Abstract
Fatty acid esters of glycidol (glycidyl esters, GE) are processing contaminants in vegetable oils and fats. GE release the carcinogenic glycidol in the gastrointestinal tract. The assessment of health risks associated with dietary GE uptake is hindered by the inaccuracy of exposure estimations based on consumption and food content data. Alternatively, the internal exposure can be approximated by monitoring of human biomarkers of glycidol, for example, the hemoglobin adduct N-(2,3-dihydroxypropyl)-valine (DHP-Val). The quantification of DHP-Val levels in blood samples showed that human adults are exposed principally by foodstuffs and tobacco smoke. Reverse dosimetry allowed calculating the mean oral exposure for 11 German adults (0.94 μg/kg body weight) and 50 Swedish adolescents (1.4 μg/kg body weight). These values exceeded the median chronic exposure estimated from dietary surveys for the adult population (0.2 μg/kg body weight) and for adolescents (0.3 μg/kg body weight), which may be due to hitherto unknown sources of glycidol/GE. Data on DHP-Val in strict raw food eaters, who do not consume food heated to more than 42 °C, suggests that DHP-Val is also formed independently from the oral exposure to GE.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Glycidol
- Glycidyl ester
- Adducts
- Hemoglobin
- Edman degradation
- UHPLC-MS/MS
- Human biomonitoring
- Biomarker
- Exposure
- Reverse dosimetry
Introduction
Occurrence and Toxicological Relevance of Glycidol
Glycidyl esters (GE) are fatty acid esters of glycidol (2,3-epoxy-1-propanol). The compounds are heat-induced food contaminants predominantly formed from acylglycerols during industrial deodorization of vegetable oils and fats involving high temperatures (Pudel et al. 2011). The highest levels of ester-bound glycidol were observed in the food groups “fats and oils” (“palm oil/fat”: 3955 μg/kg glycidol), “margarine, normal fat” (582 μg/kg glycidol), “potato crisps” (110 μg/kg glycidol), “hot surface cooked pastries” (137 μg/kg glycidol), “cookies” (134 μg/kg glycidol), and “short crusts” (149 μg/kg glycidol) (European Food Safety Authority 2016). The toxicological relevance regarding dietary exposure is due to the almost complete and rapid hydrolysis of GE by lipase activity in the gastrointestinal tract as observed in rats (Appel et al. 2013). The glycidol released is an electrophilic, alkylating agent which is prone to react with cellular nucleophiles. It induces gene mutations, chromosomal aberrations, sister chromatid exchange, and unscheduled DNA synthesis in vitro (reviewed by Bakhiya et al. (2011)). After intraperitoneal treatment with glycidol (150 mg/kg body weight), an increased micronuclei formation was observed in bone marrow of B3C3F1 mice. Glycidol is a multisite carcinogen inducing various tumors, for example, mesothelioma in tunica vaginalis and peritoneum, brain glioma, and mammary gland adenocarcinoma, after administration by gavage to B6C3F1 mice (25 and 50 mg/kg body weight) and Fischer 344 rats (37.7 and 75 mg/kg body weight) for 2 years (National Toxicology Program 1990). Glycidol is classified by the International Agency for Research on Cancer (IARC) as probably carcinogenic to humans (group 2A) (International Agency for Research on Cancer 2000) and as a category 2 carcinogen by the Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work Area (MAK) in Germany (MAK Commission 2021).
Human Exposure and Aspects of Hazard Characterization
The mean daily dietary exposure to glycidol was estimated to be about 0.2 μg/kg body weight for European adults and 0.5 μg/kg body weight for high consumers. In comparison, children (1–10 years old) have an approximately three-fold higher exposure. A mean daily exposure of 1.9 μg/kg body weight (95th percentile: 4.9 μg/kg body weight) was determined for nonbreastfed infants exclusively fed with infant milk formula (European Food Safety Authority 2016).
Due to the almost complete hydrolysis of GE in the gastrointestinal tract, it can be assumed that the health risk potentials associated with the intake of GE or of equivalent amounts of free glycidol are similar (Abraham et al. 2013). The genotoxic and carcinogenic properties of the epoxide do not permit deriving a safe threshold value. In these cases, the risk assessment is confined to the application of the margin of exposure (MoE) concept devised by the European Food Safety Authority (EFSA). An MoE is the ratio of the lowest dose inducing a significant increase of tumor incidence in animals and exposure estimates in humans (European Food Safety Authority 2005). Using the T25, the glycidol dose that results in a 25% increase of the lifetime tumor incidence above background, determined for the induction of peritoneal mesothelioma in male rats (10.2 mg/kg body weight), the MoE indicates an only low health concern if higher than 25,000. According to the EFSA exposure estimations, this is the case for median and high exposure scenarios of infants, toddlers, and other children (3–10 years) and high dietary exposure of adolescents and adults in Europe. In the particular scenario of infants receiving formula as sole diet, the MoE was 5400 and 2100, taking into account the medium and high glycidol contents in infant formulae, respectively, raising health concerns about the substitute diet (European Food Safety Authority 2016). Responding to these results, the European Commission has established maximum values for bound glycidol in vegetable oils and fats, as well as in infant and follow-on formula in 2018 (Commission Regulation (EU) 2018/290).
