. 1995). Cold shock protein (Csp)-related elicitor activity has been detected in bacterial extracts; there are many aromatic and basic side chains of csp domains that are necessary for elicitor activity; hence, RNA-binding motif RNP-1 of bacterial cold shock proteins that are highly conserved is recognized as an elicitor signal in Nicotiana sylvestris plant (Felix and Boller 2003). The two bacterial microbe-associated molecular patterns (MAMPs) are flagellin and the elongation factor Tu (EF-Tu), which are recognized by a variety of plant species (Deslandes and Rivas 2012). Botrytis cinerea and Erwinia carotovora produce a wide array of elicitors that enhance the expression of conserved plant defense-associated genes such as HrpN gene and show responses like shrinkage of cytoplasm, programmed cell death (PCD), etc. in Physcomitrella patens (de León et al. 2007). Hrp genes are crucial for HR response in plants; Wei et al. (1992) reported that hrp genes (hrpN) of Erwinia amylovora encode harpin, a proteinaceous elicitor, which shows HR necrosis in respective plants. Surfactin lipopeptide is secreted by Bacillus sp., which triggers induced systemic response in host plants and defense responses like oxidative burst, etc. (Cawoy et al. 2014). In elicitation, not only free-living or plant-associated bacteria but also animal-associated bacteria are also involved; for example, it is observed that insects named Helicoverpa zea, gut-associated bacteria, induce defenses in tomatoes indirectly by secreting a salivary elicitor that induces expression of genes of defense-related enzymes like polyphenol oxidase and jasmonic acid (JA) and suppression of pathogenesis-related genes of salicylic acid (SA) response (Wang et al. 2017). Twenty-three bacteria isolated from gut segments of Spodoptera exigua, Agrotis segetum, and Mamestra brassicae produce surfactants such as N-acylglutamine, which is recognized as a potent elicitor for plant defense response (Spiteller et al. 2000). There are several other bacterial groups identified as sources of elicitors, which are mentioned in Table 6.2.
6.2.2.3 Oomycetes
Oomycetes are taxonomically and structurally different from plants and fungi. There are several plant pathogenic oomycetes known, but genera Phytophthora and Pythium show superiority in causing disease of crop plants. The cell walls of these groups consist of several elicitor factors such as cellulose, glycan, and hydroxyproline-rich proteins. Some potent elicitors reported from oomycetes are CBEL, cryptogein, eicosapentaenoic acid, Pep-13, and INF1 (Wiesel et al. 2014). Necrosis and ethylene-inducing peptide 1 (Nep1)-like proteins (NLP) has been identified in dicot plants, which are associated with defense response in Arabidopsis thaliana (Qutob et al. 2006). In Nicotiana benthamiana, HR response is induced by INF1 elicitin of Phytophthora infestans (Kamoun et al. 1998). These responses are dependent on the receptor-like kinase SERK3/BAK1, required for multiple resistance responses in plants (Heese et al. 2007). Pathogenic species of Phytophthora release some extracellular and intracellular effectors into plants encoding protease or glucanase inhibitors to suppress pattern-triggered immunity in plants (Hein et al. 2009; Schornack et al. 2009). RXLR effector Avrblb2 of P. infestans prevents secretion of an immune-associated protease (Bozkurt et al. 2011). An intracellular RXLR effector named Avr3a of P. infestans interacts with potato E3 ubiquitin ligase CMPG1 and stabilizes it, which results in perturbation in cell death response induced by INF1 (Bos et al. 2010). The other examples of oomycete elicitors and plant defense regulations are mentioned in Table 6.2.
6.2.2.4 Virus
Among well-known elicitor-producing microbes like fungi, bacteria, and oomycetes, some viruses are also known that immunize plants and regulate their defense response. Plant virus coat proteins (CPs) can act as elicitors that triggers R-gene-mediated HR response (Moffett 2009). Several viral silencing suppressors misregulate AUXIN RESPONSE FACTOR 8, which finally causes chlorotic symptoms in plants (García and Pallás 2015). Strain-specific P3 of Soybean Mosaic Virus G7 is identified as an elicitor for Rsv1 (a single dominant resistance gene)-mediated HR response (Hajimorad et al. 2005). It is also observed that TMV replicase sequence of 126/183 kDa activates N-gene mediated hypersensitive response in tobacco plants (Padgett et al. 1997). BV1 protein of bean dwarf mosaic virus is also recognized as a determinant factor for the hypersensitive response and avirulence in French bean (Phaseolus vulgaris) (Garrido-Ramirez et al. 2000).
6.2.3 Mode of Action by Which Microbial Bioagents Bring About Plant Defense
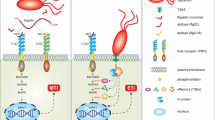
The microbial bioagents show antagonism, competition, and parasitism against different pathogenic microbes. These activities of microbial bioagents provide defense to plants directly or indirectly, such as plant defense response stimulation. These mechanisms include antimicrobial compound production, competition for niches and nutrients, elicitation of plant defenses, etc. (Jamalizadeh et al. 2011; Compant et al. 2013; Hussain et al. 2020a). Different mechanisms of biocontrol agents, which have been shown in Fig. 6.1, are described in the following sections.
6.2.3.1 Antagonisms
In antagonism, actions of one organism inhibit or obstruct the normal growth and development of other organisms appearing in its near vicinity. If these types of organisms inhibit phytopathogens, they can be used as biocontrol agents against pests and pathogens (Heydari and Pessarakli 2010). According to Shoda (2000), microorganisms that have capability to multiply in the rhizospheres are regarded as ideal biocontrol agents. Microorganisms colonize in the root of the host, produce some metabolites, and secrete into the root system, which are toxic to pathogens and directly suppress the pathogen growth. These metabolites directly offer protection to the host or sometimes trigger defense in the host plant (Nihorimbere et al. 2012; Chandran et al. 2020). The elicitation of the host plant defense system by microbial bioagents is known as direct antagonism (Ab Rahman et al. 2018).
6.2.3.2 Parasitism
In parasitism, one microorganism is ubiquitous for another. The microbial bioagents produce lytic enzymes like glucosaminidases and chitinases, which lead to the degradation of the cell wall of phytopathogens (Guigón-López et al. 2015). Urbina et al. (2016) investigated the role of enzymes synthesized by Candida oleophila (extracellular 3-glucanase exo-β-1) in biocontrol of Penicillium expansum causing apple spoilage. Moreover, many researchers reported different antagonistic microbial species (Meena et al. 2017c, d). Jeffries (1995) reported that Rhizoctonia solani could be controlled using 30 different hyperparasitic species belonging to 16 genera. Powdery mildew, which is caused by an obligate biotrophic pathogen, was controlled with eight hyperparasites by Hijmegen and Buchenauer (Hijwegen and Buchenauer 1984). In a study, it was observed that Pseudomonas flocculosa release some cell wall lytic enzymes, which cause cell collapse in powdery mildew cells (Bélanger et al. 2012). Some fungi release protease enzymes such as Pochonia chlamydosporia, which causes infection in eggs of the nematodes (Escudero et al. 2016). Rust pathogens Puccinia violae and Puccinia striiformis f. sp. tritici were tested with more than 30 hyperparasitic fungal species, including Cladosporium uredinicola and Alternaria alternata, respectively, and positive results were obtained (Zheng et al. 2017). Köhl et al. (2019) reviewed that Alternaria alternata had the capability to penetrate the urediniospore of wheat rust fungus by germ tubes; the urediniospores were completely collapsed and lost their ability to germinate. In an experiment, it was reported that the urediniospores treated with A. alternata pustules had reduced ability to germinate up to 25% as compared to untreated urediniospores pustules (80%) (Zheng et al. 2017). In Trichoderma, parasitism was observed most frequently against Pythium myriotylum and Macrophomina phaseolina (Kubicek et al. 2001). Trichoderma and Clonostachy are the most studied mycoparasites, and members belonging to these genera have a wide and varied plant pathogenic host range. These antagonistic isolates form different structures by which they attach to the host and cause infection and death of their hosts by producing cell wall degrading enzymes (Karlsson et al. 2017; Nygren et al. 2018). The synthesis of cell wall degrading substances is not constitutive. The synthesis of enzymes is triggered upon host recognition. Host contain some specific types of molecules on their surface (lectins or secondary metabolites), and these molecules trigger specific types of signaling pathways (G-protein signaling cAMP pathway, and MAPK cascades) (Zhai et al. 2017; Karlsson et al. 2017; Zehra et al. 2015; Meena et al. 2017e, f). Signaling pathways lead to upregulation and transcription of certain genes known as “molecular weapons” including lytic enzymes, which attack and cause lysis of the host. In Trichoderma, there are two types of mycoparasitism-related gene families, namely ech42 and prb1, which are overexpressed throughout mycoparasitism (Barbara et al. 2011). Mycoparasitics (Trichoderma) first release lytic enzymes; as a result, some oligosaccharides are secreted from the host that are identified by receptors and trigger increased synthesis of lytic enzymes (Karlsson et al. 2017; Meena et al. 2016a, b). This increased level of lytic enzymes results in increased permeability, degradation, and death of the host plant. These types of collaborative transcriptional results were also reported by Reithner et al. (2011) in Trichoderma atroviride in response to B. cinerea and Phytophthora capsici. In Metschnikowia fructicola, induced chitinase activity was observed to be regulated by MfChi gene due to close contact with the cell wall of yeast Monilinia fructicola (Banani et al. 2015). The same type of result was also observed with Pichia pastoris when used against Monilinia fructicola and Monilinia laxa that cause postharvest disease in peach fruits (Dukare et al. 2019).
