Abstract
The present human intervention study investigated the relation between the intake of acrylamide (AA) in diets with minimized, low, and high AA contents and the levels of urinary exposure biomarkers. As biomarkers, the mercapturic acids, N-acetyl-S-(carbamoylethyl)-l-cysteine (AAMA), and N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA) were monitored. The study was performed with 14 healthy male volunteers over a period of 9 days, under controlled conditions excluding any inadvertent AA exposure. Dietary exposure to AA was measured by determining AA contents in duplicates of all meals consumed by the volunteers. The study design included an initial washout period of 3 days on AA-minimized diet, resulting in dietary AA exposure not exceeding 41 ng/kg bw/d. Identical washout periods of 2 days each followed the AA exposure days (day 4, low exposure, and day 7, high exposure). At the respective AA intake days, volunteers ingested 0.6–0.8 (low exposure) or 1.3–1.8 (high exposure) μg AA/kg bw/d with their food. Both low and high AA intakes resulted in an AAMA output within 72 h corresponding to 58 % of the respective AA intake. At the end of the initial 3-day washout period, an AAMA baseline level of 93 ± 31 nmol/d was recorded, suggestive for an assumed net AA baseline exposure level of 0.2–0.3 μg AA/kg bw/d.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
2-Propenamide (acrylamide, AA) is classified as a genotoxic carcinogen that is formed during heat processing of foods, leading to human dietary exposure levels associated with margins of exposure (MOE) which indicate a potential human health concern (EFSA 2014). This heat-induced contaminant is known to arise during the Maillard reaction between asparagine and reducing sugars at enhanced processing temperatures (>120 °C) (Mottram et al. 2002; Stadler et al. 2002). Estimated daily AA intake of adults in Europe averages 0.3–0.9 µg/kg bw or 0.6–2.0 µg/kg bw for the 95th percentile (EFSA 2014). Ingested AA is metabolized in part by CYP450 2E1 to the epoxide 2,3-epoxypropanamide (glycidamide, GA), which is largely accepted as the ultimate genotoxic agent. It causes DNA damage by forming DNA adducts, primarily at position N7 of the DNA base guanine (Gamboa da Costa et al. 2003; Watzek et al. 2012a). Both AA and GA are electrophilic agents that avidly react with nucleophilic sites of amino acids, peptides, and proteins, including plasma proteins, haemoglobin and the major cellular detoxifying agent, glutathione (GSH). Haemoglobin adducts (AA-Hb and GA-Hb) and GSH adducts, respectively their terminal metabolites, the corresponding mercapturic acids (MAs) have been characterized and established as exposure biomarkers. Main MAs formed from AA and GA are N-acetyl-S-(carbamoylethyl)-l-cysteine (AAMA) and N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA), respectively (Berger et al. 2011; Hartmann et al. 2008; Watzek et al. 2012b). Urinary MAs reflect recent exposure to AA and can therefore be used as short-term biomarkers of exposure (EFSA 2014). An overview of the main metabolic pathways of AA is given in Fig. 1. In addition to the main reactions with biological nucleophiles (proteins and GSH), regarded to lead to detoxification, one minor detoxification pathway has been detected in humans, representing hydrolysis of GA to 2,3-dihydroxypropionamide (DHPA), also known as glyceramide. This metabolic sideway may contribute by about 10 % to overall AA metabolism (Fennell et al. 2005). However, since DHPA may also be generated from other sources, it is not considered to be a useful biomarker of AA exposure (EFSA 2014).
In a large dose–response study in rats, some indications of putative background internal exposure to AA became apparent, as evidenced by consistent background mercapturic acid excretion. This baseline MA signal was observed in the urine of control animals at the end of an initial 2-week washout period, characterized by meticulous exclusion of any dietary or inadvertent AA exposure. In this study, AA was given orally in single dosage of 0.1–10,000 µg/kg bw. On average, 37.0 ± 11.5 % of the AA dose was excreted as MAs within 16 h, the time point of maximal DNA adduct levels found in the organs analysed (Watzek et al. 2012a). The diet consumed by the control group had been proven to be ‘AA free’ with a limit of detection (LOD) of 0.5 µg/kg. Taking the LOD value as true maximum AA intake, a daily AA exposure of 0.1 μg/kg bw was estimated. Surprisingly, the output of urinary MAs accounted for a presumed ingestion of 0.6–0.7 μg AA/kg bw in the control group. This was interpreted as an indication for potential endogenous AA formation in rats by an as-yet-unknown metabolic pathway (Watzek et al. 2012a). Some support for this assumption was derived from the observation that AA haemoglobin adducts were found increased in mice treated with compounds known to induce free radicals (Tareke et al. 2008), and that formation of AA became apparent after incubation of asparagine with hydrogen peroxide under physiological conditions (Tareke et al. 2009).
The aim of the present study was to investigate the question, whether and how such presumed background internal exposure to AA may also became observable in humans. To this end, an intervention study in healthy non-smoking volunteers under tightly controlled dietary and environmental conditions was designed, characterized by exact measurement of the dietary AA intake using duplicate diet monitoring and determining urinary AAMA and GAMA output throughout washout periods and dietary intake days.
Materials and methods
Chemicals
Chemicals were of analytical grade. Acrylamide (AA) was from Sigma-Aldrich (Steinheim, Germany) and the deuterium-labelled acrylamide (D3-AA) from Toronto Research Chemicals (Toronto, Canada), as were the MAs, N-acetyl-S-(carbamoylethyl)-l-cysteine (AAMA) and N-acetyl-S-(1-carbamoyl-2-hydroxyethyl)-l-cysteine (GAMA). The deuterium-labelled MAs (N-acetyl-S-(carbamoyl-d3-ethyl)-l-cysteine (D3-AAMA) and N-acetyl-S-(1-carbamoyl-2-hydroxy-d3-ethyl)-l-cysteine) (D3-GAMA) were available from former studies (Watzek et al. 2012b).
Study design
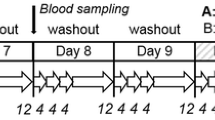
The study was approved by the Ethics Commission of Rhineland-Palatinate, Mainz (No. 837.449.12 (8600-F)). Fifteen non-smoking male volunteers of Caucasian origin in good health status were recruited. Exclusion criteria were age <20 or >44 years; body mass index (BMI) <19 or >25 kg/m2; smoking or any other tobacco consumption; being under medication and/or taking dietary supplements; performing a competitive sport; metabolic disorders; not being accustomed to the coffee consumption foreseen in the study; participation in other studies or regular blood donors. Prior to their inclusion in the study, all volunteers gave their informed consent and a medical check-up was carried out to determine body weight, height, body mass index, blood pressure, and clinical parameters including general blood count as well as creatinine, cystatin C, γ–glutamyltransferase (γ–GT), glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), and C-reactive protein (CRP). All volunteers were of good health status with a BMI of 23.1 ± 1.5 kg/m2. The study location was a well-secluded home deep in the forest with no car or other traffic, no open fire, no tobacco smoking, or any other potential source for inadvertent AA exposure. Volunteers were under supervision 24 h/d. A scheme of the study design is given in Fig. 2. Diets were equal for all volunteers; water was allowed ad libitum. At days 1, 2, 3, 5, 6, 8, and 9, an AA-minimized diet was consumed, mainly consisting of boiled potatoes, noodles, rice, boiled meat, yoghurts, vegetables, and fruits (washout phase). At day 4, a diet was given resulting in a low AA intake (low AA) and at day 7 in a high AA intake (high AA). These consisted mainly of potato crisps, French fries, fried potatoes, steak, sausages, crisp bread, biscuits, breakfast cereals, and coffee. Foods ready for consumption were divided into aliquots to be consumed and duplicates for analysis (duplicate meals). Duplicates (overall homogenates during washout, individual food items ready for consumption during AA intake days) were weighed and frozen at −20 °C until determination of AA, protein, and asparagine contents. Urine was collected at days 1, 2, and 9 over a period of 24 h, respectively. From day 3 to day 8, urine collection was split into three consecutive periods of 4 h each (8 a.m.–12 p.m., 12–4 p.m., 4–8 p.m.), followed by a 12-h overnight sampling period (8 p.m.–8 a.m.) (Fig. 2). After determination of the total urine weight, aliquots were stored at −20 °C until determination of urinary creatinine and MAs. Body weight of the volunteers was monitored daily (every morning before breakfast), and all physical activities were protocolled. One volunteer dropped out on the second study day due to dissatisfaction with the diet. Therefore, 14 volunteers (age 23 ± 3 years; BMI 23.2 ± 1.6 kg/m2) terminated the study.
Urinary excretions of AAMA (left, 1) and GAMA (right, 2) as absolute amounts (in nmol, a) and concentrations (in µmol/g creatinine, b) for each individual study day (24 h, start at 8 a.m.). Day 1, 2, 3, 5, 6, 8, and 9: washout days (21–41 ng AA/kg bw/d), day 4: low AA (0.6–0.9 µg/kg bw/d), day 7: high AA (1.3–1.8 µg/kg bw/d). Boxes represent the interquartile range (IQR), the maximum length of each whisker is 1.5 times the IQR, crosses are outliers according to the 1.5 times the IQR criterion, squares represent mean values, horizontal lines within boxes represent median values. ***significant difference (p < 0.001); **significant difference (p < 0.01); *significant difference (p < 0.05)
Experimental procedures
Food analysis (acrylamide, protein, asparagine, calorie content)
For AA determination, a stable isotope dilution method (SIDA) adapted from Watzek et al. was performed (Watzek et al. 2012a). Thawed food samples (1–30 g) were comminuted, homogenized, and diluted to 30 mL with bidistilled water. The isotopically labelled standard (D3-AA, 5 µL for foods with minor AA contents or 20 µL for foods with major AA contents, 50 μg/mL) was added. After stirring (1 h) and centrifugation (3000 g; 30 min), supernatants (10 mL) were applied to SPE columns (Isolute® ENV+; 500 mg; 6 mL from Biotage, Düsseldorf, Germany), preconditioned with methanol (4 mL) and bidistilled water (4 mL). After sample loading, columns were washed with bidistilled water (4 mL) and analytes eluted with 60 % aqueous methanol (v/v, 2 mL). The eluates were concentrated to approximately 1 mL using a vacuum centrifuge (Eppendorf, Hamburg, Germany). Aliquots were injected into the high-performance liquid chromatography–electrospray-tandem mass spectrometry (HPLC–ESI–MS/MS) system. AA determination in food with high AA was performed using an Agilent 1200 HPLC system (Agilent Technologies, Waldbronn, Germany) coupled with an API 3200 mass spectrometer (AB Sciex, Darmstadt, Germany). Minor AA contents were determined using an Agilent 1290 HPLC system (Agilent Technologies) coupled with a QTRAP 5500 mass spectrometer (AB Sciex). HPLC separation was achieved with a reversed-phase HPLC column (Luna C8(2); 150 mm × 4.6 mm; 3 µm from Phenomenex, Aschaffenburg, Germany) equipped with a guard column (Luna C8(2); 4 mm × 3 mm from the same supplier).
Using the Agilent 1200 HPLC/API 3200 MS system with electrospray ionization, the injection volume was 50 µL and the flow was 0.5 mL/min. A gradient elution was carried out with 0.05 % aqueous formic acid as solvent A and methanol as solvent B. The concentration of methanol was increased from 1 to 20 % (minute 1–12). MS measurements were performed as described earlier (Watzek et al. 2012a).
For the Agilent 1290 HPLC/QTRAP 5500 MS system, the injection volume was 5 µL and the used flow was 0.5 mL/min. Gradient elution was carried out with 0.05 % aqueous acetic acid as solvent A and methanol as solvent B. The concentration of methanol was increased from 1 to 5 % (minute 1–12). MS measurements were carried out after positive electrospray ionization using the multiple reaction monitoring (MRM) mode. Optimized MS parameters are listed in Table 1.
Calibration plots of peak area versus concentration ratios (AA/D3-AA) were linear with an R2 value of at least 0.999. Limit of detection (LOD) and quantification (LOQ), using the Agilent 1290 HPLC/QTRAP 5500 MS system, was 0.05 and 0.15 pmol, respectively.
Determination of protein contents was carried out by Eurofins Analytik GmbH (Hamburg, Germany). Asparagine contents were measured by Institut Kuhlmann GmbH (Ludwigshafen, Germany). Calorie contents of the foods consumed were calculated using the software PRODI 5.8 Expert (Nutri-Science GmbH, Hausach, Germany).
Urine analysis (mercapturic acids, creatinine, urine volumes)
Determination of mercapturic acids was carried out using the stable isotope dilution method (SIDA) published by Watzek et al. (2012b), with slight modifications. Urine aliquots (2 mL) were diluted to a total volume of 8 mL with ammonium formate buffer (50 mM; pH 2.5). D3-AAMA (40 µL; 10 µg/mL) and D3-GAMA (40 µL; 10 µg/mL) were added as internal standards. The samples were shaken and the pH adjusted to 2.5 by acidification with 4 N HCl. After centrifugation (3000g; 15 min), supernatants were applied to SPE columns (Isolute® ENV+; 100 mg; 10 mL from Biotage), preconditioned with methanol (4 mL), bidistilled water (4 mL), and HCl (pH 2.5; 4 mL). After sample loading, columns were washed first with HCl (2 mL; pH 2.5), then with HCl containing 10 % aqueous methanol (pH 2.5; 1 mL) and subsequently dried under vacuum. Analytes were eluted with methanol containing 1 % formic acid (1.85 mL). The eluates were concentrated to a volume of 300 µL using a vacuum centrifuge and the residues taken up in 0.1 % aqueous formic acid. Aliquots of 5 µL were injected into a HPLC–ESI–MS/MS (Agilent 1290 HPLC/QTRAP 5500 MS) system. For HPLC separation, a reversed-phase HPLC column (Luna C8(2); 150 mm × 4.6 mm; 3 µm from Phenomenex) equipped with a guard column (Luna C8(2); 4 mm × 3 mm from Phenomenex) was used. Solvent A was 0.1 % aqueous acetic acid and solvent B acetonitrile. The concentration of methanol was 4 % for the first 9 min and then increased up to 10 % in 11 min. Using a flow of 0.5 mL/min, peaks for AAMA and GAMA were baseline separated. After negative ionization, MS measurement was carried out in MRM mode. Optimized MS parameters are given in Table 1. Calibration plots of peak area vs. concentration ratios (AAMA/D3-AAMA and GAMA/D3-GAMA) were linear with an R2 value of at least 0.999. LOD/LOQ were 0.9/3.0 fmol for AAMA and 0.9/3.1 fmol for GAMA (absolute amounts).
Urinary creatinine concentrations were measured using a Creatinine (urinary) Assay Kit (Cayman Chemical Company, Ann Arbor, USA) following the manufacturer’s information. Total urine volumes were calculated by converting the determined urine weights into total urine volume assuming a density of 1 kg/L.
Data analysis
MS data were evaluated by Analyst 1.6 Software (AB Sciex). Further data analysis and preparation of the respective plots were carried out using Origin® 9.1 Software (Origin Lab Corporation, Northampton, USA). Statistical analysis is based on differences to the individual baseline levels. All statistical hypotheses were tested with a 5 % significance level against a two-sided alternative. Normal distribution was checked using the Shapiro–Wilk or Anderson–Darling test. When normality could not be rejected, a paired t test was applied to compare dose effects; otherwise, the Wilcoxon signed rank test was used.