Exposure Assessment of Glycidol
The value of the MoE calculation depends on the quality of the human exposure estimation, which is beset with various sources of uncertainties: (1) The number of analytical results (samples) reported to the EFSA were limited, (2) analytical data for some food groups were lacking altogether, and (3) there may be unknown levels of free glycidol in food. The data on glycidol contents collected by the EFSA are based on the analyses of GE; suitable methods for the quantification of free glycidol were not available (European Food Safety Authority 2016). In addition, the actual exposure may be altered greatly by formation of glycidol and GE during domestic food processing and by additional uptake via alternative routes (e.g., passive smoking). The problems related to the inaccuracy of external exposure assessment can be circumvented using a human biomarker, which allows monitoring the individual internal exposure to specific substances regardless of the intake route. The current chapter summarizes results from recent studies on the principal biomarker of glycidol exposure, the hemoglobin (Hb) adduct N-(2,3-dihydroxy-propyl)-valine (DHP-Val), and the significance to the exposure estimation in human studies.
Biomarkers of Glycidol Exposure: Methodological Considerations
Biomarkers of Short- and Medium-Term Exposure
As an alternative to the traditional exposure assessment by dietary surveys, the individual internal exposure to reactive substances can be estimated by specific biomarkers in different matrices. For example, mercapturic acids of electrophilic metabolites formed after the detoxifying glutathione conjugation are detectable in the urine. The amounts typically reflect the exposure to the parent compound over a short period of 1–2 days (Mathias and B’Hymer 2016). As a potential biomarker of exposure for glycidol and GE, the urinary metabolite 2,3-dihydroxypropyl mercapturic acid (DHPMA) was discussed, because it was detected in rat urine after administration of high doses of glycidol and GE (Barocelli et al. 2011). However, the urinary excretion in human (exposed to much lower doses) was found at high and constant levels, which are rather independent from the intake of glycidol and GE in normal dietary quantities, led to the conclusion that DHPMA is not a suitable biomarker of short-term exposure (Abraham et al. 2021; Monien and Abraham 2022).
The adduct levels in the blood proteins serum albumin (SA) and Hb reflect the systemic exposure to reactive substances within the last 3 weeks (SA) or the last 4 months (Hb) (Sabbioni and Day 2022). The preferred choice for protein adduct analysis in the last three decades was Hb, which is abundant (~150 mg/ml blood), readily accessible and allows cumulative (medium-term) exposure monitoring due to the lifetime of aprox. 110 days (Törnqvist et al. 2002). Glycidol reacts with the N-terminal valine in the peptide chains α and β of Hb to yield the adduct DHP-Val (Fig. 1). The protein fold of Hb supports the reaction. The amino group of free valine (pKa = 9.74; pKa = −log10 Ka, with Ka as the acid dissociation constant) is not very reactive because it is largely protonated in blood (pH = 7.4). In the environment of Hb, the valine residues are somewhat shielded. The hydrophobic environment leads to increased nitrogen acidities of the N-terminal valine in subunits α (pKa = 7.8) and subunit β (pKa = 6.8), which in turn explains the reactivity of the α-nitrogen in the valine residues, readily forming adducts with a variety of electrophilic compounds (Törnqvist et al. 2002).
Hydrolysis of glycidyl esters (GE) and reaction of glycidol with hemoglobin (Hb). Glycidol is formed by lipase-catalyzed hydrolysis of glycidyl esters (GE); R represents fatty acid side chains. The terminal epoxide carbon of glycidol reacts via an addition reaction with the N-terminal valine of one of the Hb peptide chains
Edman Degradation and Mass Spectrometric Quantification of DHP-Val in Hb
The conception for the application of Hb as a target (dosimeter) for the analysis of alkylating agents was introduced by Ehrenberg et al. (1974). The development of the modified Edman degradation by Törnqvist et al. was a major breakthrough in 1986, which allowed the reliable determination of Hb adducts on the N-terminal valine (Törnqvist et al. 1986). The sample preparation included several steps: isolation of globin from erythrocytes, cleavage of modified valine residues by pentafluorophenyl isothiocyanate (PFPITC), and derivatization of free hydroxyl groups of the resulting thiohydantoin using, for example, acetic anhydride (Landin et al. 1997). Optionally, the analytes were enriched by extraction with ether (Törnqvist et al. 1986) or by solid phase-extraction (SPE) (Fennell et al. 2005). Finally, the thiohydantoins were analyzed by gas chromatography-mass spectrometry (GC-MS) or liquid chromatography-tandem mass spectrometry (LC-MS/MS).