6.2.3.3 Competition
Competition is a mechanism in which two or more organisms utilize the same type of nutrition or space or both for their survival; therefore, the interaction becomes competitive. The microbial bioagents exploit the nutrients, prevent the pathogen growth and proliferation, and reduce the virulence of the pathogen. For a microbe to thrive in the phyllosphere or rhizosphere, it must be able to make use of accessible nutrients in the form of leachates and exudates or senescent tissue. In rhizosphere, plants release different photosynthates, which are a great source of nutrients (specific sugars, organic acids, and amino acids) for microbes; therefore, the rhizosphere works as a niche. High availability of carbon (40%) around the root surface attracts different microbes. Microbial bioagents compete with pathogens for nutrients and protect the host from disease occurrence (Degenhardt et al. 2003). This type of approach has been observed in different pathogens such as Pythium and Fusarium; these are soil-borne pathogens and cause infection by mycelial penetration. Enterobacter cloacae act as a microbial bioagent against Pythium ultimum by increasing catabolism of nutrients (van Dijk and Nelson 2000; Kageyama and Nelson 2003). Some microbial bioagents, namely Pseudomonas fluorescence, chelate iron, which is essential for Fusarium oxysporum, whereas Chryseobacterium sp. WR21 exploits root exudates and competes with Ralstonia solanacearum (Huang et al. 2017). Moreover, antagonistic fungus Pichia guilliermondii was found to show competition against certain known pathogenic fungi isolated from wounds of fruit such as apple, namely Penicillium expansum, Penicillium digitatum, Colletotrichum spp. or B. cinerea, and Aureobasidium pullulans (Spadaro and Droby 2016). It was reported that microbes compete for nitrogen sources in a carbohydrate-rich environment. Besides nitrogen, they also compete for iron because it is a limiting factor for microbial growth and also has low solubility, thus playing a vital role in antagonistic activity such as competition (Spadaro and Droby 2016). Microorganisms have the ability to produce a variety of siderophores, which are low-molecular-weight chelating compounds with a great affinity for iron (van Loon 2000). Pathogenic strains use the chelating compounds to accumulate the ions, and they can be used as microbial bioagents for disease suppression through competition with pathogenic strains that produce siderophores but with low affinity (van Loon 2000; Lugtenberg and Kamilova 2009). Pseudomonas spp. have shown siderophore-facilitated iron competition with pathogenic populations present in rhizospheres and reduced their number in soil (Raaijmakers et al. 1995). Fungal antagonists such as Trichoderma asperellum and Metschnikowia pulcherrima produce iron-binding siderophores and control the growth of Fusarium and A. alternata, B. cinerea, and P. expansum, respectively (Saravanakumar et al. 2008; Segarra et al. 2010).
6.2.3.4 Production of Antimicrobial Compounds
Active microbes and the produced allelochemicals as secondary metabolites are potent options for treating plant diseases (Puopolo et al. 2018; Zhao et al. 2021). The most common mechanism associated with biocontrol activity is the production of antibiotics. Besides that, many biocontrol strains produce antifungal enzymes like β-1,3-glucanases, chitinases, proteases, or lipases that are involved in fungal cell wall lysis, produce siderophores, and chelate iron in the rhizosphere, thus inhibiting the proliferation of pathogens (Bais et al. 2004; Latz et al. 2018; Köhl et al. 2019; Pirttilä et al. 2021).
6.2.3.5 Antibiotics
Antibiotics are small, heterogenous molecular compounds, which can inhibit the growth of pathogens at low concentrations (Huang et al. 2021). The general mechanism of antibiotic action is cell wall synthesis inhibition, disruption of cell membrane structure and function, nucleic acids structure and function inhibition, and blocking of key metabolic pathways (Wu et al. 2021). Some antibiotic-producing strains among rhizobacteria are Bacillus sp. producing surfactin and iturin A, Pseudomonas spp. producing phenazine derivatives, Erwinia sp. producing herbicolin A, Agrobacterium sp. producing agrocin 84, etc. (Viswanathan and Samiyappan 1999; Compant et al. 2005a, b; Sonigra and Meena 2021).
6.2.3.6 Siderophores
Iron is a trace element that affects the growth, germination, and virulence of a pathogen and hence the development of the pathogen (Spadaro and Droby 2016; Chen et al. 2020; Huang et al. 2021). The bacterial siderophores compete for zinc, copper, manganese, and most importantly iron. These BCA limit the availability of iron in the soil by solubilization and the competitive acquisition of Fe3+ and subsequently inhibit the plant pathogen by limiting their growth (Leong 1986; Loper and Henkels 1997; Chin-A-Woeng et al. 2003; Haas and Défago 2005; Ab Rahman et al. 2018). Bacteria produce many types of siderophores, for example, catecholate, carboxylate, hydroxamate, and salicylate (Rajkumar et al. 2010; Kumari et al. 2018a, b). Dual inoculation of Pseudomonas koreensis and B. subtilis strains have been proved to have antagonistic activity and produce siderophore in controlling Cephalosporium maydis in maize plants (Ghazy and El-Nahrawy 2021). Paenibacillus polymyxa, a siderophore producer, has been proved as a growth promoter of Lilium lancifolium and showed antifungal activity against Botryosphaeria dothidea, F. oxysporum, Fusarium fujikuroi, and B. cinerea (Khan et al. 2020).
6.2.3.7 Volatile Organic Compounds (VOCs)
VOCs are low-molecular-weight compounds that, under low normal atmospheric temperature and pressure, can evaporate below 300 Da (Vespermann et al. 2007). The main composition of VOC mixture is alcohols, esters, aldehydes, terpenes, aliphatic and aromatic hydrocarbons, nitrides, and sulfides, which exhibit strong antimicrobial effects (Strobel 2011; Lemfack et al. 2018; Huang et al. 2021). Delgado et al. (2021) developed a new consortium PUCV-VBL, composed of Hanseniaspora osmophila and Gluconobacter cerinus, to control fungal rots in the grapes. The VOCs produced by this consortium showed 86% mycelial inhibition against B. cinerea.
6.2.3.8 Lytic Enzymes
Microbial enzymes assist microbes in reproducing in a particular niche and function as biocatalysts for key biochemical reactions (Chaudhari and Patel 2021). The microbes extracellularly produce hydrolytic enzymes to prevent potential plant pathogens (Umer et al. 2021). The antagonists release various enzymes, such as lipase, cellulases, chitinases, xylanases, mannanases, laminarinase, chitosanase, glucose oxidase, protease, and betaglucosidases for biocontrol activity (Picard et al. 2000). Two novel Bacillus strains (simplex and subtilis species) have been found to produce lytic enzymes (protease and β-glucanase), which aided in the biofungicidal activity against Zymoseptoria tritici causing Septoria tritici blotch of wheat (Allioui et al. 2021).
6.3 Conclusion
During the past few years, beneficial plant microbes have received attention as a substitute for chemical fertilizers because of their sustainable plant protection property. The microbial bioagents produce different elicitors and MAMPs, which trigger induced systemic resistance. A distinctive feature of ISR-eliciting microbial bioagents is local suppression of root immune response in a cell-type specific manner. The studies of root cell-type-specific metabolome and transcriptome profiles in response to microbial bioagents will aid in providing information to develop consistent and reliable methods of crop production. Agrochemicals pose a danger to the health of living beings and the environment due to their toxicity, while elicitors have no adverse effects and leave no residues. The isolation of novel microbial bioagents with high effectiveness against plant pathogens is important and essential. The microbial bioagents with synergistic action against plant pathogens may provide desirable results.
References
Ab Rahman SFS, Singh E, Pieterse CM, Schenk PM (2018) Emerging microbial biocontrol strategies for plant pathogens. Plant Sci 267:102–111. https://doi.org/10.1016/j.plantsci.2017.11.012
Abdul Malik NA, Kumar IS, Nadarajah K (2020) Elicitor and receptor molecules: orchestrators of plant defense and immunity. Int J Mol Sci 21(3):963. https://doi.org/10.3390/ijms21030963
Allioui N, Driss F, Dhouib H, Jlail L, Tounsi S, Frikha-Gargouri O (2021) Two novel Bacillus strains (subtilis and simplex species) with promising potential for the biocontrol of Zymoseptoria tritici, the causal agent of Septoria Tritici blotch of wheat. Biomed Res Int. https://doi.org/10.1155/2021/6611657
Arseneault T, Pieterse CM, Gérin-Ouellet M, Goyer C, Filion M (2014) Long-term induction of defense gene expression in potato by Pseudomonas sp. LBUM223 and Streptomyces scabies. Phytopathology 104(9):926–932. https://doi.org/10.1094/PHYTO-11-13-0321-R
Bais HP, Park SW, Weir TL, Callaway RM, Vivanco JM (2004) How plants communicate using the underground information superhighway. Trends Plant Sci 9(1):26–32. https://doi.org/10.1016/j.tplants.2003.11.008
Banani H, Spadaro D, Zhang D, Matic S, Garibaldi A, Gullino ML (2015) Postharvest application of a novel chitinase cloned from Metschnikowia fructicola and overexpressed in Pichia pastoris to control brown rot of peaches. Int J Food Microbiol 199:54–61. https://doi.org/10.1016/j.ijfoodmicro.2015.01.002
Barbara R, Enrique IL, Robert LM, Alfredo HE (2011) Identification of mycoparasitism-related genes in Trichoderma atroviride. Appl Environ Microbiol 70:4361–4370. https://doi.org/10.1128/AEM.00129-11
Barupal T, Meena M, Sharma K (2019) Inhibitory effects of leaf extract of Lawsonia inermis on Curvularia lunata and characterization of novel inhibitory compounds by GC–MS analysis. Biotechnol Rep 23:e00335. https://doi.org/10.1016/j.btre.2019.e00335
Barupal T, Meena M, Sharma K (2020) A study on preventive effects of Lawsonia inermis L. bioformulations against leaf spot disease of maize. Biocatal Agric Biotechnol 23:101473. https://doi.org/10.1016/j.bcab.2019.101473
Basit A, Farhan M, Abbas M, Wang Y, Zhao D.G, Mridha A.U, Al-Tawaha ARMS, Bashir, MA, Arif M, Ahmed S, Alajmi RA (2021) Do microbial protein elicitors PeaT1 obtained from Alternaria tenuissima and PeBL1 from Brevibacillus laterosporus enhance defense response against tomato aphid (Myzus persicae)?. Saudi J Biol Sci 28(6):3242–3248. https://doi.org/10.1016/j.sjbs.2021.02.063
Bélanger RR, Labbé C, Lefebvre F, Teichmann B (2012) Mode of action of biocontrol agents: all that glitters is not gold. Can J Plant Pathol 34(4):469–478. https://doi.org/10.1080/07060661.2012.726649
Bos JI, Armstron MR, Gilroy EM, Boevink PC, Hein I, Taylor RM, Zhendong T, Engelhardt S, Vetukuri RR, Harrower B, Dixelius C (2010) Phytophthora infestans effector AVR3a is essential for virulence and manipulates plant immunity by stabilizing host E3 ligase CMPG1. Proc Natl Acad Sci U S A 107(21):9909–9914. https://doi.org/10.1073/pnas.0914408107
Bozkurt TO, Schornack S, Win J, Shindo T, Ilyas M, Oliva R (2011) Phytophthora infestans effector Avrblb2 prevents secretion of a plant immune protease at the haustorial interface. Proc Natl Acad Sci U S A 108:20832–20837. https://doi.org/10.1073/pnas.1112708109
Calderón AA, Zapata JM, Muñoz R, Pedreño MA, Barceló AR (1993) Resveratrol production as a part of the hypersensitive-like response of grapevine cells to an elicitor from Trichoderma viride. New Phytol 124(3):455–463. https://doi.org/10.1111/j.1469-8137.1993.tb03836.x
Cawoy H, Mariutto M, Henry G, Fisher C, Vasilyeva N, Thonart P, Dommes J, Ongena M (2014) Plant defense stimulation by natural isolates of Bacillus depends on efficient surfactin production. Mol Plant-Microbe Interact 27(2):87–100. https://doi.org/10.1094/MPMI-09-13-0262-R
Chandran H, Meena M, Barupal T, Sharma K (2020) Plant tissue culture as a perpetual source for production of industrially important bioactive compounds. Biotechnol Rep 26:e00450. https://doi.org/10.1016/j.btre.2020.e00450
Chaudhari A, Patel J (2021) Current status and future perspective on enzyme involving in biocontrol of plant pathogen. Int J Appl Sci Biotechnol 8(4):49–55. https://doi.org/10.31033/ijrasb.8.4.8
Chen T, Dong G, Zhang S, Zhang X, Zhao Y, Cao J, Zhou TWQ (2020) Effects of iron on the growth, biofilm formation and virulence of Klebsiella pneumoniae causing liver abscess. BMC Microbiol 20(1):1–7. https://doi.org/10.1186/s12866-020-01727-5
Chin-A-Woeng TF, Bloemberg GV, Lugtenberg BJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157(3):503–523. https://doi.org/10.1046/j.1469-8137.2003.00686.x
Chowdhury SP, Hartmann A, Gao X, Borriss R (2015) Biocontrol mechanism by root-associated Bacillus amyloliquefaciens FZB42 – a review. Front Microbiol 6:780. https://doi.org/10.3389/fmicb.2015.00780
Chung EJ, Hossain MT, Khan A, Kim KH, Jeon CO, Chung YR (2015) Bacillus oryzicola sp. nov., an endophytic bacterium isolated from the roots of rice with antimicrobial, plant growth promoting, and systemic resistance inducing activities in rice. Plant Pathol J 31(2):152. https://doi.org/10.5423/PPJ.OA.12.2014.0136
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005a) Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
Compant S, Reiter B, Sessitsch A, Nowak J, Clément C, Ait Barka E (2005b) Endophytic colonization of Vitis vinifera L. by plant growth-promoting bacterium Burkholderia sp. strain PsJN. Appl Environ Microbiol 71(4):1685–1693. https://doi.org/10.1128/AEM.71.4.1685-1693.2005
Compant S, Duffy B, Nowak J, Clement C, Barka EA (2013) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71(9):4951–4959. https://doi.org/10.1128/AEM.71.9.4951-4959.2005
de León IP, Oliver JP, Castro A, Gaggero C, Bentancor M, Vidal S (2007) Erwinia carotovora elicitors and Botrytis cinerea activate defense responses in Physcomitrella patens. BMC Plant Biol 7(1):1–11. https://doi.org/10.1186/1471-2229-7-52
Degenhardt J, Gershenzon J, Baldwin IT, Kessler A (2003) Attracting friends to feast on foes: engineering terpene emission to make crop plants more attractive to herbivore enemies. Curr Opin Biotech 14(2):169–176. https://doi.org/10.1016/s0958-1669(03)00025-9
Delgado N, Olivera M, Cádiz F, Bravo G, Montenegro I, Madrid A, Besoain X (2021) Volatile organic compounds (VOCs) produced by Gluconobacter cerinus and Hanseniaspora osmophila displaying control effect against table grape-rot pathogens. Antibiotics 10(6):663. https://doi.org/10.3390/antibiotics10060663
Deslandes L, Rivas S (2012) Catch me if you can: bacterial effectors and plant targets. Trends Plant Sci 17(11):644–655. https://doi.org/10.1016/j.tplants.2012.06.011
Djonović S, Pozo MJ, Dangott LJ, Howell CR, Kenerley CM (2006) Sm1, a proteinaceous elicitor secreted by the biocontrol fungus Trichoderma virens induces plant defense responses and systemic resistance. Mol Plant-Microbe Interact 19(8):838–853. https://doi.org/10.1094/MPMI-19-0838
Dukare AS, Paul S, Nambi VE, Gupta RK, Singh R, Sharma K, Vishwakarma RK (2019) Exploitation of microbial antagonists for the control of postharvest diseases of fruits: a review. Crit Rev Food Sci Nutr 59(9):1498–1513. https://doi.org/10.1080/10408398.2017.1417235
Dunlap CA, Bowman MJ, Schisler DA (2013) Genomic analysis and secondary metabolite production in Bacillus amyloliquefaciens AS 43.3: a biocontrol antagonist of Fusarium head blight. Biol Control 64(2):166–175. https://doi.org/10.1016/j.biocontrol.2012.11.002
Erbs G, Newman MA (2012) The role of lipopolysaccharide and peptidoglycan, two glycosylated bacterial microbe–associated molecular patterns (MAMPs), in plant innate immunity. Mol Plant Pathol 13(1):95–104. https://doi.org/10.1111/j.1364-3703.2011.00730.x
Escudero N, Ferreira SR, Lopez-Moya F, Naranjo-Ortiz MA, Marin-Ortiz AI, Thornton CR, Lopez-Llorca LV (2016) Chitosan enhances parasitism of Meloidogyne javanica eggs by the nematophagous fungus Pochonia chlamydosporia. Fungal Biol 120(4):572–585. https://doi.org/10.1016/j.funbio.2015.12.005
Felix G, Boller T (2003) Molecular sensing of bacteria in plants: the highly conserved RNA-binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J Biol Chem 278(8):6201–6208. https://doi.org/10.1074/jbc.M209880200
Fousia S, Paplomatas EJ, Tjamos SE (2016) Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv. tomato and induces plant defence-related genes. Phytopathology 164(4):264–270. https://doi.org/10.1111/jph.12455
García JA, Pallás V (2015) Viral factors involved in plant pathogenesis. Curr Opin Virol 11:21–30. https://doi.org/10.1016/j.coviro.2015.01.001
Garcia-Brugger A, Lamotte O, Vandelle E, Bourque S, Lecourieux D, Poinssot B, Wendehenne D, Pugin A (2006) Early signaling events induced by elicitors of plant defenses. Mol Plant-Microbe Interact 19(7):711–724. https://doi.org/10.1094/MPMI-19-0711
Garrido-Ramirez ER, Sudarshana MR, Lucas WJ, Gilbertson RL (2000) Bean dwarf mosaic virus BV1 protein is a determinant of the hypersensitive response and avirulence in Phaseolus vulgaris. Mol Plant-Microbe Interact 13(11):1184–1194. https://doi.org/10.1094/MPMI.2000.13.11.1184
Ghazy N, El-Nahrawy S (2021) Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant. Arch Microbiol 203(3):1195–1209. https://doi.org/10.1007/s00203-020-02113-5
Goswami J, Pandey RK, Tewari JP, Goswami BK (2008) Management of root knot nematode on tomato through application of fungal antagonists, Acremonium strictum and Trichoderma harzianum. J Environ Sci Health B 43(3):237–240. https://doi.org/10.1080/03601230701771164
Govindappa M, Lokesh S, Rai VR, Naik VR, Raju SG (2010) Induction of systemic resistance and management of safflower Macrophomina phaseolina root-rot disease by biocontrol agents. Arch Phytopathol Prot 43(1):26–40. https://doi.org/10.1080/03235400701652227
Gowthami L (2018) Role of elicitors in plant defense mechanism. Int J Pharmacogn Phytochem Res 7(6):2806–2812
Guigón-López C, Vargas-Albores F, Guerrero-Prieto V, Ruocco M, Lorito M (2015) Changes in Trichoderma asperellum enzyme expression during parasitism of the cotton root rot pathogen Phymatotrichopsis omnivora. Fungal Biol 119(4):264–273. https://doi.org/10.1016/j.funbio.2014.12.013
Gupta KJ, Mur LA, Brotman Y (2014) Trichoderma asperelloides suppresses nitric oxide generation elicited by Fusarium oxysporum in Arabidopsis roots. Mol Plant-Microbe Interact 27(4):307–314. https://doi.org/10.1094/MPMI-06-13-0160-R
Guzmán-Valle P, Bravo-Luna L, Montes-Belmont R, Guigón-López C, Sepúlveda-Jiménez G (2014) Induction of resistance to Sclerotium rolfsii in different varieties of onion by inoculation with Trichoderma asperellum. Eur J Plant Pathol 138(2):223–229. https://doi.org/10.1007/s10658-013-0336-y
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microb 3(4):307–319. https://doi.org/10.1038/nrmicro1129
Hadrami AE, Adam LR, Hadrami IE, Daayf F (2010) Chitosan in plant protection. Mar Drugs 8(4):968–987. https://doi.org/10.3390/md8040968
Hajimorad MR, Eggenberger AL, Hill JH (2005) Loss and gain of elicitor function of Soybean mosaic virus G7 provoking Rsv1-mediated lethal systemic hypersensitive response maps to P3. J Virol 79(2):1215–1222. https://doi.org/10.1128/JVI.79.2.1215-1222.2005
Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci U S A 104:12217–12222. https://doi.org/10.1073/pnas.0705306104
Hein I, Gilroy EM, Armstrong MR, Birch PRJ (2009) The zig-zag-zig in oomycete? Plant interactions. Mol Plant Pathol 10:547–562. https://doi.org/10.1111/j.1364-3703.2009.00547.x
Heydari A, Pessarakli M (2010) A review on biological control of fungal plant pathogens using microbial antagonists. J Biol Sci 10(4):273–290
Hijwegen T, Buchenauer H (1984) Isolation and identification of hyperparasitic fungi associated with Erysiphaceae. Neth J Plant Pathol 90(2):79–83. https://doi.org/10.1007/BF01999956
Huang J, Wei Z, Hu J, Yang C, Mei X, Shen Q, Riaz (2017) Chryseobacterium nankingense sp. nov. WR21 effectively suppresses Ralstonia solanacearum growth via intensive root exudates competition. Biol Control 62(4):567–577. https://doi.org/10.1007/s10526-017-9812-1
Huang X, Ren J, Li P, Feng S, Dong P, Ren M (2021) Potential of microbial endophytes to enhance the resistance to postharvest diseases of fruit and vegetables. J Sci Food Agric 101(5):1744–1757. https://doi.org/10.1002/jsfa.10829
Hussain T, Akthar N, Aminedi R, Danish M, Nishat Y, Patel S (2020a) Role of the potent microbial based bioagents and their emerging strategies for the ecofriendly management of agricultural phytopathogens. In: Singh J, Yadav A (eds) Natural bioactive products in sustainable agriculture. Springer, Singapore, pp 45–66. https://doi.org/10.1007/978-981-15-3024-1_4
Hussain T, Singh S, Danish M, Pervez R, Hussain K, Husain R (2020b) Natural metabolites: an eco-friendly approach to manage plant diseases and natural bioactive products. J Sustain Agric 2020:1. https://doi.org/10.1007/978-981-15-3024-1_1
Jain S, Choudhary DK (2014) Induced defense-related proteins in soybean (Glycine max L. Merrill) plants by Carnobacterium sp. SJ-5 upon challenge inoculation of Fusarium oxysporum. Planta 239(5):1027–1040. https://doi.org/10.1007/s00425-014-2032-3
Jamalizadeh M, Etebarian HR, Aminian H, Alizadeh A (2011) A review of mechanisms of action of biological control organisms against post-harvest fruit spoilage. Bull OEPP 41(1):65–71. https://doi.org/10.1111/j.1365-2338.2011.02438.x
Jeffries P (1995) Biology and ecology of mycoparasitism. Can J Bot 73(S1):1284–1290. https://doi.org/10.1139/b95-389
John RP, Tyagi RD, Prévost D, Brar SK, Pouleur S, Surampalli RY (2010) Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot 29(12):1452–1459. https://doi.org/10.1016/j.cropro.2010.08.004
Kageyama K, Nelson EB (2003) Differential inactivation of seed exudate stimulation of Pythium ultimum sporangium germination by Enterobacter cloacae influences biological control efficacy on different plant species. Appl Environ Microbiol 69(2):1114–1120. https://doi.org/10.1128/AEM.69.2.1114-1120.2003
Kamoun S, Van West P, Vleeshouwers VGAA, de Groot KE, Govers F (1998) Resistance of Nicotiana benthamiana to Phytophthora infestans is mediated by the recognition of the elicitor protein INF1. Plant Cell 10:1413–1425. https://doi.org/10.1105/tpc.10.9.1413
Karlsson M, Atanasova L, Jensen DF, Zeilinger S (2017) Necrotrophic mycoparasites and their genomes. Microbiol Spectr 5(2). https://doi.org/10.1128/microbiolspec.FUNK-0016-2016
Karthikeyan V, Sankaralingam A, Nakkeeran S (2006) Biological control of groundnut stem rot caused by Sclerotium rolfsii (Sacc.). Arch Phytopathol Plant Prot 39(3):239–246. https://doi.org/10.1080/03235400500094688
Kavitha S, Senthilkumar S, Gnanamanickam S, Inayathullah M, Jayakumar R (2005) Isolation and partial characterization of antifungal protein from Bacillus polymyxa strain VLB16. Process Biochem 40(10):3236–3243. https://doi.org/10.1016/j.procbio.2005.03.060
Khan MS, Gao J, Chen X, Zhang M, Yang F, Du Y, Zhang X (2020) Isolation and characterization of plant growth-promoting endophytic bacteria Paenibacillus polymyxa SK1 from Lilium lancifolium. Biomed Res Int 1:1–7. https://doi.org/10.1155/2020/8650957
Khokon MAR, Hossain MA, Munemasa S, Uraji M, Nakamura Y, Mori IC, Murata Y (2010) Yeast elicitor-induced stomatal closure and peroxidase-mediated ROS production in Arabidopsis. Plant Cell Physiol 51(11):1915–1921. https://doi.org/10.1093/pcp/pcq145
Köhl J, Kolnaar R, Ravensberg WJ (2019) Mode of action of microbial biological control agents against plant diseases: relevance beyond efficacy. Front Plant Sci 10:845. https://doi.org/10.3389/fpls.2019.00845
Kokalis-Burelle N, Vavrina CS, Rosskopf EN, Shelby RA (2002) Field evaluation of plant growth-promoting rhizobacteria amended transplant mixes and soil solarization for tomato and pepper production in Florida. Plant Soil 238(2):257–266. https://doi.org/10.1023/A:1014464716261
Kubicek CP, Mach RL, Peterbauer CK, Lorito M (2001) Trichoderma: From genes to biocontrol. J Plant Pathol 83(2):11–23. https://www.jstor.org/stable/41998018
Kumari P, Meena M, Gupta P, Dubey MK, Nath G, Upadhyay RS (2018a) Plant growth promoting rhizobacteria and their biopriming for growth promotion in mung bean (Vigna radiata (L.) R. Wilczek). Biocatal Agric Biotechnol 16:163–171. https://doi.org/10.1016/j.bcab.2018.07.030
Kumari P, Meena M, Upadhyay RS (2018b) Characterization of plant growth promoting rhizobacteria (PGPR) isolated from the rhizosphere of Vigna radiata (mung bean). Biocatal Agric Biotechnol 16:155–162. https://doi.org/10.1016/j.bcab.2018.07.029
Lahlali R, McGregor L, Song T, Gossen BD, Narisawa K, Peng G (2014) Heteroconium chaetospira induces resistance to clubroot via upregulation of host genes involved in jasmonic acid, ethylene, and auxin biosynthesis. PLoS One 9(4):94144. https://doi.org/10.1371/journal.pone.0094144
Latz MA, Jensen B, Collinge DB, Jørgensen HJ (2018) Endophytic fungi as biocontrol agents: elucidating mechanisms in disease suppression. Plant Ecol Divers 11(5–6):555–567. https://doi.org/10.1080/17550874.2018.1534146
Lawton MA, Lamb CJ (1987) Transcriptional activation of plant defense genes by fungal elicitor wounding and infection. Mol Cell Biol 7(1):335–341. https://doi.org/10.1128/mcb.7.1.335-341.1987
Lemfack MC, Gohlke BO, Toguem SMT, Preissner S, Piechulla B, Preissner R (2018) mVOC 2.0: a database of microbial volatiles. Nucleic Acids Res 46(D1):D1261–D1265. https://doi.org/10.1093/nar/gkx1016
Leong J (1986) Siderophores: their biochemistry and possible role in the biocontrol of plant pathogens. Annu Rev Phytopathol 24(1):187–209
Lherminier J, Benhamou N, Larrue J, Milat ML, Boudon-Padieu E, Nicole M, Blein JP (2003) Cytological characterization of elicitin-induced protection in tobacco plants infected by Phytophthora parasitica or phytoplasma. Phytopathology 93(10):1308–1319. https://doi.org/10.1094/PHYTO.2003.93.10.1308
Loper JE, Henkels MD (1997) Availability of iron to Pseudomonas fluorescens in rhizosphere and bulk soil evaluated with an ice nucleation reporter gene. Appl Environ Microb 63(1):99–105. https://doi.org/10.1128/aem.63.1.99-105.1997
Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96(3):533–542. https://doi.org/10.1111/j.1399-3054.1996.tb00469.x
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556. https://doi.org/10.1146/annurev.micro.62.081307
Mao J, Liu Q, Yang X, Long C, Zhao M, Zeng H, Liu H, Yuan J, Qiu D (2010) Purification and expression of a protein elicitor from Alternaria tenuissima and elicitor–mediated defence responses in tobacco. Ann Appl Biol 156(3):411–420. https://doi.org/10.1111/j.1744-7348.2010.00398.x
Mavrodi OV, Walter N, Elateek S, Taylor CG, Okubara PA (2012) Suppression of Rhizoctonia and Pythium root rot of wheat by new strains of Pseudomonas. Biol Control 62(2):93–102. https://doi.org/10.1016/j.biocontrol.2012.03.013
Mbarga JB, Ten Hoopen GM, KuatÚ J, Adiobo A, Ngonkeu MEL, Ambang Z, Akoa A, Tondje PR, Begoude BAD (2012) Trichoderma asperellum: A potential biocontrol agent for Pythium myriotylum, causal agent of cocoyam (Xanthosoma sagittifolium) root rot disease in Cameroon. Crop Prot 36:18–22. https://doi.org/10.1016/j.cropro.2012.02.004
Meena M, Samal S (2019) Alternaria host-specific (HSTs) toxins: an overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol Rep 6:745–758. https://doi.org/10.1016/j.toxrep.2019.06.021
Meena M, Swapnil P (2019) Regulation of WRKY genes in plant defense with beneficial fungus Trichoderma: current perspectives and future prospects. Arch Phytopathol Plant Protect 52(1–2):1–17. https://doi.org/10.1080/03235408.2019.1606490
Meena M, Prasad V, Upadhyay RS (2016a) Assessment of the bioweedicidal effects of Alternaria alternata metabolites against Parthenium species. Bull Environ Sci Res 5(1):1–7
Meena M, Zehra A, Dubey MK, Aamir M, Gupta VK, Upadhyay RS (2016b) Comparative evaluation of biochemical changes in tomato (Lycopersicon esculentum Mill.) infected by Alternaria alternata and its toxic metabolites (TeA, AOH, and AME). Front Plant Sci 7:1408. https://doi.org/10.3389/fpls.2016.01408
Meena M, Prasad V, Upadhyay RS (2017a) Evaluation of biochemical changes in leaves of tomato infected with Alternaria alternata and its metabolites. Vegetos 30:2. https://doi.org/10.5958/2229-4473.2017.00020.9
Meena M, Swapnil P, Upadhyay RS (2017b) Isolation, characterization and toxicological potential of tenuazonic acid, alternariol and alternariol monomethyl ether produced by Alternaria species phytopathogenic on plants. Sci Rep 7:8777. https://doi.org/10.1038/s41598-017-09138-9
Meena M, Swapnil P, Zehra A, Aamir M, Dubey MK, Upadhyay RS (2017c) Beneficial microbes for disease suppression and plant growth promotion. In: Singh DP, Singh HB, Prabha R (eds) Plant-microbe interactions in agro-ecological perspectives. Springer, Singapore, pp 395–432. https://doi.org/10.1007/978-981-10-6593-4_16
Meena M, Swapnil P, Zehra A, Dubey MK, Upadhyay RS (2017d) Antagonistic assessment of Trichoderma spp. by producing volatile and non-volatile compounds against different fungal pathogens. Arch Phytopathol Plant Protect 50(13–14):629–648. https://doi.org/10.1080/03235408.2017.1357360
Meena M, Gupta SK, Swapnil P, Zehra A, Dubey MK, Upadhyay RS (2017e) Alternaria toxins: potential virulence factors and genes related to pathogenesis. Front Microbiol 8:1451. https://doi.org/10.3389/fmicb.2017.01451
Meena M, Prasad V, Upadhyay RS (2017f) Evaluation of Alternaria alternata isolates for metabolite production isolated from different sites of Varanasi, India. J Agric Res 2(1):00012
Meena M, Swapnil P, Zehra A, Dubey MK, Aamir M, Patel CB, Upadhyay RS (2019) Virulence factors and their associated genes in microbes. In: Singh HB, Gupta VK, Jogaiah S (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier. https://doi.org/10.1016/B978-0-444-63503-7.00011-5
Meena M, Swapnil P, Divyanshu K, Kumar S, Harish TYN, Zehra A, Marwal A, Upadhyay RS (2020) PGPR-mediated induction of systemic resistance and physiochemical alterations in plants against the pathogens: current perspectives. J Basic Microbiol 60(8):1–34. https://doi.org/10.1002/jobm.202000370
Mei L, Liang Y, Zhang L, Wang Y, Guo Y (2014) Induced systemic resistance and growth promotion in tomato by an indole-3-acetic acid-producing strain of Paenibacillus polymyxa. Ann Appl Biol 165(2):270–279. https://doi.org/10.1111/aab.12135
Milling A, Babujee L, Allen C (2011) Ralstonia solanacearum extracellular polysaccharide is a specific elicitor of defense responses in wilt-resistant tomato plants. PLoS One 6(1):5853. https://doi.org/10.1371/journal.pone.0015853
Moffett P (2009) Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res 75:1–229. https://doi.org/10.1016/S0065-3527(09)07501-0
Montesano M, Brader G, Palva ET (2003) Pathogen derived elicitors: searching for receptors in plants. Mol Plant Pathol 4(1):73–79. https://doi.org/10.1046/j.1364-3703.2003.00150.x
Murali M, Amruthesh KN (2015) Plant growth-promoting fungus Penicillium oxalicum enhances plant growth and induces resistance in pearl millet against downy mildew disease. Phytopathology 163(9):743–754. https://doi.org/10.1111/jph.12371
Mustafa G, Randoux B, Tisserant B, Fontaine J, Magnin-Robert M, Sahraoui ALH, Reignault P (2016) Phosphorus supply, arbuscular mycorrhizal fungal species, and plant genotype impact on the protective efficacy of mycorrhizal inoculation against wheat powdery mildew. Mycorrhiza 26(7):685–697. https://doi.org/10.1007/s00572-016-0698-z
Nair A, Kolet SP, Thulasiram HV, Bhargava S (2015) Systemic jasmonic acid modulation in mycorrhizal tomato plants and its role in induced resistance against Alternaria alternata. Plant Biol 17(3):625–631. https://doi.org/10.1111/plb.12277
Navarro MO, Piva AC, Simionato AS, Spago FR, Modolon F, Emiliano J, Azul AM, Chryssafidis AL, Andrade G (2019) Bioactive compounds produced by biocontrol agents driving plant health. In: Kumar V, Prasad R, Kumar M, Choudhary DK (eds) Microbiome in plant health and disease. Springer, Singapore, pp 337–374. https://doi.org/10.1007/978-981-13-8495-015
Newman MA, Daniels MJ, Dow JM (1995) Lipopolysaccharide from Xanthomonas campestris induces defense-related gene expression in Brassica campestris. Mol Plant-Microbe Interact 8(5):778–780
Newman MA, Sundelin T, Nielsen JT, Erbs G (2013) MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front Plant Sci 4:139. https://doi.org/10.3389/fpls.2013.00139
Nihorimbere V, Cawoy H, Seyer A, Brunelle A, Thonart P, Ongena M (2012) Impact of rhizosphere factors on cyclic lipopeptide signature from the plant beneficial strain Bacillus amyloliquefaciens S499. FEMS Microbiol Ecol 79(1):176–191. https://doi.org/10.1111/j.1574-6941.2011.01208.x
Niu D, Wang X, Wang Y, Song X, Wang J, Guo J, Zhao H (2016) Bacillus cereus AR156 activates PAMP-triggered immunity and induces a systemic acquired resistance through a NPR1-and SA-dependent signaling pathway. Biochem Biophys Res Commun 469(1):120–125. https://doi.org/10.1016/j.bbrc.2015.11.081
Nygren K, Dubey M, Zapparata A, Iqbal M, Tzelepis GD, Durling MB, Karlsson M (2018) The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol Appl 11(6):931–949. https://doi.org/10.1111/eva.12609
Oclarit E, Cumagun C (2009) Evaluation of efficacy of Paecilomyces lilacinus as biological control agent of Meloidogyne incognita attacking tomato. J Plant Prot Res 49(4). https://doi.org/10.2478/v10045-009-0053-x
Onaga G, Wydra K (2016) Advances in plant tolerance to abiotic stresses. Plant Genome 10:229–272
Padgett HS, Watanabe Y, Beachy RN (1997) Identification of the TMV replicase sequence that activates the N gene-mediated hypersensitive response. Mol Plant-Microbe Interact 10(6):709–715. https://doi.org/10.1094/MPMI.1997.10.6.709
Patel CB, Singh VK, Singh AP, Meena M, Upadhyay RS (2019) Microbial genes involved in interaction with plants. In: Singh HB, Gupta VK, Jogaiah S (eds) New and future developments in microbial biotechnology and bioengineering. Elsevier, pp 171–180. https://doi.org/10.1016/B978-0-444-63503-7.00011-5
Patterson GM, Bolis CM (1997) Fungal cell wall polysaccharides elicit an antifungal secondary metabolite (phytoalexin) in the cyanobacterium Scytonema ocelutum2. J Phycol 33(1):54–60. https://doi.org/10.1111/j.0022-3646.1997.00054.x
Picard K, Ponchet M, Belin JP, Rey P, Tirily Y, Benhamou N (2000) Oligandrin. A proteinaceous molecule produced by the mycoparasite Pythium oligandrum induces resistance to Phytophthora parasitica infection in tomato plants. Plant Physiol 124:379–395. https://doi.org/10.1104/pp.124.1.379
Pieterse CM, Zamioudis C, Berendsen RL, Weller DM, Van Wees SC, Bakker PA (2014) Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375. https://doi.org/10.1146/annurev-phyto-082712-102340
Pirttilä AM, Mohammad Parast Tabas H, Baruah N, Koskimäki JJ (2021) Biofertilizers and biocontrol agents for agriculture: how to identify and develop new potent microbial strains and traits. Microorganisms 9(4):817. https://doi.org/10.3390/microorganisms9040817
Pouteau S, Grandbastien MA, Boccara M (1994) Microbial elicitors of plant defence responses activate transcription of a retrotransposon. Plant J 5(4):535–542. https://doi.org/10.1046/j.1365-313X.1994.05040535.x
Puopolo G, Tomada S, Pertot I (2018) The impact of the omics era on the knowledge and use of Lysobacter species to control phytopathogenic micro-organisms. J Appl Microbiol 124(1):15–27. https://doi.org/10.1111/jam.13607
Qutob D, Kemmerling B, Brunner F, Kufner I, Engelhardt S, Gust AA, Luberacki B, Seitz HU, Stahl D, Rauhut T, Glawischnig E (2006) Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 18(12):3721–3744. https://doi.org/10.1105/tpc.106.044180
Raaijmakers JM, Sluis LVD, Bakker PA, Schippers B, Koster M, Weisbeek PJ (1995) Utilization of heterologous siderophores and rhizosphere competence of fluorescent Pseudomonas spp. Can J Microbiol 41(2):126–135. https://doi.org/10.1139/m95-017
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28(3):142–149. https://doi.org/10.1016/j.tibtech.2009.12.002
Reithner B, Ibarra-Laclette E, Mach RL, Herrera-Estrella A (2011) Identification of mycoparasitism-related genes in Trichoderma atroviride. Appl Environ Microbiol 77(13):4361–4370. https://doi.org/10.1128/AEM.00129-11
Rosyidah A, Wardiyati T, Abadi AL, Maghfoer MD, Aini LQ (2014) Induced resistance of potato (Solanum tuberosum L.) toward Ralstonia solanacearum disease with combination of several bio-control microbes. J Biol Agri Healthc 4(2):90–98
Sahebani N, Hadavi N (2008) Biological control of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Soil Biol Biochem 40(8):2016–2020. https://doi.org/10.1016/j.soilbio.2008.03.011
Saravanakumar D, Ciavorella A, Spadaro D, Garibaldi A, Gullino ML (2008) Metschnikowia pulcherrima strain MACH1 outcompetes Botrytis cinerea, Alternaria alternata and Penicillium expansum in apples through iron depletion. Postharvest Biol Tec 49(1):121–128. https://doi.org/10.1016/j.postharvbio.2007.11.006
Sarma BK, Yadav SK, Singh S, Singh HB (2015) Microbial consortium-mediated plant defense against phytopathogens: readdressing for enhancing efficacy. Soil Biol Biochem 87:25–33. https://doi.org/10.1016/j.soilbio.2015.04.001
Schornack S, Huitema E, Cano LM, Bozkurt TO, Oliva R, Van Damme M, Schwizer S, Raffaele S, CHAPARRO-GARCIA ANGELA, Farrer R, Segretin ME (2009) Ten things to know about oomycete effectors. Mol Plant Pathol 10(6):795–803. https://doi.org/10.1111/j.1364-3703.2009.00593.x
Schwacke R, Hager A (1992) Fungal elicitors induce a transient release of active oxygen species from cultured spruce cells that is dependent on Ca2+ and protein-kinase activity. Planta 87(1):136–141. https://doi.org/10.1007/BF00201635
Segarra G, Casanova E, Avilés M, Trillas I (2010) Trichoderma asperellum strain T34 controls fusarium wilt disease in tomato plants in soilless culture through competition for iron. Microb Ecol 59(1):141–149. https://doi.org/10.1007/s00248-009-9545-5
Shoda M (2000) Bacterial control of plant diseases. J Biosci Bioeng 89(6):515–521. https://doi.org/10.1016/S1389-1723(00)80049-3
Siah A, Magnin-Robert M, Randoux B, Choma C, Rivière C, Halama P, Reignault P (2018) Natural agents inducing plant resistance against pests and diseases. Int J Antimicrob Agents. Springer, Cham:121–159. https://doi.org/10.1007/978-3-319-67045-46
Singh BN, Singh A, Singh BR, Singh HB (2014) Trichoderma harzianum elicits induced resistance in sunflower challenged by Rhizoctonia solani. J Appl Microbiol 116(3):654–666. https://doi.org/10.1111/jam.12387
Singh S, Kumar V, Dhanjal DS, Singh J (2020) Biological control agents: diversity, ecological significances, and biotechnological applications. In: Singh J, Ajar Nath Y (eds) Natural bioactive products in sustainable agriculture. Springer, Singapore, pp 31–44. https://doi.org/10.1007/978-981-15-3024-1_3
Son JS, Sumayo M, Hwang YJ, Kim BS, Ghim SY (2014) Screening of plant growth-promoting rhizobacteria as elicitor of systemic resistance against gray leaf spot disease in pepper. Agric Ecosyst Environ Appl 73:1–8. https://doi.org/10.1016/j.apsoil.2013.07.016
Song M, Yun HY, Kim YH (2014) Antagonistic Bacillus species as a biological control of ginseng root rot caused by Fusarium cf. incarnatum. J Ginseng Res 38(2):136–145
Sonigra P, Meena M (2021) Metabolic profile, bioactivities, and variations in the chemical constituents of essential oils of the Ferula genus (Apiaceae). Front Pharmacol 11:608649. https://doi.org/10.3389/fphar.2020.608649
Spadaro D, Droby S (2016) Development of biocontrol products for postharvest diseases of fruit: the importance of elucidating the mechanisms of action of yeast antagonists. Trends Food Sci Technol 47:39–49. https://doi.org/10.1016/j.tifs.2015.11.003
Spiteller D, Dettner K, Bolan W (2000) Gut bacteria may be involved in interactions between plants, herbivores and their predators: microbial biosynthesis of N-acylglutamine surfactants as elicitors of plant volatiles. Biol Chem 381(8):755–762. https://doi.org/10.1515/BC.2000.096
Strobel G (2011) Muscodor species-endophytes with biological promise. Phytochem Rev 10(2):165–172. https://doi.org/10.1007/s11101-010-9163-3
Surekha CH, Neelapu NRR, Prasad BS, Ganesh PS (2014) Induction of defense enzymes and phenolic content by Trichoderma viride in Vigna mungo infested with Fusarium oxysporum and Alternaria alternata. Int J Agric Sci 4(4):31–40
Swapnil P, Meena M, Singh SK, Dhuldhaj UP, Harish MA (2021) Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr Plant Biol 26:100203. https://doi.org/10.1016/j.cpb.2021.100203
Thakur M, Sohal BS (2013) Role of elicitors in inducing resistance in plants against pathogen infection: a review. International Scholarly Research Notices. https://doi.org/10.1155/2013/762412
Umer M, Mubeen M, Iftikhar Y, Shad MA, Usman HM, Sohail MA, Ateeq M (2021) Role of rhizobacteria on plants growth and biological control of plant diseases: a review. Plant Protect 5(1):59–73. https://doi.org/10.33804/pp.005.01.3565
Urbina CT, Prieto VG, Lopez CG, Albores FV, Reyes DB, Muniz CA, Barrios DO (2016) Purification and characterization of β-1,3-glucanase from Candida oleophila for the biocontrol of Penicillium expansum. Res Rev J Bot Sci 5(1):38–45
Van Dijk K, Nelson EB (2000) Fatty acid competition as a mechanism by which Enterobacter cloacae suppresses Pythium ultimum sporangium germination and damping-off. Appl Environ Microbiol 66(12):340–5347. https://doi.org/10.1128/AEM.66.12.5340-5347.2000
Van Loon LC (2000) Helping plants to defend themselves: biocontrol by disease-suppressing rhizobacteria. In: Developments in plant genetics and breeding, vol 6. Elsevier, pp 203–213. https://doi.org/10.1016/s0168-7972(00)80123-1
Van Loon LC, Van Strien EA (1999) The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol Mol Plant Pathol 55(2):85–97. https://doi.org/10.1006/pmpp.1999.0213
Veloso J, Alabouvette C, Olivain C, Flors V, Pastor V, García T, Díaz J (2016) Modes of action of the protective strain Fo47 in controlling verticillium wilt of pepper. Plant Pathol 65(6):997–1007. https://doi.org/10.1111/ppa.12477
Vespermann A, Kai M, Piechulla B (2007) Rhizobacterial volatiles affect the growth of fungi and Arabidopsis thaliana. Appl Environ Microb 73(17):5639–5641. https://doi.org/10.1128/AEM.01078-07
Viswanathan R, Samiyappan R (1999) Induction of systemic resistance by plant growth promoting rhizobacteria against red rot disease in sugarcane. Sugar Tech 1(3):67–76. https://doi.org/10.1016/S0261-2194(00)00056-9
Wang J, Peiffer M, Hoove K, Rosa C, Zeng R, Felton GW (2017) Helicoverpa zea gut-associated bacteria indirectly induce defenses in tomato by triggering a salivary elicitor. New Phytol 214(3):1294–1306. https://doi.org/10.1111/nph.14429
Wei ZM, Laby RJ, Zumoff CH, Bauer DW, He SY, Collmer A, Beer SV (1992) Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 257(5066):85–88. https://doi.org/10.1126/science.1621099
Wiesel L, Newton AC, Elliott I, Booty D, Gilroy EM, Birch PR, Hein I (2014) Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front Plant Sci 5:655. https://doi.org/10.3389/fpls.2014.00655
Wu S, Shan L, He P (2014) Microbial signature-triggered plant defense responses and early signaling mechanisms. Plant Sci 228:118–126. https://doi.org/10.1016/j.plantsci.2014.03.001
Wu ZH, Ma Q, Sun ZN, Cui HC, Liu HR (2021) Biocontrol mechanism of Myxococcus fulvus B25-I-3 against Phytophthora infestans and its control efficiency on potato late blight. Folia Microbiol (Praha) 66(4):555–567. https://doi.org/10.1007/s12223-021-00865-1
Yacou AJ, Gerbore N, Magnin P, Chambon MC, Dufour MF, Corio-Costet R, Guyoneaud P, Rey (2016) Ability of Pythium oligandrum strains to protect Vitis vinifera L., by inducing plant resistance against Phaeomoniella chlamydospora, a pathogen involved in Esca, a grapevine trunk disease. Biol Control 92:7–16. https://doi.org/10.1016/j.biocontrol.2015.08.005
Yadav G, Meena M (2021) Bioprospecting of endophytes in medicinal plants of Thar Desert: an attractive resource for biopharmaceuticals. Biotechnol Rep 30:e00629. https://doi.org/10.1016/j.btre.2021.e00629
Yamamoto S, Shiraishi S, Kawagoe Y, Mochizuki M, Suzuki S (2015) Impact of Bacillus amyloliquefaciens S13-3 on control of bacterial wilt and powdery mildew in tomato. Pest Manag Sci 71:722–727. https://doi.org/10.1002/ps.3837
Zehra A, Dubey MK, Tiwari A, Meena M, Kumari P, Singh VK, Gupta VK, Upadhyay RS (2015) Fungal biomolecules and their implications. In: Gupta VK, Mach RL, Sreenivasaprasad S (eds) Fungal biomolecules: source applications and recent developments. Wiley Blackwell/John Wiley & Sons Ltd., USA, pp 363–375
Zehra A, Meena M, Dubey MK, Aamir M, Upadhyay RS (2017a) Synergistic effects of plant defense elicitors and Trichoderma harzianum on enhanced induction of antioxidant defense system in tomato against Fusarium wilt disease. Bot Stud 58(1):44. https://doi.org/10.1186/s40529-017-0198-2
Zehra A, Meena M, Dubey MK, Aamir M, Upadhyay RS (2017b) Activation of defense response in tomato against Fusarium wilt disease triggered by Trichoderma harzianum supplemented with exogenous chemical inducers (SA and MeJA). Braz J Bot 40(3):651–664. https://doi.org/10.1007/s40415-017-0382-3
Zehra A, Raytekar NA, Meena M, Swapnil P (2021) Efficiency of microbial bio-agents as elicitors in plant defense mechanism under biotic stress: a review. Curr Res Microb Sci 2:100054. https://doi.org/10.1016/j.crmicr.2021.100054
Zhai X, Jia M, Chen L, Zheng CJ, Rahman K, Han T, Qin LP (2017) The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit Rev Microbiol 43(2):238–261. https://doi.org/10.1080/1040841X.2016.1201041
Zhang W, Fraiture M, Kolb D, LÖffelhardt B, Desaki Y, Boutrot FF., TÖr M, Zipfel C, Gust AA, Brunner F (2013) Arabidopsis receptor-like protein30 and receptor-like kinase suppressor of BIR1-1/EVERSHED mediate innate immunity to necrotrophic fungi. Plant Cell 25(10):4227–4241. https://doi.org/10.1105/tpc.113.117010
Zhang Q, Yong D, Zhang Y, Shi X, Li B, Li G, Liang W, Wang C (2016) Streptomyces rochei A-1 induces resistance and defense-related responses against Botryosphaeria dothidea in apple fruit during storage. Postharvest Biol Technol 115:30–37. https://doi.org/10.1016/j.postharvbio.2015.12.013
Zhao Y, Jiang T, Xu H, Xu G, Qian G, Liu F (2021) Characterization of Lysobacter spp. strains and their potential use as biocontrol agents against pear anthracnose. Microbiol Res 242:126624. https://doi.org/10.1016/j.micres.2020.126624
Zheng L, Zhao J, Liang X, Zhan G, Jiang S, Kang Z (2017) Identification of a novel Alternaria alternata strain able to hyperparasitize Puccinia striiformis f. sp. tritici, the causal agent of wheat stripe rust. Front Microbiol 87:1. https://doi.org/10.3389/fmicb.2017.00071
Zakaria H, Kassab AS, Shamseldean M, Oraby M, El-Mourshedy MMF (2013) Controlling the root-knot nematode, Meloidogyne incognita in cucumber plants using some soil bioagents and some amendments under simulated field conditions. Ann Agric Sci 58(1):77–82. https://doi.org/10.1016/j.aoas.2013.01.011
Acknowledgments
The authors are thankful to their respective departments and universities for providing the necessary facilities during the course of study.
Author Contributions
MM: Provided the general concept, conceived, and drafted part of the manuscript; writing – original draft preparation; prepared the figures and tables; conceptualization; investigation; resources; supervision; validation; visualization; writing – review & editing.
GY, PR, AN, and TM: Provided the general concept, conceived, and drafted part of the manuscript; writing – original draft preparation; prepared the figures and tables.
AZ and PS: Provided the general concept; validation; writing – review & editing.
All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No associated data marked.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding
The authors are thankful to the University Grant Commission (UGC) under Startup Research Grant (UGC Faculty Research Promotion Scheme; FRPS), New Delhi, India, for its financial assistance (No.F. 30-476/2019 (BSR) FD Diary No. 5662).
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Meena, M. et al. (2022). Role of Microbial Bioagents as Elicitors in Plant Defense Regulation. In: Wani, S.H., Nataraj, V., Singh, G.P. (eds) Transcription Factors for Biotic Stress Tolerance in Plants. Springer, Cham. https://doi.org/10.1007/978-3-031-12990-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-031-12990-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-12989-6
Online ISBN: 978-3-031-12990-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)