Abstract
The erythrocyte glutathione S-transferase (e-GST) is a member of a superfamily of inducible enzymes involved in cell detoxification that shows an increased expression in chronic kidney disease (CKD) patients. We propose a new automated analysis procedure for e-GST activity that has been validated in 72 CKD patients and 62 maintenance hemodialysis patients (MHD). Regression analysis was carried out to assess association between e-GST activity data, main clinical variables, and plasma homocysteine (Hcy), a modified sulfur amino acid known as potential risk factor for cardiovascular disease that is increased above normal levels in more than 90% of the uremic patients. An increased e-GST activity was confirmed in MHD patients (N = 62; 10.2 ± 0.4 U/gHb) compared with healthy subjects (N = 80; 5.8 ± 0.4 U/gHb), and as an original finding, a significant increase of e-GST activity was observed in pre-dialysis CKD patients with a positive correlation with disease severity weighted according to the four stages of “Kidney Disease Outcomes Quality Initiative” classification (7.4 ± 0.5, 8 ± 1, 9.5 ± 0.6, 12 ± 1 U/gHb, respectively). No correlation was found between e-GST activity and hemoglobin, transferrin, blood iron and the markers of systemic inflammation and renal function such as alpha-1 acid glycoprotein and high-sensitive C-Reactive Protein, beta-2 microglobulin and the index of malnutrition-inflammation PINI, while a significant correlation was observed for the first time between plasma Hcy and e-GST activity (r 2 = 0.64, P < 0.0001) in MHD patients. Hcy, however, was not identified as an inhibitor of e-GST enzyme. The results in this study suggest the potential for automated e-GST analysis as a valuable tool to further explore phase II-related uremic toxicity in CKD and MHD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glutathione transferases (GSTs) represent a superfamily of enzymes devoted to cell protection and detoxification (Armstrong 1997). A prominent function of these enzymes is the conjugation of glutathione (GSH) to toxic hydrophobic compounds provided by an electrophilic center (Sheehan et al. 2001). This reaction facilitates their inactivation and renal elimination (Armstrong 1997). Human cytosolic GSTs have been grouped into eight gene-independent classes termed Alpha, Mu, Pi, Theta, Sigma, Omega, Kappa, and Zeta (Hayes et al. 2005). Distinct GST isoenzymes belonging to different classes can be expressed in the same tissue and variably over-expressed as a consequence of tissue exposition to endobiotic or xenobiotic compounds (Hayes et al. 2005). On the other hand, red blood cells express almost exclusively a single GST isoenzyme (e-GST) which has been identified as GSTP1-1 (Awasthi et al. 1994) and represents more than 95% of the erythrocyte GST pool. Over-expression of e-GST has been found only in uremic patients under maintenance hemodialysis (MHD) (Galli et al. 1999) and in subjects affected by hyperbilirubinemia (Carmagnol et al. 1981). This phenomenon was not influenced by vitamin E supplementation (Galli et al. 1999), thus suggesting possible independence from cellular oxidative stress (Galli et al. 1999). In any case, e-GST expression has been proposed as possible marker to check the accumulation of uremic toxins and to probe the efficiency of dialytic procedures (Galli et al. 1999). In these previous studies, e-GST levels have been checked only in a few chronic kidney disease (CKD) patients under conservative therapy, and the expression of this enzyme appeared not different from the one found in the controls. The main aim of this study is to develop a simple method for the determination of e-GST activity to re-evaluate this on large populations of CKD patients, also assessing the correlation with the severity of kidney disease that was identified according to K-DOQI staging (K-DOQI Clinical practice guidelines 2004). High-sensitive C-reactive protein (hs-CRP) (Jacobs et al. 2010), prognostic inflammatory nutritional index (PINI) (Dessì et al. 2009), alpha-1 acid glycoprotein, fibrinogen, and beta-2 microglobulin (Bethea and Forman 1990) were also measured to verify a possible correlation between e-GST over-activity versus systemic inflammation (Kendrick and Chonchol 2008).
