Abstract
-
Spasmodic dysphonia is a neurological central motor processing disorder with unknown etiology that leads to severe problems in verbal communication. Two general types of spasmodic dysphonia are distinguished: adductor spasmodic dysphonia (AdSD) and abductor spasmodic dysphonia (AbSD). Of these, AdSD is the more common type.
-
The main challenge is to prevent the recurrence of symptoms.
-
Botulinum toxin injection is the current gold standard of therapy. Temporarily, the voice quality can improve to an average of 80%–90% of normal vocal function after an injection.
-
Surgical procedures could potentially offer a more stable and long-lasting beneficial effect than botulinum toxin treatment.
-
The three most frequently used surgical techniques for AdSD over the past 10 years are selective laryngeal adductor denervation–reinnervation (SLAD-R), thyroplasty type II, and endoscopic laser thyroarytenoid myoneurectomy.
-
However, to date, none of the surgical procedures has led to a broad consensus on modifying the current gold standard of botulinum toxin treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
FormalPara Key Points-
Two general types of spasmodic dysphonia are distinguished: adductor spasmodic dysphonia (AdSD) and abductor spasmodic dysphonia (AbSD). Of these, AdSD is the more common type.
-
Botulinum toxin injection is the current gold standard of therapy. Temporarily, the voice quality can improve to an average of 80%–90% of normal vocal function after an injection.
-
Surgical procedures could potentially offer a more stable and long-lasting beneficial effect than botulinum toxin treatment.
-
The three most frequently used surgical techniques for AdSD over the past 10 years are selective laryngeal adductor denervation–reinnervation (SLAD-R), thyroplasty type II, and endoscopic laser thyroarytenoid myoneurectomy.
20.1 Introduction
-
Definition
-
Historical background (psychogenic versus organic approach)
-
Etiology (fMRI, gamma-motor neurons)
-
Adductor/abductor spasmodic dysphonia
-
Symptomatology
-
Diagnostic assessment
Spasmodic dysphonia is a rare voice disorder with unknown etiology that leads to severe problems in verbal communication. Historically, most clinicians viewed spasmodic dysphonia as a hysterical conversion reaction wherein some psychic conflict becomes somatized to the laryngeal sphincter. This theory prevailed for several decades, but nowadays spasmodic dysphonia is classified as a focal (laryngeal) dystonia. Dystonia is a neurological disorder of central motor processing with many different clinical manifestations. The neuroanatomical substrates for laryngeal dystonia are still only partly understood [1]. A potential etiology is an imbalance of sensory and motor signalling originating in the basal ganglia [2]. However, evidence from functional magnetic resonance imaging (fMRI) suggests a more heterogeneous localization in the brain, e.g., the somatosensory cortex [3]. More recently, authors have theorized that spasmodic dysphonia is a sensory disorder, induced by high activity of gamma-motor neurons [4]. Gamma-motor neurons regulate the innervation of the intrafusal fibers of the muscle spindle thereby increasing the firing rate and sensitivity of the afferent neuron [4]. Consensus on the precise etiology has not been reached yet.
Generally, two different types of spasmodic dysphonia are recognized: adductor spasmodic dysphonia (AdSD) and abductor spasmodic dysphonia (AbSD) [5]. AdSD and AbSD differ in their acoustic characteristics [6]. In AdSD, hyperactivity of the adductor muscles results in voice breaks and consequent alterations in phonation and pitch [6]. The most commonly involved laryngeal adductor muscles are the thyroarytenoid and the lateral cricoarytenoid [7]. The AdSD symptoms are a result of intermittent and involuntary contraction of these adductor muscles during phonation, which leads to tense vocal folds that are pressed against each other and to an increased glottic resistance [8]. In AbSD, the spasms occur in the posterior cricoarytenoid muscles resulting in breathy, segmented speech [6]. Patients with AdSD have greater difficulty with voiced sounds and AbSD with unvoiced sounds [6]. Mixed spasmodic dysphonia involves characteristics of both types [6]. Some authors believe that all of the patients are a mixed adductor/abductor with a predominance of one form [9]. The symptoms typically progress over 1–2 years and then remain chronic. A reduction or relief of symptoms is seen during uncontrolled voicing such as laughing, singing, coughing, whispering, humming, or with alcohol intake [10]. However, stress, fatigue, and communication through telephone often leads to an increase of complaints [11]. Spasmodic dysphonia patients often suffer from severe problems in communication, which has a huge impact on their quality of life [12, 13].
About one-third of the spasmodic dysphonia patients exhibit a co-occurring dystonic voice tremor, which often complicates the diagnosis and clinical management [14]. Therefore, the correct diagnosis is made by a team of experienced neurologists, otorhinolaryngologists, and speech therapists. This chapter focuses on the management of adductor spasmodic dysphonia (AdSD), the more common form of spasmodic dysphonia in approximately 90% of the cases. AbSD will be discussed briefly.
20.2 Treatment Options for AdSD
-
Nonsurgical treatment
-
Botulinum toxin: gold standard therapy
-
Voice therapy
-
Oral medication
-
-
Surgical treatment
-
Historical background
-
Selective laryngeal adductor denervation–reinnervation (SLAD-R)
-
Thyroplasty type II
-
Endoscopic laser thyroarytenoid myoneurectomy
-
Anecdotal/experimental procedures
-
There is no known cure for adductor spasmodic dysphonia. Various treatment modalities have been described over the past decades and they all focus on symptom management. Regardless of the treatment modality, the main challenge is to prevent the recurrence of symptoms.
20.2.1 Nonsurgical Treatment
20.2.1.1 Botulinum Toxin: The Current Gold Standard of Therapy
The concept of using “botulinum toxin” to treat patients with disorders of muscle function is credited to Dr. Alan B. Scott. He published the results of his first clinical trial with injection of botulinum toxin type A in patients with strabismus in 1980 [15]. Shortly after, in 1984, Blitzer was the first who injected botulinum toxin A into the vocal folds as treatment for AdSD [16]. Botulinum toxin A is a neurotoxin produced by Clostridium botulinum. Strains of Clostridium botulinum produce seven distinct neurotoxins (types A–G botulinum toxin) which act primarily on peripheral cholinergic synapses, but only botulinum toxin types A and B are currently available for clinical use [17, 18]. The mechanism of action of these potent neurotoxins is direct inhibition of the injected muscles by blocking the release of the neurotransmitter acetylcholine at the neuromuscular junction [17]. The result is a chemical denervation causing a dose-related muscle weakness, lasting several months. Recovery occurs by muscular reinnervation with smaller collateral nerve sprouts and an increase in the number of postsynaptic acetylcholine receptors [19]. The objective in AdSD patients is to inject botulinum toxin in the presumed main laryngeal adductor muscles affected in AdSD (the thyroarytenoid muscles) in the outpatient setting. This causes a temporary chemical denervation of these injected adductors. Currently, botulinum toxin A (BTX) injections in the adductor musculature are considered gold standard of care for AdSD patients.
Though widely accepted, BTX treatment for AdSD is not standardized; because no clear guidelines exist, protocols in BTX treatment vary among physicians [20]. There are different injection strategies. The most frequently used approach is the percutaneous route through the cricothyroid membrane under EMG control (Fig. 20.1), as described by Blitzer et al. [21].
EMG-guided injection of the thyroarytenoid muscle for adductor spasmodic dysphonia. Image from Sulica L, Blitzer A, Oper Tech Otolaryngol 15:76–80, 2004 © Elsevier, adapted and reprinted with permission [22]
In our center, the patient is placed in a supine position with the neck extended. A modified 1.5-inch hollow-bore 27-gauge Teflon-coated EMG needle is used both as a monopolar electrode to locate the thyroarytenoid muscle and as a port for injection of the botulinum toxin. The needle is placed through the skin and cricothyroid membrane and angled superiorly and slightly laterally into the thyroarytenoid muscles. Correct position of the needle tip is confirmed by the presence of crisp action potentials on phonation. The botulinum toxin is then injected in the muscles. Topical anesthesia is not necessary. The main disadvantage of this approach is that the needle tip can easily be placed more posteriorly or laterally than intended, injecting the lateral cricoarytenoid or cricothyroid muscles. Alternatively, botulinum toxin may also be injected percutaneously transcartilaginous through the thyroid cartilage, wherein the needle position is confirmed by flexible laryngoscopy [23]. Although less frequently used, an indirect laryngoscopic peroral approach or injection through an operative channel of a flexible laryngoscope have also been suggested [24, 25]. The duration of benefit of unilateral injections appear to be less than bilateral injections [26]. Therefore, most patients receive bilateral injections. However, if patients cannot tolerate the side effects of bilateral injections, a unilateral injection is offered [26]. Preference and/or prior experience of the patient/physician may influence that decision. Injection dosages range widely in literature. In a recent survey among 70 American laryngologists, a mode of 1.25 units (range 0.5–5.0 units) per injection side was described [20]. Decisions regarding dose of the injection are generally based on the experience and empirical judgement of the otolaryngologist and the patient. Good results can be accomplished with botulinum injections: voice quality can improve to an average of 80%–90% of normal vocal function [26, 27]. However, there are several drawbacks to BTX injection therapy. The main disadvantage is the need for repeated injections, since the BTX effect is temporary. The duration of improvement of symptoms varies by individual, but lasts about 3–4 months [28]. Thus, if patients want to maintain improved voice, they usually return 3–4 times a year for injections [28]. Second, an initial period of breathiness as a side effect of the injection usually leads to a decline in voice quality [27]. This breathiness typically starts immediately after the injection and lasts for 2–3 weeks before the optimal BTX voice quality is reached. As a result of either awaiting the full therapeutic effect or experiencing a therapeutic decline, optimal voicing is achieved during only 30% of the injection cycle [27]. Third, there is a lack of uniform responses to the injections among and within patients, causing wide cycle-to-cycle differences. This results in an unpredictable voice and emotional stress with high impact on quality of life [13]. Besides the breathy dysphonia, the most frequently reported side effects of botulinum toxin treatment are swallowing problems. Swallowing difficulties (i.e., mild choking on fluids) appear as early as the breathy voice and usually resolves within 2 weeks. Less common complications include hyperventilation, a “sore throat” feeling, and diplophonia. In the literature, there are no major complications reported [26].