In early studies on DHP-Val, Landin et al. (1997) and Honda et al. (2014) used the technique of PFPITC-cleavage from isolated globin samples with subsequent GC-MS analysis. Recently, the analysis of DHP-Val was improved greatly by implementing the FIRE procedure™ for a modified Edman degradation (Rydberg et al. 2009). The valine adduct is cleaved with fluorescein-5-isothiocyanate (FITC), resulting in the formation of N-(2,3-dihydroxypropyl)-valine fluorescein thiohydantoin (DHP-Val-FTH, Fig. 2). This analyte is quantified using an ultra-high performance liquid chromatography-tandem mass spectrometry system (UHPLC-MS/MS) and DHP-Val-d7-FTH as reference standard (Hielscher et al. 2017). The technique surpasses the former methods of DHP-Val analysis due to its simplicity, sensitivity, and accuracy. The tedious globin isolation is obsolete, because the FITC-mediated valine cleavage is feasible using erythrocytes or even whole blood sample, and the resulting thiohydantoin DHP-Val-FTH (Fig. 2) does not require further derivatization for the analysis by UHPLC-MS/MS. The limit of detection (LOD; 0.7 pmol/g Hb) and the limit of quantification (LOQ; 1.4 pmol/g Hb) of DHP-Val analysis after cleavage with the FIRE procedure™ were about three times lower compared to those of the GC-MS methods reported previously by Landin et al. (LOD: 2 pmol/g globin) (Landin et al. 1996).
A general problem of Hb adduct quantification is that the cleavage efficiency of the isothiocyanate-mediated Edman degradation is unknown, leading to a method-inherent underestimation of Hb adduct levels. To improve the accuracy of the quantification, Monien et al. synthesized the peptide N-(2,3-dihydroxypropyl)-Val-Leu-anilide (DHP-Val-Leu-An), which was used to determine the Edman reaction turnover in separate samples. The average Edman efficiency for the cleavage of DHP-Val from DHP-Val-Leu-An in nine independent experiments was between 67.0% and 85.3% (mean ± SD = 77.7 ± 4.3%). The application of these values to the correction of DHP-Val levels determined in the set of regular erythrocyte samples analyzed on the same day improved greatly the accuracy of the quantification by isotope-dilution UHPLC-MS/MS (Monien et al. 2020).
Applications of DHP-Val as Biomarker of Glycidol Exposure
DHP-Val in Blood Samples from Rats and Humans
The adduct was first described by Landin et al. (1996), who observed that DHP-Val levels were 50% higher in blood samples of rats fed with fried animal feed compared to those observed in the control group (Landin et al. 2000). Also, direct oral administration of a high dose of glycidol or ester-bound glycidol (50 mg/kg body weight) in rats was shown to lead to DHP-Val levels in the range of about 13 nmol/g globin (Appel et al. 2013). Honda et al. observed that application of four different doses of glycidol to male rats led to an approximately linear increase of DHP-Val concentrations in blood samples retrieved 24 h after administration. The slope of the linear regression was 0.055 pmol DHP-Val/g Hb per μg glycidol/kg body weight (Honda et al. 2014).
Landin et al. compared DHP-Val levels in different groups of nonsmokers, smokers, and industry worker exposed to epichlorohydrin, a potential precursor of DHP-Val in Hb (Landin et al. 1997). In three subgroups of nonsmokers, mean DHP-Val levels were 7.3 pmol/g Hb (German industry workers, n = 8), 6.8 pmol/g Hb (German office clerks from the same company as the industry workers, n = 3), and 2.1 pmol/g Hb (control group of Swedish adults, n = 6). The data did not support the hypothesis that DHP-Val results from occupational exposure to the reactive epichlorohydrin. Instead, Landin et al. stated later that DHP-Val may originate from dietary intake of glycidol and GE formed by heat-treatment of food (Landin et al. 2000). Higher mean DHP-Val levels were observed in blood samples from smokers, 21.1 pmol/g Hb (German industry workers, n = 7), 13.1 pmol/g Hb (German office clerks, n = 8), and 9.5 pmol/g Hb (Swedish adults, n = 4), indicating that the inhalation by cigarette smoke contributes significantly to the overall glycidol exposure (Landin et al. 1997).
Honda et al. investigated the formation of DHP-Val in nonsmokers consuming GE-rich refined cooking oil. The mean adduct levels in participants (7.3 pmol/g Hb, n = 14) exposed to a specific oil with a high GE content and in the control population without specified exposure to the indicated oil (6.9 pmol/g Hb, n = 42) were not statistically different. Due to missing data on consumption of the cooking oil in question and the resulting overall glycidol exposure of the study participants, the study of Honda et al. did not provide the basis for a clear interpretation (Honda et al. 2012).
In more recent studies, the FITC-mediated Edman degradation was used for the quantification of DHP-Val. In blood samples of 11 nonsmoking adults, Abraham et al. determined median levels of 4.0 (range: 3.2–5.1) pmol/g Hb (n = 11) (Abraham et al. 2019). In children (n = 50), Aasa et al. observed a mean DHP-Val level of 7.3 ± 2.5 (range: 4.420) pmol/g Hb. In the same study, the mean DHP-Val level of 23.4 (range: 18.1–31.4) pmol/g Hb observed in smoking adults (n = 6) was much higher compared to that of nonsmoking adults 10.3 ± 2.7 (range: 6.3–14.0) pmol/g Hb (Aasa et al. 2019).
DHP-Val in Cord and Maternal Blood: Prenatal Exposure to Glycidol
The technique of DHP-Val quantification was used by Monien et al. to clarify the question if unborn children are exposed by prenatal transfer of glycidol from the circulation of their mothers. For this sake, 100 paired cord and maternal blood samples from the Belgian birth cohort ENVIRONAGE (ENVIRonmental influence ON AGEing in early life (Janssen et al. 2017)) were analyzed. Median levels of DHP-Val were 5.4 (range: 2.3–29.2) and 1.6 (range: < LOD–8.9) pmol/g Hb in samples of mother and newborns, respectively. In blood samples of mothers who smoked during pregnancy and in the cord blood samples of their newborns (n = 6), the median DHP-Val levels were 16.7 (range: 6.4–29.2) and 6.2 (range: < LOD–8.6) pmol/g Hb, respectively. The Spearman correlation coefficient of DHP-Val levels in cord and maternal blood samples was 0.63 (p < 0.001) among all mother-newborn pairs and 0.59 (p < 0.001) among mother-newborn pairs of nonsmoking mothers. The median ratio of DHP-Val levels of cord to maternal blood was 0.35 (range: 0.19–1.14). The data indicated that unborn children are exposed to glycidol due to a relatively unhindered passive transfer through the placental barrier (Monien et al. 2020).
Estimation of External Glycidol Exposure Using DHP-Val (Reverse Dosimetry)
Most of the studies on DHP-Val in humans focused on identifying influencing factors of glycidol exposure in different population groups, for example, smoking (in adults) or transplacental exposure (in unborn infants). The studies allowed drawing conclusions about the relative exposure, without permitting an estimation of the actual glycidol intake. However, the external dietary exposure to GE/glycidol is required to improve the risk assessment. Reverse dosimetry is the discipline to approximate external exposure from biomarker data (Clewell et al. 2008; Skipper and Tannenbaum 1990). Using a kinetic model, it is possible to estimate the mean exposure to a reactive substance from a single measurement of a specific Hb adduct assuming that a continuous exposure leads to a steady-state level of the adduct and that the adduct is chemically stable (Fennell et al. 1992). Under these circumstances, the daily dose (D) on a body weight basis can be calculated as follows:
with H as the adduct level (pmol/g Hb), τ as the lifetime of the adduct, and k, a proportionality constant (daily increase of adduct level per dose on a body weight basis). For the case of glycidol and DHP-Val formation, the constant k was determined in a recent study at the Federal Institute for Risk Assessment (BfR), in which 11 participants took part in a controlled intervention with a commercially available palm fat containing a relatively high amount of ester-bound glycidol (8.7 mg glycidol/kg) over 28 days. The consumption of a daily portion of 36 g fat (individual glycidol doses between 2.7 and 5.2 μg/kg body weight per day) led to an increase in the mean adduct levels from 4.0 pmol DHP-Val/g Hb before the start of the intervention to 12.2 pmol DHP-Val/g Hb after 28 days. Fitting of the data with the model described by Fennel et al. (1992) allowed determining the mean value of k (0.082 pmol DHP-Val/g Hb per μg glycidol/kg body weight) and the mean adduct lifetime τ (104 days), which corresponds to the estimated lifetime of erythrocytes (~ 110 ± 21 days) (Bentley et al. 1974). The value of k derived in this study is valid for an oral exposure to GE, but is expected to be different in case of inhalational exposure to glycidol.
With the value of the adduct level per dose ratio k at hand, the external dietary glycidol exposure was calculated from the mean DHP-Val background observed in the 11 study participants (4.0 pmol/g Hb) to be 0.94 μg/kg body weight (Abraham et al. 2019). This value exceeds significantly the median value of the mean chronic exposure to glycidol from GE across dietary surveys estimated for the adult population in Europe by the EFSA (0.2 μg glycidol/kg body weight) (European Food Safety Authority 2016).
Also Aasa et al. used the value of k to calculate the mean glycidol exposure from mean DHP-Val levels in adolescents (7.3 pmol/g Hb, n = 50, 2014) and in nonsmoking (10.3 pmol/g Hb, n = 6, 1997) and smoking adults (23.4 pmol/g Hb, n = 6, 1997). The estimated mean dose levels of adolescents (1.4 μg glycidol/kg body weight), nonsmoking adults (2.0 μg glycidol/kg body weight), and smoking adults (4.9 μg glycidol/kg body weight) were even higher than those calculated for German adults by Abraham et al. (Aasa et al. 2019). It must be noted that the sampling time may be an influential parameter. The blood samples from German adults (n = 11) were from 2017 (Abraham et al. 2019), while those of Swedish adolescents and of nonsmoking and smoking adults were from 2014 and 1997, respectively. The effect of the mitigation strategies of GE formation in plant and vegetable oils studies in the last decade (Oey et al. 2019) on the human exposure may be monitored using DHP-Val as a biomarker.
Important Considerations for the Application of DHP-Val as Biomarker of Glycidol Exposure
Unknown Exposure Sources of Glycidol
The discrepancy between the external exposure values calculated from the mean background of DHP-Val in German adults (n = 11) (Abraham et al. 2019) or estimated using occurrence and consumption data of food in Europe (European Food Safety Authority 2016) may have different reasons. It is conceivable that there are oral or inhalational glycidol sources, which are currently not identified by the exposure estimation. Some foods with relevant levels of GE may not be identified yet, and there is hardly any data on the formation of GE by domestic food preparation procedures (Inagaki et al. 2016). In addition, the inhalational exposure, for example, from passive smoking, was not considered by the dietary exposure estimate of the EFSA.