Hyperhomocysteinemia is found in more than 90% of the MHD patient population (Bostom and Lathrop 1997; van Guldener 2006). In non-uremic subjects, hyperhomocysteinemia is often caused by genetic defects of metabolic enzymes (Welch and Loscalzo 1998; Kluijtmans et al. 1996) such as 5,10-methylenetetrahydrofolate reductase. In renal patients, the total plasma homocysteine (Hcy) is significantly increased regardless of genetic defects (Welch and Loscalzo 1998). Elevated plasma Hcy may promote endothelial dysfunction, which is probably the consequence of the oxidative inactivation of endothelium-derived nitric oxide (NO) (Welch and Loscalzo 1998). Hyperhomocysteinemia may also cause oxidative stress by inhibiting the expression or the activity of cellular antioxidant enzymes such as glutathione peroxidase-1 (GPx-1) (Lubos et al. 2007). Thus, the second target of this study is to verify a possible causal relationship between the increase of plasma Hcy level and e-GST activity in uremic MHD patients. The results found here disclose an interesting scenario where e-GST may act as a new bio-marker complementary or substitutive of other procedures to weight uremic toxicity and its derived comorbididty in CKD and MHD patients.
Methods
Patients and study design
The study protocol complied with the declaration of Helsinki and was appointed by the Ethical Committee of the University of Rome ‘Tor Vergata’. A written fully informed consent was provided by all patients and healthy subjects before enrolment into the studies.
Blood samples were obtained from 72 CKD patients under conservative therapy (33 men, 39 women, mean age 54.5 years, range 24–80 years), 62 MHD patients (29 men, 33 women, mean age 58.0 years, range 36–81 years) were on maintenance renal replacement therapy since six months at least, and 80 healthy controls (35 men, 45 women, mean age 46.1 years, range 23–75 years) with normal renal function and no history of diabetes mellitus. K-DOQI CKD staging system was used to group pre-dialysis patients according to their estimated glomerular filtration rate (GFR) assessed by Modification of Diet in Renal Diseases Study (MDRD) equation (Levey et al. 1999). Exclusion criteria for both patients and control subjects were hyperbilirubinemia, a clinical history of virus hepatitis B and C or serum aspartate transaminase and/or alanine transaminase ≥ twice the upper limit of normal values, morbid obesity, rheumatologic disorders such as systemic lupus erythematosus and active malignancy.
All 62 MHD patients were treated with either standard bicarbonate dialysis with 1.5–2.0 m2 hollow fiber low-flux polysulphone membranes (or ‘on line’ hemofiltration with 1.5–2.0 m2 hollow fiber high-flux polysulphone (B. Braun GMBH, Melsungen, Germany)) 4 h, three times weekly through a well-functioning native A–V fistula or a cuffed internal jugular indwelling venous catheter. The vascular access performance was satisfactory with a blood flow of at least 300 ml/min and K t /V ratio of >1.2. Underlying disease was nephroangiosclerosis in 20 patients, chronic primary glomerulonephritis in 23 patients, chronic interstitial nephritis in 10 patients, polycystic kidney disease in 6 patients, and diabetes mellitus in 3 patients.
Analytical procedures
All reagents in this study were from Sigma–Aldrich (St. Louis, USA) and used without further purification. Blood samples were collected from the antecubital vein in healthy subjects and in CKD patients under conservative therapy from the arterial site of the vascular access before dialysis in MHD patients at the end of the long-interval for Hcy determinations. Blood samples were collected into K3 EDTA vacutainer tubes as well, put on ice and immediately centrifuged and stored at −20°C until analysis. For e-GST activity determinations, blood drawn was collected in EDTA and stored until use at 4°C for not more than 4 days. One volume (20 μl) of whole blood was diluted in 25 volumes (0.5 ml) of bi-distilled water and after 5 min introduced into the Modular P800 (Roche, Switzerland) automated apparatus for GST activity determination. After dilution, the hemolysis is almost complete within a few seconds (Jacobs 1932), as also confirmed by microscopic examination. GST activity was assessed spectrophotometrically at 340 nm (37°C) using 20 μl of hemolyzed sample in 0.2 ml final volume containing 1 mM GSH, 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) in 0.1 M potassium phosphate buffer, pH 6.5. Time run was 1 min. The automated analyzer uses an optical path length of 0.5 cm and it can perform up to 800 tests per hour. The results were compared with the standard spectrophotometric assay method for GST described by Habig et al. (1974).