Results
Food analysis (acrylamide, protein, asparagine, calories)
During the 9-day human intervention study, all volunteers consumed the same amount of foods, selected for minimized AA intake at the washout days (1, 2, 3, 5, 6, 8, and 9) and for medium and high AA contents at the AA intake days (4 and 7). The AA contents of the foods with quantifiable AA amounts are listed in Table 2. AA contents of all other analysed foods and drinks were below LOQ.
AA contents determined in duplicate meals were used to calculate the daily AA intake of the volunteers. On day 4 (low AA), the total AA intake was 54.0 µg (0.6–0.9 µg/kg bw), and on day 7 (high AA), it was 108.4 µg (1.3–1.8 µg/kg bw). To assess the maximum AA intake on the washout days (1, 2, 3, 5, 6, 8, and 9), an upper bound estimate (UB) was applied. All AA contents found between LOD and LOQ were accepted as respective concentration at the LOQ. Likewise, all results below the LOD were presumed to be true value at the respective LOD concentration. On this basis, a maximum AA intake of 2.2 ± 0.005 µg (21–41 ng/kg bw) was estimated for the washout days.
During the washout phases, an average protein intake of 103 ± 29 g and an average asparagine intake of 1.0 ± 0.5 g were determined (day 1, 2, 3, 5, 6, 8, and 9). On day 4 (low AA), a protein intake of 164 g and an asparagine intake of 1.4 g were measured, and on day 7 (high AA), the respective values were 123 g (protein intake) and 1.6 g (asparagine intake). Daily calorie intake was 2472 ± 72 kcal when the diet was AA-minimized (day 1, 2, 3, 5, 6, 8, and 9), 2634 kcal on day 4 (low AA), and 2837 kcal on day 7 (high AA).
Urine analysis (mercapturic acids, creatinine, urine volumes)
The total urine volume was collected throughout the study over the different collecting periods. This allowed to calculate the urinary mercapturic acids as amounts per total urine volume as well as concentrations related to the creatinine concentration (Fig. 3; Table 3).
At day 3, the end of the initial 3-day washout period, the total AAMA and GAMA amounts excreted were 93 ± 31 and 23 ± 8 nmol/24 h (0.05 ± 0.02 and 0.01 ± 0.004 µmol/g creatinine), respectively. Implying that within 24 h about 30 % of ingested AA is excreted as AAMA, the AAMA level at day 3 appeared equivalent to a total AA uptake of 311 nmol corresponding to 22.1 µg/d (0.3–0.4 µg/kg bw/d). Subtraction of the estimated maximal dietary AA uptake during washout (2.2 ± 0.005 µg/24 h) results in a corrected AA baseline level on day 3 of the initial washout period of 19.9 µg/d (0.2–0.3 µg/kg bw/d).
After the low AA intake (0.6–0.9 µg/kg bw) on day 4, the AAMA excretion over 24 h increased to 225 ± 37 nmol (0.11 ± 0.01 µmol/g creatinine) and the GAMA excretion to 29 ± 7 nmol (0.01 ± 0.003 µmol/g creatinine), accounting for 30 and 4 % of the consumed AA, respectively. Including day 5 and 6, the excretion over 72 h was 58 % of the AA intake for AAMA and 10 % for GAMA. After the washout period following low AA intake, the AAMA output observed on day 6 was 24 % above the baseline value reached on day 3 of the initial washout period (Table 3). High AA intake (1.3–1.8 µg/kg bw) on day 7 resulted in an increased 24 h output of AAMA (404 ± 78 nmol or 0.17 ± 0.04 µmol/g creatinine) and GAMA (40 ± 9 nmol or 0.02 ± 0.004 µmol/g creatinine). Excretion of AAMA and GAMA within 24 h accounted for 27 and 3 %, respectively, of the AA intake. Within 72 h, the respective total output reached 58 % for AAMA and 7 % for GAMA. After high AA intake, the cumulative AAMA excretion on day 9 (at the end of the 2-day washout period) exceeded the baseline value reached on day 3 of the initial washout period by 59 % (Table 3).
Excretion kinetics of MAs within a time period of 72 h after low (0.6–0.8 µg/kg bw) and high (1.3–1.8 µg/kg bw) dietary AA intake are illustrated in Fig. 4 (additional information in Table 4). The areas under the curve (AUC) were calculated after subtraction of presumed baseline values defined as final washout concentrations at baseline (−12 to 0 h, Fig. 4). The low dietary AA intake resulted in an AUC of 0.58 4 h × µmol/g creatinine for AAMA, with a maximum concentration (c max) of 0.15 µmol/g creatinine. High AA intake resulted in an AUC of 1.72 4 h × µmol/g creatinine and a c max of 0.29 µmol/g creatinine. The c max was reached earlier after AA intake on day 4 (t max 8–12 h) compared to day 7 (t max 24–28 h). This shift of t max reflects prolonged consumption of AA containing foods on day 7 in comparison with day 4. On day 4, the main AA intake (with crisp bread, steak, vegetable stew, biscuits, yoghurt, and coffee) took place between 4 and 8 h after the beginning of the AA intake period, resulting in a total intake of 0.5 µg AA/kg bw within that time period. In contrast, the main AA sources on day 7 consisted of potato crisps were consumed 12 h after the beginning of the AA consumption period. This resulted in an AA intake of 0.6 µg/kg bw (12–24 h).
Discussion
Acrylamide
This study was carried out under strictly controlled conditions. Volunteers stayed together in a remote, well-secluded home under continuous supervision by the research team. Deep in the forest, there was no car or other traffic, no open fire, no tobacco smoking, or any other potential source for inadvertent AA exposure. Dietary exposure to AA was measured in duplicates of the respective daily meals ready for consumption. Main AA sources were potato crisps, French fries, fried potatoes, crisp bread, biscuits, and breakfast cereals, with contents mostly as described in the literature (Table 2). The exceptions were potato fried products (French fries 59 ± 1 µg/kg; fried potatoes 23 ± 1 µg/kg) with AA contents found lower than those reported in the literature (308 µg/kg) (EFSA 2014).
AA intake on day 4 (low AA 0.6–0.9 µg/kg bw) and on day 7 (high AA 1.3–1.8 µg/kg bw) was in the range of the average (0.3–0.9 µg/kg bw/d) and the 95th percentile (0.6–2.0 µg/kg bw/d) of adults in Europe (EFSA 2014). During the washout phases (day 1, 2, 3, 5, 6, 8, and 9), we aimed to keep AA intake as low as possible, mainly offering boiled potatoes, noodles, rice, boiled meat, vegetables, and fruits (all <LOQ). Only one item (vanilla yoghurt) was above LOQ (1.00 ± 0.02 µg/kg AA). This ensured a maximum daily intake of 21–41 ng/kg bw/d during the washout days.
Protein and asparagine contents of all foods were also determined to examine their potential impact on a possible endogenous AA formation. However, no clear correlation between daily protein and asparagine intakes and MA excretion was observed. An isocaloric intake was achieved during the washout periods. On the AA intake days, the calorie intake was somewhat elevated, reflecting enhanced caloric contribution by foods with elevated AA contents (fried potato products, biscuits).
Mercapturic acids
Acrylamide- and glycidamide-associated mercapturic acids (AAMA and GAMA) were monitored in all urine samples collected during the 9-day human intervention study (Fig. 3; Table 3). After intake of 0.6–0.8 µg AA/kg bw on day 4 (low AA) and of 1.3–1.8 µg AA/kg bw on day 7 (high AA), the excretion of AAMA reached 58 % of the respective ingested AA amounts within 72 h. The respective values for GAMA reached 10 % after low AA intake (day 4) and 7 % after high AA intake (day 7). This compares well to the 52 % found excreted as AAMA and 5 % found as GAMA found in 46-h urine after intake of a single dose (1 mg) of deuterium-labelled AA (Boettcher et al. 2006). After consumption of potato crisps (0.94 mg AA) by six male volunteers, the AA equivalent found excreted within 72 h was 48 % for AAMA and 5 % for GAMA (Fuhr et al. 2006).
At the end of the initial 3-day washout period, baseline concentrations of AAMA and GAMA were 0.05 ± 0.02 and 0.01 ± 0.004 µmol/g creatinine on day 3, respectively. The 2-day washout periods after low AA (day 4) and high AA (day 7) intake were too short to reach the AAMA and GAMA baseline level of day 3 (Fig. 3), indicating some residual MA still excreted in the urine after the 2-day washout periods due to the previous AA intake. MA baseline levels after the initial 3 days washout are comparable to those described in the literature at different time points. For instance after 11 h of fasting, pre-dose concentrations of 0.05 ± 0.02 µmol AAMA/g creatinine and of 0.02 ± 0.01 µmol GAMA/g creatinine have been reported in a human study with five male volunteers (Watzek et al. 2012b).
Our results have shown that after dietary AA uptake, about 30 % of AA is excreted as AAMA within 24 h. On this premise, the observed baseline level on day 3 of the initial washout period would be equivalent to a total AA uptake of 22.1 µg/d (0.3–0.4 µg/kg bw/d) the day before. If the assumed maximal dietary AA uptake during washout by consumption of the AA-minimized diet is subtracted (2.2 ± 0.005 µg/24 h), a net AA baseline level of 19.9 µg/d (0.2–0.3 µg/kg bw/d) would result. However, there may have been some substantial dietary AA intake before the start of the initial 3-day washout period in some volunteers (not controlled for). Thus, some residual MA excretion resulting from potentially substantial dietary AA intake 3 days ago may well have contributed to this apparent urinary baseline level. The results therefore do not allow us yet to conclude on the true nature of the AAMA excreted on day 3 of the initial washout period. It may reflect dietary intake 3 days before the initial washout or may indeed indicate a true baseline level. The latter assumption is supported by experimental observations from an extended rat study reporting a background of urinary MA excretion even after 2 weeks of washout suggesting an endogenous AA exposure equivalent to ingestion of 0.6–0.7 μg AA/kg bw (Watzek et al. 2012a).
This will be further investigated in a follow-up study with extended washout periods, using stable isotope-labelled AA to clarify toxicokinetics at the low levels of present day average human dietary exposure.
Conclusion
AA contents determined in duplicate meals of quantified servings were used to calculate the daily AA intake of volunteers at present day levels of human dietary exposure. Total AA intake was 54.0 µg (0.6–0.9 µg/kg bw) for low and 108.4 µg (1.3–1.8 µg/kg bw) for high uptake. A maximum AA intake of 2.2 ± 0.005 µg (21–41 ng/kg bw) was estimated for the washout days. Within 72 h after dietary AA uptake, the excretion of AAMA reached 58 % of the respective ingested AA, whereas within 24 h, about 30 % of AA was found excreted as AAMA. The baseline level observed on day 3 of the initial washout period appeared equivalent to a hypothetical net AA baseline level of 19.9 µg/d (0.2–0.3 µg/kg bw/d). The results do not allow us yet to conclude on the true nature of the AAMA excreted on day 3 of the initial washout period. It may reflect dietary intake 3 days before the initial washout or may indeed indicate a true baseline level. A follow-up study with stable isotope-labelled AA and extended washout periods is in preparation.
References
Berger FI, Feld J, Bertow D, Eisenbrand G, Fricker G, Gerhardt N, Merz K-H, Richling E, Baum M (2011) Biological effects of acrylamide after daily ingestion of various foods in comparison to water: a study in rats. Mol Nutr Food Res 55:387–399
Boettcher MI, Bolt HM, Drexler H, Angerer J (2006) Excretion of mercapturic acids of acrylamide and glycidamide in human urine after single oral administration of deuterium-labelled acrylamide. Arch Toxicol 80:55–61
EFSA CONTAM Panel (EFSA panel on contaminants in the food chain) (2014) Draft scientific opinion on acrylamide in food. http://www.efsa.europa.eu/de/consultations/call/140701.pdf
Fennell TR, Sumner SC, Snyder RW, Burgess J, Spicer R, Bridson WE, Friedman MA (2005) Metabolism and hemoglobin adduct formation of acrylamide in humans. Toxicol Sci 85:447–459
Fuhr U, Boettcher MI, Kinzig-Schippers M, Weyer A, Jetter A, Lazar A, Taubert D, Tomalik-Scharte D, Pournara P, Jakob V (2006) Toxicokinetics of acrylamide in humans after ingestion of a defined dose in a test meal to improve risk assessment for acrylamide carcinogenicity. Cancer Epidemol Biomark 15:266–271
Gamboa da Costa G, Churchwell MI, Hamilton LP, Von Tungeln LS, Beland FA, Marques MM, Doerge DR (2003) DNA adduct formation from acrylamide via conversion to glycidamide in adult and neonatal mice. Chem Res Toxicol 16:1328–1337
Hartmann EC, Boettcher MI, Schettgen T, Fromme H, Drexler H, Jr Angerer (2008) Hemoglobin adducts and mercapturic acid excretion of acrylamide and glycidamide in one study population. J Agric Food Chem 56:6061–6068
Mottram DS, Wedzicha BL, Dodson AT (2002) Food chemistry: acrylamide is formed in the Maillard reaction. Nature 419:448–449
Stadler RH, Blank I, Varga N, Robert F, Hau J, Guy PA, Robert M-C, Riediker S (2002) Food chemistry: acrylamide from Maillard reaction products. Nature 419:449–450
Tareke E, Lyn-Cook B, Robinson B, Ali SF (2008) Acrylamide: a dietary carcinogen formed in vivo? J Agric Food Chem 56:6020–6023
Tareke E, Heinze TM, Gamboa da Costa G, Ali S (2009) Acrylamide formed at physiological temperature as a result of asparagine oxidation. J Agric Food Chem 57:9730–9733
Watzek N, Bohm N, Feld J, Scherbl D, Berger F, Merz KH, Lampen A, Reemtsma T, Tannenbaum SR, Skipper PL, Baum M, Richling E, Eisenbrand G (2012a) N7-glycidamide-guanine DNA adduct formation by orally ingested acrylamide in rats: a dose-response study encompassing human diet-related exposure levels. Chem Res Toxicol 25:381–390
Watzek N, Scherbl D, Feld J, Berger F, Doroshyenko O, Fuhr U, Tomalik-Scharte D, Baum M, Eisenbrand G, Richling E (2012b) Profiling of mercapturic acids of acrolein and acrylamide in human urine after consumption of potato crisps. Mol Nutr Food Res 56:1825–1837
Acknowledgments
We thank our volunteers for their participation in the study and the members of the research group as well as the study director Dr. Jens Galan for conducting the study. We also thank Dorothea Schipp for the statistical evaluation and Sylvia Schmidt, Patricia Podsiadly, Byron Baus, and Johannes Keller for supporting laboratory work. This study has been supported by Institute for Scientific Information on Coffee (ISIC), Entre deux Villes 10-1814 La Tour de Peliz-Switzerland.
Conflict of interest
G. Eisenbrand serves as scientific advisor of ISIC.
Ethical standards
The study was approved by the Ethics Commission of Rhineland-Palatinate, Mainz (No. 837.449.12 (8600-F)) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruenz, M., Bakuradze, T., Eisenbrand, G. et al. Monitoring urinary mercapturic acids as biomarkers of human dietary exposure to acrylamide in combination with acrylamide uptake assessment based on duplicate diets. Arch Toxicol 90, 873–881 (2016). https://doi.org/10.1007/s00204-015-1494-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1494-9