Abstract
Liver cancer is the second leading cause of cancer-related death worldwide. Hepatocellular carcinoma (HCC) accounts for approximately 90% of liver cancer-related deaths. The liver is a central immunomodulator that ensures its protection through immunotolerance. Liver immunotolerance results from complex interactions between liver-resident cells and peripheral leukocytes. Several cells of immunity are involved in the mechanism of hepatocarcinogenesis: macrophages, myeloid-derived suppressor cells, Kupffer cells, neutrophils, dendritic cells and natural killer cells. The interactions are maintained by proinflammatory cytokines (IL-2, IL-7, IL-12, IL-15 and IFN-γ) and anti-inflammatory cytokines (IL-10, IL-13 and TGF-β). Also, the effects of chemokines and their receptors in HCC range from promoting to inhibiting tumor growth. They regulate the recruitment of white blood cells and play a crucial role in many events, such as angiogenesis, Th1/Th2 development, inflammatory diseases and tumor. They seem to be strictly associated with HCC and correlate with distant organ and lymph node metastasis, even though the exact mechanism is still unknown. Dysregulation of this immunological network plays a key role in the development and progression of chronic liver diseases and HCC.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Immunotolerance
- Innate immune system
- Adaptive immune system
- Macrophages
- Neutrophils
- Dendritic cells
- Kupffer cells
- Natural killer cells
- Interleukins
- Chemokines
1 General Aspects
Liver cancer is the second leading cause of cancer-related deaths worldwide. Hepatocellular carcinoma (HCC) accounts for approximately 90% of liver cancer-related deaths [1, 2]. The liver has considerable capacity to remove gut-derived microbial compounds and pathogens from the circulation and is involved in the detection and clearance of blood-borne infectious organisms [3].
This is reflected in the multitude of innate and adaptive immune cells in the liver.
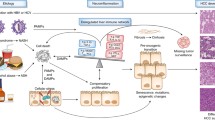
Dysregulation of immunological networks plays a key role in the development and progression of chronic liver diseases and HCC. In chronic viral hepatitis, alcoholic and metabolic liver disease, chronic inflammation and an altered immune response are all associated with the development of HCC [4,5,6].
Hepatocarcinogenesis can arise from various different factors promoting tumor antigen tolerance, such as decreased recognition of malignant cells, suppression of immunity and chronic inflammation [7–8].
In necroinflammation, altered survival and proliferation signals are generated and these result in cellular DNA damage. The proliferation of damaged hepatocytes leads to neoplastic transformation [5, 9,10,11].
Pro-inflammatory cytokines, such as IL-6 and TNF which activate transcription factors, play an important role in the development and progression of HCC. Moreover, the innate and adaptive immune systems are important in the detection and elimination of transformed cells; their alteration is associated with disease progression (Fig. 3.1).
The liver is a major immunomodulator, its protective function being liver-modulated immune tolerance. Liver immune tolerance results from complex interactions between liver-resident cells and peripheral leukocytes. The interactions are maintained by pro-inflammatory cytokines (IL-2, IL-7, IL-12, IL-15 and IFN-γ) and anti-inflammatory cytokines (IL10, IL-13 and TGF-β) [8]. Thus, understanding this immunological network is crucial to identifying new and increasingly effective treatments.
2 Innate Immune System
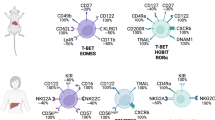
Several immune cells are involved in the mechanism of hepatocarcinogenesis: macrophages, myeloid-derived suppressor cells, Kupffer cells (KCs), neutrophils, dendritic cells (DCs) and natural killer cells (NKs).
2.1 Macrophages
Macrophages exert a phagocyte function and play a critical role in pro-inflammatory response and pathogen clearance. They also induce the cytotoxicity of target cells, critical for anticancer immunity.
Tumor-associated macrophages (TAMs) are mainly derived from monocytes, from the bone marrow and spleen and they constitute the main inflammatory cells [12,13,14,15]. In infiltrating tumors, TAMs develop an M2 phenotype characterized by expression of immunomodulatory cytokines (IL-10 and TGF-β) and poor antigen presentation capacity. M2 macrophages produce tumor-promoting and immunosuppressive cytokines and growth factors related to tissue regeneration and angiogenesis. In particular, in HCC IL-6 and TGF-β promote tumor growth, IL-1, TNF-α and IL-6 are involved in invasion and metastasis and TGF-β and IL-20 reduce the anti-tumor immune response. TAMs M2 increase the recruitment and development of regulatory T cells (Tregs) through the activation of a T helper type 2 immune response [16,17,18].
2.2 Myeloid-Derived Suppressor Cells
In HCC, monocyte-derived macrophages contribute to the recognition and clearance of senescent hepatocytes, preventing tumor development. When, however, these cells acquire a myeloid-derived suppressor cell phenotype, they support tumor growth. They suppress T cell infiltration in the tumor and dendritic cell function and promote the expansion of Tregs through the up-regulation of free radicals, arginase activity and production of TGF-β [18, 19].
2.3 Kupffer Cells
Kupffer cells (KCs), the liver’s resident macrophages, are involved in chemical carcinogenesis-induced hepatocarcinogenesis. They are central to pathogen capture as they clear bacteremia and recruit immune cells to the liver. KCs express an array of scavenger receptors in order to internalize pathogens. At later stages of disease, dying hepatocytes may release danger signals (danger-associated molecular patterns, DAMPs) triggering activation of KCs through Toll-like receptors. Activated KCs produce anti-inflammatory cytokines in response to bacterial endotoxins and downregulate the action of antigen-presenting cells [20, 21]. In addition, KCs can be activated by hypoxic conditions stimulating inflammation also by production of IL-6.
2.4 Neutrophils
Neutrophils are the most common tumor-infiltrating immune cells and, when in large numbers, they are predictive of a poorer outcome. Neutrophils can promote hepatocarcinogenesis by enhancing cell growth, angiogenesis and metastasis through production of growth factors HGF and VEGF. Moreover, neutrophils suppress anti-tumor immunity by producing many pro-oncogenic ligands. In HCC, tumor-associated neutrophils interact closely with KCs and recruit Tregs and macrophages, resulting in immune tolerance [22, 23].
2.5 Natural Killer Cells
Natural killer (NK) cells are a key part of the innate immune response against viruses and tumors. They exert cytotoxic activity and regulate immune cell functions through cytokine release (IFN-γ in particular) [24]. Their role is similar to that of cytotoxic T lymphocytes (CTLs); however, NK cells react more quickly during the immune reaction and they can also recognize target cells in the absence of MHC. This potential is especially important because cancer cells missing MHC I molecules can only be killed by NK cells [25]. More recently it has been shown that NK cells participate in the adaptive immune response through crosstalk with dendritic cells and T cells. In HCC patients, there is a reduced presence of NK cells with impaired activity in the liver. There is also a depletion in peripheral blood with reduced levels of IFN-γ secretion.
2.6 Dendritic Cells
Dendritic cells (DCs) act as a messenger between the innate and the adaptive immune systems. DCs recognize, process and present tumor antigen and are thus essential for the immune response against tumor. Failed HCC-associated antigen presentation by DCs can lead to a weak T cell immune response. This lack of function of DCs may be due to a decreased expression of human leukocyte antigen class-I molecules and maturation defects determining an alteration in cytokine production, in particular a reduction of IL-12 production and an increased release of IL-10 and TGF-β [26, 27]. Thus, defects in DCs promote immune tolerance and are associated with the initiation and progression of HCC. There are fewer activated DCs in the liver tissues of patients with HCC and these are unable to infiltrate cancer nodules, resulting in a reduced recruitment of specific lymphocytes. In addition, DCs indirectly promote proliferation of transformed hepatocytes through their inhibitory effect on CD8+ T cells.
3 Adaptive Immune System
Immune cells, such as T lymphocytes, are present in HCC and are crucial in the surveillance and clearance of tumor cells. An abundance of both CD4+ T helper cells and CD8+ cytotoxic T cells correlates with a favorable prognosis in many cancers. CD8+ CTLs recognize tumor antigens carried by antigen presenting cells (APCs) via MHC class I molecules, and kill them by direct lysis or by secretion of cytokines (IFN-γ and TNF-α). Many studies report a significant decrease in CD4+ T cells and an exhausted phenotype of CTLs in HCC patients; these findings are associated with poor prognosis. Continuous antigen presentation in the liver in the absence of CD4+ cells and monocytes derived IL-10 induces antigen-specific tolerance. A memory-like virus-specific CD8+ T cell subset with features of T cell exhaustion has been observed during chronic infection with HCV, persisting even after chronic antigen stimulation ended [28].
A decrease in the ratio of T helper/T suppressive cells is seen in the peripheral blood of patients with cirrhosis and HCC, while a high CD4+/CD8+ T cell ratio is significantly associated with lower recurrence of HCC after liver transplant. A subset of T helper cells, the follicular T-helper cells (Tfh), supports in-germinal center B-cell activation and maturation in plasma cells. Impairment in these cells appears to be associated with disease progression in HBV-related HCC.
The presence of Tregs, a small sub-population of CD4+ T cells in the tumor microenvironment, is involved in tumor cells escaping immune surveillance and clearance. Tregs inhibit effector B and T cell function after antigen response. Infiltrating Tregs gradually increase during the progression of carcinogenesis.
We can therefore say that the progression of liver diseases correlates with a dysregulated cellular immune response [29, 30].
3.1 Interleukins and Chemokines
Immune suppression in the liver is predominantly mediated by cytokines. Liver immune tolerance results from interactions between liver resident cells and peripheral leukocytes. This environment is maintained by pro-inflammatory cytokines (IL-2, IL-7, IL-12, IL-15, IFN-γ) and anti-inflammatory cytokines (IL-6, IL-10, IL-13, TGF-β). As long as HCC is a typical inflammation-related cancer, interleukin molecules can play crucial roles in the development and progression of the disease [31].
Interleukins (ILs) are cytokines that regulate inflammatory and immune responses. They activate and regulate immune cells and participate in an inflammatory cascade. Th1 cells are involved in cell-mediated immune responses, the Th2 cells in humoral-mediated immunity. Hepatocytes express receptors for several cytokines, rendering them susceptible to their action.
In the presence of IL-12 and IFN-γ, naïve CD4+ cells (activated through MHC class II recognition) differentiate into Th1 cells, and activated CD8+ cells. In the presence of IL-4, CD4+ T cells differentiate in Th2 cells. Expression of the Th1 cytokine (IL-1, IL-2, IFN-γ) in tumor tissue is associated with a good prognosis, whereas expression of the Th2 cytokine (IL-4, IL-5, and IL-10) is associated with vascular invasion or metastases. In liver cancer cells, the cytokine milieu often switches to a Th2 profile, which inhibits the tumor-specific CD8 T-cell response, boosts anti-inflammatory cytokines and lowers pro-inflammatory ones. The causative factor of this shift is still unknown. In spite of these findings, we often find overexpression of Th1 cytokines in HCC.
The best known anti-inflammatory cytokines in HCC are IL-6 and IL-10. They suppress T-cell activation and their levels often result increased in HCC. These higher levels seem to correlate with disease progression and a poor prognosis. Specifically, IL-10 downregulates the major histocompatibility complex, facilitating T cell tolerance. IL-6 and TNF, which activate HCC progenitor cells, are mostly produced by resident macrophages [7, 16, 18]. IL-17, a pro-inflammatory mediator, has pro-tumorigenic effects, promoting cirrhosis progression and HCC development. It is associated with a poor prognosis. IL-22, released by Th 22 cells, is high in HCC patients, a finding which correlates to disease progression.
The roles of chemokines and their receptors in HCC range from promoting to inhibiting tumor growth.
A family of small soluble proteins, chemokines by regulating the recruitment of white blood cells play a crucial role in many events, including angiogenesis, Th1/Th2 development, inflammatory diseases and tumor. They seem to be strictly associated with HCC and correlate with distant organ and lymph node metastasis, even though the exact mechanism is still unknown.
High expression of CXCL12-CXCR4 is found in HCC and surrounding tissues. The CXCL12-CXCR4 axis is implicated in angiogenesis, promoting growth, invasion and metastasis, while the CCL21-CCR7 axis correlates with lymph node metastasis. The CCL20-CCR6 axis is associated with tumor progression; in particular, HCC cell lines with high expression of CCR6 correlate with formation of pseudopodia, augmented intrahepatic metastasis and poor disease-free survival. The expression levels of some chemokine receptors, such as CXCR3 in tumor-infiltrating cells, is higher than in peripheral lymphocytes, suggesting a role in addressing migration of effector T cells into the tumor.
Not all chemokines promote tumor growth. High expression of fractalkine/CX3CL1 in particular correlates with better prognosis in HCC patients, probably by the direct killing of tumor cells [32].
4 Conclusions
HCC is the most common type of liver cancer. It occurs in the setting of chronic liver inflammation and is most closely linked to chronic viral hepatitis infection (hepatitis B or C) or exposure to toxins such as alcohol, aflatoxin, or pyrrolizidine alkaloids. Certain diseases, such as hemochromatosis and alpha 1-antitrypsin deficiency, markedly increase the risk of developing HCC. Metabolic syndrome and NASH are also increasingly recognized as risk factors for HCC. Deregulation of controlled immunological network inevitably leads to liver disease, including chronic infection, autoimmunity and tumor development. Persistent upregulation of inflammatory signals due to chronic liver damage leads to necroinflammation (activation of immune cells, altered immunological, survival and proliferation signals and promotion of liver fibrosis) and, subsequently, the induction of tumorigenesis.
The innate and adaptive immune systems are important for the detection and elimination of transformed cells. However, this process is dysregulated in necroinflammation, and anti-inflammatory cytokines (e.g., IL-10 and TGF-β) suppress proper anti-tumor immune responses. The study of these mechanisms is crucial, as early and sustained elimination of the underlying chronic liver damage is key to reducing the risk of HCC and end-stage liver disease. Furthermore, the highly immunotolerant environment and tightly controlled protective mechanisms in the liver make the development of effective immunotherapies for HCC challenging [5]. The identification and validation of immunological biomarkers in HCC and the clinical characterization of patients will be crucial for the generation of favorable responses to novel immunotherapies.
References
Yao SY, Liang B, Chen YY, et al. Clinical stages of recurrent hepatocellular carcinoma: a retrospective cohort study. World J Clin Cases. 2021;9(27):8020–6.
Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52(12):1898–907.
Øie CI, Wolfson DL, Yasunori T, et al. Liver sinusoidal endothelial cells contribute to the uptake and degradation of entero bacterial viruses. Sci Rep. 2020;10(1):898. https://doi.org/10.1038/s41598-020-57652-0.
Cho HJ, Cheong JY. Role of immune cells in patients with hepatitis B virus-related hepatocellular carcinoma. Int J Mol Sci. 2021;22(15):8011. https://doi.org/10.3390/ijms22158011.
Ringelhan M, Pfister D, O’Connor T, et al. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–32.
Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. https://doi.org/10.1038/s41698-018-0048-z.
Sachdeva M, Chawla YK, Arora SK. Immunology of hepatocellular carcinoma. World J Hepatol. 2015;7(17):2080–90.
Bian J, Lin J, Long J, et al. T lymphocytes in hepatocellular carcinoma immune microenvironment: insights into human immunology and immunotherapy. Am J Cancer Res. 2020;10(12):4585–606.
Alqahtani A, Khan Z, Alloghbi A, et al. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas). 2019;55(9):526. https://doi.org/10.3390/medicina55090526.
Lin H, Huang YS, Fustin JM, et al. Hyperpolyploidization of hepatocyte initiates preneoplastic lesion formation in the liver. Nat Commun. 2021;12(1):645. https://doi.org/10.1038/s41467-020-20572-8.
Ramakrishna G, Rastogi A, Trehanpati N, et al. From cirrhosis to hepatocellular carcinoma: new molecular insights on inflammation and cellular senescence. Liver Cancer. 2013;2(3–4):367–83.
Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32(19–20):1267–84.
Laviron M, Boissonnas A. Ontogeny of tumor-associated macrophages. Front Immunol. 2019;10:1799. https://doi.org/10.3389/fimmu.2019.01799.
Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86(5):1065–73.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Capece D, Fischietti M, Verzella D, et al. The inflammatory microenvironment in hepatocellular carcinoma: a pivotal role for tumor-associated macrophages. Biomed Res Int. 2013;2013:187204. https://doi.org/10.1155/2013/187204.
Nouri-Vaskeh M, Mirza-Aghazadeh-Attari M, Pashazadeh F, et al. Prognostic impact of monocyte to lymphocyte ratio in clinical outcome of patients with hepatocellular carcinoma: a systematic review and meta-analysis. Galen Med J. 2020;9:e1948. https://doi.org/10.31661/gmj.v9i0.1948.
Zhu XD, Zhang JB, Zhuang PY, et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol. 2013;31(24):3049.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74.
Roderburg C, Wree A, Demir M, et al. The role of the innate immune system in the development and treatment of hepatocellular carcinoma. Hepat Oncol. 2020;7(1):HEP17. https://doi.org/10.2217/hep-2019-0007.
Koh MY, Gagea M, Sargis T, et al. A new HIF-1α/RANTES-driven pathway to hepatocellular carcinoma mediated by germline haploinsufficiency of SART1/HAF in mice. Hepatology. 2016;63(5):1576–91.
Li X, Xing YF, Lei AH, et al. Neutrophil count is associated with myeloid derived suppressor cell level and presents prognostic value of for hepatocellular carcinoma patients. Oncotarget. 2017;8(15):24380–8.
Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11(3):224–31.
Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57(4):1654–62.
Zhang DY, Friedman SL. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56(2):769–75.
Matsui M, Machida S, Itani-Yohda T, Akatsuka T. Downregulation of the proteasome subunits, transporter, and antigen presentation in hepatocellular carcinoma, and their restoration by interferon-gamma. J Gastroenterol Hepatol. 2002;17(8):897–907.
Ninomiya T, Akbar SM, Masumoto T, et al. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31(2):323–31.
Budhu A, Forgues M, Ye QH, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on a unique immune response signature of the liver microenvironment. Cancer Cell. 2006;10(2):99–111.
Hou J, Zhang H, Sun B, Karin M. The immunobiology of hepatocellular carcinoma in humans and mice: basic concepts and therapeutic implications. J Hepatol. 2020;72(1):167–82.
Nishida N, Kudo M. Immunological microenvironment of hepatocellular carcinoma and its clinical implication. Oncology. 2017;92(Suppl 1):40–9.
Robinson MW, Harmon C, O’Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13(3):267–76.
Huang F, Geng XP. Chemokines and hepatocellular carcinoma. World J Gastroenterol. 2010;16(15):1832–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Taibi, C., Vincenzi, L., D’Offizi, G. (2023). Role of the Immune System in Hepatocellular Carcinoma. In: Ettorre, G.M. (eds) Hepatocellular Carcinoma. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-09371-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-09371-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-09370-8
Online ISBN: 978-3-031-09371-5
eBook Packages: MedicineMedicine (R0)