GST activity was expressed as enzyme units (U) per gram of Hb. One unit represents the amount of enzyme that catalyzes the conjugation of 1 μmole of GSH to CDNB in 1 min at 37°C. Recombinant human GSTP1-1 expressed in E. coli and purified as described previously (Lo Bello et al. 1995) was used as internal standard. Hemoglobin was determined with an automated haematology analyzer XE-2100 (Sysmex, Kobe, Japan).
Clinical tests (in-routine) reported in this paper are utilized within a hospital setting. Analytical systems of clinical chemistry are controlled by IQA (internal quality assessment) and EQA (external quality assessment) according to the international recognized standards (UNI EN ISO 9001:2008 and UNI EN ISO 15189:2007).
Routine laboratory measurements performed by turbidimetric methods used Modular P800. Fibrinogen was quantified by phototurbimetric method (Ca 7000 Sysmex, Japan).
Plasma total Hcy was assayed by a fully automated HPLC method using reversed-phase separation and fluorescence detection as reported previously (Pastore et al. 2006). Creatinine was determined by an automated method using the Modular P800 (Roche) apparatus. The assay is based on the colorimetric reaction between creatinine and picric acid at 510 nm. Supplementary analysis on clinical and inflammation markers (e.g., alpha-1 acid glycoprotein, beta-2 microglobulin, albumin, pre-albumin, hs-CRP) was performed using BN II nephelometer (Siemens healthcare diagnostics, Milan, Italy).
Statistical analysis
Data are expressed as mean ± standard deviation of the mean (SEM). Unpaired t test was employed for statistical analysis of results. A P value of <0.05 was considered significant. Data were processed using statistical software MedCalc (Mariakerke, Belgium). Passing–Bablock regression analysis was performed according to the original protocol (Passing and Bablock 1984).
Results
Simplified procedure for e-GST activity determination
Previous studies finalized to quantify e-GST activity needed time-consuming erythrocyte isolation and conspicuous blood volumes (Awasthi et al. 1994; Galli et al. 1999). Our simplified procedure, adapted to an automated apparatus (Modular P800), requires only 20 μl of whole blood and no erythrocyte purification step. Actually, the amount of extra-erythrocyte GST is always negligible and no relevant spectrophotometric artifacts occur in the presence of broken erythrocytes. Furthermore, GSTP1-1 represents more than 95% of all GST isoenzymes found in the red blood cells. This enzyme homogeneity makes the activity value directly related to the level of expression of GSTP1-1.
Experiments shown in Fig. 1a, performed on the automated apparatus using blood samples implemented by authentic GSTP1-1, demonstrate an excellent linearity (r 2 = 0.998, P < 0.0001) in a concentration range between 1 and 25 U/gHb (blood of dialyzed and healthy subjects shows e-GST levels ranging from 2 to 18 U/gHb) and a good recovery (98–100%) (Fig. 1c). The lower limit of detection is 0.3 mU/ml in the final volume of the activity test, while the lower limit of quantitation is 0.8 mU/ml (approximately, the e-GST of healthy subjects, i.e., 5.8 U/gHb, corresponds to about 3.2 mU/ml in the final volume of the activity test).
Linearity of the automated procedure and comparison with the Habig assay. a Blood samples (in case implemented with variable amounts of recombinant GSTP1-1) were assayed for GST activity on the automated Modular P800 apparatus set at 340 nm (37°C). Data were compared with those obtained with the manual Habig procedure (Habig et al. 1974) (panel b). c Recovery experiments of GSTP1-1 have been performed using the standard assay procedure on the automated Modular apparatus. Five whole blood samples implemented with recombinant GSTP1-1 were used. The unit values of the endogenous GSTP1-1 have been subtracted in each sample. Experimental points represent the mean ± SD of at least three different measurements
Comparison of the automated procedure for e-GST with the traditional manual spectrophotometric assay procedure by Habig et al. (1974) (Fig. 1a, b) shows a very good correlation (r 2 = 0.998). In addition, the Passing–Bablock regression analysis of the results obtained with the two methods indicated that the intercept of 95% confidence interval included zero value (not shown). Thus, the two methods can be used interchangeably.
Intra-day precision and inter-day precision data calculated on seven different days were evaluated using four samples of whole blood stored at 4°C. e-GST activity was measured six times for each sample and the relative SEM was 2.9 and 3.5% for intra-day and inter-day assay, respectively.