Biomarker Specificity: Other Xenobiotic Precursors of DHP-Val?
For the estimation of the dietary exposure to glycidol from the steady-state level of DHP-Val, it is also relevant to clarify the specificity of the biomarker: Is it possible that DHP-Val may be formed from reactive substances other than glycidol? Various compounds were considered as precursors for DHP-Val, that is, 3-monochloropropane-1,2-diol (3-MCPD), epichlorohydrin (Landin et al. 1997), 1-bromopropane (Ishidao et al. 2002), and allyl alcohol (Honda et al. 2012). However, none of these hypotheses were confirmed, and 3-MCPD and epichlorohydrin were shown to not contribute significantly to DHP-Val formation (Aasa et al. 2017; Ishidao et al. 2002; Landin et al. 1997; Wollin et al. 2014). In a recent study, Shimamura et al. administered relatively high single oral doses of glycidol, glycidol oleate, and the alternative precursors, 3-MCPD, epichlorohydrin, propylene oxide, 1-bromopropane, allyl alcohol, fructose, and glyceraldehyde dissolved in soybean oil by gavage in male mice and analyzed DHP-Val in blood samples 24 h afterwards. DHP-Val was detected (LOD 10 pmol/g Hb) only in mice treated with glycidol and glycidol oleate (Shimamura et al. 2020). In summary, there are no indications so far of reactive metabolites of xenobiotics other than glycidol that form the adduct DHP-Val in relevant amounts.
DHP-Val from Endogenous Exposure to Glycidol or Other Reactants?
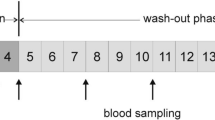
A recent review summarized indications for endogenous sources contributing to the internal exposure to various heat-induced food contaminants independent from the dietary intake (Rietjens et al. 2022). For example, the mercapturic acid N-acetyl-S-(carbamoylethyl)-L-cysteine (AAMA), the main metabolite of the food carcinogen acrylamide, is not only formed as a consequence of external acrylamide exposure. In a group of 14 adults on an acrylamide free diet, Ruenz et al. observed high amounts of urinary AAMA after day 3 of the washout period, which were equivalent to a daily acrylamide exposure of 0.2–0.3 μg/kg body weight. Considering the EFSA estimation of the median adult exposure of acrylamide (0.4–0.6 μg/kg body weight), the authors followed that roughly one third of the AAMA excretion is not due to the dietary intake of acrylamide. To explain this, Ruenz et al. suggested that there is an endogenous exposure source of acrylamide (Ruenz et al. 2016). This raises the question whether there is another source of endogenous metabolites leading to the formation of DHP-Val. This was hypothesized first by Honda et al., however, without suggesting possible sources (Honda et al. 2012, 2014).
To answer the question whether DHP-Val formation occurs independently from the dietary glycidol uptake, we quantified DHP-Val levels in 27 nonsmoking adults with a conventional omnivore diet recruited in the “Risks and Benefits of a Vegan Diet” (RBVD) study (Weikert et al. 2020) and in 16 nonsmoking strict raw food eaters (Abraham et al. 2022), who did not consume any food heated to higher temperatures than 42 °C for at least 4 months (Fig. 3). The levels of DHP-Val in blood samples of strict raw food eaters (median 1.9, range 1.2–1.9 pmol/g Hb) were significantly lower (p < 0.001) compared to those of omnivores (median 4.1, range 1.9–10.5 pmol/g Hb). Comparison of the data suggests that under current conditions in Germany, about half of the DHP-Val levels in nonsmoking omnivores are formed independently from external exposure to glycidol and GE by the diet. Taking this into account, the median exposure to free and bound glycidol can be estimated to be 0.48 μg/kg body weight in the group of nonsmoking omnivores (n = 27). This is relatively close to the EFSA estimate of the dietary exposure to bound glycidol in adults (0.2 μg/kg body weight) (European Food Safety Authority 2016). However, as pointed out above, the EFSA estimate did not consider free glycidol in foodstuffs or GE and glycidol formed in the domestic food preparation.
DHP-Val in omnivores and strict raw food eaters. Levels of DHP-Val in blood samples from nonsmoking omnivores with conventional dietary habits including heated foodstuffs (n = 27, red box) and nonsmoking strict raw food eaters (n = 16, green box). Lines and boxes represent median values and the lower and upper quartiles, respectively, and the error bars represent the 10th and 90th percentiles (*** p < 0.001, Mann-Whitney rank-sum test)
Mini Dictionary
-
Glycidyl esters (GE) are fatty acid esters formed during the refinement of vegetable oils. After ingestion, these oils and fats release glycidol in the gastrointestinal tract.
-
Glycidol (2,3-epoxy-1-propanol) is an electrophilic, alkylating agent prone to react with cellular nucleophiles, such as DNA and proteins. It was classified as probably carcinogenic to humans (group 2A) by the International Agency for Research on Cancer (IARC).
-
The margin of exposure (MoE) is the ratio between the lowest glycidol dose leading to a significant increase of tumor incidence in animals and the exposure estimates in humans.
-
Hemoglobin (Hb) adducts, that is, the valine residues at the N-termini of Hb peptide chains modified by reactive compounds/metabolites, are used as biomarkers of medium-term exposure.
-
N-(2,3-dihydroxypropyl)-valine (DHP-Val) is the adduct formed by the reaction of N-terminal valine residues in Hb with glycidol.
-
The Edman degradation with isothiocyanates is used to cleave the modified valine adducts from the N-termini in Hb.
-
Ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) is a powerful analytical technique for the quantification of Hb adducts and other analytes using isotope-labeled standard compounds.
-
Reverse dosimetry allows estimating of the external exposure to a toxicologically relevant substance from the quantitative information of a biomarker, for example, a metabolite excreted in the urine or a Hb adduct.
Key Facts of Valine Adducts in Hb
-
Nitrogen atoms at the N-terminal valine residues in Hb are prone to react with electrophilic compounds.
-
The modified valine residues are cleaved with isothiocyanates (Edman degradation) and the resulting thiohydantoin analytes are quantified using isotope-dilution mass spectrometry.
-
Based on the Edman degradation, various biomonitoring methods for the determination of N-terminal adducts of acrylamide, ethylene oxide, epichlorohydrin, glycidol, glycidamide, benzyl chloride, and others were validated and are routinely used in occupational medicine.
-
The valine adducts in Hb reflect the internal exposure to a reactive compound over a period of approximately 4 months corresponding to the lifetime of human erythrocytes in adults.
-
A reverse dosimetry model was developed by Fennel et al. This allows calculating the daily external exposure to a reactive substance with a proportionality constant k, which describes the increase of the valine adduct level (pmol/g Hb) per dose (μg/kg body weight).
-
The adduct per dose ratio k was determined in a controlled intervention study, monitoring the increase intervals of Hb adduct levels in a group of human participants exposed to defined amounts of ester-bound glycidol.
Summary
The exposure to the heat-induced food contaminant glycidol and its fatty acid esters (GE) leads to the formation of DHP-Val in Hb.
The absence of detectable DHP-Val amounts in blood samples of mice treated with alternative precursors, 3-MCPD, epichlorohydrin, propylene oxide, 1-bromopropane, allyl alcohol, fructose, and glyceraldehyde supported the notion that DHP-Val is a specific biomarker of glycidol exposure.
The quantification of DHP-Val levels in blood samples from different groups of study participants (mother/newborns, smokers/nonsmokers, omnivores/raw food eaters) allowed conclusions about the exposure sources of glycidol and GE.
Principal sources of glycidol exposure in adults are food (glycidol/GE) and tobacco smoke (glycidol), whereas unborn infants are exposed via placental transport of glycidol.
Using the adduct increase per dose ratio k (0.082 pmol DHP-Val/g Hb per μg glycidol/kg body weight) for reverse dosimetry, the mean oral exposure to glycidol was calculated for German adults (0.94 μg/kg body weight, n = 11) and Swedish adolescents (1.4 μg/kg body weight, n = 50).
The exposure data estimated from DHP-Val levels exceeded the median values of the mean chronic exposure to glycidol from GE across dietary surveys estimated for the adult population (0.2 μg glycidol/kg body weight) and for adolescents (0.3 μg glycidol/kg body weight) in Europe by the EFSA (European Food Safety Authority 2016).
The discrepancy between the results from reverse dosimetry calculation and estimations using occurrence and consumption data of food in Europe may be due to unknown sources of glycidol exposure not considered by EFSA, for example, yet unidentified foodstuffs with relevant levels of GE, the formation of GE by domestic food preparation procedures, and inhalational exposure of glycidol (e.g., from passive smoking).
The comparison of DHP-Val in blood samples from nonsmoking omnivores and raw food eaters suggests that about half of the DHP-Val levels in nonsmoking omnivores are formed independently from external exposure to glycidol and GE by the diet under current conditions in Germany.
Abbreviations
- DHP-Val:
-
N-(2,3-dihydroxypropyl)-valine
- DHP-Val-FTH:
-
N-(2,3-dihydroxypropyl)-valine fluorescein thiohydantoin
- EFSA:
-
European Food Safety Authority
- FITC:
-
Fluorescein-5-isothiocyanate
- FTH:
-
Fluorescein thiohydantoin
- GC-MS:
-
Gas chromatography-mass spectrometry
- GE:
-
Glycidyl esters
- Hb:
-
Hemoglobin
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- SPE:
-
Solid-phase extraction
- UHPLC-MS/MS:
-
Ultra-high performance liquid chromatography-tandem mass spectrometry
References
Aasa J, Abramsson-Zetterberg L, Carlsson H, et al. The genotoxic potency of glycidol established from micronucleus frequency and hemoglobin adduct levels in mice. Food Chem Toxicol. 2017;100:168–74. https://doi.org/10.1016/j.fct.2016.12.022.
Aasa J, Vryonidis E, Abramsson-Zetterberg L, et al. Internal doses of glycidol in children and estimation of associated cancer risk. Toxics. 2019;7:7. https://doi.org/10.3390/toxics7010007.
Abraham K, Appel KE, Berger-Preiss E, et al. Relative oral bioavailability of 3-MCPD from 3-MCPD fatty acid esters in rats. Arch Toxicol. 2013;87:649–59. https://doi.org/10.1007/s00204-012-0970-8.
Abraham K, Hielscher J, Kaufholz T, et al. The hemoglobin adduct N-(2,3-dihydroxypropyl)-valine as biomarker of dietary exposure to glycidyl esters: a controlled exposure study in humans. Arch Toxicol. 2019;93:331–40. https://doi.org/10.1007/s00204-018-2373-y.
Abraham K, Hielscher J, Kuhlmann J, et al. Urinary excretion of 2/3-Monochloropropanediol (2/3-MCPD) and 2,3-Dihydroxypropylmercapturic acid (DHPMA) after a single high dose of fatty acid esters of 2/3-MCPD and glycidol: a controlled exposure study in humans. Mol Nutr Food Res. 2021;65:e2000735. https://doi.org/10.1002/mnfr.202000735.
Abraham K, Trefflich I, Gauch F, et al. Nutritional intake and biomarker status in strict raw food eaters. Nutrients. 2022;14:1725.
Appel KE, Abraham K, Berger-Preiss E, et al. Relative oral bioavailability of glycidol from glycidyl fatty acid esters in rats. Arch Toxicol. 2013;87:1649–59. https://doi.org/10.1007/s00204-013-1061-1.
Bakhiya N, Abraham K, Gurtler R, et al. Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol Nutr Food Res. 2011;55:509–21. https://doi.org/10.1002/mnfr.201000550.
Barocelli E, Corradi A, Mutti A, et al. Comparison between 3-MCPD and its palmitic esters in a 90-day toxicological study. EFSA J. 2011;1–131. https://www.efsa.europa.eu/en/supporting/pub/en-187
Bentley SA, Lewis SM, White JM. Red cell survival studies in patients with unstable haemoglobin disorders. Br J Haematol. 1974;26:85–92.
Clewell HJ, Tan YM, Campbell JL, et al. Quantitative interpretation of human biomonitoring data. Toxicol Appl Pharmacol. 2008;231:122–33. https://doi.org/10.1016/j.taap.2008.04.021.
Ehrenberg L, Hiesche KD, Osterman-Golkar S, et al. Evaluation of genetic risks of alkylating agents: tissue doses in the mouse from air contaminated with ethylene oxide. Mutat Res. 1974;24:83–103.
European Food Safety Authority. Opinion of the Scientific Committee on a request from EFSA related to a harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005;282:1–31.
European Food Safety Authority. Risks for human health related to the presence of 3-and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016;14:4426–584. https://doi.org/10.2903/j.efsa.2016.4426.
Fennell TR, Sumner SC, Walker VE. A model for the formation and removal of hemoglobin adducts. Cancer Epidemiol Biomark Prev. 1992;1:213–9.
Fennell TR, Sumner SC, Snyder RW, et al. Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci. 2005;85:447–59. https://doi.org/10.1093/toxsci/kfi069.
Hielscher J, Monien BH, Abraham K, et al. An isotope-dilution UPLC-MS/MS technique for the human biomonitoring of the internal exposure to glycidol via a valine adduct at the N-terminus of hemoglobin. J Chromatogr B. 2017;1059:7–13. https://doi.org/10.1016/j.jchromb.2017.05.022.
Honda H, Fujii K, Yamaguchi T, et al. Glycidol exposure evaluation of humans who have ingested diacylglycerol oil containing glycidol fatty acid esters using hemoglobin adducts. Food Chem Toxicol. 2012;50:4163–8. https://doi.org/10.1016/j.fct.2012.07.058.
Honda H, Törnqvist M, Nishiyama N, et al. Characterization of glycidol-hemoglobin adducts as biomarkers of exposure and in vivo dose. Toxicol Appl Pharmacol. 2014;275:213–20. https://doi.org/10.1016/j.taap.2014.01.010.
Inagaki R, Hirai C, Shimamura Y, et al. Formation of glycidol fatty acid esters in meat samples cooked by various methods. J Food Process Technol. 2016;7:557–62. https://doi.org/10.4172/2157-7110.1000557.
International Agency for Research on Cancer. Glycidol in some industrial chemicals. In: IARC monographs on the evaluation of carcinogenic risks to humans, vol. 77. Lyon: International Agency for Research on Cancer; 2000. p. 469–86.
Ishidao T, Kunugita N, Fueta Y, et al. Effects of inhaled 1-bromopropane vapor on rat metabolism. Toxicol Lett. 2002;134:237–43. https://doi.org/10.1016/s0378-4274(02)00171-6.
Janssen BG, Madhloum N, Gyselaers W, et al. Cohort profile: the ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol. 2017;46:1386–7. https://doi.org/10.1093/ije/dyw269.
Landin HH, Osterman-Golkar S, Zorcec V, et al. Biomonitoring of epichlorohydrin by hemoglobin adducts. Anal Biochem. 1996;240:1–6. https://doi.org/10.1006/abio.1996.0322.
Landin HH, Grummt T, Laurent C, et al. Monitoring of occupational exposure to epichlorohydrin by genetic effects and hemoglobin adducts. Mutat Res. 1997;381:217–26.
Landin HH, Tareke E, Rydberg P, et al. Heating of food and haemoglobin adducts from carcinogens: possible precursor role of glycidol. Food Chem Toxicol. 2000;38:963–9.
MAK Commission. List of MAK and BAT values 2021, vol. 57. MAK Collection. Publisso. 2021. https://doi.org/10.34865/mbwl_2021_eng.
Mathias PI, B’Hymer C. Mercapturic acids: recent advances in their determination by liquid chromatography/mass spectrometry and their use in toxicant metabolism studies and in occupational and environmental exposure studies. Biomarkers. 2016;21:293–315. https://doi.org/10.3109/1354750X.2016.1141988.
Monien BH, Abraham K. Levels of 2,3-dihydroxypropyl mercapturic acid (DHPMA) in human urine do not reflect the exposure to 3-chloro-1,2-propanediol (3-MCPD) or glycidol. Environ Res. 2022;211:112977. https://doi.org/10.1016/j.envres.2022.112977.
Monien BH, Abraham K, Nawrot TS, et al. Levels of the hemoglobin adduct N-(2,3-Dihydroxypropyl)-valine in cord and maternal blood: prenatal transfer of glycidol in the ENVIRONAGE birth cohort. Toxicol Lett. 2020;332:82–7. https://doi.org/10.1016/j.toxlet.2020.06.013.
National Toxicology Program. Toxicology and carcinogenesis studies of Glycidol (CAS No. 556-52-5) in F344/N rats and B6C3F1 mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 1990;374:1–229.
Oey SB, van der Fels-Klerx HJ, Fogliano V, et al. Mitigation strategies for the reduction of 2-and 3-MCPD esters and Glycidyl esters in the vegetable oil processing industry. Compr Rev Food Sci Food Saf. 2019;18:349–61. https://doi.org/10.1111/1541-4337.12415.
Pudel F, Benecke P, Fehling P, et al. On the necessity of edible oil refining and possible sources of 3-MCPD and glycidyl esters. Eur J Lipid Sci Technol. 2011;113:368–73. https://doi.org/10.1002/ejlt.201000460.
Rietjens I, Michael A, Bolt HM, et al. The role of endogenous versus exogenous sources in the exposome of putative genotoxins and consequences for risk assessment. Arch Toxicol. 2022;96(5):1297–352. https://doi.org/10.1007/s00204-022-03242-0.
Ruenz M, Bakuradze T, Eisenbrand G, et al. Monitoring urinary mercapturic acids as biomarkers of human dietary exposure to acrylamide in combination with acrylamide uptake assessment based on duplicate diets. Arch Toxicol. 2016;90:873–81. https://doi.org/10.1007/s00204-015-1494-9.
Rydberg P, von Stedingk H, Magner J, et al. LC/MS/MS analysis of N-Terminal protein adducts with improved sensitivity: a comparison of selected edman isothiocyanate reagents. Int J Anal Chem. 2009;2009:153472. https://doi.org/10.1155/2009/153472.
Sabbioni G, Day BW. Quo vadis blood protein adductomics? Arch Toxicol. 2022;96:79–103. https://doi.org/10.1007/s00204-021-03165-2.
Shimamura Y, Inagaki R, Honda H, et al. Does external exposure of glycidol-related chemicals influence the forming of the hemoglobin adduct, N-(2,3-dihydroxypropyl)valine, as a biomarker of internal exposure to glycidol? Toxics. 2020;8(4):119. https://doi.org/10.3390/toxics8040119.
Skipper PL, Tannenbaum SR. Protein adducts in the molecular dosimetry of chemical carcinogens. Carcinogenesis. 1990;11:507–18.
Törnqvist M, Mowrer J, Jensen S, et al. Monitoring of environmental cancer initiators through hemoglobin adducts by a modified Edman degradation method. Anal Biochem. 1986;154:255–66.
Törnqvist M, Fred C, Haglund J, et al. Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:279–308.
Weikert C, Trefflich I, Menzel J, et al. Vitamin and mineral status in a vegan diet. Dtsch Arztebl Int. 2020;117:575–82. https://doi.org/10.3238/arztebl.2020.0575.
Wollin KM, Bader M, Müller M, et al. Assessment of long-term health risks after accidental exposure using haemoglobin adducts of epichlorohydrin. Toxicol Lett. 2014;231:378–86. https://doi.org/10.1016/j.toxlet.2014.07.020.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry
Monien, B.H., Abraham, K. (2023). The Carcinogen Glycidol and Use of N-(2,3-Dihydroxypropyl)-valine in Hemoglobin as a Biomarker of Exposure. In: Patel, V.B., Preedy, V.R., Rajendram, R. (eds) Biomarkers in Toxicology. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-07392-2_65
Download citation
DOI: https://doi.org/10.1007/978-3-031-07392-2_65
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07391-5
Online ISBN: 978-3-031-07392-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences