Abstract
The erythrocyte glutathione transferase (e-GST) is the most abundant GST isoenzyme in the red blood cells. This enzyme is able to inactivate numerous different toxic compounds, and many studies indicate its possible role as a biomarker in environmental toxicology since it is hyper-expressed in case of exposure to pollutants in humans as well as in animals. This chapter summarizes most of these studies, revealing also a peculiarity of this biomarker: many toxins induce hyper-expression of e-GST with consequent increased activity, but a few toxins are actually inhibitors of e-GST and cause a decrease in activity. Studies involving inhabitants, mammalians reared in polluted areas, miners, or other workers exposed to a variety of toxic chemicals, demonstrate that a simple, rapid, and inexpensive analysis of e-GST activity may provide an early alarm signal of contamination that can be verified with more traditional chemical analyses to characterize the nature of the contaminants.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Biomarker
- Glutathione transferase
- Erythrocyte glutathione transferase
- Blood toxicity
- Environmental toxicology
- Pollutant
- Animal model
- Detoxifying activity
- Antioxidants
- Xenobiotics
- Enzyme inhibition
Introduction
The discovery of glutathione S-transferases (GSTs; E.C. 2.5.1.18), an enzyme that is active in the metabolism of carcinogens and other toxic compounds dates back to Booth et al. (1961). The GSTs comprise a complex enzyme superfamily that has been subdivided further into a number of classes based on a variety of criteria, including amino acid sequence, and immunological, kinetics, and tertiary-quaternary structural properties. Today, the mammalian cytosolic GSTs are grouped into seven classes: alpha, zeta, theta, mu, pi, sigma, and omega (Oakley 2011). Interclass sequence identities are around 25% or less. Despite the low sequence identities, all cytosolic GSTs share a common fold, which is also largely conserved in mitochondrial GSTs. Mammalian cytosolic GSTs are dimeric enzymes; usually, the dimers are made from identical chains, but heterodimers made of two different chains from the same class are also known.
GSTs are able to inactivate a wide range of xenobiotics and endogenous metabolic by-products, thus promoting their conjugation to glutathione (GSH). The GSH-toxin conjugation makes many toxins less reactive and more water-soluble, which facilitates their excretion via the phase III detoxification pathway (Salinas and Wong 1999). A few isoenzymes also display a glutathione-dependent peroxidase activity and catalyze isomerization reactions (Hayes et al. 2005).
Interestingly, human GSTP1-1 has been shown to catalyze the isomerization of 13-cis-retinoic acid to all-trans-retinoic acid (Chen and Juchau 1998). Since this isomerization is independent of GSH, it is distinct from most other isomerization reactions reported for GSTs, which usually require GSH.
A few typical reactions catalyzed by GSTs are shown in Fig. 1.
Some examples of reactions catalyzed by GSTs. (a) Conjugation of GSH with 1-chloro-2,4-dinitrobenzene (CDNB). (b) Glutathione peroxidase activity toward cumene hydroperoxide. (c) Reduction of trinitroglycerin. (d) Isomerization of maleylacetoacetate. (e) Isomerization of prostaglandin H2 (PGH2) to prostaglandin D2 (PGD2). (f) Thiolysis of 4-nitrophenylacetate. (Figure modified from Hayes et al. (2005))
Nonenzymatic activity is also involved as GSTs may act like “ligandins” binding toxins and promoting their excretion (Sheehan et al. 2001). Mammalian GSTs, in particular the GSTP1-1, have intracellular roles as modulators of Jun N-terminal kinase signalling pathways, protecting cells against hydrogen peroxide–induced apoptosis (Tew and Ronai 1999).
During the last decades, a number of reviews have collected and organized the immense number of scientific studies on GSTs, and a few of them are mentioned in the reference section of this chapter (Allocati et al. 2018; Armstrong 1997; Dirr et al. 1994; Eaton and Bammler 1999; Edwards et al. 2000; Hayes and Pulford 1995; Hayes et al. 2005; Salinas and Wong 1999; Sheehan et al. 2001; Wilce and Parker 1994).
GSTs and Toxin Exposure
GST enzymes are known to respond to exposures to toxins. Many studies reported activity alterations of GSTs in various organs of different living organisms after exposure to industrial metals or in soils contaminated with pesticides (Aly and Schröder 2008; Bocedi et al. 2019; Enayati et al. 2005; Gonçalves et al. 2021; Hellou et al. 2012; Saint-Denis et al. 2001). Recent studies highlighted GSTs as responders to xenobiotics from many chemical classes, including both inorganic and organic compounds, and in particular agrochemicals (Bundy et al. 2008; Stott et al. 2001). This review chapter focuses more on the erythrocyte GSTP1-1 (e-GST) and in particular on its emerging role as a biomarker in environmental toxicology.
The Peculiar Molecular Properties of GSTP1-1
GSTP1-1 is a dimeric protein composed of two identical subunits with a total of about 23,000 Da (Fig. 2). Each subunit contains a binding site for GSH, named the G-site, and a second site (the H-site) able to bind many different electrophilic toxic organic compounds. A third site, at the dimer interface, is involved in the binding and transport of toxins out of the cell. The rate-limiting step of the most common catalyzed reaction, i.e., the conjugation of GSH to dangerous organic compounds, is represented by the nucleophilic attack of the sulfur atom of GSH to the electrophilic center, promoted by a lowered pKa of the sulfhydryl group of GSH, which is shifted from 9.1 to 6.5. This unusually low pKa is caused by the proximity of a Tyr residue, which forms a hydrogen bond with the sulfhydryl group of GSH. X-ray crystallographic data and molecular dynamic simulations demonstrate that a high flexibility of the alpha helix 2, flanking the G-site, is crucial for the conjugation of GSH to the co-substrate (Fig. 2). A singular molecular feature of GSTP1-1 is the presence of four cysteine residues, which remain in the reduced form in the native enzyme. The role of all these residues is not yet clear, but Cys47 displays unusual properties. It is characterized by a very low pKa (3.5), which makes it possible for the thiolate ion to form a strong ion pair with the protonated ε-amino group of Lys54 at physiological pH values (Lo Bello et al. 1993). This link seems to be essential for the correct structure of helix 2. Chemical modification of this cysteine as well as its oxidation to form a disulfide with Cys101 causes a drastic loss of enzyme activity (Fabrini et al. 2014; Sluis-Cremer et al. 1996).
Figure 1 shows the reaction between GSH and 1-chloro-2,4-dinitrobenzene (CDNB), the most common co-substrate used for kinetic studies on GSTP1-1. The kinetic pathway follows a random sequential mechanism. This reaction, which can be followed spectrophotometrically at 340 nm where the product absorbs (ε = 9600 M−1 cm−1), is universally used to measure GST activity in biological samples (Habig and Jakoby 1981). Alternatively, a fluorometric procedure has been also developed using 4-chloro-7-nitrobenzofurazan as co-substrate (Ricci et al. 1994). Among the GST superfamily, GSTP1-1 is one of the most studied isoenzymes. During the last 40 years, crystallographic, NMR, fluorescence, and kinetics studies have been applied to reveal the peculiar molecular properties of this homodimer enzyme. Of particular interest is the tight binding of the two subunits (Kd < 10–10 M) and their cooperative interaction, which have important physiological consequences (Fabrini et al. 2009). Since 1989, the unusual nonequivalence of the two identical subunits in their reaction with sulfhydryl reagents has been studied and assigned to an inter-subunit communication effect. However, only in 1995 was it demonstrated that the mutation of Cys47 or Lys54 into alanine disclosed a true positive cooperativity in GSTP1-1 in its interaction with GSH. This cooperative modulation is latent in the native enzyme, but it becomes evident in the case of point mutations or under particular temperature-stress conditions (Caccuri et al. 1999). In fact, GSTP1-1 is also present in the upper layers of the epidermis, where it plays a protective role against carcinogens and toxic compounds that may get in contact with the skin or is exposed to low or high temperatures that can alter its catalytic efficiency (Caccuri et al. 1999). A sophisticated mechanism of temperature adaptation has been developed for this enzyme during evolution and finalized to maintain an efficient catalytic activity of GSTP1-1. In fact, above 35 °C, this enzyme displays positive cooperativity for GSH binding, whereas negative cooperativity occurs below 25 °C. This homotropic mechanism minimizes changes in GSTP1-1 affinity for GSH due to temperature fluctuation. This is likely an advantage for epithelial skin cells, which are naturally exposed to temperature variations and, incidentally, also to carcinogenic compounds that always need efficient detoxifying systems (Caccuri et al. 1999). As a whole, GSTP1-1 represents the first enzyme, which displays such temperature-dependent homotropic regulation of its substrate (e.g., GSH) binding.