A surprising property of e-GST, useful for analytical purposes, is its strong stability in whole blood samples. The loss of enzyme activity does not exceed 5% even after 14 days at 4°C and at 8°C the activity is unchanged for 3 days (Fig. 2a). We also explored the possibility that e-GST may be more exposed to inactivation after hemolysis under dilute conditions; it has been described that under these conditions, GSTP1-1 undergoes a remarkable biphasic inactivation due to active site solvatation (Adams and Sikakana 1990). Indeed, in blood samples diluted with bi-distilled water (1:25) and stored at 25°C, e-GST displays a complete inactivation after 4 days, but it remains fully active for about 6 h (Fig. 2b). e-GST in diluted samples stored at 4°C does not change at all its original activity for 4 days (Fig. 2b). Intriguingly, while blood samples at −80°C display a stable activity at least for 1 month, storage at −20°C causes a rapid 30% inactivation which does not increase any further after 1 month (Fig. 2c). The original activity is also unmodified after three freeze–thaw cycles (Fig. 2c, inset).
e-GST stability under different conditions. a Five different blood samples with e-GST ranging from 3 to 12 U/gHb were stored as such at 4, 8, and 25°C. b The same samples were diluted 1:25 with bi-distilled water and stored at 4 and 25°C. c The same samples stored at −20 and −80°C or after one and three freeze–thaw treatments (inset). Experimental points represent the mean ± SD of determinations made for all five samples
Evaluation of e-GST activity in renal failure patients
The aforementioned procedure was successfully employed to evaluate the e-GST activity in CKD patients under conservative therapy, in MHD patients, and in healthy subjects. Mean clinical features and laboratory findings of healthy subjects, MHD patients, and CKD patients under conservative therapy are summarized in Table 1. e-GST is highly increased in MHD patients, according to the previous studies (Galli et al. 1999; Carmagnol et al. 1981). However, for the first time, we observed an increased e-GST activity also in pre-dialysis patients, and this seems to be related to CKD stage (Table 1; Fig. 3). The difference was statistically significant between the healthy subjects and all CKD patients (P < 0.0001 for groups I–IV and P < 0.0001 vs. MHD patients). Among CKD patients, the difference was significant between groups I and III (P = 0.016), I and IV (P < 0.0001), II and IV (P = 0.015) and III and IV (P = 0.04). e-GST activity was significantly higher in MHD patients than in CKD patients of groups I and II (P < 0.0001 and P = 0.016), and in healthy controls (P < 0.0001); unexpectedly, e-GST activity was significantly lower in MHD patients than in CKD patients of group IV (P = 0.034).
e-GST activity does not correlate with inflammation markers
When e-GST activity in CKD patients was related to markers commonly used to monitor the progress and gravity of the renal disease, we invariably observed a parallel increase (Table 1). However, a more stringent statistical analysis demonstrated the absence of any direct correlation between e-GST activity and conventional markers of either acute phase (i.e., alpha-1 acid glycoprotein) or chronic inflammation (i.e., hs-CRP), or chronic inflammation/kidney disease (i.e., beta-2 microglobulin) and malnutrition-inflammation (i.e., PINI) (Table 1).
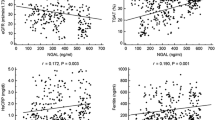
e-GST activity correlates with plasma Hcy in MHD patients
According to the previous studies (Bostom and Lathrop 1997; van Guldener 2006), almost all MHD patients display increased levels of plasma Hcy (Table 2). Interestingly, we found that either mean e-GST activity and mean plasma Hcy were significantly increased in MHD patients compared with controls (10.2 ± 0.4 U/gHb vs. 5.8 ± 0.4 U/gHb, P < 0.0001; 52 ± 4 μmol/l vs. 13.6 ± 0.8 μmol/l, P < 0.0001) (Table 2). Furthermore, a significant direct correlation was also found between plasma Hcy and e-GST activity, as represented in Fig. 4 (r 2 = 0.64, P < 0.0001). To verify if a causal relation between Hcy and e-GST occurs, we checked a possible direct interaction between this enzyme and Hcy. However, e-GST is unable to use Hcy as substrate as demonstrated by the absence of any transferase activity using 1 mM CDNB and 1 mM Hcy. On the other hand, no detectable inhibition has been found using up to 1 mM Hcy or Hcy thiolactone in the presence of the classical GST substrates (1 mM GSH and 1 mM CDNB) (not shown). Previous studies have shown a significant relationship between uremic toxins (such as beta-2 microglobulin) and residual renal function (Fry et al. 2007). This has also been established for Hcy (Anwar et al. 2001). Therefore, residual renal function might be an important confounder for the observed relation between e-GST and plasma Hcy. However, multi-regression analysis did not show any correlation between e-GST activity and residual renal function assessed on the basis of MDRD equation or cystatine C (r 2 = 0.338 and 0.230 for cystatine C and GFR calculated by MDRD equation, respectively).
Finally, we did not find any correlation between e-GST activity in MHD patients and hemoglobin (r 2 = 0.026) or markers of iron metabolism such as transferrin (r 2 = 0.034), ferritin (r 2 = 0.042), blood iron (r 2 = 0.0048).
Discussion
A simple and fast procedure to measure the activity of the e-GST on an automated spectrophotometric apparatus has been optimized and employed to evaluate e-GST activity in CKD patients under conservative therapy and in MHD patients. The use of very small amounts of whole blood that avoid the time-consuming erythrocyte isolation represents a certain improvement over the usual procedures. This simplification is possible because the presence of extra-erythrocyte GST in the blood is always negligible, and broken erythrocyte membranes in the assay mixture do not perturb the spectrophotometric measurements. The simplified assay requires only 20 μl of whole blood which can be collected after a non-invasive pinprick. Furthermore, e-GST activity does not change appreciably during prolonged storage of the blood samples (14 days at 4°C without dilution and 4 days after hemolysis, see Fig. 2). Overall, the assay procedure appears very sensitive and reproducible (Fig. 1). The good linearity and sensitivity (Fig. 1), as well as the low intra-day and inter-day variations, also indicate that this assay may routinely be used to test e-GST activity as a surrogate measure of the exposure of erythroid cells to uremic toxins and other stressors associated with dialysis therapy and uremic comorbidity. The efficiency of different dialytic procedures in correcting the extent of e-GST overexpression will be evaluated. In this context, preliminary results indicate that patients using convective dialysis display a lower e-GST activity than that found in patients under diffusive modality, suggesting the former procedure to be more efficient for blood detoxification.
Actually, our results confirm the previous reports of an enhanced e-GST activity in MHD patients (Galli et al. 1999; Carmagnol et al. 1981); in CKD patients under conservative therapy, we also observed for the first time a significant and progressive increase of e-GST activity that likely related to the K-DOQI stage (Fig. 3; Table 1). Interestingly, we found a significantly higher activity of e-GST in pre-dialysis patients of K-DOQI stage IV compared with MHD patient. This finding is possibly explained by considering that GST expression reflects the abundance of circulating uremic toxins and that the hemodialytic procedure likely lowers the level of these toxic compounds.
Both e-GST and inflammation/malnutrition markers are all significantly increased in CKD and MHD patients as compared to controls (Table 1). However, we did not find any significant correlation between the increased level of e-GST and all these markers. This finding is not surprising. In fact, not all toxins ignite chronic or acute inflammation, and GSTs are enzymes able to sweep or inactivate a lot of toxic compounds irrespectively of their inflammatory action. In this line, the level of e-GST may fulfill a snapshot of the amount of circulating toxins complementary to the classical markers used to test the severity of chronic inflammation, malnutrition, and kidney dysfunction.
The correlation between the increased level of e-GST and plasmatic Hcy concentration in MHD patients (Fig. 4) is of particular interest. Hyperhomocysteinemia is considered a cardiovascular risk factor and it is often associated with renal failure by a defective renal and extra-renal metabolism (van Guldener 2006). The autoxidation of this sulfur-containing amino acid produces hydrogen peroxide and high levels of Hcy reduce the bioavailability of nitric oxide forming S-nitrosohomocysteine and inhibiting NOS activity. Thus, it has been proposed that high Hcy levels are deleterious leading to endothelial dysfunctions and oxidative stress (Welch and Loscalzo 1998), which suggested a role of uremic toxin for this modified amino acid. However, a very different scenario is possible where high levels of plasma Hcy are not the cause, but the consequence, of increased blood toxicity. Indeed, no effect on mortality and cardiovascular diseases had been found in MHD patients after lowering Hcy level with folic acid treatment (Wrone et al. 2004). A paradoxical negative relationship has been reported between Hcy levels and mortality in non-treated patients (Wrone et al. 2004).
Increased levels of e-GST are certainly a consequence (and not the cause) of an increased toxicity of the uremic environment on erythroid cell precursors. Present data do not support any causal relation between hyperhomocysteinemia and e-GST overexpression. In fact, we found that Hcy or Hcy thiolactone does not interact directly with e-GST both like inhibitor and substrate (see “Results”). In addition, the possible oxidative interaction of Hcy with the essential sulfhydryl group of e-GST (Cys47) would be signaled by an inverse relation between Hcy concentration and GST activity, given that any chemical modification of this residue strongly inhibits the transferase activity (Ricci et al. 1989), in contrast with what we found (Fig. 3). Furthermore, the anemic status or metabolic iron dysfunctions does not appear the origin of elevated e-GST activity.
In conclusion, the correlation found in this study between hyperhomocysteinemia and levels of e-GST activity indicates that high levels of Hcy may merely reflect the presence of high levels of other circulating toxins challenging nucleated elements in the bone marrow. In addition, our observation that the increase of e-GST in CKD patients does not correlate with inflammatory markers parallels a similar observation found for Hcy (Kalantar-Zadeh et al. 2004), paving the way for the exploration of e-GST inducers between non-inflammatory solutes that accumulate in the uremic blood and tissues. Being GST a GSH-dependent phase II detoxification enzyme (Hayes et al. 2005), these are expected to be endobiotics with electrophilic properties that are produced as a consequence of the impaired metabolisms and depurative function of the kidney.
Whatever the primary cause(s) of increased levels of e-GST and Hcy in MHD patients, the present findings suggest that e-GST could be a good marker of uremic toxicity, which parallels the severity of renal damage in CKD and eventually the efficacy of dialytic procedures in MHD patients. Under this light, e-GST activity could be further investigated as a new biomarker useful in assessing the risk of developing severe comorbidity of CKD and MHD patients, mainly reflecting the exposure to phase II-related endobiotics and xenobiotics generated by the uremic condition and therapy protocols. Identification of uremic solutes influencing e-GST activity will help us to better characterize the possible pathogenic roles of this detoxification response associated with CKD.
References
Adams PA, Sikakana CN (1990) Factors affecting the inactivation of human placental glutathione S-transferase pi. The kinetic mechanism and pH-dependence of solvational and 1-chloro-2,4-dinitrobenzene-mediated inactivation of the enzyme. Biochem Pharmacol 39:1883–1889
Anwar W, Guéant JL, Abdelmouttaleb I, Adjalla C, Gérard P, Lemoel G, Erraess N, Moutabarrek A, Namour F (2001) Hyperhomocysteinemia is related to residual glomerular filtration and folate, but not to methylenetetrahydrofolate-reductase and methionine synthase polymorphisms, in supplemented end-stage renal disease patients undergoing hemodialysis. Clin Chem Lab Med 39:747–752
Armstrong RN (1997) Structure, catalytic mechanism, and evolution of the glutathione transferases. Chem Res Toxicol 10:2–18
Awasthi YC, Sharma R, Singhal SS (1994) Human glutathione S-transferase. Int J Biochem 26:295–308
Bethea M, Forman DT (1990) Beta 2-microglobulin: its significance and clinical usefulness. Ann Clin Lab Sci 20:163–168
Bostom AG, Lathrop L (1997) Hyperhomocysteinemia in end-stage renal disease: prevalence, etiology, and potential relationship to arteriosclerotic outcomes. Kidney Int 52:495–502
Carmagnol F, Sinet PM, Rapin J, Jerome H (1981) Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: hyperbilirubinemia and impaired renal function. Clin Chem Acta 117:209–217
Dessì M, Noce A, Agnoli A, De Angelis S, Fuiano L, Tozzo C, Taccone-Gallucci M, Fuiano G, Federici G (2009) The usefulness of the prognostic inflammatory and nutritional index (PINI) in a haemodialysis population. Nutr Metab Cardiovasc Dis 19:811–815
Fry AC, Singh DK, Chandna SM, Farrington K (2007) Relative importance of residual renal function and convection in determining beta-2-microglobulin levels in high-flux haemodialysis and on-line haemodiafiltration. Blood Purif 225:295–302
Galli F, Rovidati S, Benedetti S, Buoncristiani U, Covarelli C, Floridi A, Canestrai F (1999) Overexpression of erythrocyte glutathione-S-transferase in uremia and dialysis. Clin Chem 45:1781–1788
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases: the first enzymatic step in mercapturic acid formation. J Biol Chem 22:7130–7139
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Ann Rev Pharmacol Toxicol 45:51–88
Jacobs MH (1932) Osmotic properties of the erythrocyte. Biol Bull 62:178–194
Jacobs LH, van de Kerkhof JJ, Mingels AM, Passos VL, Kleijnen VW, Mazairac AH, van der Sande FM, Wodzig WK, Konings CJ, Leunissen KM, van Dieijen-Visser MP, Kooman JP (2010) Inflammation, overhydration and cardiac biomarkers in haemodialysis patients: a longitudinal study. Nephrol Dial Transplant 25:243–248
Kalantar-Zadeh K, Block G, Humphreys MH, McAllister CJ, Kopple JD (2004) A low, rather than a high, total plasma homocysteine is an indicator of poor outcome in hemodialysis patients. J Am Soc Nephrol 15:442–453
K-DOQI (2004) Clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43(5 Suppl 1):S1–S290
Kendrick J, Chonchol MB (2008) Non traditional risk factors for cardiovascular disease in patients with chronic kidney disease. Nat Clin Pract Nephrol 4:672–681
Kluijtmans LA, van den Heuvel LP, Boers GH, Frosst P, Stevens EM, van Oost BA, den Heijer M, Trijbels FJ, Rozen R, Blom HJ (1996) Molecular genetic analysis in mild hyperhomocysteinemia: a common mutation in the methylenetatrahydrofolate reductase gene in a genetic risk factor for cardiovascular disease. Am J Hum Genet 58:35–41
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D (1999) A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130:461–470
Lo Bello M, Battistoni A, Mazzetti AP, Board PG, Muramatsu M, Federici G, Ricci G (1995) Site-directed mutagenesis of human glutathione transferase P1–1: spectral, kinetic and structural properties of Cys-47 and Lys-54 mutants. J Biol Chem 270:1249–1253
Lubos E, Loscalzo J, Handy DE (2007) Homocysteine and glutathione peroxidase-1. Antioxid Redox Signal 9:1923–1940
Passing H, Bablock W (1984) Comparison of several regression procedures for method comparison studies and determination of sample size. Application of linear regression procedures for method comparison studies in clinical chemistry. Part II. J Clin Chem Clin Biochem 22:431–445
Pastore A, De Angelis S, Casciani S, Ruggia R, Di Giovamberardino G, Noce A, Splendiani G, Cortese C, Federici G, Dessi M (2006) Effects of folic acid before and after vitamin B12 on plasma homocysteine concentrations in hemodialysis patients with known MTHFR genotypes. Clin Chem 52:145–148
Ricci G, Del Boccio G, Pennelli A, Aceto A, Whitehead EP, Federici G (1989) Nonequivalence of the two subunits of horse erythrocyte glutathione transferase in their reaction with sulfhydryl reagents. J Biol Chem 264:5462–5467
Sheehan D, Meade G, Foley VM, Dowd CA (2001) Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J 360:1–16
van Guldener C (2006) Why is homocysteine elevated in renal failure and what can be expected from homocysteine-lowering? Nephrol Dial Transplant 21:1161–1166
Welch GN, Loscalzo J (1998) Homocysteine and atherothrombosis. N Engl J Med 338:1042–1050
Wrone EM, Hornberger JM, Zehnder JL, McCann LM, Coplon NS, Fortmann SP (2004) Randomized trial of folic acid for prevention of cardiovascular events in end-stage renal disease. J Am Soc Nephrol 15:420–426
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding authors
Additional information
M. Dessì, A. Noce, and K. F. Dawood equally contributed to this work.
Rights and permissions
About this article
Cite this article
Dessì, M., Noce, A., Dawood, K.F. et al. Erythrocyte glutathione transferase: a potential new biomarker in chronic kidney diseases which correlates with plasma homocysteine. Amino Acids 43, 347–354 (2012). https://doi.org/10.1007/s00726-011-1085-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-011-1085-x