Abstract
Introduction and Aims: Inhibition of the IL-1 pathway has been the target of treatments for several inflammatory conditions. This clinical review aims to summarize the risk of infectious complications associated with the use of interleukin-1 (IL-1) targeted therapy.
Methods: A narrative review was undertaken utilizing computer-based MEDLINE searches for each IL-1 targeting agent. Literature review was focused on available meta-analysis, randomized controlled trials (RCT), and open-label long-term extension studies.
Content: Among adults and children with autoimmune diseases receiving IL-1-targeted therapy (anakinra, canakinumab, rilonacept, gevokizumab), a small to moderate risk of infections are associated with these agents. The risk of serious and life-threatening infections is significantly increased among older patients, those with baseline comorbidities and concomitant therapy with corticosteroids. The most commonly reported infections are upper respiratory tract infections, which are generally reported as mild to moderate in severity and do not require discontinuation of therapy. Reported serious infections consist of pneumonia, cellulitis, and urinary tract infections. The risk of tuberculosis reactivation appears to be low with IL-1 therapy. However, the evidence remains limited with these agents. Opportunistic infections are rarely reported with IL-1 therapy.
Conclusion: Anti-IL-1 therapy has been demonstrated to be generally safe and not associated with a significant risk of infection. However, more data in older individuals with comorbidities, particularly with regard to canakinumab, is needed. Specific infection prevention strategies such as age-appropriate vaccination, optimal chronic disease management, and close clinical monitoring constitute a reasonable approach to managing patients receiving anti-IL-1 therapy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Interleukin-1
- Monoclonal antibody
- Infection
- Autoimmune
- Anakinra
- Canakinumab
- Rilonacept
- Gevokizumab
- Biologics
Introduction
Interleukin-1 (IL-1) was initially discovered in the mid-1980s under various names such as leukocyte endogenous mediator, endogenous pyrogen, and osteoclast-activating factor [1], indicating multiple biological functions attributed to this cytokine. In the past two decades, several other IL-1 members were identified. Currently, 11 family members of IL-1 cytokines and 10 IL-1 receptors (IL-R) have been identified [2]. This review will focus mainly on IL-1α and IL-1β since these represent the best studied cytokines [3].
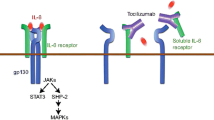
IL-1α and IL-1β are two cytokines that have similar biological activities [1]. Once they bind to their receptors, they trigger a cascade of inflammatory mediators such as chemokine and cytokine production, neutrophil activation, and the appearance of fever [2]. IL-1α is found in epithelial cells and mucosal membranes throughout the body [4]. IL-1β is predominantly found in innate immune cells such as monocytes and tissue macrophages [1, 5]. IL-1β is secreted systemically, while IL-1α is activated locally in the cell membrane [1]. In the setting of inflammation, IL-1α migrates toward the cell surface activating adjacent cells by binding with IL-1R [6, 7]. During ischemia and cell death, IL-1α and its precursor are released from cells inducing sterile inflammation of neutrophilic predominance [8,9,10]. This generates tissue destruction at the site of injury [4]. Once IL-1α binds to its receptors on resident macrophages, IL-1β precursor is synthesized by them. The IL-1β precursor is then activated by the pro-inflammatory protease caspase-1 [4, 5]. Activation of IL-1β is stimulated by several additional factors including microbial products, tumor necrosis factor (TNF), and IL-1β itself [4]. The active IL-1β binds to endothelial receptors, promoting monocyte migration and opening of endothelial intracellular junctions resulting in capillary leak [4]. IL-1Ra is an inhibitory cytokine of the IL-1 family as it binds to IL-1R but does not induce an intracellular pro-inflammatory response [11].
Inhibition of the IL-1 pathway (Fig. 9.1) has been the target of treatments for several inflammatory conditions such as rheumatoid arthritis (RA) [12], juvenile idiopathic arthritis (JIA) [13, 14], adult-onset Still’s disease (AOSD) [13], autoinflammatory syndromes including cryopyrin-associated periodic fever syndrome (CAPS) [15], TNF-associated periodic syndrome (TRAPS) [16], familial Mediterranean fever (FMF) [15], and mevalonate kinase deficiency (hyper-IgD syndrome) [15, 17]. IL-1 agents are also used off label for the treatment of gout [18,19,20,21], refractory pericarditis [22], Bechet’s disease [23, 24], pyoderma gangrenosum [25], and neutrophilic dermatosis (Sweet’s syndrome) [26].
Available IL-1-Targeting Agents
Anakinra is a recombinant IL-1Ra approved by the American Food and Drug administration (FDA) in 2001 [4]. It is similar to the structure of the natural IL-1Ra but differs by an extra methionine residue manufactured from Escherichia coli [3]. Anakinra is approved for treatment of RA, JIA, AOSD, and CAPS [2, 3]. Canakinumab is a fully human IL-1β antagonist that blocks IL-1β’s interaction with IL-1R. It is approved for treatment of CAPS, TRAPS, mevalonate kinase deficiency, and AOSD [2]. Rilonacept is a soluble decoy receptor that binds to IL-1 thereby inhibiting the binding of IL-1 to IL-1R. Rilonacept is currently approved for CAPS [2]. Similar to canakinumab, gevokizumab is a potent humanized IL-1β antagonist that has not yet been FDA approved [2, 3].
Infectious Complications of Interleukin-1 (IL-1)-Targeted Agents
Anakinra
Tables 9.1 and 9.2 summarize the risk of infection reported in clinical trials and the described infections for each drug, respectively. A meta-analysis of seven randomized controlled trials (RCTs) and three extension studies demonstrated no increased risk of infections when anakinra was compared to placebo, with a pooled relative risk (RR) of 1.06 (CI 0.94–1.20) [12]. Multiple placebo-controlled RCTs have evaluated long-term safety of anakinra in RA [27,28,29,30]. Cohen et al. evaluated the efficacy and safety of anakinra for 24 weeks and demonstrated no serious infections in both groups assigned to methotrexate (MTX) and placebo vs. MTX and anakinra [27]. Similarly, Nuki et al. demonstrated no increased risk of infection with anakinra compared to placebo on evaluation of almost 500 patients with RA for a total period of 76 weeks, with an incidence rate (IR) of 0.91, 1.0, 1.1, and 1.4 events per 100 patient-years for the 30 mg, 75 mg, and 150 mg of anakinra and the placebo groups, respectively [28]. Schiff et al. conducted a post hoc analysis of an RCT, comparing safety of anakinra versus placebo in patients with RA and coexisting comorbidities [31]. Comorbidities were defined as having had at least one cardiovascular, pulmonary, or central nervous system events; infection; renal insufficiency; diabetes; or malignancy [31]. The incidence of serious infections was similar between high-risk patients receiving anakinra (2.5%), compared to all the patients receiving anakinra in the study (2.1%) [31].
Another meta-analysis included 74 RCTs evaluating the safety of multiple interleukin (IL) inhibitors, of which 8 RCTs evaluated anakinra [45]. After stratifying risk for serious infections for each IL inhibitor, an increased odd of serious infection was associated with anakinra compared to placebo (odds ratio 2.67; CI 1.03–6.90). Fleishmann et al. evaluated the safety of anakinra compared to placebo in an RCT, followed by an open-label extension trial for 3 years [29, 30]. A total of 1414 patients were recruited. Serious infections (defined as infections requiring hospitalization and the use of intravenous antibiotics) were observed in 23 patients in the anakinra group (2.1%) vs. only one patient in the placebo group (0.4%); P = 0.068 [29]. Pneumonia was the most common serious infection followed by cellulitis, in ten patients and three patients, respectively [29]. Five patients had underlying chronic pulmonary disease and three patients had a history of prior pneumonia [29]. Additionally, out of three patients with cellulitis, two had underlying diabetes and one had a toe ulcer at baseline. Of note, none of these serious infections were fatal. However, 6 out of 23 patients permanently discontinued anakinra due to infection [29]. Organisms isolated in pneumonia and
cellulitis cases were Streptococcus pneumoniae and Staphylococcus aureus, respectively. None of the patients developed tuberculosis (TB) or opportunistic infections [29]. The 3-year open-label extension trial that included 1346 patients reported a higher incidence of serious infections with anakinra compared to placebo, with adjusted event rates of 5.37 vs. 1.65 per 100 patient-years, respectively [30]. Pneumonia was again the most common infection (1.50 events per 100 patient-years), followed by cellulitis (1.20 events per 100 patient-years). Rates of infections were significantly lower in patients who did not receive corticosteroids at baseline (2.87 events per 100 patient-years), with an incidence rate of pneumonia of 0.96 events per 100 patient-years and of cellulitis of 0.21 events per 100 patient-years [30]. Overall, the event rate of serious infections was consistently low throughout the entire treatment period [30].
Many of the autoinflammatory conditions for which anti-IL-1 therapy has been studied affect children [3].
In an observational study of 18 patients, the use of anakinra in neonatal-onset multisystem inflammatory disease (NOMID) was assessed. Fifteen patients had upper respiratory tract infections (URTI), and two patients had urinary tract infections (UTI). None of the infections required drug discontinuation [40]. A similar cohort evaluated the use of anakinra for 5 years and found similar results, with URTI being the most common infection [46]. The only two serious infections reported were wound infections, and none of these required drug discontinuation [46].
Although many studies demonstrated no increased risk of infection, some studies did find an increased rate of infection in patients treated with anakinra. Nevertheless, the majority of infections reported were not serious, suggesting an overall good safety profile of anakinra [31].
Canakinumab
Two RCTs assessed the safety and efficacy of canakinumab in gout [18, 19]. Schlesinger et al. evaluated the efficacy and safety of canakinumab vs. daily colchicine in 432 patients [18]. Overall, the incidence of infections was slightly increased with canakinumab use compared to colchicine (18% vs. 12%, respectively) [18]. Additionally, six serious infections (pneumonia, erysipelas, gangrene, sepsis, tonsilitis, and ear infection) were reported in canakinumab vs. none reported in the colchicine group. Similarly, a 12-week RCT followed by a 12-week double blind extension study, β-RELIEVED and β-RELIEVED-II, denoted increased risk of infections in patients receiving canakinumab compared to placebo (20% vs. 12%, respectively), mostly reported as mild infections [19]. Four serious infections occurred in the canakinumab group (1.8%)—jaw abscess, arm abscess, pneumonia, and gastroenteritis—all requiring hospitalization, and three requiring antibiotic therapy [19].
More recently, the CANTOS trial, a placebo-controlled RCT that recruited more than 10,000 patients, evaluated canakinumab use in the treatment of atherosclerosis. In contrast to other trials studying biologic therapies, CANTOS provided the opportunity to observe the risk of infections in patients who have no prior or current history of autoimmune disease and/or receipt of immunosuppression [32]. Infection rates of canakinumab vs. placebo were similar, 3.14 vs. 2.86 events per 100 patient-years, respectively, (P = 0.14) [32]. However, fatal infections or sepsis were higher in the canakinumab group vs. placebo, with an IR of 0.31 vs. 0.18 per 100 patient-years, respectively (P = 0.02) [32]. Individuals who had fatal infections were more likely to be older and have diabetes [32].
In the pediatric age group, a canakinumab placebo-controlled RCT of sJIA followed by an open-label extension phase [33, 34] demonstrated no differences in the incidence of infections at 29 days [33]. Similarly, serious infections were similar between the two groups in the open-label phase, with 4% in each group [33]. Patients from this study were able to enter an open-label long-term extension phase for 5 years [34]. Serious infections occurred at an incidence rate (IR) of 10.28 per 100 patient-years. The most common infection was gastroenteritis (1.05 per 100 patient-years), followed by pneumonia (0.84 per 100 patient-years) [34]. Other infections included varicella, septic shock, subcutaneous abscess, and streptococcal tonsilitis, all with equivalent rates of 0.42 per 1000 patient-years [34]. In autoinflammatory diseases, a three-part double-blind, placebo-controlled, randomized withdrawal study of patients (n = 35) with CAPS demonstrated an increased risk of infection in patients receiving canakinumab compared to placebo (12 vs. 9 patients; P = 0.03) [47].
Rilonacept
Rilonacept has been studied for the treatment of gout, pericarditis, and autoinflammatory disorders.
In the RESURGE study, a multicenter placebo-controlled trial that evaluated 1315 patients with gout for a period of 20 weeks, the incidence of serious infections was similar between rilonacept and placebo groups, 0.5% and 0.9%, respectively [35].
Recently, the RHAPSODY trial recruited 86 patients with recurrent pericarditis in a placebo-controlled RCT [22]. Rilonacept demonstrated a significantly lower recurrence of pericarditis. Infections were more frequent in the rilonacept group (23%) compared to placebo (0%). However, all infections were mild to moderate URTI, which did not require drug discontinuation [22].
In autoinflammatory conditions, Hoffman et al. conducted a placebo-controlled RCT on 44 patients with CAPS [36]. Overall, the incidence of infections was more frequent in the rilonacept arm compared to placebo (48% vs. 17%, respectively) with URTI being the most common infection, reported in 26% for rilonacept and 4% for placebo. One case of severe bronchitis was reported with rilonacept, but there have been no reports of opportunistic infections associated with this agent [36]. In addition to the 44 patients recruited in the Hoffman et al. RCT, an additional 57 patients entered the open-label phase (101 patients total) [37]. Two severe infections (pneumococcal meningitis and tooth abscess) were reported in the open-label phase [37]. Additionally, one death from pneumococcal meningitis was reported in a 71-year-old female patient with a history of recurrent skin infections [37]. The investigator deemed this infection to be unrelated to rilonacept therapy [37]. A placebo-controlled RCT of sJIA patients demonstrated similar rates of infections between rilonacept and placebo (46% and 61%, respectively) [38]. Four serious infections were reported in the rilonacept group (varicella, viral URTI, Salmonella gastroenteritis, streptococcal pharyngitis) [38].
Gevokizumab
Given that this monoclonal antibody is not yet approved, there is limited data of its safety and risk of infections. Cavelti-Weder et al. evaluated the efficacy and safety of gevokizumab in patients with type 2 diabetes in a dose-escalation RCT [48]. Gevokizumab was administered either as a single dose intravenously (0.01–3.0 mg/kg) or as single or multiple subcutaneous doses (0.03–0.3 mg/kg). No serious infectious adverse events were observed at any dose of gevokizumab [48]. More recently, Tugal-Tutkun et al. performed a placebo-controlled RCT followed by an open-label extension phase that evaluated the use of gevokizumab in Bechet’s uveitis [39]. This study evaluated 83 patients for a total duration of 420 days. Infections were similar between placebo and gevokizumab (46% vs. 51%, respectively); most common infections were nasopharyngitis and URTI [39]. Positive interferon-gamma released assay (IGRA) was reported in two patients in the gevokizumab group. Both patients received prophylactic TB therapy with either isoniazid or rifampin, with no reported cases of active TB [39].
Tuberculosis
There is scarce and weak evidence regarding the risk of TB with anakinra use. Two cases of pulmonary TB and TB pyomyositis have been reported in association with combined anakinra and corticosteroid use for treatment of RA [41, 42]. Additionally, data from a Canadian RA registry that included over 110,000 patients showed no statistically significant increased risk of TB in patients receiving anakinra, with an adjusted rate ratio (ARR) 1.3 events per 1000 patient-years (CI 0.8–2.1) [49].
Only six cases of TB were confirmed in individuals treated with canakinumab, all reported in the CANTOS trial. The same rate of TB was reported in both arm of the trial (0.06% each), five of those cases occurred in India and one case in Taiwan [32]. It is important to recognize that most RCTs evaluating IL-1-targeted therapies to date have taken place in low TB prevalence areas [3].
Opportunistic Infections
Opportunistic infections have only been reported in four patients with RA receiving anakinra, one case of nontuberculous mycobacteria infection in a patient receiving concomitant prednisone and MTX, one case of esophageal candidiasis in a patient with cirrhosis and on concomitant prednisone, and one case of histoplasmosis [30]. Additionally, one case of CMV hepatitis has been reported in a patient with JIA treated with anakinra [44]. In an observational cohort of 35 patients with systemic juvenile idiopathic arthritis (sJIA) and AOSD, one case of visceral leishmaniasis and two cases of varicella were identified [43]. Visceral leishmaniasis occurred 6 months after anakinra therapy in a child with sJIA. Of note, the child lived in an endemic area, in France, prior to starting therapy [43].
Four cases of opportunistic infections were identified with canakinumab use for sJIA including toxoplasmosis, CMV infection, Salmonella gastroenteritis, and adenovirus infection [34].
Conclusions
IL-1 inhibition has emerged as an important therapy for many patient groups over the last two decades. These biologic agents have been demonstrated to be generally safe, and although there may be an increased risk of infection, when infections do occur, these appear to be mostly mild to moderate in severity with the most common infections being URTIs, pneumonia, and cellulitis. The risk of severe infections associated with anti-IL-1 therapy may be increased in older patients with comorbidities, particularly with canakinumab, but more data is needed. Rare cases of TB and other opportunistic infections have been reported in association with IL-1 therapy, but the exact contribution of the IL-1 therapy to the development of these infections remains unclear.
References
Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102.
Davis JS, Ferreira D, Paige E, Gedye C, Boyle M. Infectious complications of biological and small molecule targeted immunomodulatory therapies. Clin Microbiol Rev. 2020;33(3):e00035–19.
Winthrop KL, Mariette X, Silva JT, Benamu E, Calabrese LH, Dumusc A, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [II]: agents targeting interleukins, immunoglobulins and complement factors). Clin Microbiol Infect. 2018;24:21–40.
Dinarello CA, Simon A, van der Meer JWM. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–52.
Lopez-Castejon G, Brough D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011;22(4):189–95.
Kaplanski G, Farnarier C, Kaplanski S, Porat R, Shapiro L, Bongrand P, et al. Interleukin-1 induces interleukin-8 secretion from endothelial cells by a juxtacrine mechanism. Blood. 1994;84(12):4242–8.
Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985;82(4):1204–8.
Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13(7):851–6.
Cohen I, Rider P, Carmi Y, Braiman A, Dotan S, White MR, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci. 2010;107(6):2574–9.
Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, et al. IL-1α and IL-1β recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol. 2011;187(9):4835–43.
Arend WP, Malyak M, Guthridge CJ, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55.
Nikfar S, Saiyarsarai P, Tigabu BM, Abdollahi M. Efficacy and safety of interleukin-1 antagonists in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatol Int. 2018;38(8):1363–83.
Feist E, Quartier P, Fautrel B, Schneider R, Sfriso P, Efthimiou P, et al. Efficacy and safety of canakinumab in patients with Still’s disease: exposure-response analysis of pooled systemic juvenile idiopathic arthritis data by age groups. Clin Exp Rheumatol. 2018;36(4):668–75.
Horneff G, Schulz AC, Klotsche J, Hospach A, Minden K, Foeldvari I, et al. Experience with etanercept, tocilizumab and interleukin-1 inhibitors in systemic onset juvenile idiopathic arthritis patients from the BIKER registry. Arthritis Res Ther. 2017;19(1):256.
De Benedetti F, Gattorno M, Anton J, Ben-Chetrit E, Frenkel J, Hoffman HM, et al. Canakinumab for the treatment of autoinflammatory recurrent fever syndromes. N Engl J Med. 2018;378(20):1908–19.
Gentileschi S, Rigante D, Vitale A, Sota J, Frediani B, Galeazzi M, et al. Efficacy and safety of anakinra in tumor necrosis factor receptor-associated periodic syndrome (TRAPS) complicated by severe renal failure: a report after long-term follow-up and review of the literature. Clin Rheumatol. 2017;36(7):1687–90.
van der Hilst JCH, Bodar EJ, Barron KS, Frenkel J, Drenth JPH, van der Meer JWM, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine. 2008;87(6):301–10.
Schlesinger N, Mysler E, Lin H-Y, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Ann Rheum Dis. 2011;70(7):1264–71.
Schlesinger N, Alten RE, Bardin T, Schumacher HR, Bloch M, Gimona A, et al. Canakinumab for acute gouty arthritis in patients with limited treatment options: results from two randomised, multicentre, active-controlled, double-blind trials and their initial extensions. Ann Rheum Dis. 2012;71(11):1839–48.
Thueringer JT, Doll NK, Gertner E. Anakinra for the treatment of acute severe gout in critically ill patients. Semin Arthritis Rheum. 2015;45(1):81–5.
Tran AP, Edelman J. Interleukin-1 inhibition by anakinra in refractory chronic tophaceous gout. Int J Rheum Dis. 2011;14(3):e33–7.
Klein AL, Imazio M, Cremer P, Brucato A, Abbate A, Fang F, et al. Phase 3 trial of interleukin-1 trap rilonacept in recurrent pericarditis. N Engl J Med. 2020;384:31–41.
Cantarini L, Talarico R, Generali E, Emmi G, Lopalco G, Costa L, et al. Safety profile of biologic agents for Behçet’s disease in a multicenter observational cohort study. Int J Rheum Dis. 2017;20(1):103–8.
Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Guerriero S, et al. Interleukin (IL)-1 inhibition with anakinra and canakinumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017;36(1):191–7.
Acquitter M, Plantin P, Kupfer I, Auvinet H, Marhadour T. Anakinra improves pyoderma gangrenosum in psoriatic arthritis: a case report. Ann Intern Med. 2015;163(1):70–1.
Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet’s syndrome. Dermatology. 2011;222(2):123–7.
Cohen S, Hurd E, Cush J, Schiff M, Weinblatt ME, Moreland LW, et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin-1 receptor antagonist, in combination with methotrexate: Results of a twenty-four-week, multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(3):614–24.
Nuki G, Bresnihan B, Bear MB, McCabe D. Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: Extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(11):2838–46.
Fleischmann RM, Schechtman J, Bennett R, Handel ML, Burmester GR, Tesser J, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist (r-metHuIL-1ra), in patients with rheumatoid arthritis: a large, international, multicenter, placebo-controlled trial. Arthritis Rheum. 2003;48(4):927–34.
Fleischmann RM, Tesser J, Schiff MH, Schechtman J, Burmester GR, Bennett R, et al. Safety of extended treatment with anakinra in patients with rheumatoid arthritis. Ann Rheum Dis. 2006;65(8):1006–12.
Schiff MH, DiVittorio G, Tesser J, Fleischmann R, Schechtman J, Hartman S, et al. The safety of anakinra in high-risk patients with active rheumatoid arthritis: six-month observations of patients with comorbid conditions. Arthritis Rheum. 2004;50(6):1752–60.
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–31.
Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat N, Horneff G, et al. Two randomized trials of canakinumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367(25):2396–406.
Ruperto N, Brunner HI, Quartier P, Constantin T, Wulffraat NM, Horneff G, et al. Canakinumab in patients with systemic juvenile idiopathic arthritis and active systemic features: results from the 5-year long-term extension of the phase III pivotal trials. Ann Rheum Dis. 2018;77(12):1710–9.
Sundy JS, Schumacher HR, Kivitz A, Weinstein SP, Wu R, King-Davis S, et al. Rilonacept for gout flare prevention in patients receiving uric acid-lowering therapy: results of RESURGE, a phase III, international safety study. J Rheumatol. 2014;41(8):1703–11.
Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum. 2008;58(8):2443–52.
Hoffman HM, Throne ML, Amar NJ, Cartwright RC, Kivitz AJ, Soo Y, et al. Long-term efficacy and safety profile of rilonacept in the treatment of cryopryin-associated periodic syndromes: results of a 72-week open-label extension study. Clin Ther. 2012;34(10):2091–103.
Ilowite NT, Prather K, Lokhnygina Y, Schanberg LE, Elder M, Milojevic D, et al. Randomized, double-blind, placebo-controlled trial of the efficacy and safety of rilonacept in the treatment of systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66(9):2570–9.
Tugal-Tutkun I, Pavesio C, De Cordoue A, Bernard-Poenaru O, Gül A. Use of gevokizumab in patients with Behçet’s disease uveitis: an international, randomized, double-masked, placebo-controlled study and open-label extension study. Ocul Immunol Inflamm. 2018;26(7):1023–33.
Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1β inhibition. N Engl J Med. 2006;355(6):581–92.
Settas LD, Tsimirikas G, Vosvotekas G, Triantafyllidou E, Nicolaides P. Reactivation of pulmonary tuberculosis in a patient with rheumatoid arthritis during treatment with IL-1 receptor antagonists (anakinra). J Clin Rheumatol. 2007;13(4):219–20.
Migkos MP, Somarakis GA, Markatseli TE, Matthaiou M, Kosta P, Voulgari PV, et al. Tuberculous pyomyositis in a rheumatoid arthritis patient treated with anakinra. Clin Exp Rheumatol. 2015;33(5):734–6.
Lequerré T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset still disease: preliminary experience in France. Ann Rheum Dis. 2008;67(3):302–8.
Ilowite N, Porras O, Reiff A, Rudge S, Punaro M, Martin A, et al. Anakinra in the treatment of polyarticular-course juvenile rheumatoid arthritis: safety and preliminary efficacy results of a randomized multicenter study. Clin Rheumatol. 2009;28(2):129–37.
Bilal J, Berlinberg A, Riaz IB, Faridi W, Bhattacharjee S, Ortega G, et al. Risk of infections and cancer in patients with rheumatologic diseases receiving interleukin inhibitors: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10):e1913102.
Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three- and five-year outcomes. Arthritis Rheum. 2012;64(7):2375–86.
Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med. 2009;360(23):2416–25.
Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, et al. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35(8):1654–62.
Brassard P, Kezouh A, Suissa S. Antirheumatic drugs and the risk of tuberculosis. Clin Infect Dis. 2006;43(6):717–22.
Acknowledgment
Figure created with support from Servier medical art (https://smart.servier.com/).
Conflicts of Interest
The authors have no conflicts of interest to declare regarding the publication of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alam, M., Mah, A., Belga, S. (2022). Interleukin-1 Targeted Agents. In: Cervera, C., Aguado, J.M. (eds) Infectious Complications in Biologic and Targeted Therapies. Springer, Cham. https://doi.org/10.1007/978-3-031-11363-5_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-11363-5_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-11362-8
Online ISBN: 978-3-031-11363-5
eBook Packages: MedicineMedicine (R0)