A second cooperative interaction was described for GSTP1-1 after observing the interaction of this enzyme with the dinitrosyl-diglutathionyl-iron complex (DNDGIC), a natural carrier and storage molecule of nitric oxide. This complex is formed when NO is overproduced, but it is also toxic for the cells. GSTP1-1 and other GST isoenzymes are able to protect the cell binding DNDGIC with extraordinary high affinity (Kd = 10–9 M) and thus making it harmless. However, binding of this complex to GSTP1-1 causes a strong inhibition of this enzyme. A complete inactivation is limited by a negative cooperative mechanism; i.e., when a first subunit has bound the DNDGIC, the second adjacent subunit becomes much less affine. This self-preservation ability has been observed even in the presence of other toxic compounds, and it represents one of the most plausible examples of utility given by a negative cooperativity mechanism in enzymes (Bocedi et al. 2016a).
Activity Detection of GSTP1-1 in Biological Samples: Critical Points
The method most used to quantify the activity for GSTP1-1 is the one described by Habig and Jakoby (1981). This procedure uses CDNB as co-substrate and is based on the spectrophotometric absorbance at 340 nm of the conjugation product between GSH and CDNB, the S-(2,4-dinitrophenyl)glutathione (ε = 9600 M−1 cm−1), a reaction catalyzed by all GSTs (Fig. 1). In this assay, the GSH level is saturating (1 mM), while CDNB is under-saturating given that its concentration in the assay (1 mM) is similar to the Km value. Thus, the enzyme rate cannot exceed 50% of the maximum velocity obtainable under saturating conditions. Unfortunately, higher CDNB concentrations cannot be used due to its scarce solubility in aqueous solutions. Thus, the Habig procedure needs special attention because even minor variations in the CDNB concentrations give significant differences in the activities observed.
A second particular crucial point comes from a few studies indicating possible spectrophotometric measurements of GST in serum (Habdous et al. 2002). A careful re-examination of the procedure demonstrated that the level of the enzyme calculated using this method turned out to be much higher than that found using RIA or ELISA procedures. Actually pH-dependent artifacts strongly affect the results. In fact, the spectral changes previously interpreted as a measure of GST activity are mainly due to an increase of the spontaneous reaction between the two substrates (Fabrini et al. 2012a).
In conclusion, the GST activity in normal serum cannot be correctly determined with this spectrophotometric assay because of the very low enzyme concentration and the pH-dependent artifacts. Despite this re-examination, some researchers have continued to use the Habdous procedure, thus providing an unspecified number of erroneous results.
GSTP1-1 Polymorphism
The Pi class contains only one enzyme, the GSTP1-1 (one gene, GSTP1); four alleles in human genetic polymorphisms (GSTP*A, GSTP*B, GSTP*C, and GSTP*D) and 11 different alterations in gene or in nucleotides like A313, C341, C555, G313, T555, T341 and others. The well-characterized polymorphism of amino acid 105 substitution (i.e., Ile105Val) affects the substrate-binding site, resulting in the reduction of substrate affinity (Hayes et al. 2005).
GSTP1-1: An Enzyme Dedicated to Biomedical Applications
In many studies, it was observed that GSTP1-1 is hyper-expressed in tumor cells and closely involved in carcinogenesis, tumor formation, and resistance toward chemotherapy (Aliya et al. 2003; Chen et al. 2013; Kural et al. 2019; Lee et al. 2014; Parker et al. 2017; Sawers et al. 2014).
This enzyme apparently fulfills a protective role for tumor cells through weakening the efficacy of chemotherapeutic agents by causing their extrusion from cell and by inhibiting the MAPK pathway. Thus, a number of anticancer drugs have been designed to inhibit GSTP1-1 in order to increase the efficiency of chemotherapy. They include ethacrynic acid and its analogs (Burg et al. 2002; Li et al. 2012), TLK117 and TLK199 (Mahadevan and Sutton 2015; Raza et al. 2009), and NBDHEX (Ricci et al. 2005; Rotili et al. 2015).
Hyper-Expression of GSTP1-1
The levels of GSTP1-1 as well as other cytosolic GSTs (e.g., alpha, mu, and theta) are up-regulated by exogenous compounds (Higgins and Hayes 2011). In particular, a study on rat showed that expression of GSTP1-1 was induced preferentially by polycyclic aromatic hydrocarbons, phenolic antioxidants, and thiol-reactive chemopreventive agents. Rats treated with a diet enriched with different xenobiotics showed induction of liver GST subunits as determined by immunoblotting. GSTP1-1 was more expressed by rats in which ethoxyquin, butylated hydroxyanisole, coumarin, diethyl maleate, benzyl isothiocyanate, β-naphthoflavone, and trans-stilbene oxide were administered (Higgins and Hayes 2011).
Tissue Distribution of GSTP1-1 in Humans
The importance of the GSTPi class is underlined by the widespread tissue distribution of this enzyme. GSTP1-1 expression was quantified in human tissues by immunohistochemical staining studies, RNA sequence database, HPLC, mass spectrometry, and immunoblotting. Interestingly, GSTPi is widely diffused in all human tissues, but it is mainly present in the bladder, skin, lung, small intestine, kidney, ovary, placenta, and thyroid, whereas in liver only, there is a rather small amount of this GST class (Buratti et al. 2021; Dhanani and Awasthi 2007; Rowe et al. 1997; Terrier et al. 1990). GSTP1-1 is the most abundant GST in erythrocytes, representing the 95% of the entire GST pool.
GSTP1-1 in Human Pathologies
GSTP1-1 hyper-expression is associated with a variety of human pathologies. As mentioned above, GSTPi levels increase in tumors of non-small cell lung, breast, colon, and pancreas (Bocedi et al. 2019). Moreover, the GSTPi is overexpressed in tumor cell lines resistant to chemotherapy treatments. Expression of GSTPi is also relevant in benign prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma (Chatterjee and Gupta 2018). An increased activity of GSTP1-1 was described in the oral mucosa in which the development of the squamous cell carcinoma is active (Chatterjee and Gupta 2018). The involvement of GSTP1-1 in liver diseases is only definite in animal models like rats for hepatocellular carcinoma and liver fibrosis (Bocedi et al. 2019). The hyperbilirubinemia is also characterized by an increased level of the erythrocyte GSTP1-1 in young human patients (Carmagnol et al. 1981). The GSTP1-1 expression is also important in neurodegenerative disorders; in Alzheimer’s and Parkinson’s diseases and other psychiatric disorders like autism and schizophrenia, a linkage between the levels of GSTP1-1 and the severity of the pathology has been reported recently (Bocedi et al. 2019).