20.2.1.2 Oral Medication
The utility of treatment of AdSD with oral medication (muscle relaxants, tranquilizers, etc.) is often limited by significant central nervous system side effects like sedation and memory loss [22]. It usually results in an incomplete response and is frequently unsuccessful. After a report showing that more than half of patients with SD experienced dramatically improved voice quality after ingestion of alcohol, Rumbach et al. have focused on the use of GABA receptor agonists, which produce effects through mechanisms similar to the action of alcohol, to improve symptoms [10]. A metabolite of sodium oxybate has been found to improve SD symptoms in 82% of patients who had improvement following alcohol ingestion [14].
20.2.1.3 Voice Therapy
Voice therapy has shown little therapeutic effect in AdSD patients, but can be useful in differentiating between the diagnosis of AdSD and voice disorders of other origins [29]. Additionally, behavioral treatment approaches may enhance the effectiveness of other AdSD treatment strategies, by reducing hyperfunctional vocal behaviors such as hard glottal attacks and excessive laryngeal tension [30]. These hyperfunctional behaviors are often compensatory in nature and are developed over time by individuals with AdSD as a means of countering vocal spasms and may persist even after the spasms are eliminated via injection [30, 31].
20.2.2 Surgical Treatment
Surgical procedures could potentially offer a more stable and long-lasting beneficial effect than botulinum toxin treatment. In 1976, Dedo introduced recurrent laryngeal nerve sectioning (RLNS) [32]. He hypothesized that “if a recurrent laryngeal nerve were paralyzed in a patient with spastic dysphonia, the other vocal cord might prove to be ‘precompensated’ so that its excessively strong adduction would carry it across the midline to the deliberately paralyzed cord, giving a relatively normal phonation” (p. 453) [32]. In the following years, several institutes reported their results after RLNS. Unfortunately, after initially promising outcomes, it showed disappointing long-term results with reoccurrence of symptoms [33,34,35,36]. This recurrence of spasmodic closure has been attributed to increased function of the opposite vocal fold and/or to regeneration of the resected laryngeal nerve [37, 38]. Due to the disappointing results, RLNS is nowadays abandoned. Currently, different surgical modalities are used. However, there is no consensus as to which procedure gives the best results. Therefore, the most commonly performed surgical modalities are highlighted below. The aim of every surgical treatment is to permanently reduce the endolaryngeal constriction activity during phonation.
20.2.2.1 Selective Laryngeal Adductor Denervation–Reinnervation (SLAD-R)
Berke presented the SLAD-R procedure in 1999 [39]. The concept is to produce thyroarytenoid and lateral cricoarytenoid muscle paralysis in patients with AdSD, by selectively denervating the recurrent laryngeal nerve (RLN) branches to these muscles [39]. In order to prevent unwanted reinnervation by RLN efferents and preserve muscle tone, a problem that limited the effectiveness of the previously described RLNS, the thyroarytenoid nerve branch is reinnervated with a branch of the ansa cervicalis [39]. The procedure does not affect the third adductor of the larynx, the interarytenoid muscle, which receives innervation from a nerve branch that divides more proximally from the RLN [40].
The steps of the SLAD-R procedure are shown in Figs. 20.2, 20.3, 20.4. After a low transverse incision in the neck, an inferiorly based window of approximately 14 × 18 mm is made in the thyroid lamina as a cartilaginous flap [39]. The distal portion of the anterior branch of the RLN is identified and followed distally [39]. The thyroarytenoid branch is located beneath the perichondrium and ligated 3 mm from its termination [39]. Generally, the sternohyoid branch of the ansa nerve is appropriately sized for thyroarytenoid reinnervation [39]. The ansa is sutured to the distal thyroarytenoid nerve stump to maintain muscle tone and bulk and also to presumably prevent regeneration of RLN axons to the neuromuscular endplates of the thyroarytenoid and lateral cricoarytenoid muscles [39, 40]. The proximal stump of the thyroarytenoid nerve branch is ligated with a 2–0 silk suture and sutured outside the cartilage window to the posterior lamina [39]. Initially, Berke added a lateral cricoarytenoid myotomy after several recurrences after thyroarytenoid SLAD-R in some patients, to provide maximal reduction of adductory force [39, 40]. Although lateral cricoarytenoid myotomy appeared to increase the potential for long-term success, it also increased the risk of severe breathiness because of incomplete posterior commissure closure [40]. Therefore, this additional step has been abandoned [40].
“Lateral oblique view of right thyroid cartilage. The hashed lines show the trapdoor laryngotomy window, crossing the oblique line after removing inferior constrictor muscle attachments. The posterior limb is just anterior to the inferior cornu, the anterior limb passes through the inferior tubercle, and the superior limb is below the vertical midpoint of the cartilage.” [41] Image from Long J, Berke G, Oper Tech Otolaryngol 23: 183–187, 2012 © Elsevier, adapted and reprinted with permission
“Intralaryngeal course of the right recurrent laryngeal nerve (RLN). The thyroid cartilage laryngotomy window is drawn with the long hashed lines. The short dashed lines represent nerves behind cartilage. The characteristic oblique anterosuperior course of the adductor nerve within the window is shown, destined for the thyroarytenoid muscle (TA) fibers. Other branches to the posterior cricoarytenoid (PCA) and interarytenoid (IA) muscles are not encountered during the dissection. Lateral cricoarytenoid (LCA) branch may be seen, although it is short and fine.” [41] Image from Long J, Berke G, Oper Tech Otolaryngol 23: 183–187, 2012 © Elsevier, adapted and reprinted with permission
“The postoperative arrangement of laryngeal nerves, after closure of laryngotomy trapdoor (outlined with long hashed lines). The ansa cervicalis (AC) enters through the cartilage window and anastomoses the distal stump of the adductor branch serving the thyroarytenoid muscle (TA) and the lateral cricoarytenoid (LCA) muscles. The proximal adductor branch from the recurrent laryngeal nerve (RLN) is divided and secured out of the larynx. The branches to the posterior cricoarytenoid (PCA) and the interarytenoid (IA) are unaltered.” [41] Image from Long J, Berke G, Oper Tech Otolaryngol 23: 183–187, 2012 © Elsevier, adapted and reprinted with permission
Titanium bridges (various sizes are available) are put in place to fix the position of the cartilage. The device consists of bilateral receptors that grasp the cartilage edges and a connector between them to enable adjustment of the distance of separation. The optimal distance is usually +/−4 mm. Schematic image of the vocal cords and the ‘imperfect close of the glottis’ (dotted line) by reshaping the laryngeal framework [48]
Berke achieved excellent preliminary results with the SLAD-R: he reported that 19 of 21 consecutive SLAD-R patients had moderate to severe dysphonia prior to the operation and mild voice symptoms postoperatively [39, 40]. From the same research group, Chettri et al. reported good long-term results of 86/136 patients in 2006 (50 lost to follow-up) [40]. They showed a high degree of patient satisfaction, with 91% agreeing that their voice is more fluent after the surgery [40]. Additionally, the group of Berke et al. compared the postoperative SLAD-R voice outcomes (i.e., VHI-10, voice questionnaire, and panel voice ratings) with the voice outcomes of a patient cohort 5–8 weeks after BTX treatment [42]. They described 77 participating SLAD-R patients (out of 157 patients who underwent a SLAD-R procedure between 1995 and 2007) and the prospectively collected results of 28 patients receiving BTX treatment [42]. A majority of the postoperative SLAD-R patients had a stable, long-lasting resolution of spasmodic voice breaks and voice outcomes equal or superior to those after BTX treatment [40, 42]. Besides a case series, there no large studies on the SLAD-R procedure reported by other research groups other than Berke/Chettri et al. [43] Reported side effects are postoperative breathiness [40]. Aside from one aspiration pneumonia, no major surgical complications were noted [40, 44].
20.2.2.2 Thyroplasty Type II (TP II)
TP II as treatment for AdSD was initially suggested by Isshiki in 1998 [45]. His “basic idea behind this type of surgery is that any wide surgical intervention into the vocal folds should be avoided, and the vocal fold position and tension can be set optimally for the voice by reshaping the laryngeal framework” (p. 1761). The concept of the TP II in AdSD patients was to create “an imperfect closure of the glottis” to prevent their excessive glottic adduction [46]. It was thought that this would provide a longer-lasting result without the adverse effects of botulinum toxin injection [47]. The advantages of the surgery include the following: (1) optimal glottal closure for phonation can be adjusted, (2) no recurrence is likely to occur, (3) no damage is induced on the physiological function of phonation such as paralysis, (4) it is reversible if it were found ineffective intraoperatively, and (5) readjustment is possible when needed [46].
The procedure, as described initially by Isshiki and later modified by Sanuki, is performed under local anesthesia [47, 48]. A 4-cm horizontal skin incision is made at approximately the level of the vocal folds [47]. The anterior third of the thyroid cartilage is fully cut at the midline with a BP 11 scalpel [47]. The incised edges are pulled apart laterally by 3–4 mm, to test the effect of lateralization on the voice [47]. The required width of midline cartilage separation is determined and was initially maintained by a silicone shim [47]. The shim was fixed to the incised edges of the thyroid cartilage with mattress sutures [47]. However, the suture fixation of the silicone shim to a fragile cartilage edge was not easy, requiring considerable time and caution [48]. Therefore, nowadays a titanium bridge of different sizes, as described by Sanuki et al., is used to fix the position of the cartilage (Figs. 20.5 and 20.6) [48].
Reported results in literature vary. Isshiki and Sanuki described excellent results in relieving vocal stress–strain [48,49,50,51]. Nearly 70% of the patients judged their postoperative voice as normal [49]. In a more recent case series, roughly 30% of the patients who received thyroplasty type 2 reported that they were completely relieved of their AdSD voice symptoms (strangulation, interruption, and tremor) [51]. Concerning the symptom of strangulation, 25.8% of patients were completely relieved after surgery, 22.6% reported little strangulation, and 51.6% answered that they still sometimes experienced strangulation [51]. Only 1 of the 47 patients who underwent TP II with an titanium bridge had disease recurrence [51].
However, Chan et al. stated that only about 30% of patients had moderate to good improvements in their symptom severity and vocal effort 1 year after TP II [52]. No major complications were reported in literature. A total of 5.6% patients needed a revision surgery [49].
20.2.2.3 Endoscopic Laser Thyroarytenoid Myoneurectomy (TA Myoneurectomy)
TA myoneurectomy, in which an endoscopic CO2 laser myectomy of the thyroarytenoid muscle is combined with neurectomy of the thyroarytenoid branch of the recurrent laryngeal nerve, was defined by Su and Tsuji et al. [8, 53]. The concept of TA myoneurectomy is to mimic the effect of botulinum toxin injections on the thyroarytenoid muscle, by removing this muscle and its innervating nerves. By removal of the thyroarytenoid muscle, potential muscle reinnervation by regeneration of the resected laryngeal nerves is very unlikely (Figs. 20.7, 20.8, 20.9, 20.10) [31].
The concept of thyroarytenoid (TA) myoneurectomy consists of a triangular resection of this muscle. The medial border of this triangle is just lateral of the vocal ligament. The lateral boundary of this triangle is the inner perichondrium of the thyroid lamina. The posterior border of the triangle is formed by the insertion of the thyroarytenoid muscle, lateral at the level of the vocal process. The deepest part of the resection is the fascia of the lateral cricoarytenoid (LCA). The neurovascular bundle, which enters the larynx inferolaterally, is coagulated. Schematic image of the vocal cords
The TA myoneurectomy is performed under general anesthesia. In our center, we perform a modification of the procedure earlier described by Tsuij et al. [8]. And as in most studies, in general, we perform TA myoneurectomy bilaterally. A triangular piece of the thyroarytenoid muscle is resected with a CO2 laser through a transoral approach [54]. To get a clear view of the resection area, partial resection of the ventricular fold is performed, with preservation of the mucosal strip in the apex of the ventricle. This is followed by a delineation of the triangular resection plane. The medial edge of the resection is located just lateral of the vocal ligament. The internal perichondrium of the thyroid cartilage is considered the lateral limit. The posterior/base edge of this triangular resection plane is formed by the insertion of the thyroarytenoid muscle, at the level just lateral of the vocal process. Guided by the inner perichondrium of the thyroid, the muscle fibers of the thyroarytenoid are resected, until the fascia of the lateral cricoarytenoid muscle is visible. Inferolaterally, the neurovascular thyroarytenoid bundle enters the endolarynx, which is coagulated. The inferior limit of the resection is defined by palpation of the free caudal edge of the thyroid, the fascia of the lateral cricoarytenoid muscles, and the edge of the elastic cone.
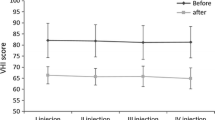
The results of different studies suggest that the long-term outcomes of TA myoneurectomy are encouraging. Several authors describe significant long-term improvement in voice quality in terms of reduced speech brakes, effort, and strain in voice [8, 55, 56]. Su et al. reported on 29/52 patients who were followed 12 months or more [55]. Approximately 90% (26/29) of these patients achieved moderate and marked vocal improvement [55]. This was confirmed by Tsuij et al., who prospectively analyzed 15 patients and described a postoperative median VHI-10 score improvement in 80%, after a median of 31 months follow-up postoperatively [8]. Schuering et al. were the first who conducted a comparative study, which compared the results after a TA myoneurectomy versus botulinum toxin in the same patient [54]. They found an initially improved stable voice quality after TA myoneurectomy in all 22 patients [54]. The postoperative voice quality was comparable to the best voice with botulinum toxin in the same patient [54]. However, after good results initially, voice deterioration was seen in 45% (10/22) of the patients during a follow-up of >12 months due to reoccurrence of symptoms. All these patients underwent a second procedure with varying success [54]. Nomoto et al. compared the outcomes of 35 TP II patients with 30 TA myoneurectomy patients [54, 57]. Voice quality improved in both procedures, but significant differences in severity outcomes favoring TA myoneurectomy were found in strangulation, interruption, tremor, and grade [57]. Postoperative VHI-10 scores did not differ significantly between the two procedures [57]. Given favorable improvement rates, both surgical procedures were considered effective [57].
Other than surgical removal of granuloma tissue in a few patients, there were no major complications or side effects reported in literature [54].
20.2.2.4 Anecdotal/Experimental Therapies
Anecdotal case reports on bipolar radiofrequency-induced thermotherapy (RFITT) with varying positive results have been described (Fig. 20.11). And again, “The goal is to weaken the force of laryngeal closure during spasms by creating fibrosis of the terminal branches of one recurrent nerve through coagulation” [58]. It consists of transoral coagulation of the terminal branches of the recurrent nerve using RFITT [4, 58, 59].
Drawing of the transoral approach with insertion of the RFITT probe into the vocal fold externally and anteriorly to the vocal process. The location of the region of maximal stimulation is usually just lateral and anterior to the vocal process of the arytenoids [58]
Pitman et al. presented a report on the use of an implantable electrical stimulation device to treat spasmodic dysphonia via neuromodulation of the muscle spindle gamma loop [4]. This would modulate the afferent nerve signal, in contrast to all previously mentioned treatments where the efferent nerve signal is modulated. This is based on theory that SD is likely due to a sensory dysfunction, with the muscle spindle playing a central role [4]. Accordingly, the benefit of botulinum toxin injections may be in their modulation of the gamma loop of the muscle spindle via inhibition of the c-motor neuron and not via induction of muscle weakness [4]. They hypothesized that neuromodulation of the c-motor neuron and/or the 1a afferent neuron of the muscle spindle via electrical stimulation can inhibit these nerves, modulate the gamma loop, and mitigate the symptoms of spasmodic dysphonia [4]. In a small test case series, the left thyroarytenoid muscle of five human study participants was stimulated on 5 consecutive days below the level of alpha-motor neuron activation [4]. The spasmodic dysphonia symptoms were reduced in four out of five patients and no major complications were reported. However, no firm conclusions can be drawn from this concept study.
20.3 AbSD Treatment
AbSD is a rare form of spasmodic dysphonia, in which the posterior cricoarytenoid muscles are affected. Treatment of AbSD with botulinum injections weakens these posterior cricoarytenoid muscles. Initially, there was a concern that this could potentially lead to a compromised airway. However, there are now several studies describing successful therapy with botulinum injection with either unilateral or bilateral injection [60,61,62]. The injection can be applied through a transcricoid or retrocricoid approach (see Figs. 20.12 and 20.13). An average posttreatment voice function of 70% has been achieved [60].
Transcricoid EMG-guided injection of the posterior cricoarytenoid muscle for abductor spasmodic dysphonia. Image from Sulica L, Blitzer A, Oper Tech Otolaryngol 15:76–80, 2004 © Elsevier, adapted and reprinted with permission [22]
Retrocricoid EMG-guided injection of the posterior cricoarytenoid muscle for abductor spasmodic dysphonia. Image from Sulica L, Blitzer A, Oper Tech Otolaryngol 15:76–80, 2004 © Elsevier, adapted and reprinted with permission [22]
For refractory AbSD cases, different surgical techniques have been mentioned in literature [63]. Case reports of posterior cricoarytenoid myoplasty with medialization thyroplasty and endoscopic partial posterior cricoarytenoid myectomy have been described as treatment for AbSD [63, 64]. In some AbSD patients, decreased adductor muscle activity contributes to their symptoms and may be corrected by conventional medialization (type I) thyroplasty or bilateral medialization laryngoplasty [64, 65]. However, long-term outcomes in larger cohorts studies for these surgical procedures are rarely reported [63].
20.4 Concluding Remarks
Spasmodic dysphonia is a rare voice disorder with unknown etiology. The most common subtype is adductor spasmodic dysphonia, in which hyperactivity of the laryngeal adductor muscles occurs. Patients suffer from severe problems in communication, resulting in a significant decrease in quality of life. The current standard of therapy is injection with botulinum toxin in the main laryngeal adductor muscle: the thyroarytenoid muscle. Excellent voice quality can be achieved with an injection in the outpatient setting. However, the temporary effect and the unpredictable dose–response relationship between and within patients are serious disadvantages of botulinum injection treatment. A surgical procedure could potentially offer a more stable and long-lasting beneficial effect than botulinum toxin treatment. To achieve this, there are several surgical procedures described in literature. The three most frequently used techniques over the past 10 years are the SLAD-R, TP II, and TA myoneurectomy. Based on the current literature, no definitive conclusions can be drawn about what would be the best surgical procedure.
20.5 Recommendations for the Future
Botulinum toxin injections currently remain the gold standard for the treatment of AdSD as there is disagreement as to whether a surgical procedure is an equivalent alternative and, if this is the case, which procedure yields the best results. However, for some patients experiencing severe disadvantages of the temporal and unpredictable injection effect, a surgical procedure can offer the possibility of having more stable and long-lasting results. Currently, it is difficult to determine which surgical procedure is best. Most reported studies on postoperative results are based on small single-center case series, all using different outcome parameters. To be able to make a better assessment of the advantages and disadvantages of each surgical procedure, further research is needed. A single-center study comparing different surgical modalities in a randomized setting, or a multicenter study using standardizes diagnostic and outcome measurement protocols, is recommended.
References
Neychev VK, et al. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42(2):185–201.
Izdebski K. Symptomatology of adductor spasmodic dysphonia: a physiologic model. J Voice. 1992;6(4):306–19.
Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an fMRI study. Cereb Cortex. 2010;20(11):2749–59.
Pitman MJ. Treatment of spasmodic dysphonia with a neuromodulating electrical implant. Laryngoscope. 2014;124(11):2537–43.
Cannito MP, Kahane JC, Chorna L. Vocal aging and adductor spasmodic dysphonia: response to botulinum toxin injection. Clin Interv Aging. 2008;3(1):131–51.
Whurr R, Lorch M. Review of differential diagnosis and management of spasmodic dysphonia. Curr Opin Otolaryngol Head Neck Surg. 2016;24(3):203–7.
Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111(4 Pt 2 Suppl 97):1–47.
Tsuji DH, et al. Endoscopic laser thyroarytenoid myoneurectomy in patients with adductor spasmodic dysphonia: a pilot study on long-term outcome on voice quality. J Voice. 2012;26(5):666.e7–12.
Cannito MP, Johnson JP. Spastic dysphonia: a continuum disorder. J Commun Disord. 1981;14(3):215–33.
Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015;262(6):1548–56.
Barkmeier JM, Case JL, Ludlow CL. Identification of symptoms for spasmodic dysphonia and vocal tremor: a comparison of expert and nonexpert judges. J Commun Disord. 2001;34(1–2):21–37.
Hintze JM, et al. Spasmodic dysphonia: a review. Part 1: pathogenic factors. Otolaryngol Head Neck Surg. 2017;157(4):551–7.
Paniello RC, Barlow J, Serna JS. Longitudinal follow-up of adductor spasmodic dysphonia patients after botulinum toxin injection: quality of life results. Laryngoscope. 2008;118(3):564–8.
Rumbach AF, et al. An open-label study of sodium oxybate in Spasmodic dysphonia. Laryngoscope. 2017;127(6):1402–7.
Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology. 1980;87(10):1044–9.
Blitzer A, et al. Electromyographic findings in focal laryngeal dystonia (spastic dysphonia). Ann Otol Rhinol Laryngol. 1985;94(6 Pt 1):591–4.
Hambleton P. Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. J Neurol. 1992;239(1):16–20.
Cha W, et al. Liquid-type botulinum toxin type a in adductor spasmodic dysphonia: a prospective pilot study. J Voice. 2017;31(3):378 e19–24.
Wong DL, et al. Effect of neuromuscular activity on the response to botulinum toxin injections in spasmodic dysphonia. J Otolaryngol. 1995;24(4):209–16.
Shoffel-Havakuk H, et al. Common practices in botulinum toxin injection for spasmodic dysphonia treatment: a national survey. Laryngoscope. 2019;129(7):1650–6.
Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108(10):1435–41.
Sulica L, Blitzer A. Botulinum toxin treatment of spasmodic dysphonia. Oper Tech Otolaryngol Head Neck Surg. 2004;15(2):76–80.
Green DC, et al. Point-touch technique of botulinum toxin injection for the treatment of spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1992;101(11):883–7.
Ford CN, Bless DM, Lowery JD. Indirect laryngoscopic approach for injection of botulinum toxin in spasmodic dysphonia. Otolaryngol Head Neck Surg. 1990;103(5 ( Pt 1)):752–8.
Rhew K, Fiedler DA, Ludlow CL. Technique for injection of botulinum toxin through the flexible nasolaryngoscope. Otolaryngol Head Neck Surg. 1994;111(6):787–94.
Blitzer A. Spasmodic dysphonia and botulinum toxin: experience from the largest treatment series. Eur J Neurol. 2010;17(Suppl 1):28–30.
Novakovic D, et al. Botulinum toxin treatment of adductor spasmodic dysphonia: longitudinal functional outcomes. Laryngoscope. 2011;121(3):606–12.
Zhao K, Guillaud M, Hu A. Factors associated with failure of botulinum toxin injection in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2020;129(10):996–1002.
Silverman EP, et al. Combined modality treatment of adductor spasmodic dysphonia. J Voice. 2012;26(1):77–86.
Murry T, Woodson GE. Combined-modality treatment of adductor spasmodic dysphonia with botulinum toxin and voice therapy. J Voice. 1995;9(4):460–5.
Pearson EJ, Sapienza CM. Historical approaches to the treatment of Adductor-Type Spasmodic Dysphonia (ADSD): review and tutorial. NeuroRehabilitation. 2003;18(4):325–38.
Dedo HH. Recurrent laryngeal nerve section for spastic dysphonia. Ann Otol Rhinol Laryngol. 1976;85(4 Pt 1):451–9.
Aronson AE, De Santo LW. Adductor spastic dysphonia: three years after recurrent laryngeal nerve resection. Laryngoscope. 1983;93(1):1–8.
Biller HF, Som ML, Lawson W. Laryngeal nerve crush for spastic dysphonia. Ann Otol Rhinol Laryngol. 1983;92(5 Pt 1):469.
Fritzell B, et al. Long-term results of recurrent laryngeal nerve resection for adductor spasmodic dysphonia. J Voice. 1993;7(2):172–8.
Weed DT, et al. Long-term follow-up of recurrent laryngeal nerve avulsion for the treatment of spastic dysphonia. Ann Otol Rhinol Laryngol. 1996;105(8):592–601.
Blitzer A, Brin MF. Laryngeal dystonia: a series with botulinum toxin therapy. Ann Otol Rhinol Laryngol. 1991;100(2):85–9.
Ludlow CL, et al. Spasmodic dysphonia: botulinum toxin injection after recurrent nerve surgery. Otolaryngol Head Neck Surg. 1990;102(2):122–31.
Berke GS, et al. Selective laryngeal adductor denervation-reinnervation: a new surgical treatment for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108(3):227–31.
Chhetri DK, et al. Long-term follow-up results of selective laryngeal adductor denervation-reinnervation surgery for adductor spasmodic dysphonia. Laryngoscope. 2006;116(4):635–42.
Long JL, Berke GS. Selective laryngeal adductor denervation-reinnervation surgery for spasmodic dysphonia. Oper Tech Otolaryngol Head Neck Surg. 2012;23(3):183–7.
Mendelsohn AH, Berke GS. Surgery or botulinum toxin for adductor spasmodic dysphonia: a comparative study. Ann Otol Rhinol Laryngol. 2012;121(4):231–8.
Hussain A, Shakeel M. Selective lateral laser thyroarytenoid myotomy for adductor spasmodic dysphonia. J Laryngol Otol. 2010;124(8):886–91.
Allegretto M, et al. Selective denervation: reinnervation for the control of adductor spasmodic dysphonia. J Otolaryngol. 2003;32(3):185–9.
Isshiki N. Vocal mechanics as the basis for phonosurgery. Laryngoscope. 1998;108(12):1761–6.
Isshiki N, et al. Thyroplasty for adductor spasmodic dysphonia: further experiences. Laryngoscope. 2001;111(4 Pt 1):615–21.
Isshiki N, et al. Midline lateralization thyroplasty for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109(2):187–93.
Isshiki N, Yamamoto I, Fukagai S. Type 2 thyroplasty for spasmodic dysphonia: fixation using a titanium bridge. Acta Otolaryngol. 2004;124(3):309–12.
Sanuki T, Isshiki N. Overall evaluation of effectiveness of type II thyroplasty for adductor spasmodic dysphonia. Laryngoscope. 2007;117(12):2255–9.
Sanuki T, Isshiki N. Outcomes of type II thyroplasty for adductor spasmodic dysphonia: analysis of revision and unsatisfactory cases. Acta Otolaryngol. 2009;129(11):1287–93.
Sanuki T, Yumoto E. Long-term evaluation of type 2 Thyroplasty with titanium bridges for adductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2017;157(1):80–4.
Chan SW, et al. Long-term results of type II thyroplasty for adductor spasmodic dysphonia. Laryngoscope. 2004;114(9):1604–8.
Tsuji DH, et al. Impact in vocal quality in partial myectomy and neurectomy endoscopic of thyroarytenoid muscle in patients with adductor spasmodic dysphonia. Braz J Otorhinolaryngol. 2006;72(2):261–6.
Schuering JHC, et al. Adductor spasmodic dysphonia: botulinum toxin a injections or laser thyroarytenoid myoneurectomy? A comparison from the patient perspective. Laryngoscope. 2020;130(3):741–6.
Su CY, et al. Transoral laser ventricular fold resection and thyroarytenoid myoneurectomy for adductor spasmodic dysphonia: long-term outcome. Laryngoscope. 2010;120(2):313–8.
Nakamura K, et al. Surgical treatment for adductor spasmodic dysphonia—efficacy of bilateral thyroarytenoid myectomy under microlaryngoscopy. Acta Otolaryngol. 2008;128(12):1348–53.
Nomoto M, et al. The comparison of thyroarytenoid muscle myectomy and type II thyroplasty for spasmodic dysphonia. J Voice. 2015;29(4):501–6.
Remacle M, et al. Bipolar radiofrequency-induced thermotherapy (rfitt) for the treatment of spasmodic dysphonia. A report of three cases. Eur Arch Otorhinolaryngol. 2005;262(10):871–4.
Kim HS, et al. Radiofrequency thyroarytenoid myothermy for treatment of adductor spasmodic dysphonia: how we do it. Clin Otolaryngol. 2008;33(6):621–5.
Blitzer A, et al. Abductor laryngeal dystonia: a series treated with botulinum toxin. Laryngoscope. 1992;102(2):163–7.
Woodson G, Hochstetler H, Murry T. Botulinum toxin therapy for abductor spasmodic dysphonia. J Voice. 2006;20(1):137–43.
Klein AM, et al. Vocal outcome measures after bilateral posterior cricoarytenoid muscle botulinum toxin injections for abductor spasmodic dysphonia. Otolaryngol Head Neck Surg. 2008;139(3):421–3.
Benito DA, Ferster APO, Sataloff RT. Bilateral posterior cricoarytenoid myoneurectomy for abductor spasmodic dysphonia. J Voice. 2020;34(1):127–9.
Shaw GY, Sechtem PR, Rideout B. Posterior cricoarytenoid myoplasty with medialization thyroplasty in the management of refractory abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2003;112(4):303–6.
Postma GN, Blalock PD, Koufman JA. Bilateral medialization laryngoplasty. Laryngoscope. 1998;108(10):1429–34.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Schuering, J.H.C., Sjögren, E.V., Langeveld, A.P.M. (2022). Spasmodic Dysphonia. In: Remacle, M., Eckel, H.E. (eds) Textbook of Surgery of Larynx and Trachea. Springer, Cham. https://doi.org/10.1007/978-3-031-09621-1_20
Download citation
DOI: https://doi.org/10.1007/978-3-031-09621-1_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09620-4
Online ISBN: 978-3-031-09621-1
eBook Packages: MedicineMedicine (R0)