Abstract
Last decade has witnessed tremendous growth in the new promising treatment options based on mRNA, RNAi, antisense RNA, and RNA aptamers, the four classes of RNA-based therapeutics. Among these, mRNA-based therapy is centered on producing proteins within the cells to supplant deficient or abnormal proteins and in vaccination to a target pathogen. The potential of mRNA therapeutics is evident from the two major mRNA vaccines approved for COVID-19: developed by Moderna and by Pfizer. Nonetheless, mRNA therapeutic potential extends far beyond this, such as in treating genetic diseases, cancers, and other infectious diseases. Given the potential of mRNA therapeutics, this chapter is written to provide the reader an insight into the features of several synthetic mRNA platforms, production, purification; strategies to increase the stability and reduce the immunogenicity of therapeutic mRNA molecules; delivery methods of these mRNAs in vivo; and their applications, safety, and efficacy.
Graphical abstract

Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Chemically modified mRNA
- In vitro transcription
- mRNA therapeutics
- mRNA vaccines
- Immunogenicity
- Immunostimulatory
- In vivo delivery
- Cell-mediated immunity
1 Introduction
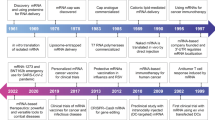
Ever since its discovery in 1961, the mRNA molecule has been under research and discussion. With the advancement in technology, in vitro transcribed (IVT) mRNA was investigated for its properties, assorted uses, and functions. Of the various applications, the use of IVT mRNA in the replacement of protein and vaccinations for cancers and several infectious diseases have been investigated. Some characteristics that make IVT mRNA a better candidate for therapeutics as compared to DNA are as follows:
-
(i)
IVT mRNA can be translated into desired protein immediately upon its arrival into the cytoplasm without any prerequisite to enter the nucleus.
-
(ii)
It will not lead to insertional mutations as it doesn’t get incorporated into the host genome and hence, it is fast-acting and safe (Sahin et al. 2014; Pardi et al. 2018).
-
(iii)
It is active for a short duration and can easily be degraded, which makes it suitable in various pharmaceutical applications.
-
(iv)
It can be produced cost-effectively with ease within the specified time (Sahin et al. 2014; Zhong et al. 2018).
-
(v)
It can also be used to produce pluripotent stem cells (Sergeeva et al. 2016).
Additionally, its in vivo efficacy is well documented. The transfected liver cells might attain 100% efficacy (Sergeeva et al. 2016). Besides, mRNA can also encode zinc-finger nucleases (ZFN), transcription activator-like effector nucleases (TALEN), and even CRISPR-Cas9 and can further be employed in genome editing (Sergeeva et al. 2016). IVT mRNA-based vaccines are a promising alternative to conventional vaccines as they can induce immune modulation with an increase in humoral as well as cell-mediated immunity (CMI) due to their self-adjuvanting nature (Sahin et al. 2014). mRNA vaccines are a safe platform and proved to be very effective as in the case of COVID-19 vaccines. They elicited elevated B-cell responses, CD4+ Type 1 T helper cell responses, and strong interferon-gamma (IFN-γ), and interleukin-2 (IL-2) producing CD8+ cytotoxic T-cell (TC) responses (Walsh et al. 2020; Bettini and Locci 2021).
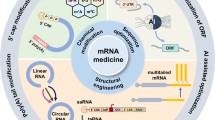
Formulation of mRNA into carrier molecules can lead to efficient in vivo delivery. Moreover, mRNA vaccines can be administered multiple times, as there is no anti-vector immunity because mRNA is the smallest genetic vector (Pardi et al. 2018). The mRNA is usually manufactured either from a linearized plasmid DNA (pDNA) template or from a PCR product in a cell-free system by in vitro transcription with the help of T7 or SP6 RNA polymerase and is capped enzymatically. IVT mRNA undergoes translation process in vivo and forms the protein which in turn undergoes post-translational modification achieving its bioactive configuration. The pharmacokinetics of mRNA-based therapeutics largely depends on the half-life of IVT mRNA as well as of the encoded protein, and the pharmacodynamics depends on the different processing pathways encountered by the encoded protein. Several other factors of the encoded protein like the biological functions, mode of action, among others are responsible for determining the total amount of IVT mRNA dose required for a particular therapeutic regimen (Sahin et al. 2014). IVT mRNA-based therapeutics have undergone leaps and bounds due to immense potential to deliver personalized intervention permitting patients to synthesize therapeutic proteins within themselves, eliminating the need for purification, glycosylation, and other solubility barriers linked with the conventional recombinant protein therapies.
Three major hurdles allied with mRNA are its short half-life, unpropitious immunogenicity, and in vivo delivery (Sahin et al. 2014). Structural modifications of IVT mRNA can enhance stability and encapsulating mRNA with suitable carrier molecules will ease their delivery and lead to rapid uptake. Codon optimization and nucleoside modifications can alleviate the immunogenicity to varying degrees and also affect the secondary structure of mRNA, kinetics, and accuracy of translation and proper folding (Sergeeva et al. 2016).
2 Production of IVT mRNA
For therapeutic uses, mRNA is produced synthetically by in vitro transcription of either a linear pDNA or a PCR template containing a promoter of bacteriophage origin. IVT requires modified nucleotides and an RNA polymerase (RNAP) enzyme usually derived from a bacteriophage (T7, SP6, or T3) which recognizes the bacteriophage promoter present on the DNA template to be transcribed. Capping and tailing of the synthetically produced mRNA are carried out as the translation of mRNA in the eukaryotic cell requires the presence of a 5′ cap and a 3′ poly(A) tail. A mature IVT mRNA contains a 5′ cap, a 5′ untranslated region (UTR), an open-reading frame (ORF), a 3′ UTR, and a 3′ poly(A)/polyadenylation tail. This kind of production process leads to the formation of certain by-products resulting in increased immunogenicity, which must be removed through the purification steps (see Sect. 5) (Sergeeva et al. 2016).
Recently, a novel, simple, and scalable method has been developed to synthesize functional mRNA with reduced immunogenicity in vivo. This method makes use of high temperatures, a thermostable T7 RNAP, and template-encoded poly(A) tail. All these features together prevented the formation of dsRNA—an unwanted by-product (Wu et al. 2020).
3 Immunogenicity of IVT mRNA
The immune response elicited depends on the size and type of the carrier of IVT mRNA. Several components like RNA sensors in cells and structural parts of mRNA involved in the activation of the immune system have been identified. When the IVT mRNA is recognized by pattern recognition receptors (PRRs) like toll-like receptors (TLRs), protein kinases (PKRs), or the retinoic acid-inducible gene 1 (RIG-1)-like receptors (RLRs), an immune response is generated. TLR3 and PKR identify dsRNA, whereas TLR7 and TLR8 identify ssRNA and upon activation, both the sets induce IFNs secretion (Sahin et al. 2014; Sergeeva et al. 2016). The most effective inducer of IFN is Poly(U) which acts through binding with TLR7 (Sahin et al. 2014).
Several applications like protein-replacement therapies do not require activation of the innate immune system which rather is a drawback (Sahin et al. 2014). A unique approach to curb the innate immune response makes use of innate immune inhibitors which either avert the identification of synthetic mRNA by PRRs or block several downstream mediators in the TLR-3, TLR-7, RIG-1 pathways. Some inhibitors for released IFNs and cytokines have been recognized. Chloroquine, an anti-malarial drug, was the first candidate to reduce tumor-necrosis factor-alpha (TNF-α), ILs, and IFNs type-I. Although it was anticipated that TLRs present in the endosomes do not get activated as chloroquine is capable of averting endosomal acidification, it was reported to be ineffective in the human foreskin fibroblast (HFF) cell line. Other inhibitors like trichostatin A, Pepinh-TRIF, and Pepinh-MYD are also partially effective. Trichostatin A blocks the nuclear translocation of IRF7 (Interferon Regulatory Factor 7), Pepinh-TRIF blocks the interaction of TRIF (TIR-domain-containing adapter-inducing interferon-β) with TLR3 and Pepinh-MYD blocks the interaction of MYD88 (Myeloid differentiation primary response 88) with TLR7/8. Two appealing small inhibitors—BAY11, an anti-inflammatory compound, and BX975, an aminopyrimidine compound inhibits Nuclear Factor (NF-κB) and IRF3/7 activation respectively, thereby curbing the immunogenicity of synthetic mRNA (Zhong et al. 2018).
4 Strategies to Increase the Stability and Reduce the Immunogenicity of IVT mRNA
The stability, translational efficiency, and immunogenicity of mRNA can be regulated by modifying its structural elements as per the requirement (Sahin et al. 2014).
4.1 Capping (m7GpppN or m7Gp3N)
The addition of a 5′ cap (m7GpppN, also called cap-0) through a 5′-5′ triphosphate bond is important as it stabilizes the mRNA by abolishing the free phosphate groups and preventing its degradation by the nucleases. It is also important for the initiation of translation, helps avoid the recognition of the synthetically produced mRNA as a foreign entity by innate immune sensors and activities like splicing, transport, and translation of mRNA. Cap-0 is later on methylated in the cytoplasm on the first and second nucleotides (nucleoside-2′-O) of mRNA to generate cap-1 and cap-2 structures. 2′-O-methylated cap is required for efficient translation of mRNA (Sergeeva et al. 2016; Zhong et al. 2018; Xu et al. 2020). The cap is added either during in vitro transcription called co-transcriptional capping or post-transcription. Several editions of 5′ cap such as anti-reverse cap analogs (ARCAs) or synthetic caps can be added with the help of vaccinia virus capping enzymes (Pardi et al. 2018). ARCA is a cap analog that is modified with the replacement of the 3′ OH group with the −OCH3 group. The initiation of transcription with the remaining hydroxyl group forces ARCA incorporation only in the forward orientation. Therefore, ARCA results in 100% of the transcripts produced with the capping at the 5′ end, and all such transcripts are translatable in the cell.
In 2018, a co-transcriptional CleanCap® capping method was developed that made use of an initiating capped trimer to generate cap-1 on the 5′ end of IVT mRNA. This resulted in the production of naturally occurring 5′ cap with increased capping efficiency reaching up to 90–99% (Xu et al. 2020). 100% efficacy is not possible when capping is done in vitro. Hence, 5′ triphosphates of mRNA transcripts must be removed with the help of phosphatase, thereby decreasing the immunogenicity as well as degradation of IVT mRNA (Sergeeva et al. 2016). Remaining uncapped or incompletely capped mRNA molecules can lead to the production of unnatural ends or anomalous RNAs such as dsRNAs produced by self-complementarity and extension resulting from self-priming (Schlake et al. 2019). In the cell cytosol, mRNA is decapped by the decapping enzymes of the cellular machinery, leading to instability. To reduce the pace of the decapping, reduce immunogenicity, and increase stability, chemically modified analogs of 5′ cap can be used (Grudzien-Nogalska et al. 2007).
-
(i)
Methylation/removal of the 3′ OH group which wedges N7-methylated guanosine (m7G) residue elongation (Sergeeva et al. 2016).
-
(ii)
Addition of a phosphorothioate group results in a twofold reduction in retinoic acid-inducible gene 1 (RIG-1) activation by ssRNA and adjusts the binding between the cap-region and mRNA degrader protein Dcp2 (Sergeeva et al. 2016). Phosphorothioate modification of ARCA further enhances stability and translational efficiency (Xu et al. 2020).
-
(iii)
Addition of 2-thiouridine (S2U), N6-methyladenosine (m6A), 5-methyluridine (m5U), 5-methoxyuridine (mo5U), 5-methylcytidine (m5C), pseudouridine (Ψ), and N-methyl pseudouridine (m Ψ) could bring down the activation of TLRs, PKRs, and RIG-1 receptors and downregulate type-1 IFN signaling. As a consequence of m6A addition, there is an interaction of YT521-B homology (YTH) family proteins that reduces the half-life of mRNA by inciting its degradation. So, this modification should be avoided. The addition of Ψ enhances the translational rate, stabilizes secondary structure, and does not lead to any kind of toxicity since they are found naturally (Sergeeva et al. 2016).
-
(iv)
Capping with m7GmppSpG (β-S-ARCA) increased the stability and translational efficiency appreciably in the immature dendritic cells (iDCs) (Xu et al. 2020).
-
(v)
2S analogs which are derived from the combination of 1,2—dithiophosphate modification, ARCA, and an elongated polyphosphate chain outshines any other capping methodology (Xu et al. 2020).
4.2 Tailing
There are many different ways to achieve polyadenylation of the synthetic mRNA, namely post-transcriptional tailing by the enzyme poly(A)polymerase or by introducing a poly(T) sequence into the DNA template that encodes for poly(A) tail. Also, while using the PCR template for synthetic mRNA production, poly(T) primers which bind at the 3′ end of the insert have been successfully used. Poly(A) tail shows a co-operative effect with other elements like the 5′ cap, the internal ribosomal entry site (IRES), poly(A)-binding proteins (PABPs), translation initiation factor eIF4G, etc., to regulate the stability and translatability of mRNA (Sahin et al. 2014). The cap and the tail circularize the mRNA and form an association with PABPs and eIF4G. This leads to a development in binding to the ribosome and further prevents the destruction of mRNA. To reduce the deadenylation through the activity of poly(A)-specific nucleases, modified nucleotides must be integrated into the poly(A) tail (Sahin et al. 2014; Sergeeva et al. 2016). But some of the modifications were a disappointment, e.g., cordycepin (3ʹ‑deoxyadenosine) could not increase the half-life of mRNA probably due to its failure to fully incorporate at the 3′ end as it is a chain terminator (Sahin et al. 2014). Nonetheless, cordycepin can be readily converted to cordycepin 5′ triphosphate which can further be used for 3′ end labeling. The estimated length of the tail capable of plummeting mRNA immunogenicity is between 120 and 150 nucleotides. But in many cases, short tails work better. The length of the poly(A) tail should be appropriate for the binding of PABPs, both at the poly(A) tail and the cap, leading to mRNA circularization. Therefore, one must be very careful about the choice made on the tail length (Sahin et al. 2014; Sergeeva et al. 2016).
4.3 Untranslated Regions (UTRs)
IVT mRNA has UTRs at 5′ as well as 3′ ends flanking the coding region. 5′ UTR has a direct influence on the translational process of the downstream sequence of mRNA. Certain sequences like CC-(A/G)-CCAUGG can be incorporated to augment the stability and translational efficiency of mRNA. Some studies revealed that short and loose 5′ UTR is much more favorable for ribosomal binding compared to that of over-stabilized secondary structures (Xu et al. 2020).
The 3′ UTRs of many IVT mRNAs are derived from α- and β-globin mRNAs having certain sequence elements like iron-responsive elements (IRE; bounded by iron regulatory proteins—IRPs) that enhance the stability and translation of mRNA. When two human β-globin 3′ UTRs are set in head‑to‑tail orientation, the stability is amplified to a greater extent. Likewise, human heat shock proteins (Hsps) and several viral UTRs have also been employed to augment the expression of the protein. UTRs have some unstable regions (AU-rich or GU-rich regions) which must be substituted by stable structured sequences. This boosts the half-life of the mRNA (Sahin et al. 2014; Sergeeva et al. 2016; Xu et al. 2020).
A novel 3′ UTR motif has been identified with the help of cell culture-based systematic selection process. As compared to the 3′ UTR of the human β-globin, this motif could synthesize ~ threefold higher protein. These sequences are obtained from viral or eukaryotic genes and further can be altered through a method of systemic enrichment of RNA sequences that exist naturally. This increases the efficiency of mRNA and also its half-life (von Niessen et al. 2019).
4.4 Coding Region
The coding region in IVT mRNA may or may not be codon-optimized. Optimization of rare codons with frequent similar/synonymous codons is believed to enhance the translation efficiency. They do not alter the amino acid sequence, but can still increase the stability of mRNA and in some cases affect its secondary structure as well (Wang et al. 2021). The expression is increased by ~ 1.6 fold in a human T lymphocyte cell line with codon optimization of gag protein of HIV-1 (Ngumbela et al. 2008). Codon-optimized mRNA-encoding angiotensin-converting enzyme 2 (ACE2) exhibiting enhanced expression were transfected into A549 and HepG2 cells (Schrom et al. 2017; Schlake et al. 2019). Nonetheless, it should not be ignored that some proteins rely on slow translation for proper folding which can be guaranteed by the rare codons only. Some of the IVT mRNA vaccines work better with original ORF (Kimchi-Sarfaty et al. 2007).
5 Purification of Synthetic mRNA
The IVT mRNA has to be purified to remove DNA template, RNA polymerase, and unincorporated RNTPs. After the capping of the purified mRNA, the capping machinery also needs to be removed. Synthetic IVT mRNA may also be contaminated with short abortive RNA fragments, RNA-RNA hybrids, etc., which are immunostimulatory in nature and reduce the efficiency of translation. Hence, all these elements ought to be removed to reduce the magnitude of innate immunity responses. During the commercial production of IVT mRNA, initial purification is done by commercial purification kits succeeded by precipitation methods. Several methods are being used for the purification process which includes LiCl2 precipitation, silica membrane columns, etc., among others (Zhong et al. 2018). However, these methods fail to remove dsRNAs and other short abortive RNA fragments completely, and therefore another more efficient method such as reverse-phase high-performance liquid chromatography (HPLC) is used to reduce the immunogenicity of mRNA (Sahin et al. 2014; Sergeeva et al. 2016; Schlake et al. 2019). But this method is efficient for small length mRNAs only. Hence, other techniques such as size-exclusion chromatography, cross-flow filtration have emerged as better options for the purification of IVT mRNAs (Edelmann et al. 2014). Chromatographic techniques such as anion-exchange chromatography have also been successfully used for the purification of smaller synthetic mRNA (less than 500 nucleotides) (Zhong et al. 2018). Different techniques in combination may also be used to enhance the purity of synthetic mRNA. One such example is the use of hydroxyapatite chromatography (a common method used for separating nucleic acids and proteins) in conjunction with cellulose chromatography (Urayama et al. 2015).
6 Synthetic mRNA Platforms and their Features
There are various platforms for synthetic mRNA which are discussed below along with their advantages and disadvantages.
6.1 Unmodified mRNA
Unmodified mRNA are the mRNA molecules that do not have any modifications before in vivo delivery. These are recognized by the PRRs, and innate immune responses are induced by activating several such receptors (see Sect. 3). The unmodified mRNAs are also recognized by the nod-like receptors (NLRs), which can lead to pyroptosis mediated by caspase-1. By the activation of PRRs such as TLR, expression of type-I interferons such as IFN-α, IFN-β, and pro-inflammatory cytokines such as IL-12 are induced. These, unmodified mRNA molecules used as vaccines can act as brilliant self-adjuvants due to their ability to induce robust cellular and humoral immune response. However, these innate immune responses may be so severe that it may inhibit translation and lead to mRNA degradation by means of molecules such as dsRNA-dependent PKR, 2, 5-oligoadenylate synthetase (OAS) and adenosine deaminases (ADARs). Therefore, there should be a perfect balance between the mRNA expression and the innate immune responses. Unnecessary immune stimulation by the synthetic mRNA is a side effect of several protein-replacement therapies, reducing the efficacy of the treatment. Although some approaches are being used to reduce the immunogenicity of the unmodified mRNA (see Sect. 3) so that its full potential may be exploited for therapeutic usage, yet more exploration is required in this area (Zhong et al. 2018).
6.2 Modified mRNA
Addition of m5C/S2U/Ψ/m6A/m5U, etc., to the 5′ cap decreases the immune stimulation. Despite reduced immune responses against the modified mRNA, the level of protein expression may vary from cell to cell. Modified mRNA resulted in escalated protein expression in RAW 264.7 cells (Uchida et al. 2013), but lesser protein expression in HuH7 and MEFs cells which may be attributed to the differences in PRR activity in these cells. Another reason could be that modified mRNAs have lesser ability to be translated than their unmodified counterparts, due to the alteration of their secondary structure, leading to a decrease in the protein binding affinity to regulatory sequence elements located within the UTRs involved in the modulation of translation and stability of the synthetic mRNA molecule (Zhong et al. 2018).
The advantage of having reduced immunostimulatory action is the escalation in the production of the encoded protein. But, it is important to highlight that the modification of the mRNA does not always guarantee an increase in the expression levels as it may also depend on the nature of the target cell and the delivery system.
Apart from modified nucleotides, mRNAs may be modified by the use of specific regulatory sequences in the 5′ and 3′ UTRs. For example, the 5′ and 3′ UTRs of 17-β-hydroxysteroid dehydrogenase 4 can be incorporated within the synthetic mRNA to increase its efficacy. Some other UTRs used for modification of mRNA include UTRs of α- and β-globin, viral UTRs of Venezuelan equine encephalitis virus (VEEV) and Sindbis virus (SINV), etc. The addition of a histone stem-loop after the poly (A) tail has also been reported to increase the efficacy of synthetic mRNA (Zhong et al. 2018).
6.3 Sequence-Optimized Unmodified mRNA
Some studies have indicated that sequence-optimized unmodified mRNA is non-immunostimulatory with excellent levels of expression in vivo. Sequence-optimized unmodified mRNA coding for the protein erythropoietin was non-immunogenic and exhibited protein expression several times better than the ψ-modified mRNA (Zhong et al. 2018). Another approach for producing sequence-optimized unmodified mRNA can be the replacement of codons poor in GC content by similar codons which are rich in GC content. This leads to a decrease in the AU-rich codons resulting in lesser immune responses as AU-rich codons act as decay signals and are recognized by the TLR-3 and 7. Also, since uridine is the most commonly modified nucleoside in eukaryotic mRNA, a decrease in the uridine content also means reduced chances of PRRs recognizing the synthetic mRNA as a foreign entity (Meng and Limbach 2006; Zhong et al. 2018).
6.4 Replicon RNA
As the name suggests, replicon RNAs are self-amplifying RNAs containing the gene of interest, the sequence coding for viral replicase, and promoters (both genomic and subgenomic). These replicon RNAs can be used for the production of multiple proteins at a time. The most commonly used replicon RNAs are derived from viruses belonging to Alphaviruses such as SINV, VEEV, Kunjin virus (KUNV) (Zhong et al. 2018).
These replicons act through some basic steps such as translation of the gene-encoding viral replicase complex upon delivery into the cell cytoplasm, transcription of replicon RNA by replicase complex into a (−) sense complementary RNA strand, further used by replicase complex to generate a large number of (+) sense RNA, encoding the protein of interest. As observed with unmodified mRNA, replicon RNAs are also immunostimulatory (Akhrymuk et al. 2016). pDNA vectors can also be used for launching replicon RNA. These pDNA vectors have the gene coding for replicon RNA and hence helps achieve additional amplification as a result of the production of several copies of replicon for each pDNA molecule. These pDNA-launched replicon RNA are reported to be more immunostimulatory than replicon RNAs (Johansson et al. 2012; Zhong et al. 2018). Also, during the amplification process, a large number of single-stranded DNA and double-stranded RNA species are produced, eliciting immunity (Akhrymuk et al. 2016; Zhong et al. 2018). Hence, replicons may be beneficial for creating RNA vaccines. Several studies have suggested that non-structural proteins (NSPs) forming the replicase complex of alphaviruses cause severe cytopathic effect (CPE), resulting in cell death (Petrakova et al. 2005). This might raise the replicon RNA vaccine’s efficacy due to the production of certain immunostimulatory factors as well as encoded intracellular antigens in the extracellular environment. These are then taken up by the antigen presenting cells (APCs) and presented to the respective major histocompatibility complex (MHC)—molecules (Leitner et al. 2004). Certainly, when replicon RNAs are employed for protein therapies, such untimely death of target cells is not at all required as this would cause an obstruction in the overall protein production. To prevent the CPE and untimely death of the target cells, amino acid substitution is frequently done in the NSP2 protein.
7 In Vivo Delivery Strategies of Exogenous mRNA
As mRNA molecules are not very stable, it requires the assistance of a suitable carrier for its delivery in vivo. Approaches to the in vivo delivery of synthetic mRNA include direct uptake of naked mRNA, cationic-liposome-mediated RNA transfection or cationic nanoemulsion, peptide-based delivery, electroporation and nucleoporation, gene gun-mediated delivery of mRNA, use of polymer nanomaterials, virus-like replicon particle-based delivery of mRNA, and some other lesser-known techniques.
7.1 Delivery of Naked mRNA
This approach was first described already in 1990, which demonstrated temporal transgene expression after injection of naked mRNA into the skeletal muscle of mice (Rhoads 2016). Reversal of condition in diabetic rats with mRNA-encoding vasopressin, decrease in viral load in the lungs after delivery of naked mRNA expressing antigens against influenza A virus, respiratory syncytial virus (RSV) and louping ill virus are some examples of the efficacy of naked mRNA (Jirikowski et al. 1992; Fleeton et al. 2001). Delivery of naked mRNAs involves the direct injection of the mRNA in solution (commonly used mRNA solutions include Ringer’s solution and lactated Ringer’s solution) (Wang et al. 2021). Although it is assumed that naked mRNA are not able to freely cross the cellular membranes, a number of studies have proposed some hypotheses pertaining to its uptake mechanism. One of such studies hypothesizes that the uptake of naked mRNA molecule by the DCs in the central nervous system (CNS), after an intranodal injection, involves macropinocytosis, which allows the expression of the antigen-encoding mRNA along with the activation of T cells/DC (Wang et al. 2021). Intradermal injection of synthetic mRNA-encoding several antigens such as telomerase, survivin, human EGF2, in 30 patients resulted in benefits to a few patients (Rittig et al. 2011). Intradermal injection of naked mRNA-encoding influenza virus hemagglutinin (HA) antigen in animals such as mouse, pigs, resulted in these animals gaining immunity to influenza A infections (Petsch et al. 2012).
7.2 Cationic Liposome-Mediated or Cationic Nanoemulsion (CNE)-based RNA Transfection
The use of complexing agents for the delivery of mRNA has an advantage over naked mRNA, as it helps mRNA resist the degrading action of nucleases. Also, by binding to the self-amplifying RNAs, it potentiates the mRNA vaccine. The most important component for achieving delivery via this method is the cationic lipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOTAP). In the very first attempt, incorporation of IVT mRNA-encoding luciferase enzyme into cationic liposome exhibited a considerable response in transfected mouse NIH 3T3 cells, where the luciferase expression was demonstrated to be linearly correlated to dose (Rhoads 2016). Furthermore, a liposome-protected mRNA vaccine-encoding human carcinoembryonic (HCE) tumor antigen has been developed (Conry et al. 1995). Intramuscular injection of this vaccine into mice produced a good immune response against the HCE antigen. Injection of a liposome-protected mRNA vaccine-encoding melanoma antigen glycoprotein 100 (gp100) into the spleen led to delayed tumor growth (Zhou et al. 1999). Also, immunization by injecting mRNA into the skin has been done. They isolated mRNA from spindle cell tumors (S1509a) and encapsulated it with the cationic lipid dioleoyl-3-trimethylammonium propane (DOTAP), and injected it into mice epidermal cells. Upon challenge with alive S1509a cells, these mice exhibited a greatly reduced rate of tumor formation (Granstein et al. 2000). Nucleoside-modified mRNA, encapsulated within cationic lipid, Lipofectamine 2000, was used for the expression of therapeutically significant proteins (Kormann et al. 2011). Based on all these studies, it can be concluded that CNE has the potential to be evaluated and subjected to human clinical trials.
7.3 Peptide-based Delivery
The mRNA, being negatively charged, can be easily delivered by adsorption onto the surface of cationic peptides, as a result of electrostatic interactions between them. The positive charge is due to the presence of basic amino acids such as lysine and arginine. The amount of mRNA which gets adsorbed onto the cationic peptide is dependent upon the negative:positive charge ratio (Wang et al. 2021).
7.3.1 Protamine-Complexed mRNA
Protamine is one of the positively charged peptides which has been evaluated for the delivery of mRNA. Two important properties make this peptide an important delivery agent. First, protamines have the ability to protect mRNA from degradation by host nucleases. Second, protamine is an adjuvant and helps achieve heightens immune response (Wang et al. 2021).
The β-galactosidase-encoding mRNA condensed with protamine into a negatively charged nanoparticle complex, and further encapsulated in a cationic lipid liposome was demonstrated to be effective in mice. This complex resists the mRNA degradation inside the cell and could express the protein in vivo followed by T-cell response and production of anti-β-galactosidase IgG antibodies. Protected mRNA persists longer than naked mRNA and hence remains immunogenic for a longer period. Protamine-complex mRNA encoding for proteins such as tyrosinase, gp100, melan-A was tested in clinical trials in seven metastatic melanoma patients, out of which, one patient showed a promising response (Rhoads 2016).
7.3.2 Cationic Cell-Penetrating Peptides (CPP)
These are small peptides, which are positively charged and are composed of 8–30 amino acid residues. They act as an important delivery agent for the synthetic mRNA due to two major reasons. Firstly, these peptides possess low charge densities. Secondly, they are able to disrupt the membranes for an endosomal escape. This is an important aspect with respect to synthesis of proteins. In a recent experiment, three CPP-mRNA platforms, viz. RALA (WEARLARALARALARHLARALARALRACEA), LAH4 (KKALLALALHHLAHLALHLALALKKA), and LAH4-L1 (KKALLAHALHLLALLALHLAHALKKA) were compared. All these three cationic peptides complexed with the mRNA were introduced into the dendritic cells, and elicited both innate as well as adaptive immune responses. But, the LAH4-L1 complex with mRNA exhibited the best protein expression. It is noteworthy that the uptake and other intracellular activities for such CPP-mRNA complex involve processes such as clathrin-mediated endocytosis and phagocytosis (Coolen et al. 2019). In a different set of experiments, attempts were made to combine the cationic characteristics of protamine and cell-penetrating characteristic by formulating a fused protamine-CPP protein, which was then used to deliver mRNA to human cell lines (Wang et al. 2021).
7.4 Electroporation and Nucleoporation
To increase the uptake of naked mRNA by cell, the electroporation approach has been widely used. A significant increase in both the cell-mediated and humoral immune response was recorded when SFV vector RNA-encoding β-galactosidase was electroporated intradermally into mice (Zhong et al. 2018). Electroporation technique aided in enhancing the expression levels as well as the immune response to intramuscularly injected VEEV chimeric replicon RNA coding for two different proteins, viz. secreted embryonic alkaline phosphatase (SEAP) and HIV envelope protein (Cu et al. 2013). Gain and loss of function studies in live animals may be performed by in vivo electroporation of synthetic mRNA in brain tissue (Bugeon et al. 2017).
Nucleoporation is similar to electroporation, but it is based on the use of proprietary nucleofection reagents. The synthetic mRNA is introduced into both the nucleus and cytosol (Lenz et al. 2003). It is a milder process than electroporation, and cells are found to recover faster than electroporation.
7.5 Gene Gun-Mediated Delivery of mRNA
Also known as the biolistic method, it helps in the cellular uptake of larger molecules as a result of membrane damage. Gene gun mediated in vivo delivery led to an increase in uptake efficacy and delivery of synthetic mRNA encoding for human α-1 antitrypsin and produced a significant immune response against the expressed antigen in rodents (Qiu et al. 1996). Gold microcarrier particles coated with the infectious Flavivirus RNA delivered through the gene gun method exhibited good efficiency of infection in mice (Mandl et al. 1998). In another significant experiment, synthetically produced Bovine viral diarrhea virus (BVDV) mRNA was introduced in cows and sheep using gene gun, which led to a robust immune response against BVDV, at levels similar to natural exposure to the virus (Vassilev et al. 2001).
7.6 Use of Polymer Nanomaterials
Polymer Nanomaterials are usually made up of synthetic polymeric materials such as polylactic acid (PLA), chitosan, gelatin, polycaprolactone, etc. These materials are very much stable in nature, and they show the ability to encapsulate both hydrophilic as well as hydrophobic compounds, apart from proteins and other biomolecules. They are also instrumental in the tuned delivery of therapeutic compounds to the target site (Damase et al. 2021). These polymeric substances can be utilized for the purpose of producing nanoparticles that can be delivered via injections. The injections can be done via intravenous, intramuscular, intraperitoneal, or subcutaneous routes (Molina et al. 2015). For instance, cationic polyethyleneimine-stearic acid (PSA) copolymer has been developed for the delivery of mRNA coding for HIV-1 gag, into the dendritic cells and a 6-8 week-old female BALB/c mice (Zhao et al., 2016). This modified mRNA (encapsulated within PSA) was used to immunize the mice against the HIV-1 gag antigen and to detect the titers of antibodies specific to the HIV-1 gag (anti-HIV-1 gag antibodies) along with the levels of CD8+ and CD4+ T-cell responses.
7.7 Virus-like Replicon Particle (VRP)-based Delivery of mRNA
Virus-like particles have the ability to encapsulate mRNA and deliver it into the target cell, in a manner similar to that of a virus infection. The synthesis of viral structural proteins takes place in vitro followed by the encapsulation of the antigen-encoding mRNA (Li et al. 2017). In a study, Venezuelan equine encephalitis VRP encapsulated mRNA, coding for different kinds of Dengue virus E-antigen, viz. subviral particles [prME] and soluble E dimers [E85] were used for immunization, which displayed induction of protection against the encoded antigens. Moreover, E85 antigen-VRP gained importance in terms of both speed and magnitude of immunity (White et al. 2013). In another study, HIV-derived mRNA that encoded clade C trimeric envelope glycoprotein was used as a VRP source. The complex triggered cellular immune responses in rhesus macaques (Bogers et al. 2015).
Recently, it has been shown that lipid inorganic nanoparticles (LIONs) encapsulated alphavirus-derived replicon RNA coding for SARS-CoV-2 spike (S) protein when administered intramuscularly into mice and primates, increased the titers of anti SARS-CoV-2 S protein IgG antibodies (Erasmus and Khandar 2020).
7.8 Other Lesser-Known Methods
Several other methods can be used for the delivery of mRNA into the cells but are not as common as the above-mentioned ones. The use of lipid nanoparticles for the delivery of mRNA into the cells is being exploited as a technique for delivering self-amplifying RNA vaccines (Rhoads 2016). Lipid nanoparticles (LNP) are excellent delivery platforms. They are negatively charged in nature and can be categorized as cholesterol, ionizable amino lipids, phospholipids, and polyethylene glycol. The interaction between the essential ionizable amino lipids and ionizable amino lipids as well as the endosomal membrane helps mRNA escape from the endosome. The advantages of using LNP-based systems for the delivery of mRNA are twofold. First, LNPs shield the mRNA from being degraded by the enzymes present within the endosome, which ensure high efficiency of encapsulation. The second major advantage that LNPs confer is their decent biocompatibility, by means of a series of bioprocesses for the delivery of mRNAs to be expressed (Wang et al. 2021).
Cationic oil-in-water nanoemulsions for the effective delivery of mRNA into the cells have also been documented (Brito et al. 2014). The use of polymeric mRNA nanomicelles, for the delivery of mRNA in vivo, was a significant breakthrough in the field of molecular therapy. In that method, polyethylene glycol polyaspartamide (PEG-PAsp) polymer was used to form small 50–100 nm in diameter nanomicelles with mRNA (Kataoka et al., 2012). The use of PEG-polyamino acid polymers has demonstrated continuous expression of the protein in cerebrospinal fluid for a week when mRNA-encoding luciferase with modified nucleosides was administered into mice CNS intrathecally (Uchida et al. 2013).
8 Applications of mRNA Therapeutics
Synthetic mRNAs find several applications in the field of medicine and genetic engineering such as protein-replacement therapies, vaccines against several diseases and genome editing.
8.1 mRNA as a Therapeutic Agent for Replacement of Defective Protein within the Cell
Several genetic diseases such as hemophilia B, cystic fibrosis are characterized by the presence of a defective protein or defective translation of mRNA encoding that particular protein (Huang et al. 2020). Such conditions can be treated by the delivery of a functional protein-encoding mRNA into the cell, known as protein-replacement therapy. But, unlike mRNA vaccines, delivery of mRNA intended for such therapy should not be immunostimulatory as this may lead to mRNA degradation (Rhoads 2016). This method is more effective when compared to conventional protein therapy, as a single mRNA molecule can be used to produce a considerable amount of protein produced in the cell during the course of treatment (Warren et al. 2010). Several studies have been conducted which supplement the application of mRNA for protein-replacement therapy. As early as 1992, naked IVT mRNA coding for the hormone vasopressin was demonstrated to be effective in diabetes insipidus in Brattleboro rats. The synthetic mRNA injection showed promising results within 5 h of its injection into the hypothalamus, expressing vasopressin in magnocellular neurons (Jirikowski et al. 1992). Modified synthetic mRNA coding for surfactant protein B has been demonstrated to be effective in mouse models suffering from congenital lung disease (Kormann et al. 2011). Lipid nanoparticle-packaged mRNA coding for cystic fibrosis transmembrane conductance regulator (CFTR), for the treatment of cystic fibrosis, which is an inherited genetic disorder resulting due to a mutation in the chloride channel, the CFTR. The disruption of CFTR leads to the accumulation of a thick mucous layer in various organs such as the pancreas and the lungs. The LNP-packaged mRNA has been tested in knockout mice with intranasal delivery and recorded an efficacy equivalent to the presently used drug ivacaftor (Robinson et al. 2018). Apart from this, MRT5005 (Translate Bio) is being developed for the treatment of cystic fibrosis, which codes for a functional CFTR protein, and delivered into the lung epithelia via nebulization (TranslateBio 2019).
An mRNA drug has been formulated for treating citrin deficiency, caused by a mutation occurring in the SLC25A13 gene-encoding citrin. Citrin is a mitochondrial membrane transport protein which has a role in the urea cycle. The deficiency of citrin leads to hyperammonemia and neuropsychiatric disturbances. Upon administration of an mRNA coding for human citrin in SLC25A13 knockout mice, it was observed that there was a drastic reduction in the hepatic citrulline and blood ammonia levels succeeding an oral sucrose challenge and reduced aversion of sucrose, which is a hallmark of citrin deficiency (Cao et al. 2019). Also, promising data were obtained from the pre-clinical studies of an mRNA therapy for the fabry disease, caused as a result of mutations in the GLA gene coding for enzyme α-galactosidase, which is very essential for the utilization of the glycolipids. In the Fabry disease, glycolipid derivatives such as globotriaosylceramide, globotriaosylsphingosine tend to accumulate within a number of tissues with time leading to a wide range of clinical manifestations. Administration of a single dose of GLA-encoding mRNA in GLA-deficient mice models leads to a drastic reduction in the accumulation of globotriaosylsphingosine in plasma and tissues. It is important to note here that this beneficial effect of mRNA administration was observed for up to 6 weeks after the dose (Zhu et al. 2019).
8.2 mRNA as Vaccines Against Cancer
As discussed earlier, mRNA-based vaccines against cancer have significantly more advantages over DNA vaccines and conventional peptide-based or protein-based vaccines. Also, the adaptability of these mRNA molecules to both dividing and resting cells makes them more favorable for therapeutic purposes (Huang et al. 2020). Proteasome, a protein complex, degrades the mRNA-encoded proteins and presents the resulting peptides to the MHC-I molecules which in turn activates the CD8+ TC cells. Generally, the MHC-II processing pathway is out of reach of intracellular proteins. So, TH2 cell responses are not efficiently stimulated. But with the help of certain secretion signals engineered into mRNA, the protein can be directed to the extracellular secretion pathways activating the effective TH2 responses (Sahin et al. 2014). Upon delivery, these tumor antigen-encoding mRNAs are expressed into the APCs and are presented majorly with MHC class I molecules, activating T-cell mediated response required for effective tumor clearance (Fiedler et al. 2016). Tumor-associated antigens (TAAs) are expressed abundantly in cancerous cells; hence, mRNAs coding for these antigens have been exploited as anti-cancer vaccines. Some studies by CureVac demonstrated that upon intradermal injection of cationic-liposome-protected mRNA, a sufficiently good amount of CMI and antibody-mediated immune response was induced (Hoerr et al. 2000). Also, results from some other clinical trials showed that the injection of mRNA complexed with protamine intradermally produced strong T-cell and B-cell responses and are highly safe and effective (Rittig et al. 2011). Since mRNA encoding a single antigen may not always be sufficient for inducing a robust immune response, mRNA vaccines based on multiple antigens or “antigen-cocktail” have been validated for enhancing the immunogenicity of vaccines (Vansteenkiste et al. 2016). Some of the therapeutics developed by Moderna, intended for the treatment of cancer include mRNA2416, mRNA-2752, and MEDI1191. These therapeutics are immunomodulatory in nature. mRNA2416 codes for OX40 ligand (OX40L), a membrane-bound co-stimulatory protein involved in the enhancement of expansion, function and survival of T-lymphocytes for mounting a robust immune response against the cancer cells. After delivery of this molecule into the tumor (by means of an intratumor injection), the tumor cells express it on their surface, which attracts a stronger T-cell response against these tumor cells. mRNA2416 is also being investigated by Moderna for abscopal effect in metastatic cancer, i.e., whether a localized injection into the target tumor cell would elicit secondary immune response and also show an effective result in the surrounding metastases or not (Moderna 2020). Another therapeutic ligand, mRNA2752, is based on delivery of OX40L into the tumors, in addition to the immunostimulatory cytokines IL-23- and IL-36γ-encoding mRNA for promoting T-cell-mediated cellular cytotoxicity. The therapeutic drug MEDI1191 can be administered for the solid tumors also, and it encodes IL-12, one of the most potent cytokines which is involved in mediating antitumor activity (Tugues et al., 2015).
8.3 Dendritic Cell (DC) Vaccines
Dendritic cells are an excellent vaccine target. The primary reason for this is that, as professional APCs, they tend to take up the antigen, subject it to processing and present it to the cells of the immune system, which leads to generation of a strong adaptive immune response (Beck et al. 2021).
Loading of DC-based mRNA vaccines can be achieved both in situ and ex vivo. Dendritic cells are first obtained from the peripheral blood of the patient. After the DCs undergo maturation, they are subjected to loading with mRNA encoding the desired antigen and the loaded DCs are returned back to the patients. There are multiple ways in which the loading of antigen-encoding mRNAs into mature DCs can be performed including electroporation, lipofection, nucleofection, and sonoporation. Among these, the most frequently utilized technique is electroporation (Ahmed et al. 2020; Wang et al. 2021). For achieving the delivery of mRNA into DCs in situ, the antigen-encoding mRNAs, in a complex with TriMix, can be directly injected into the lymph nodes. For instance, the first clinical trial of a TriMix-DC complex vaccine was NTC01066390 in patients suffering from advanced melanoma (Wang et al. 2021). As part of a phase 1b clinical trial in melanoma patients, it was noted that the administration of DCs electroporated with mRNA coding for the tumor antigen and TriMix showed extended progression-free survival time (Wilgenhof et al. 2013).
Targeting tumor antigen-encoding mRNAs into the monocyte-derived DCs ex vivo, and then re-injecting them in the animal stimulates effective immune responses against the tumor cells. It was shown that cationic lipid mRNA coding for ovalbumin pulsed into autologous DCs and re-injected in mice, protected them against the cancer cells expressing ovalbumin (Boczkowski et al. 1996). Injection of FOXP3-encoding mRNA into the dendritic cells led to an effective T-cell-mediated immune response against breast cancer cells (Nair et al. 2013). One of the most famous DC vaccines for viral diseases is the HIV-1 vaccine. In this, the patients suffering from HIV-1 infection are administered with DCs having mRNA vaccines coding for multiple antigens of HIV-1. The delivery of the mRNA is done by electroporation due to its high delivery efficacy. Evaluation of the elicited cellular immune responses displayed antigen-specific T cells action with not much clinical benefits (Wang et al. 2021).
8.4 mRNA Vaccines in Prevention of Diseases
mRNA vaccines can be administered for a number of infectious as well as non-infectious diseases. Such vaccines have been tested for infectious diseases such as Influenza virus infection, Rabies, Zika virus, COVID-19 infection. Apart from infectious diseases, a number of non-infectious diseases such as cardiovascular diseases, neurological disorders have the potential to be corrected by the use of mRNA vaccines. With a greater number of developments expected on the mRNA modification technology and delivery strategies, it is expected that the applications of such vaccines would gain more popularity and its use would become more widespread (Li et al. 2021).
8.4.1 mRNA Vaccines Against Infectious Diseases
mRNA vaccines have been tested for the prevention of infectious diseases caused by different pathogens such as Influenza viruses (Petsch et al. 2012), Zika virus (Feldman et al. 2019), Rabies virus (Schnee et al. 2016), Dengue virus (Roth et al. 2019), and SARS-CoV-2 infection (Kaur and Gupta 2020a, b). Influenza A was the first viral infection against which an mRNA vaccine was investigated (Borch and Svane 2016). Subcutaneous injection of liposome-encapsulated mRNA-encoding Neuraminidase (N)—antigen of Influenza A in mice elicited immune response similar to the response to natural infection in terms of specificity and strength of immune response (Martinon et al. 1993). An unmodified IVT mRNA Influenza A vaccine-encoding Hemagglutinin (HA) and N antigens led to a robust antibody-mediated as well as cell-mediated immune response upon intradermal injection into animal models such as mice, ferrets, and pigs that were similar to the immune response induced by a licensed vaccine against Influenza A virus in pigs (Petsch et al. 2012). A self-amplifying mRNA, in a complex with LNP-encoding HA antigen of Influenza A virus, was delivered into mice models intramuscularly. After two weeks of a second dose, mice developed satisfactory immunity, which was thought to be sufficient to impart protection (Hekele et al. 2013).
COVID-19 is the first viral disease for which mRNA-based vaccines gained approvals for use in human beings. Currently, two such vaccines are in use to impart effective immunity against COVID-19, mRNA-1273 by Moderna and BNT162b1 by Pfizer. The mRNA-1273 vaccine is an LNP-capsulated mRNA encoding for the S protein of SARS-CoV-2 and elicits a strong antiviral response against the S antigen. In phase-I clinical trials, it was found that the participants who received 25 μg dose of viral S mRNA had neutralizing Ab (nAb) levels which were comparable to the convalescent sera, whereas the participants who received 100 μg dose of mRNA had an Ab level surpassing the levels in convalescent sera. The vaccine was well tolerated by the patients who received both 25 μg and 100 μg doses, but grade 3 systemic symptoms were shown by three volunteers who received 250 μg doses. The BNT162b1 vaccine is a codon-optimized mRNA vaccine coding for the SARS-CoV-2 receptor-binding region (RBD) of the S protein, encapsulated in ionizable cationic LNPs. In the vaccinated individuals, RBD-specific IgG levels were found to be higher than the levels in convalescent serum. The levels of SARS-CoV-2 neutralizing antibodies (nAbs) were also found to be significantly higher than the levels found in convalescent serum (Kaur and Gupta 2020a, 2020b).
The efficacy of mRNA vaccines is not only limited to respiratory pathogens, such as influenza and coronaviruses but also rabies in rodents and pigs. It has been shown that the vaccine (CV7202)-encoding rabies viral glycoproteins (RVG) can elicit adaptive immune response (CD4+ T cells) in the body which is comparatively more than the vaccines already in use. Throughout the observation period of one year, there was no change in the nAb titer value in mice. CV7202 is currently being studied in a phase 1 trial of CureVac for its safety, reactivity, immune response, and immunogenicity (Schnee et al. 2016). Also in another study, the amplified mRNA vaccine encoding the RVG formulated with LNPs showed a promising effect against rabies virus. They made a comparison among frequently used cationic lipids and on the basis of that chose the most efficient cationic lipids-DOTAP or dimethyldioctadecylammonium (DDA). These two were capable of inducing efficient antigen expression for animal evaluation of LNPs designed (Lou et al. 2020).
The idea of mRNA vaccines for protection against the Zika virus was published in 2017 (Richner et al. 2017). Synthetic modified mRNA-encoding prM/M-E antigens of the virus were produced. Apart from a modified nucleoside, a molecule of S-adenosylmethionine was added to the capped end of the mRNA to increase the translatability of the mRNA and coated with LNPs before administration in the mice models. Results suggested that the modified synthetic mRNA vaccine provided sufficient immunity against the virus in animal models and protected the mice against Zika-virus-mediated congenital disease (Richner et al. 2017; Huang et al. 2020). A cytomegalovirus (CMV) vaccine has also been developed for the prevention of CMV infection in pregnant women and in patients who have undergone transplantation. The constituents of the vaccine were six modified mRNA which coded for CMV glycoprotein and pentameric complexes. Delivery of the modified mRNA vaccine (encapsulated within LNPs) was done by means of an intramuscular injection. It was noted that a single dose of the vaccine was able to induce strong immune responses in mice and non-human primates. As a result, the vaccine underwent clinical trials, sponsored by Moderna (mRNA-1647) (John et al. 2018).
8.4.2 mRNA-based Therapeutics Against Non-Infectious Diseases
A number of recent studies have shown that many of the previously “undruggable” pathways which are involved in the progression and development of cardiovascular diseases could be targeted by mRNA. AZD-8601 developed by Moderna coding for vascular endothelial growth factor-A (VEGF-A) was intended to be used during coronary artery bypass surgery, delivered via an epicardial injection. It was thought to reduce myocardial ischemia, along with improvement in left ventricular systolic function in people suffering from ischemic heart disease, by means of enhancement of local angiogenesis (Carlsson et al. 2018). A phase II clinical trial conducted by AstraZeneca is currently evaluating the efficacy of this drug. This trial is randomized, double-blind, placebo-controlled, as well as multicenter. The trial is being conducted in patients who have moderate contractile dysfunction and are undergoing coronary artery bypass surgery. With the aid of epicardial injections, patients are randomly assigned doses of 0, 3, or 30 mg of mRNA-encoding VEGF-A in a citrate buffer. If this study shows effective results, it would suggest that there is an improvement in the blood flow and function when mRNA is injected directly into an ischemic tissue (Anttila et al. 2020).
With respect to neurological disorders, administration of mRNA proves to be an effective approach because they can offer native proteins and peptides to the target site perpetually, allowing a synchrony between the dynamics of signal receptor expression and the availability of the bioactive factor. However, direct delivery of mRNA to neural tissues in vivo has proven to be difficult because of the instability and high immunogenicity of introduced mRNA, limiting the studies and attempts to target neural tissue.
Recently, an mRNA formulated with cationic liposomes has been developed for the treatment of chronic disorders. This was a novel approach in which nucleic acids were employed for the treatment of neurological disorders. The cationic liposome used for the delivery of the therapeutic mRNA was composed of DOTAP, dipalmitoylphosphatidylcholine (DPPC) and cholesterol. The potential for the delivery of such mRNA to the brain via the nasal passage has been evaluated in mice models, and the results suggested that the delivery of non-mRNA into the brain via the intranasal route is feasible for the treatment of neurological disorders (Dhaliwal et al. 2020).
8.5 mRNA-Mediated Genome Editing
Several tools are available for editing the genome, including ZFNs, TALENs, and CRISPR/Cas system (Sergeeva et al. 2016). But a point of major concern is their off-target effects. To reduce the off-target effects, mRNAs coding for ZFNs, TALENs, and CRISPR/Cas systems can be produced to achieve a transient expression of these nucleases, only requiring a short period for their action. A delivery system based on zwitterionic amino lipid (ZAL) to co-deliver mRNA coding for Cas9 and single-guide RNA (sgRNA) has been developed. As a result, a 95% reduction in the protein expression was observed along with permanent editing of DNA (Miller et al. 2017). LNP-coated mRNA coding for Cas9, along with sgRNA for editing transerythrin gene found in mouse liver reduced transerythrin protein levels in the serum, for at least 12 months after the administration of the synthetic mRNA (Finn et al. 2018). Targeting TTR and PCSK9 genes by an LNP-coated mRNA coding for ZFNs in mice significantly reduced the gene expression (Conway et al. 2019). All these experiments suggest that LNP-based mRNA delivery systems for genome editing are very promising and should be explored further.
8.6 Generation of Induced Pluripotent Stem Cells (iPSCs) using mRNA
Somatic cells can be successfully converted into stem cells by the introduction of DNA or mRNA coding for transcription factors into the cell. Shinya Yamanaka and co-workers demonstrated that “mature cells can be re-programmed to become pluripotent” (Takahashi et al. 2007). Although their work was based on DNA vectors, the same can be achieved by using mRNA (Rhoads 2016). The re-programmed cells are now being widely explored for the treatment of various diseases such as diabetes, muscular dystrophies (Okano et al. 2013; Fox et al. 2014).
A nucleoside-modified cationic lipid-mRNA capped with ARCA encoding four transcription factors, viz. KLF4, c-MYC, OCT4, and SOX2, has been developed. It was introduced into different cells such as human epidermal keratinocytes, and reprogramming of cells took place to produce iPSCs, proving that the use of mRNA for the generation of iPSCs was highly efficient (Warren et al. 2010). The most interesting fact is that these induced stem cells are quite similar to the embryonic stem cells (ESC) with respect to their ability to self-renewal and to differentiate into all three germ layers of the body (endoderm, mesoderm, and ectoderm). Therefore, it can be ascertained that these human iPSCs can be used as an alternative option for human ESCs, which also helps nullify the associated ethical concerns. This discovery provided a transformation for the field of regenerative medicine, as patient-specific iPSCs can be differentiated and their derivatives can be used as therapeutic cells. The major advantage which comes with the use of such cells is that these cells can be transplanted into the patient, with minimal risk with respect to genetic incompatibility of transplanted cells or even immune rejection (Chanda et al. 2021). Using this technique, differentiation of these iPSCs into terminally differentiated myogenic cells was successfully achieved. Cationic lipid-mRNA with a poly(A) tract capped with ARCA, coding for the same four transcription factors mentioned above was successfully used to reprogram HFF, leading to the generation of iPSCs expressing alkaline phosphatase (AKP) enzymes and various embryonic stem cell markers (Yakubov et al. 2010).
Viral vectors (such as retroviral, lentiviral or even adenoviral vectors) can be used for generation of iPSCs, but there is a risk of genomic integration, which tends to limit the clinical application of iPSCs produced in such manner. In order to lessen the risks associated with viral vectors, a number of integration-free approaches have been developed, which include the Sendai virus, cell permeating recombinant proteins, non-integrating plasmids, or episomal DNA. Though these techniques presented minimal risk of genome integration, the observed efficiency of generation of iPSC was very low (Chanda et al. 2021).
9 Safety of mRNA Therapeutics
As seen across different studies, the clinical use of mRNA for therapeutic purposes has demonstrated that they are safe, tolerable, and pose no major risks for the subject (Sahin et al. 2014). In the majority of cases, for example, in the case of mRNA-based protein-replacement therapies, apart from studies by independent researchers, there have been no clinical trials conducted for checking the safety and efficacy in larger groups of organisms. Due to this reason, there is a lack of evidence that proves or shows the nature of safety problems and challenges that may be posed as a result of these therapies, hence leaving the scientific community at a point of doubt (Sahin et al. 2014). The production of IVT mRNA is relatively simple and cost-effective than all other methods documented to date. It is also known that the product quality is uniform in nature and also, quality control is easier in this case. Since the production of synthetic mRNA (IVT mRNA) does not include any cellular or animal component, the safety issues and other risks are far lower than alternative methods (Sahin et al. 2014). However, despite having an edge over other therapeutic methods, the risks should also be seriously evaluated.
9.1 The Safety Concern Over the Use of Non-Natural Nucleotides/Nucleosides in IVT mRNA
RNAses are found within extracellular spaces in abundance. These enzymes act as control mechanisms that are responsible for regulating the levels of RNA molecules (Sahin et al. 2014). To date, there are no reports of safety concerns associated with IVT mRNA consisting of unmodified nucleotides (natural nucleotides) with respect to their absorption, metabolism, excretion profile, etc. This is because the human body breaks down and excretes out much higher amounts of mRNA daily. But the aforementioned conditions may not apply to the IVT mRNA composed of non-natural nucleotides. There is still no significant data that explains the mechanism of the breakdown and excretion of such unnatural nucleotides. Also, the toxic effects and associated risks are still unknown. Besides the mRNA, compounds resulting from the breakdown of mRNA composed of unnatural nucleotides may be toxic to the cell as evidenced from their association with unusual mitochondrial toxicities (Lewis 2003) linked with the roles of nucleoside transporters (Sahin et al. 2014). Significant clinical toxicities such as myopathy, lactic acidosis, pancreatitis, lipodystrophy were found in HIV-positive patients treated with nucleoside reverse transcriptase inhibitors. Mitochondrial dysfunction was solely attributed to the deleterious effects of such unnatural nucleoside analogs on the DNA polymerase γ, leading to its inhibition and hence, blocking mitochondrial DNA replication (Sahin et al. 2014). Hence, safety aspects should be strictly looked upon after the administration of modified mRNA with unnatural nucleotides/nucleosides. The organs under high risk should be diligently monitored after the administration of such drugs for prolonged use.
9.2 Safety Considerations Regarding the Encoded Protein
Other safety concerns are related to the nature and application of the protein encoded by the mRNA. There should be strict and diligent monitoring of the action of these IVT mRNA molecules in vivo that it only performs and executes the function for which it was designed, on a case-specific basis. Another main area of concern is dosing. The dose for a specific inject should be carefully determined and should be decided in accordance with the need for achieving the objectives of a particular therapy (Sahin et al. 2014).
10 Conclusions
Various studies evidenced that IVT mRNA-based therapeutics hold the noteworthy potential to be used in medicine. Despite some safety concerns, they are now being looked upon as agents which hold tremendous potential to revolutionize the field of regenerative medicine. Their efficacy in expressing the proteins of interest in the host cell may be of great therapeutic significance and can be extended for wide use in humans after necessary clinical trials. Treatment of many genetic disorders, viral infections, cancers can now be visualized to be one step easier with the help of synthetic mRNA molecules. The advancement in delivery systems for IVT mRNA needs more inclusive research efforts, and many such studies are already in progress. Though a significant advancement in the utility of synthetic mRNA molecules has been made, the possible challenges posed by these molecules need to be evaluated more comprehensively in clinical trials.
Abbreviations
- ACE2:
-
Angiotensin-converting enzyme 2
- ADAR:
-
Adenosine deaminases acting on RNA
- AKP:
-
Alkaline phosphatase
- APC:
-
Antigen presenting cell
- ARCA:
-
Anti-reverse cap analog
- BVDV:
-
Bovine viral diarrhea virus
- CFTR:
-
Cystic fibrosis transmembrane conductance regulator
- CLRs:
-
C-type lectin receptors
- CMI:
-
Cell-mediated immunity
- CMV:
-
Cytomegalovirus
- CNE:
-
Cationic nanoemulsion
- CNS:
-
Central nervous system
- COVID:
-
Coronavirus disease
- CPE:
-
Cytopathic effect
- CPP:
-
Cationic cell-penetrating peptides
- CRISPR/Cas:
-
Clustered regularly interspaced short palindromic repeats/CRISPR Associated Protein
- DDA:
-
Dimethyldioctadecylammonium
- DOTAP:
-
1,2-Dioleoyl-sn-glycero-3-phosphocholine
- DPPC:
-
Dipalmitoylphosphatidylcholine
- ESC:
-
Embryonic stem cells
- HA-antigen:
-
Hemagglutinin-antigen of Influenza Virus
- HCE:
-
Human carcinoembryonic
- HFF:
-
Human foreskin fibroblast
- HPLC:
-
High-performance liquid chromatography
- HSP:
-
Heat shock protein
- iDCs:
-
Immature Dendritic Cells
- IFN:
-
Interferon
- IgG:
-
Immunoglobulin gamma
- IL-2:
-
Interleukin-2
- iPSC:
-
Induced pluripotent stem cell
- IRE:
-
Iron-responsive element
- IRES:
-
Internal ribosomal entry site
- IRF:
-
Interferon regulatory factor
- IVT:
-
In vitro Transcribed
- KUNV:
-
Kunjin virus
- LDNP:
-
Lipidoid nanoparticle
- LIONs:
-
Lipid inorganic nanoparticles
- LNP:
-
Lipid Nanoparticle
- m Ψ:
-
N-methyl pseudouridine
- m5C:
-
5-Methylcytidine
- m5U:
-
5-Methyluridine
- mo5U:
-
5- Methoxyuridine
- m6A:
-
N6-methyladenosine
- m7G:
-
N7-methylated guanosine
- MHC:
-
Major histocompatibility complex
- MYD88:
-
Myeloid differentiation primary response 88
- NAb:
-
Neutralizing antibody
- N-Antigen:
-
Neuraminidase antigen
- NF-κB:
-
Nuclear factor-κB
- NLR:
-
NOD-like receptor
- NSP:
-
Non-structural protein
- OAS:
-
2,5-Oligoadenylate synthetase
- ORF:
-
Open reading frame
- PABPs:
-
Poly(A) binding proteins
- PCR:
-
Polymerase chain reaction
- pDNA:
-
Plasmid DNA
- PEG-PAsp:
-
Polyethylene glycol polyaspartamide
- PKR:
-
Protein kinase
- PLA:
-
Polylactic acid
- PRR:
-
Pattern recognition receptor
- PSA:
-
Polyethyleneimine-stearic acid
- Ψ:
-
Pseudouridine
- RBD:
-
Receptor-binding region
- RE:
-
Restriction enzyme
- RIG – 1:
-
Retinoic acid-inducible gene I
- RNAP:
-
RNA polymerase
- RNTP:
-
Ribonucleoside triphosphate
- RLR:
-
Retinoic acid-inducible gene I like receptor
- RSV:
-
Respiratory syncytial virus
- RVG:
-
Rabies viral glycoproteins
- SEAP:
-
Secreted embryonic alkaline phosphatase
- sgRNA:
-
Single-guide RNA
- SINV:
-
Sindbis virus
- S protein:
-
Spike protein
- S2U:
-
2-Thiouridine
- TAA:
-
Tumor-associated antigen
- TALEN:
-
Transcription activator-like effector nuclease
- TH:
-
T helper
- TLR:
-
Toll-like receptor
- TNF-α:
-
Tumor-necrosis factor-alpha
- TRIF:
-
TIR-domain-containing adapter-inducing interferon-β
- UTR:
-
Untranslated region
- VEEV:
-
Venezuelan equine encephalitis virus
- VEGF-A:
-
Vascular endothelial growth factor-A
- VRP:
-
Virus-like replicon particle
- YTH:
-
YT521-B homology
- ZAL:
-
Zwitterionic amino lipid
- ZFN:
-
Zinc finger nuclease
References
Ahmed R, Sayegh N, Graciotti M et al (2020) Electroporation as a method of choice to generate genetically modified dendritic cell cancer vaccines. Curr Opin Biotechnol 65:142–155
Akhrymuk I, Frolov I, Frolova EI (2016) Both RIG-I and MDA5 detect alphavirus replication in concentration-dependent mode. Virology 487:230–241
Anttila V, Saraste A, Knuuti J (2020) Synthetic mRNA encoding VEGF-A in patients undergoing coronary artery bypass grafting: design of a phase 2a clinical trial. Mol Ther Methods Clin 18:464–472
Beck JD, Reidenbach D, Salomon N et al (2021) mRNA therapeutics in cancer immunotherapy. Mol Cancer 20:69
Bettini E, Locci M (2021) SARS-CoV-2 mRNA vaccines: immunological mechanism and beyond. Vaccines 9:147
Boczkowski D, Nair SK, Snyder D et al (1996) Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med 184:465–472
Bogers WM, Oostermeijer H, Mooij P et al (2015) Potent immune responses in rhesus macaques induced by nonviral delivery of a self-amplifying RNA vaccine expressing HIV type 1 envelope with a cationic nanoemulsion. J Infect Dis 211:947–955
Borch TH, Svane IM (2016) Synthetic mRNA. Methods Mol Biol 1428:245–259
Brito LA, Chan M, Shaw CA et al (2014) A cationic nanoemulsion for the delivery of next-generation RNA vaccines. Mol Ther 22:2118–2129
Bugeon S, De Chevigny A, Boutin C et al (2017) Direct and efficient transfection of mouse neural stem cells and mature neurons by in vivo mRNA electroporation. Development 144:3968–3977
Cao J, An D, Galduroz M et al (2019) mRNA therapy improves metabolic and behavioral abnormalities in a murine model of citrin deficiency. Mol Ther 27:1242–1251
Carlsson L, Clarke JC, Yen C et al (2018) Biocompatible, Purified VEGF-A mRNA Improves cardiac function after intracardiac injection 1 week post-myocardial infarction in swine. Mol Ther Methods Clin Dev 9:330–346
Chanda PK, Sukhovershin R, Cooke JP (2021) mRNA-enhanced cell therapy and cardiovascular regeneration. Cells 10:187
Conry RM, LoBuglio AF, Wright M et al (1995) Characterization of a messenger RNA polynucleotide vaccine vector. Cancer Res 55:1397–1400
Conway A, Mendel M, Kim K et al (2019) Non-viral delivery of zinc finger nuclease mRNA enables highly efficient in vivo genome editing of multiple therapeutic gene targets. Mol Ther 27:866–877
Coolen AL, Lacroix C, Mercier-Gouy P et al (2019) Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 195:23–37
Cu Y, Broderick K, Banerjee K et al (2013) Enhanced delivery and potency of self-amplifying mRNA vaccines by electroporation in situ. Vaccines 1:367–383
Damase TR, Sukhovershin R, Boada C et al (2021) The limitless future of RNA therapeutics. Front Bioeng Biotechnol 9:628137
Dhaliwal HK, Fan YF, Kim JH (2020) Intranasal delivery and transfection of mRNA therapeutics in the brain using cationic liposomes. Mol Pharmaceut 17:1996–2005
Edelmann FT, Niedner A, Niessing D (2014) Production of pure and functional RNA for in vitro reconstitution experiments. Methods 65(3):333–341
Erasmus JH, Khandhar AP (2020) An alphavirus-derived replicon RNA vaccine induces SARS-CoV-2 neutralizing antibody and T cell responses in mice and nonhuman primates. Sci Transl Med 2:eabc9396
Feldman RA, Fuhr R, Smolenov I et al (2019) mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine 37:3326–3334
Fiedler K, Lazzaro S, Lutz J et al (2016) mRNA cancer vaccines. Recent Results Cancer Res 209:61–85
Finn JD, Smith AR, Patel MC et al (2018) A single administration of CRISPR/Cas9 lipid nanoparticles achieves robust and persistent in vivo genome editing. Cell Rep 22:2227–2235
Fleeton MN, Chen M, Berglund P et al (2001) Self-replicative RNA vaccines elicit protection against influenza A virus, respiratory syncytial virus, and a tickborne encephalitis virus. J Infect Dis 183:1395–1398
Fox IJ, Daley GQ, Goldman et al (2014) Use of differentiated pluripotent stem cells in replacement therapy for treating disease. Science 345:1247391
Granstein RD, Ding W, Ozawa H (2000) Induction of anti-tumor immunity with epidermal cells pulsed with tumor-derived RNA or intradermal administration of RNA. J Invest Dermatol 114:632–636
Grudzien-Nogalska E, Jemielity J, Kowalska J et al (2007) Phosphorothioate cap analogs stabilize mRNA and increase translational efficiency in mammalian cells. RNA 13:1745–1755
Hekele A, Bertholet S, Archer J et al (2013) Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg 2:e52
Hoerr I, Obst R, Rammensee HG et al (2000) In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol 30:1–7
Huang L, Zhang L, Li W et al (2020) Advances in development of mRNA-based therapeutics. Curr Top Microbiol Immunol 1–20
Jirikowski GF, Sanna PP, Maciejewski-Lenoir D et al (1992) Reversal of diabetes insipidus in Brattleboro tats: intrahypothalamic injection of vasopressin mRNA. Science 255:996–998
Johansson DX, Ljungberg K, Kakoulidou M et al (2012) Intradermal electroporation of naked replicon RNA elicits strong immune responses. PLoS ONE 7:e29732
John S, Yuzhakov O, Woods A et al (2018) Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 36:1689–1699
Kataoka K, Harada A, Nagasaki Y (2012) Block copolymer micelles for drug delivery: DESIGN, characterization and biological significance. Adv Drug Deliv Rev 64:37–48
Kaur SP, Gupta V (2020a) COVID-19 vaccine: a comprehensive status report. Virus Res 288:98114
Kaur SP, Gupta V (2020b) SARS-CoV-2 vaccine: reconnoitering the prospects. Vaccine Res Dev 1:1–5
Kimchi-Sarfaty C, Oh JM, Kim IW et al (2007) A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315:525–528
Kormann MSD, Hasenpusch G, Aneja MK et al (2011) Expression of therapeutic proteins after delivery of chemically modified mRNA in mice. Nat Biotechnol 29:154–157
Leitner WW, Hwang LN, Bergmann-Leitner ES et al (2004) Apoptosis is essential for the increased efficacy of alphaviral replicase-based DNA vaccines. Vaccine 22:1537–1544
Lenz P, Bacot SM, Frazier-Jessen MR et al (2003) Nucleoporation of dendritic cells: efficient gene transfer by electroporation into human monocyte-derived dendritic cells. FEBS Lett 538:149–154
Lewis W (2003) Defective mitochondrial DNA replication and NRTIs: pathophysiological implications in AIDS cardiomyopathy. Am J Physiol Heart Circ Physiol 284:H1–H9
Li M, Li Y, Li S et al (2021) The nano delivery systems and applications of mRNA. Eur J Med Chem 227:113910
Li W, Ma L, Guo LP et al (2017) West Nile virus infectious replicon particles generated using a packaging-restricted cell line is a safe reporter system. Sci Rep 7:3286
Lou G, Anderluzzi G, Schmidta ST et al (2020) Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: the impact of cationic lipid selection. J Contr Release 325:370–379
Mandl CW, Aberle JH, Aberle SW et al (1998) In vitro-synthesized infectious RNA as an attenuated live vaccine in a flavivirus model. Nat Med 4:1438–1440
Martinon F, Krishnan S, Lenzen G et al (1993) Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol 23:1719–1722
Moderna (2020) mRNA-2416 | Moderna, Inc Available online https://www.modernatx.com/pipeline/mrna-2416. (Accessed 28 Dec 2021)
Meng Z, Limbach PA (2006) Mass spectrometry of RNA: linking the genome to the proteome. Brief Funct Genomic Proteomic 5:87–95
Miller JB, Zhang S, Kos P et al (2017) Non-viral CRISPR/Cas gene editing in vitro and in vivo enabled by synthetic nanoparticle co-delivery of Cas9 mRNA and sgRNA. Angew Chem Int Ed Eng 56:1059–1063
Molina M, Asadian-BM BJ et al (2015) Stimuli-responsive nanogel composites and their application in nanomedicine. Chem Soc Rev 44:6161–6186
Nair S, Aldrich AJ, McDonnell E et al (2013) Immunologic targeting of FOXP3 in inflammatory breast cancer cells. PLoS ONE 8:e53150
Ngumbela KC, Ryan KP, Sivamurthy R et al (2008) Quantitative effect of suboptimal codon usage on translational efficiency of mRNA encoding HIV-1 gag in intact T cells. PLoS ONE 3:e2356
Okano H, Nakamura M, Yoshida K et al (2013) Steps toward safe cell therapy using induced pluripotent stem cells. Circ Res 112:523–533
Orlandini von Niessen AG, Poleganov MA, Rechner C et al (2019) Improving mRNA-based therapeutic gene delivery by expression-augmenting 3′ UTRs identified by cellular library screening. Mol Ther 27:824–836
Pardi N, Hogan MJ, Porter FW et al (2018) mRNA vaccines—a new era in vaccinology. Nat Rev Drug Discov 17:261–279
Petrakova O, Volkova E, Gorchakov R et al (2005) Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in Mammalian cells. J Virol 79:7597–7608
Petsch B, Schnee M, Vogel AB et al (2012) Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza A virus infection. Nat Biotechnol 30:1210–1216
Qiu P, Ziegelhoffer P, Sun J et al (1996) Gene gun delivery of mRNA in situ results in efficient transgene expression and genetic immunization. Gene Ther 3:262–268
Rhoads RE (2016) Synthetic mRNA: production, introduction into cells, and physiological consequences. Methods Mol Biol 1428:3–27
Richner JM, Himansu S, Dowd KA et al (2017) Modified mRNA vaccines protect against zika virus infection. Cell 168:1114–1125
Rittig SM, Haentschel M, Weimer KJ et al (2011) Intradermal vaccinations with RNA coding for TAA generate CD8 and CD4 immune responses and induce clinical benefit in vaccinated patients. Mol Ther 19(5):990–999
Robinson E, MacDonald KD, Slaughter K et al (2018) Lipid nanoparticle-delivered chemically modified mRNA restores chloride secretion in cystic fibrosis. Mol Ther 26:2034–2046
Roth C, Cantaert T, Colas C et al (2019) A modified mRNA vaccine targeting immunodominant NS epitopes protects against dengue virus infection in HLA class I transgenic mice. Front Immunol 10:1424
Sahin U, Kariko K, Tureci O (2014) mRNA-based therapeutics—developing a new class of drugs. Nat Rev Drug Discov 13:759–780
Schlake T, Thran M, Fiedler K et al (2019) mRNA: a novel avenue to antibody therapy. Mol Ther 27:773–784
Schnee M, Vogel AB, Voss D (2016) An mRNA vaccine encoding rabies virus glycoprotein induces protection against lethal infection in mice and correlates of protection in adult and newborn pigs. PLoS Negl Trop Dis 10:e0004746
Schrom E, Huber M, Aneja M et al (2017) Translation of angiotensin-converting enzyme 2 upon liver- and lung-targeted delivery of optimized chemically modified mRNA. Mol Ther Nucleic Acids 7:350–365
Sergeeva OV, Koteliansky VE, Zatsepin TS (2016) mRNA-based therapeutics—advances and perspectives. Biokhimiia 81:709–722
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872
TranslateBio (2019) mRNA therapeutics and vaccines | translate bio | pipeline transl bio Available online at https://translate.bio/pipeline/. (Accessed 28 Dec 2021)
Tugues S, Burkhard SH, Ohs I et al (2015) New insights into IL-12-mediated tumor suppression. Cell Death Differ 22:237–246
Uchida S, Itaka K, Uchida H et al (2013) In Vivo messenger RNA introduction into the central nervous system using polyplex nanomicelle. PLoS ONE 8:e56220
Urayama SI, Yoshida-Takashima Y, Yoshida M et al (2015) A new fractionation and recovery method of viral genomes based on nucleic acid composition and structure using tandem column chromatography. Microbes Environ 30:199–203
Vansteenkiste JF, Cho BC, Vanakesa T et al (2016) Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 17:822–835
Vassilev VB, Gil LHVG, Donis RO (2001) Microparticle-mediated RNA immunization against bovine viral diarrhea virus. Vaccine 19:2012–2019
Walsh EE, Frenck RW Jr, Falsey AR et al (2020) Safety and immunogenicity of two RNA-based covid-19 vaccine candidates. N Engl J Med 383:2439–2450
Wang Y, Zhang Z, Luo J (2021) mRNA vaccine: a potential therapeutic strategy. Mol Cancer 20:33
Warren L, Manos PD, Ahfeldt T et al (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618–630
White LJ, Sariol CA, Mattocks MD et al (2013) An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J Virol 87:3409–3424
Wilgenhof S, Van Nuffel AM, Benteyn D et al (2013) A phase IB study on intravenous synthetic mRNA electroporated dendritic cell immunotherapy in pretreated advanced melanoma patients. Ann Oncol 24:2686–2693
Wu MZ, Asahara H, Tzertzinis G et al (2020) Synthesis of low immunogenicity RNA with high-temperature in vitro transcription. RNA 26:345–360
Xu S, Yang K, Li R et al (2020) mRNA vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci 21:6582
Yakubov E, Rechavi G, Rozenblatt S et al (2010) Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun 394:189–193
Zhao M, Li M, Zhang Z et al (2016) Induction of HIV1 gag specific immune responses by cationic micelles mediated delivery of gag mRNA. Drug Deliv 23:2596–2607
Zhong Z, Mc-Cafferty S, Combes F et al (2018) mRNA therapeutics deliver a hopeful message. Nano Today 23:16–39
Zhou WZ, Hoon DSB, Huang SKS et al (1999) RNA melanoma vaccine: Induction of antitumor immunity by human glycoprotein 100 mRNA immunization. Hum Gene Ther 10:2719–2724
Zhu X, Yin L, Theisen M et al (2019) Systemic mRNA therapy for the treatment of fabry disease: preclinical studies in wildtype mice, fabry mouse model, and wild-type non-human primates. Am J Hum Genet 104:625–637
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jain, S., George, A.J., Sharma, V., Singh, G., Gupta, V. (2022). Messenger RNA Therapeutics: Start of a New Era in Medicine. In: Jurga, S., Barciszewski, J. (eds) Messenger RNA Therapeutics. RNA Technologies, vol 13. Springer, Cham. https://doi.org/10.1007/978-3-031-08415-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-08415-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08414-0
Online ISBN: 978-3-031-08415-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)