Pathologies like autoimmune diseases (e.g., scleroderma, systemic lupus erythematosus, and rheumatoid arthritis) are often characterized by systemic organ damages and are possibly due to exposure to toxins, as observed in industrialized countries with a high pollution threshold. Notably, GSTP1-1 is hyper-expressed in scleroderma, and the expression correlates with the severity of this disease (Bocedi et al. 2019; Chikezie 2015).
The most studied pathologies involving systemic toxicological problems are the kidney diseases, in which this organ, which filters and cleans the blood from circulating toxins, is somehow damaged (Bocedi et al. 2016b).
The e-GST has been studied extensively in correlation with kidney diseases. The first studies that reported an unusual overexpression of e-GST in nephropathic patients were made on newborns with hyperbilirubinemia and in patients in hemodialysis (Carmagnol et al. 1981; Galli et al. 1999). More recently, many studies have confirmed that e-GST levels in red blood cells (RBCs) increase in chronic kidney diseases (CKD), in patients in pre-dialysis under conservative therapy, and also in hemodialysis. Thus, the enzymatic expression of e-GST is a useful biomarker to verify the blood toxicity in CKD patients; the e-GST level is not influenced by vitamin E supplementation or erythropoietin therapy but only by circulating toxins (e.g., high-molecular-weight toxins, protein-bound toxins). The e-GST expression varies in patients treated with pure diffusive and convective dialytic procedures and increases linearly along the stages of kidney disease progression. An enhancement was also found in kidney transplanted patients (Bocedi et al. 2016b, 2018; Dessì et al. 2012).
The e-GST level may also be used to monitor the systemic toxicity in healthy organisms not affected by pathologies but only subject to chronic or acute exposure to dangerous compounds (Bocedi et al. 2019). In the following paragraphs, the application primarily of e-GST as an environmental biomarker is reported for humans, mammalians, fishes, and other animal species.
e-GST as a Long-Term as Well as a Short-Term Biomarker of Blood Toxicity
e-GST is an atypical biomarker because the exposure of a living organism to toxic and dangerous compounds may produce either an increase or a decrease in activity. Many toxins cause an overexpression of e-GST without interfering with the catalytic mechanism of this enzyme. In this case, an enhancement in activity is always observed. Importantly, an increase in activity should always be referred to an increase in e-GST levels and not to the presence of some hypothetic enzyme activators never described before (Bocedi et al. 2016b; Galli et al. 1999). The maximum effect for this overexpression is expected after about 2–5 months of chronic exposure to the toxin. In fact, this corresponds to the life span of erythrocytes in mammals, and e-GST is only synthetized during erythropoiesis and not later because mature erythrocytes do not synthetize de novo proteins. In this way, e-GST appears as a biomarker of chronic exposure to toxins, which resembles the behavior of glycated hemoglobin in the retrospective monitoring of blood glucose. An increase in activity observed after shorter exposition times probably only represents a qualitative underestimated indication and not a precise quantitative value.
The presence of toxins that display inhibitory interference toward the catalytic mechanism of e-GST causes a decrease in activity that can be observed even after a very short exposition time. In this case, e-GST may represent a biomarker of acute toxicity.
Human e-GST as Biomarker in Environmental Toxicology
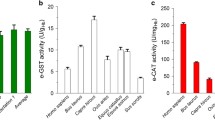
The normal values of e-GST expressed in IU/gHb varies from two in dogs to six to seven in humans (Bocedi et al. 2016b; Kurata et al. 1993). Higher values of 10, 15, and 20 are found in hamster, rhesus monkey, and cat, respectively (Kurata et al. 1993). Moreover, the RBC life spans in different mammalian species are 51 days for mice, 67 for rats, 150 for sheep and humans, and 175 for cattle (Kurata et al. 1993). A careful examination of many studies on the effects of environmental pollutants on human e-GST is reported below and in Fig. 3.
Effect of toxins on the human e-GST activity as reported in several studies. Percentage change of e-GST activity (abscissa axis) in subjects exposed to various pollutants (ordinate axis). Experimental data from different studies were obtained from tables or digitalizing graphs and then calculating the percentage values with respect to the control group present in each study. The blue line represents the control value (100%). The percentage increase of the e-GST level is shown in red, while its decrease is shown in green. (Data derived from the following references: (1) Possamai et al. (2010), (2) Wilhelm Filho et al. (2010), (3) Sharma et al. (2013), (4) Akkam et al. (2020), (5) Dutta et al. (2019), (6) Fabrini et al. (2012b), (7) Schins et al. (1997), (8) Kilpikari and Savolainen (1984), (9) Tuluce et al. (2011), (10) Almutairi et al. (2020), (11) Dobrakowski et al. (2016), (12) Primavera et al. (2008), and (13) Brucker et al. (2013))
An interesting study on the contamination produced by an electric power plant compared the e-GST activity in three groups of persons: one group was only indirectly exposed (working at the office), a second group was directly exposed (working at the burning area), and the third group was composed of residents living near the power plant (residents). The levels of e-GST compared to the control group (100%) were +270% for indirect exposure, +220% for direct exposure, and +112% for residents, respectively (Possamai et al. 2010) (Fig. 3). This somewhat surprising result (the indirect exposure causing more damage than direct exposure) might reflect the level of protection applied. Probably, the workers who are directly exposed to dust and smoke have a better protection (filters) than office workers.
Another study, made in the Brazilian district of Santa Catarina demonstrated that contaminants in the environment such as heavy metals and dioxins, produced by coal mining and hospital waste incineration, had an impact on the local population. Compared to a control group living in an uncontaminated area (defined as 100%), the e-GST values were +157% for residents living in the vicinity of the mine, +79% for miners directly exposed underground, and +58% for miners indirectly exposed by working on the surface. Furthermore, the e-GST values for residents living near the incineration site and for the workers in the same plant were +112% and +63%, respectively (Wilhelm Filho et al. 2010) (Fig. 3). These results suggest that the residents near the mine are more contaminated than the workers; probably, the workers were protected by the use of safety equipment (filters).
The pesticides represent a second category of pollutants that are spread in the environment. Studies on agriculturist in the North India and the Bengal regions reported that a widespread use of organophosphorous compounds and carbamate pesticides has altered the levels of e-GST. Today, the intensive use of agrochemicals to protect tea plantations and other crops from insects is an important environmental problem in that part of the world. As a consequence, the e-GST levels in agricultural workers were increased by up to 109% in Northern Indian subjects with respect to a control group (Sharma et al. 2013) and a +55% increase has been measured for Bengalese workers (Dutta et al. 2019) (Fig. 3).
A new form of pollution typical of the contemporary society comes from the daily use of electric devices. A study conducted in the Middle Eastern region of Jordan revealed an increase in the e-GST level of +72% (compared to a control group) in subjects exposed to electromagnetic pollution generated by cell phone towers (Akkam et al. 2020) (Fig. 3).
An important Italian study of the contribution of e-GST as a biomarker of pollution in a geographical region with cities, villages, industrial sites, and landfills was made on 500 healthy volunteers living and working in the Sacco river valley, a region south of Rome, which is polluted by illegal industrial dumps. Eight areas were tested, and in six of them, an increased e-GST level was observed, spanning from 18% to 44% higher than the control group. The highest values corresponded to the most polluted areas as reported in Fig. 3 (Fabrini et al. 2012b).
The e-GST has been tested as possible biomarker for subjects exposed to industrial toxicants. However, in a study on workers exposed to hot rubber fumes, it was found that the e-GST activity actually decreased significantly compared to the untreated control (Kilpikari and Savolainen 1984).
Other studies were performed on subjects employed in industrial sites or in mines. A modest increase in e-GST (+4%), close to control value, was observed for 66 coal miners not affected by coal workers’ pneumoconiosis, chronic bronchitis, and lung function decrease (Schins et al. 1997). A second contribution comes from a study on workers exposed to furnace coal dust particles derived from a central heating system; this study reported a value of −21% below the control group (Tuluce et al. 2011) (Fig. 3). A group of 36 males employed in periodic maintenance of blast furnaces, and thus occupationally exposed to lead for around 40 days, showed a decreased level of e-GST of −44% below the control group (Dobrakowski et al. 2016) (Fig. 3). Thus, it appears that prolonged exposition to blast furnaces, even for maintenance, has a relevant inhibitory effect on e-GST.
Petrochemical workers exposed to low doses of 1,3-butadiene (an oxidizing compound that contaminates the air of the industrial site) showed an impaired e-GST activity, resulting in an inhibition of −55% with respect to the control (Primavera et al. 2008) (Fig. 3).
Air pollution is one of the major problems in great urban agglomerates. People who live in towns or metropolis are exposed to a complex mixture of particulate matters (PMs), ozone, hydrogen sulfide, carbon monoxide, nitrogen dioxide, ammonia, methane, nitrogen oxides, sulfur dioxide, carbon dioxide, and non-methane hydrocarbon.
Two recent studies reported the inhibition of e-GST(−38% below the control) in the resident population of a town in Kuwait. The inhabitants are exposed mainly to PM10 and sulfur dioxide (Almutairi et al. 2020). The taxi drivers in Porto Alegre (Rio Grande do Sul, Brazil) are instead daily exposed to PM2.5 polycyclic aromatic hydrocarbons (benzo[a]pyrene), corresponding to −58% below the control (Brucker et al. 2013) (Fig. 3).
Mammalian e-GST as Biomarker in Environmental Toxicology
The e-GST was considered a reliable biomarker in farm animals exposed to environmental pollutants and in laboratory animal models like rat and mice. The Sacco river valley (Italy) is a highly polluted region due to illegal industrial dumping as reported above, and it represents a sort of extended open laboratory to experiment possible biomarkers. In fact, e-GST was found to be overexpressed in cows reared in farms located in this polluted area (+68%) compared to the cows reared in unpolluted areas (Bocedi et al. 2016c). Very recently, it was observed that ewes are animals involved in environmental surveillance. The ewes reared in the contaminated area express higher e-GST levels (+44%) with respect to the ewes in uncontaminated area (Bocedi et al. 2022) (Fig. 4).
Effect of toxins on the mammalian e-GST activity as reported in several studies. Percentage change of e-GST activity (abscissa axis) in animals exposed to various pollutants (ordinate axis). Experimental data from different studies were obtained from tables or digitalizing graphs and then calculating the percentage values with respect to the control group present in each study. The blue line represents the control value (100%). The percentage increase of the e-GST level is shown in red, while its decrease is shown in green. (Data derived from the following references: (1) Bocedi et al. (2016c), (2) Bocedi et al. (2022), (3) Yaman et al. (2016), (4) Oyagbemi et al. (2017), (5) Canli et al. (2017), (6) de Oliveira et al. (2018), (7) Sachdeva et al. (2013), (8) Rotimi et al. (2018), (9) Ebokaiwe et al. (2018), (10) Temel and Taysi (2019), and (11) Apaydın et al. (2018))
Aflatoxins are contaminants in food and feed products for humans and animals. Rats fed on rice contaminated with four types of aflatoxins (B1, B2, G1, and G2) at different percentages showed an e-GST value +21% higher compared to rats fed with basal diet without aflatoxins (Yaman et al. 2016) (Fig. 4). Conversely, in a more recent study, rats were exposed to pure aflatoxin B1 added to a protein diet (normal or low-protein diet) causing a decrease of −46% e-GST(−50% in low-protein diet) below the two control groups (Rotimi et al. 2018) (Fig. 4). A possible explanation of these contradictory results may be that the inhibitory effect of B1 is compensated and even overwhelmed by an induced overexpression of e-GST by other aflatoxins.
Other important contaminants are the arsenic derivatives, and in many case, they persist in the environment especially in countries without government laws that prevent indiscriminate dumping of heavy metals. Sodium arsenite was supplied in water to rats for 4 weeks at three different doses: 10, 20, and 40 mg/kg/day. The e-GST values were very similar for the three treatments, and a calculated average value of +12% with respect to untreated rats (control group) is reported (Oyagbemi et al. 2017) (Fig. 4). This probably represents an underestimate incremental value as determined only after 4 weeks of exposition.
In an another study, rats were fed with oral supplementation of metal derivatives (Al2O3, CuO, and TiO2 nanoparticles), and after 14 days, e-GST level was measured. Three doses for each nanoparticles were administered, and the three e-GST average values were calculated. Cupric oxide showed +8%, titanium dioxide +5%, and aluminum oxide −5% values very close to control group (Canli et al. 2017) (Fig. 4). Also in this case, the modest variation of e-GST may be caused by a very short time of exposition compared to the life span of the rat erythrocytes.
The most widespread air pollutants are PMs; researchers exposed old male mice to ultrafine PM2.5 containing polycyclic aromatic hydrocarbons for 5 days/week and for a total of 3 months. The value of e-GST after this exposition differs for only +3% with respect to control (de Oliveira et al. 2018) (Fig. 4).
Tungsten is present in industrial and military manufacturing, becoming during the last years a new contaminant. Male rats were administered for 14 days with an amount of 119 and 238 mg/kg of sodium tungstate orally or with 20 and 41 mg/kg via intraperitoneal route. Interestingly, an inhibitory effect on e-GST was found for sodium tungstate correlated with the administered quantities of this compound. e-GST values were −20% and −48% for 20 and 41 mg/kg of intraperitoneal sodium tungstate and −32% and −65% for 119 and 238 mg/kg for oral doses (Sachdeva et al. 2013) (Fig. 4).
One of the greatest problems in the field of environmental pollution is represented by the contamination of potable water sources by anthropogenic and geological activities; in particular, water sources are subjected to heavy metals’ presence, becoming a first line of exposure to dangerous compounds. Six groups of rats (one was the control group) were fed with borehole water from five different locations. Except the control group, the water from boreholes was polluted and gave a similar effect on e-GST, i.e., an e-GST average levels of −50% with respect to the control group (Ebokaiwe et al. 2018) (Fig. 4).
The mercury is the most dangerous among heavy metals. The exposure to mercury and mercury derivatives may permanently damage many organs like the brain and kidneys and cause cancer diseases. Male rats were fed for 10 days with mercury chloride (0.01 g/kg/day); at the end of the protocol, the e-GST level was estimated. A value of −59% with respect to the control group was found (Temel and Taysi 2019) (Fig. 4).
Bendiocarb is a pesticide of the class carbamate and is a reversible inhibitor of acetylcholinesterase activity but also of GSTP1-1. In fact, rats were fed with bendiocarb (0.8 mg/kg 1/50 LD50) for an experimental period of 28 days, and at the end, the e-GST showed −67% with respect to the control (Apaydın et al. 2018) (Fig. 4).
Antibiotics are a further important class of molecules that, as reported by many studies, are inhibitors of different enzymes involved in cellular physiology. The inhibitory effect of three new generation of cephalosporins was tested on e-GST from rats. The rats were divided into different groups and injected with intraperitoneal amounts of cefazolin (single dose of 50 mg/kg), cefuroxime (single dose of 25 mg/kg), and cefoperazone sodium (single dose of 100 mg/kg). The inhibition of 50% of e-GST activity was reached in 4.65 h for cefazolin and cefoperazone sodium while in only 1.95 h for cefuroxime (Türkan et al. 2019) (Fig. 4).
e-GST in Fish Erythrocytes
Water represents 70% of the earth’s surface and the major system for climate regulation, life sustainment, and the environment for great part of animal and plant life species. Moreover, water is the primary solvent of inorganic molecules, organic molecules, and bio-macromolecules. Water is also the physical storage system of pesticides, heavy metals, chemical contaminants from industrial plants, antibiotics, and many other dangerous compounds. Aquatic ecosystems are particularly affected by such substances, and the consequences are the decrease of water quality (potable water), impact on life species, and the disruption of ecological balance of the environments (Gonçalves et al. 2021). Organisms who live in water ecosystem are the first to be affected by this type of pollution, and therefore, they represent an in vivo model for the environment surveillance. The detoxifying enzymes of fishes are the primary targets for ecotoxicological studies, and among them, the GSTP1-1 may represent an important biomarker.
Notably, two studies were conducted on e-GST from fishes. In the first one, two groups of Carassius auratus were exposed for 96 h to 3.9 and 7.5 mg/L of Mn2+, while a third group served as control. The erythrocytes were obtained from blood samples, and e-GST was measured; e-GST levels were +156% and +68% for fishes exposed to 7.5 and 3.9 mg/L Mn2+ with respect to the control group (Aliko et al. 2018). These results are highly surprising because the exposition to Mn2+ was only for 96 h, a very short time incompatible with erythrocytes turnover.
e-GST as Biomarker in Environmental Toxicology in Other Animal Species
Besides mammals and fishes, many other species can exhibit e-GST as the major constituent of the GST pool in the erythrocytes, and thus, this enzyme could represent a possible biomarker in these species as suggested by experiments on birds.
The e-GST activity of wild birds like griffon vultures (Gyps fulvus) polluted with different metals spread in the environment was evaluated. The lead contamination caused an increased average value of e-GST of +26% in the birds present in the most contaminated area. This increased activity could be considered like a response of antioxidant mechanisms in griffon vultures against the higher pollutant levels (Espín et al. 2014a).
Finally, a very interesting study compared the e-GST activities from Eurasian eagle owl (Bubo bubo) from three areas (agricultural and rural area, industrial area, and mining area) in the south of Spain. The e-GST activity was lower in the industrial area(−13%) and in mining area(−24%) with respect to the agricultural and rural areas assumed as a sort of control group (Espín et al. 2014b). These data indicate the presence of contaminants with inhibitory activity toward e-GST.
Future Perspective and Clinical Applications
One of the major potential application of e-GST as biomarker is linked to the variations of its activity in conditions associated with chronic or acute exposure to endogenous or environmental stressors or toxins, such as environmental pollutants in urban areas, working places, or wild places affected by contaminants and polluted food or drinking water. A very peculiar characteristic of this biomarker is that the signal of dangerous contamination can be provided by both an increase and a decrease in activity depending on whether or not the contaminant is an inhibitor of e-GST. We believe that a decrease in activity is the indicator of a more dangerous contaminant as inhibiting the activity of the e-GST decreases the defense potential against toxic substances in the erythrocyte but probably also in other organs.
It must be underlined that an increase of e-GST is the consequence of chronic exposition to toxins of 2 or more months, while a decrease of e-GST activity may be also the result of a very recent contamination.
Indeed, some important advantages must be underlined:
-
a.
The e-GST assay can be performed with a simple spectrophotometric apparatus and requires only 2–3 min/analysis. The test has a negligible cost because GSH and CDNB are inexpensive reagents.
-
b.
The test is performed on whole blood and requires only 5–10 μL of blood.
-
c.
The enzyme is stable for a few hours at 25 °C and 48 h at 4 °C, but the whole blood cannot be frozen as e-GST loses part of its activity.
Obviously, any alteration of e-GST cannot be referred to a specific toxin, and further chemical analyses must be made to characterize the contaminant. In this way, e-GST remembers the behavior of the white blood cells that are mainly produced in case of bacterial infection, but subsequent analyses are necessary to identify the nature of the bacterium and which organ is invaded.
It is hoped that kits for e-GST analysis on automated equipment will soon be available and supplied by pharmaceutical industries.
A clinical laboratory with automated devices to perform large-scale screening on humans and animals may be useful in the future to detect hidden contaminations in well-defined geographical area.
In conclusion, a first European laboratory exclusively dedicated to the analysis of e-GST in humans and animals will be established at the University of Rome “Tor Vergata.”
Applications to Prognosis and Diseases
One of the most important planetary emergencies is the presence of toxic compounds in the soil, water, and air that can be not identified representing a “silent and hidden pollution.” In fact, there are not always available simple methods that reveal precociously the presence of these dangerous compounds. The epidemiological analysis, highlighting anomalous peaks of specific pathologies, allows to point out situations of harmful contamination, although in many cases the damage to human health has already partially occurred. As described in this chapter, many studies suggest e-GST as a toxicological hazard warning “device,” which may reveal early a dangerous situation for humans and animals. The fact that some kidney or systemic diseases such as sclerodermia lead to an increase in e-GST does not represent an obstacle to the use of this enzyme as a biomarker of environmental toxicity. In fact, environmental contamination causes an alteration of the activity of e-GST in several individuals or animals, as opposed to the pathology.
Mini-dictionary
-
e-GST: Enzyme expressed in red blood cells devoted to toxic compound detoxifications.
-
Erythrocyte: Blood cell type that contains the glutathione transferase Pi isoform.
-
Glutathione: Tripeptide γ-L-glutamyl-L-cysteinylglycine that serves as reducing agent in biochemical reactions.
-
Glutathione transferases: Dimeric isoenzymes encoded by three families of genes, divided into cytosolic, microsomal, and mitochondrial transferases. These enzymes catalyze nucleophilic attack by GSH on nonpolar compounds with an electrophilic carbon, nitrogen, or sulfur atom.
-
Pollutant: Contaminant with natural or anthropogenic origin.
Key Facts of Glutathione Transferase
-
GSTs are isoenzymes evolutionary correlated with a common dimeric structure.
-
GSTPi is widely studied in humans, other mammalians, fishes, and other animal species.
-
GSTP1-1 is expressed in animal tissues and in particular in the erythrocytes.
-
e-GST is a useful biomarker for environmental toxicology because of its enzymatic properties.
-
e-GST is overexpressed or inhibited after chronic or acute exposure of the organism to endogenous or exogenous toxic compounds.
Summary Points
-
GSTPi is a detoxifying enzyme with dimeric structure and peculiar enzymatic properties like negative cooperativity, temperature adaptation, and the formation of an inactive form due to an intramolecular disulfide bond.
-
e-GST is hyper-expressed in chronic kidney diseases and other systemic diseases but even in case of exposition to toxic compounds.
-
e-GST expression represents a biomarker in the retrospective assessment of the systemic exposure to environmental toxic compounds.
-
e-GST activity in humans differs in subjects exposed to toxicants in working places, industrial sites, mines, and urban areas.
-
e-GST activity varies with the animals feed, in contaminated areas, and in animals treated with toxic compounds.
-
e-GST levels from fish erythrocytes treated with toxins are sensible to presence of dangerous compounds in water ecosystems.
-
e-GST is also studied in other animal species (like birds) for ecotoxicological purposes.
Abbreviations
- CDNB:
-
1-Chloro-2,4-dinitrobenzene
- CKD:
-
Chronic kidney disease
- DNDGIC:
-
Dinitrosyl-diglutathionyl-iron complex
- e-GST:
-
Erythrocyte glutathione transferase
- GSH:
-
Glutathione
- GSTP1-1:
-
Glutathione transferase class P1-1
- GSTPi:
-
Glutathione transferase class P isoforms
- GSTs:
-
Glutathione transferases
- PMs:
-
Particulate matters
- RBCs:
-
Red blood cells
References
Akkam Y, Al-Taani AA, Ayasreh S, Almutairi A, Akkam N. Correlation of blood oxidative stress parameters to indoor radiofrequency radiation: a cross sectional study in Jordan. Int J Environ Res Public Health. 2020;17:4673. https://doi.org/10.3390/ijerph17134673.
Aliko V, Qirjo M, Sula E, Morina V, Faggio C. Antioxidant defense system, immune response and erythron profile modulation in gold fish, Carassius auratus, after acute manganese treatment. Fish Shellfish Immunol. 2018;76:101–9. https://doi.org/10.1016/j.fsi.2018.02.042.
Aliya S, Reddanna P, Thyagaraju K. Does glutathione S-transferase Pi (GST-Pi) a marker protein for cancer? Mol Cell Biochem. 2003;253:319–27. https://doi.org/10.1023/a:1026036521852.
Allocati N, Masulli M, Di Ilio C, Federici L. Glutathione transferases: substrates, inhibitors and pro-drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7:8. https://doi.org/10.1038/s41389-017-0025-3.
Almutairi AM, Akkam Y, Alajmi MF, Akkam N. Effect of air pollution on glutathione S-transferase activity and total antioxidant capacity: cross sectional study in Kuwait. J Health Pollut. 2020;10:200906. https://doi.org/10.5696/2156-9614-10.27.200906.
Aly MA, Schröder P. Effect of herbicides on glutathione S-transferases in the earthworm, Eisenia fetida. Environ Sci Pollut Res Int. 2008;15:143–9. https://doi.org/10.1065/espr2007.02.385.
Apaydın FG, Pandır D, Kalender S, Baş H, Kalender Y. Hematoprotective effect of vitamins C and E against subchronic toxicity of bendiocarb: biochemical evidences. J Food Biochem. 2018;42:1–9. https://doi.org/10.1111/jfbc.12659.
Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol. 1997;10:2–18. https://doi.org/10.1021/tx960072x.
Bocedi A, Fabrini R, Bello ML, Caccuri AM, Federici G, Mannervik B, Cornish-Bowden A, Ricci G. Evolution of negative cooperativity in glutathione transferase enabled preservation of enzyme function. J Biol Chem. 2016a;291:26739–49. https://doi.org/10.1074/jbc.M116.749507.
Bocedi A, Noce A, Fabrini R, Di Daniele N, Galli F, Ricci G. Erythrocyte glutathione transferase as a biomarker in kidney health and disease. In: Pateel VB, Preedy VR, editors. Biomarkers in kidney disease. Dordrecht: Springer; 2016b. p. 577–98.
Bocedi A, Fabrini R, Lai O, Alfieri L, Roncoroni C, Noce A, Pedersen JZ, Ricci G. Erythrocyte glutathione transferase: a general probe for chemical contaminations in mammals. Cell Death Discov. 2016c;2:16029. https://doi.org/10.1038/cddiscovery.2016.29.
Bocedi A, Noce A, Rovella V, Marrone G, Cattani G, Iappelli M, De Paolis P, Iaria G, Sforza D, Gallù M, Tisone G, Di Daniele N, Ricci G. Erythrocyte glutathione transferase in kidney transplantation: a probe for kidney detoxification efficiency. Cell Death Dis. 2018;9:288. https://doi.org/10.1038/s41419-018-0289-3.
Bocedi A, Noce A, Marrone G, Noce G, Cattani G, Gambardella G, Di Lauro M, Di Daniele N, Ricci G. Glutathione transferase P1-1 an enzyme useful in biomedicine and as biomarker in clinical practice and in environmental pollution. Nutrients. 2019;11:1741. https://doi.org/10.3390/nu11081741.
Bocedi A, Lai O, Cattani G, Roncoroni C, Gambardella G, Notari S, Tancredi F, Bitonti G, Calabrò S, Ricci G. Animal Biomonitoring for the Surveillance of Environment Affected by the Presence of Slight Contamination by β-HCH. Antioxidants. 2022;11:527. https://doi.org/10.3390/antiox11030527.
Booth J, Boyland E, Sims P. An enzyme from rat liver catalysing conjugations with glutathione. Biochem J. 1961;79:516–24. https://doi.org/10.1042/bj0790516.
Brucker N, Moro AM, Charão MF, Durgante J, Freitas F, Baierle M, Nascimento S, Gauer B, Bulcão RP, Bubols GB, Ferrari PD, Thiesen FV, Gioda A, Duarte MM, de Castro I, Saldiva PH, Garcia SC. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci Total Environ. 2013;463–464:884–93. https://doi.org/10.1016/j.scitotenv.2013.06.098.
Bundy JG, Sidhu JK, Rana F, Spurgeon DJ, Svendsen C, Wren JF, Stürzenbaum SR, Morgan AJ, Kille P. “Systems toxicology” approach identifies coordinated metabolic responses to copper in a terrestrial non-model invertebrate, the earthworm Lumbricus rubellus. BMC Biol. 2008;6:25. https://doi.org/10.1186/1741-7007-6-25.
Buratti FM, Darney K, Vichi S, Turco L, Di Consiglio E, Lautz LS, Béchaux C, Dorne JCM, Testai E. Human variability in glutathione-S-transferase activities, tissue distribution and major polymorphic variants: meta-analysis and implication for chemical risk assessment. Toxicol Lett. 2021;337:78–90. https://doi.org/10.1016/j.toxlet.2020.11.007.
Burg D, Filippov DV, Hermanns R, van der Marel GA, van Boom JH, Mulder GJ. Peptidomimetic glutathione analogues as novel gammaGT stable GST inhibitors. Bioorg Med Chem. 2002;10:195–205. https://doi.org/10.1016/s0968-0896(01)00269-3.
Caccuri AM, Antonini G, Ascenzi P, Nicotra M, Nuccetelli M, Mazzetti AP, Federici G, Lo Bello M, Ricci G. Temperature adaptation of glutathione S-transferase P1-1. A case for homotropic regulation of substrate binding. J Biol Chem. 1999;274:19276–80. https://doi.org/10.1074/jbc.274.27.19276.
Canli EG, Atli G, Canli M. Response of the antioxidant enzymes of the erythrocyte and alterations in the serum biomarkers in rats following oral administration of nanoparticles. Environ Toxicol Pharmacol. 2017;50:145–50. https://doi.org/10.1016/j.etap.2017.02.007.
Carmagnol F, Sinet PM, Rapin J, Jerome H. Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: hyperbilirubinemia and impaired renal function. Clin Chim Acta. 1981;117:209–17. https://doi.org/10.1016/0009-8981(81)90040-1.
Chatterjee A, Gupta S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018;433:33–42. https://doi.org/10.1016/j.canlet.2018.06.028.
Chen H, Juchau MR. Recombinant human glutathione S-transferases catalyse enzymic isomerization of 13-cis-retinoic acid to all-trans-retinoic acid in vitro. Biochem J. 1998;336:223–6. https://doi.org/10.1042/bj3360223.
Chen C, Wu C, Lu X, Yan Z, Gao J, Zhao H, Li S. Coniferyl ferulate, a strong inhibitor of glutathione S-transferase isolated from radix Angelicae sinensis, reverses multidrug resistance and downregulates P-glycoprotein. Evid Based Complement Alternat Med. 2013;2013:639083. https://doi.org/10.1155/2013/639083.
Chikezie PC. Glutathione S-transferase activity in diagnostic pathology. Metabolomics. 2015;5:153. https://doi.org/10.4172/2153-0769.1000153.
de Oliveira AAF, de Oliveira TF, Dias MF, Medeiros MHG, Di Mascio P, Veras M, Lemos M, Marcourakis T, Saldiva PHN, Loureiro APM. Genotoxic and epigenotoxic effects in mice exposed to concentrated ambient fine particulate matter (PM2.5) from São Paulo city, Brazil. Part Fibre Toxicol. 2018;15:40. https://doi.org/10.1186/s12989-018-0276-y.
Dessì M, Noce A, Dawood KF, Galli F, Taccone-Gallucci M, Fabrini R, Bocedi A, Massoud R, Fucci G, Pastore A, Manca di Villahermosa S, Zingaretti V, Federici G, Ricci G. Erythrocyte glutathione transferase: a potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids. 2012;43:347–54. https://doi.org/10.1007/s00726-011-1085-x.
Dhanani S, Awasthi YC. Glutathione S-transferase isozyme composition of human tissues. In: Awasthi YC, editor. Toxicology of glutathione transferases. New York: Taylor & Francis Group; 2007. p. 321–38.
Dirr H, Reinemer P, Huber R. X-ray crystal structures of cytosolic glutathione S-transferases. Implications for protein architecture, substrate recognition and catalytic function. Eur J Biochem. 1994;220:645–61. https://doi.org/10.1111/j.1432-1033.1994.tb18666.x.
Dobrakowski M, Pawlas N, Hudziec E, Kozłowska A, Mikołajczyk A, Birkner E, Kasperczyk S. Glutathione, glutathione-related enzymes, and oxidative stress in individuals with subacute occupational exposure to lead. Environ Toxicol Pharmacol. 2016;45:235–40. https://doi.org/10.1016/j.etap.2016.06.008.
Dutta T, Nayak C, Bhattacharjee S. Acetylcholinesterase, butyrylcholinesterase and glutathione S-transferase enzyme activities and their correlation with genotypic variations based on GST M1 and GST T1 loci in long term-pesticide-exposed tea garden workers of sub-Himalayan West Bengal. Toxicol Environ Health Sci. 2019;11:63–72. https://doi.org/10.1007/s13530-019-0389-1.
Eaton DL, Bammler TK. Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci. 1999;49:156–64. https://doi.org/10.1093/toxsci/49.2.156.
Ebokaiwe AP, Omaka ON, Okorie U, Oje O, Egedeigwe C, Ekwe A, Nnaji NJ. Assessment of heavy metals around Abakaliki metropolis and potential bioaccumulation and biochemical effects on the liver, kidney, and erythrocyte of rats. Hum Ecol Risk Assess Int J. 2018;24:1233–55. https://doi.org/10.1080/10807039.2017.1410695.
Edwards R, Dixon DP, Walbot V. Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 2000;5:193–8. https://doi.org/10.1016/s1360-1385(00)01601-0.
Enayati AA, Ranson H, Hemingway J. Insect glutathione transferases and insecticide resistance. Insect Mol Biol. 2005;14:3–8. https://doi.org/10.1111/j.1365-2583.2004.00529.x.
Espín S, Martínez-López E, Jiménez P, María-Mojica P, García-Fernández AJ. Effects of heavy metals on biomarkers for oxidative stress in Griffon vulture (Gyps fulvus). Environ Res. 2014a;129:59–68. https://doi.org/10.1016/j.envres.2013.11.008.
Espín S, Martínez-López E, León-Ortega M, Martínez JE, García-Fernández AJ. Oxidative stress biomarkers in Eurasian eagle owls (Bubo bubo) in three different scenarios of heavy metal exposure. Environ Res. 2014b;131:134–44. https://doi.org/10.1016/j.envres.2014.03.015.
Fabrini R, De Luca A, Stella L, Mei G, Orioni B, Ciccone S, Federici G, Lo Bello M, Ricci G. Monomer-dimer equilibrium in glutathione transferases: a critical re-examination. Biochemistry. 2009;48:10473–82. https://doi.org/10.1021/bi901238t.
Fabrini R, Bocedi A, Massoud R, Federici G, Ricci G. Spectrophotometric assay for serum glutathione transferase: a re-examination. Clin Biochem. 2012a;45:668–71. https://doi.org/10.1016/j.clinbiochem.2012.02.017.
Fabrini R, Bocedi A, Del Grosso E, Morici L, Federici G, Palleschi A, Ricci G. Erythrocyte glutathione transferase: a novel biomarker to check environmental pollution hazardous for humans. Biochem Biophys Res Commun. 2012b;426:71–5. https://doi.org/10.1016/j.bbrc.2012.08.037.
Fabrini R, Bocedi A, Camerini S, Fusetti M, Ottaviani F, Passali FM, Topazio D, Iavarone F, Francia I, Castagnola M, Ricci G. Inactivation of human salivary glutathione transferase P1-1 by hypothiocyanite: a post-translational control system in search of a role. PLoS One. 2014;9:e112797. https://doi.org/10.1371/journal.pone.0112797.
Galli F, Rovidati S, Benedetti S, Buoncristiani U, Covarelli C, Floridi A, Canestrari F. Overexpression of erythrocyte glutathione S-transferase in uremia and dialysis. Clin Chem. 1999;45:1781–8.
Gonçalves AMM, Rocha CP, Marques JC, Gonçalves FJM. Enzymes as useful biomarkers to assess the response of freshwater communities to pesticide exposure – a review. Ecol Indic. 2021;122:107303. https://doi.org/10.1016/j.ecolind.2020.107303.
Habdous M, Vincent-Viry M, Visvikis S, Siest G. Rapid spectrophotometric method for serum glutathione S-transferases activity. Clin Chim Acta. 2002;326:131–42. https://doi.org/10.1016/S0009-8981(02)00329-7.
Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. https://doi.org/10.1016/s0076-6879(81)77053-8.
Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. https://doi.org/10.3109/10409239509083491.
Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. https://doi.org/10.1146/annurev.pharmtox.45.120403.095857.
Hellou J, Ross NW, Moon TW. Glutathione, glutathione S-transferase, and glutathione conjugates, complementary markers of oxidative stress in aquatic biota. Environ Sci Pollut Res Int. 2012;19:2007–23. https://doi.org/10.1007/s11356-012-0909-x.
Higgins LG, Hayes JD. Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents. Drug Metab Rev. 2011;43:92–137. https://doi.org/10.3109/03602532.2011.567391.
Kilpikari I, Savolainen H. Decreased erythrocyte glutathione S-transferase activity in rubber workers. Int Arch Occup Environ Health. 1984;53:299–302. https://doi.org/10.1007/BF00380668.
Kural C, Kaya Kocdogan A, Şimşek GG, Oğuztüzün S, Kaygın P, Yılmaz I, Bayram T, Izci Y. Glutathione S-transferases and cytochrome P450 enzyme expression in patients with intracranial tumors: preliminary report of 55 patients. Med Princ Pract. 2019;28:56–62. https://doi.org/10.1159/000494496.
Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life-span in mammals. Comp Biochem Physiol B Comp Biochem. 1993;106:477–87. https://doi.org/10.1016/0305-0491(93)90121-k.
Lee WH, Joshi P, Wen R. Glutathione S-transferase pi isoform (GSTP1) expression in murine retina increases with developmental maturity. Adv Exp Med Biol. 2014;801:23–30. https://doi.org/10.1007/978-1-4614-3209-8_4.
Li T, Liu G, Li H, Yang X, Jing Y, Zhao G. The synthesis of ethacrynic acid thiazole derivatives as glutathione S-transferase pi inhibitors. Bioorg Med Chem. 2012;20:2316–22. https://doi.org/10.1016/j.bmc.2012.02.011.
Lo Bello M, Parker MW, Desideri A, Polticelli F, Falconi M, Del Boccio G, Pennelli A, Federici G, Ricci G. Peculiar spectroscopic and kinetic properties of Cys-47 in human placental glutathione transferase. Evidence for an atypical thiolate ion pair near the active site. J Biol Chem. 1993;268:19033–8.
Mahadevan D, Sutton GR. Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes. Expert Opin Investig Drugs. 2015;24:725–33. https://doi.org/10.1517/13543784.2015.1021003.
Oakley A. Glutathione transferases: a structural perspective. Drug Metab Rev. 2011;43:138–51. https://doi.org/10.3109/03602532.2011.558093.
Oyagbemi AA, Omobowale TO, Asenuga ER, Afolabi JM, Adejumobi OA, Adedapo AA, Yakubu MA. Effect of arsenic acid withdrawal on hepatotoxicity and disruption of erythrocyte antioxidant defense system. Toxicol Rep. 2017;4:521–9. https://doi.org/10.1016/j.toxrep.2017.09.006.
Parker LJ, Bocedi A, Ascher DB, Aitken JB, Harris HH, Lo Bello M, Ricci G, Morton CJ, Parker MW. Glutathione transferase P1-1 as an arsenic drug-sequestering enzyme. Protein Sci. 2017;26:317–26. https://doi.org/10.1002/pro.3084.
Possamai FP, Júnior SÁ, Parisotto EB, Moratelli AM, Inácio DB, Garlet TR, Dal-Pizzol F, Filho DW. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ Toxicol Pharmacol. 2010;30:175–80. https://doi.org/10.1016/j.etap.2010.05.006.
Primavera A, Fustinoni S, Biroccio A, Ballerini S, Urbani A, Bernardini S, Federici G, Capucci E, Manno M, Lo Bello M. Glutathione transferases and glutathionylated hemoglobin in workers exposed to low doses of 1,3-butadiene. Cancer Epidemiol Biomark Prev. 2008;17:3004–12. https://doi.org/10.1158/1055-9965.EPI-08-0443.
Raza A, Galili N, Smith S, Godwin J, Lancet J, Melchert M, Jones M, Keck JG, Meng L, Brown GL, List A. Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome. Blood. 2009;113:6533–40. https://doi.org/10.1182/blood-2009-01-176032.
Ricci G, Caccuri AM, Bello ML, Pastore A, Piemonte F, Federici G. Colorimetric and fluorometric assays of glutathione transferase based on 7-chloro-4-nitrobenzo-2-oxa-1,3-diazole. Anal Biochem. 1994;218:463–5. https://doi.org/10.1006/abio.1994.1209.
Ricci G, De Maria F, Antonini G, Turella P, Bullo A, Stella L, Filomeni G, Federici G, Caccuri AM. 7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione S-transferases. Mechanism of action of potential anticancer drugs. J Biol Chem. 2005;280:26397–405. https://doi.org/10.1074/jbc.M503295200.
Rotili D, De Luca A, Tarantino D, Pezzola S, Forgione M, Morozzo Della Rocca B, Falconi M, Mai A, Caccuri AM. Synthesis and structure–activity relationship of new cytotoxic agents targeting human glutathione-S-transferases. Eur J Med Chem. 2015;89:156–71. https://doi.org/10.1016/j.ejmech.2014.10.033.
Rotimi OA, Rotimi SO, Oluwafemi F, Ademuyiwa O, Balogun EA. Oxidative stress in extrahepatic tissues of rats co-exposed to aflatoxin B1 and low protein diet. Toxicol Res. 2018;34:211–20. https://doi.org/10.5487/TR.2018.34.3.211.
Rowe JD, Nieves E, Listowsky I. Subunit diversity and tissue distribution of human glutathione S-transferases: interpretations based on electrospray ionization-MS and peptide sequence-specific antisera. Biochem J. 1997;325:481–6. https://doi.org/10.1042/bj3250481.
Sachdeva S, Kushwaha P, Flora SJ. Effects of sodium tungstate on oxidative stress enzymes in rats. Toxicol Mech Methods. 2013;23:519–27. https://doi.org/10.3109/15376516.2013.787132.
Saint-Denis M, Narbonne JF, Arnaud C, Ribera D. Biochemical responses of the earthworm Eisenia fetida andrei exposed to contaminated artificial soil, effects of lead acetate. Soil Biol Biochem. 2001;33:395–404.
Salinas AE, Wong MG. Glutathione S-transferases – a review. Curr Med Chem. 1999;6:279–309.
Sawers L, Ferguson MJ, Ihrig BR, Young HC, Chakravarty P, Wolf CR, Smith G. Glutathione S-transferase P1 (GSTP1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. Br J Cancer. 2014;111:1150–8. https://doi.org/10.1038/bjc.2014.386.
Schins RPF, Keman S, Borm PJA. Blood antioxidant status in coal dust induced respiratory disorders: a longitudinal evaluation of multiple biomarkers. Biomarkers. 1997;2:45–50. https://doi.org/10.1080/135475097231968.
Sharma RK, Upadhyay G, Siddiqi NJ, Sharma B. Pesticides-induced biochemical alterations in occupational North Indian suburban population. Hum Exp Toxicol. 2013;32:1213–27. https://doi.org/10.1177/0960327112474835.
Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. https://doi.org/10.1042/0264-6021:3600001.
Sluis-Cremer N, Naidoo N, Dirr H. Class-pi glutathione S-transferase is unable to regain its native conformation after oxidative inactivation by hydrogen peroxide. Eur J Biochem. 1996;242:301–7. https://doi.org/10.1111/j.1432-1033.1996.0301r.x.
Stott WT, Gollapudi BB, Rao KS. Mammalian toxicity of 1,3-dichloropropene. Rev Environ Contam Toxicol. 2001;168:1–42. https://doi.org/10.1007/978-1-4613-0143-1_1.
Temel Y, Taysi MŞ. The effect of mercury chloride and boric acid on rat erythrocyte enzymes. Biol Trace Elem Res. 2019;191:177–82. https://doi.org/10.1007/s12011-018-1601-x.
Terrier P, Townsend AJ, Coindre JM, Triche TJ, Cowan KH. An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue. Am J Pathol. 1990;137:845–53.
Tew KD, Ronai Z. GST function in drug and stress response. Drug Resist Updat. 1999;3:143–7. https://doi.org/10.1054/drup.1999.0086.
Tuluce Y, Ozkol H, Koyuncu I, Ine H. Increased occupational coal dust toxicity in blood of central heating system workers. Toxicol Ind Health. 2011;27:57–64. https://doi.org/10.1177/0748233710381889.
Türkan F, Huyut Z, Demir Y, Ertaş F, Beydemir Ş. The effects of some cephalosporins on acetylcholinesterase and glutathione S-transferase: an in vivo and in vitro study. Arch Physiol Biochem. 2019;125:235–43. https://doi.org/10.1080/13813455.2018.1452037.
Wilce MC, Parker MW. Structure and function of glutathione S-transferases. Biochim Biophys Acta. 1994;1205:1–18. https://doi.org/10.1016/0167-4838(94)90086-8.
Wilhelm Filho D, Avila S Jr, Possamai FP, Parisotto EB, Moratelli AM, Garlet TR, Inácio DB, Torres MA, Colepicolo P, Dal-Pizzol F. Antioxidant therapy attenuates oxidative stress in the blood of subjects exposed to occupational airborne contamination from coal mining extraction and incineration of hospital residues. Ecotoxicology. 2010;19:1193–200. https://doi.org/10.1007/s10646-010-0503-2.
Yaman T, Yener Z, Celik I. Histopathological and biochemical investigations of protective role of honey in rats with experimental aflatoxicosis. BMC Complement Altern Med. 2016;16:232. https://doi.org/10.1186/s12906-016-1217-7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 Springer Nature Switzerland AG
About this entry
Cite this entry
Bocedi, A., Gambardella, G., Cattani, G., Notari, S., Pedersen, J.Z., Ricci, G. (2023). Erythrocyte Glutathione Transferase P1-1 as a Biomarker in Environmental Toxicology: A New Narrative. In: Patel, V.B., Preedy, V.R., Rajendram, R. (eds) Biomarkers in Toxicology. Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-07392-2_25
Download citation
DOI: https://doi.org/10.1007/978-3-031-07392-2_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07391-5
Online ISBN: 978-3-031-07392-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences