Abstract
Phenolic compounds (PC) are a diverse class of phytochemicals that are thought to be among the major responsible for the association between high intake of fruits and vegetables and low risk of chronic diseases. Despite promising protective effects in vitro and in animal models, human studies associating PC intake to beneficial health effects have showed more variable results. The accurate assessment of dietary intake of the different classes of PC is a critical step in epidemiological or intervention studies and biomarkers of PC intake are an alternative to the traditional dietary assessment tools. This chapter focuses on the relation between PC intake and the concentration of PC and their metabolites in body fluids (plasma and urine). The biotransformation of PC during digestive process and their kinetics following exposure, as well as the methods used for estimating dietary intake of PC are also discussed.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Plasma
- Urine
- Biotransformation
- Nuclear magnetic resonance spectroscopy
- Mass spectrometry
- Microbiota
- Digestive process
- Flavonoids
- Tannins
- Phenolic acids
- Stilbenes
- Lignans
- Coumarins
Introduction
Fruits and vegetables have been usually associated with a decreased risk of nontransmissible chronic diseases, such as cancer and cardiovascular diseases. Phenolic compounds (PC) are the most likely bioactive compounds associated to these health benefits. These non-nutrient phytochemicals are widely found in the plant kingdom and comprise more than 8000 structures characterized by one or more hydroxyl groups attached to at least one benzene ring (Crozier et al. 2009).
In order to establish firm evidence for the health effects of PC consumption, it is essential to have accurate quantitative information regarding their dietary intake. Despite in vitro and animal experiments have provided strong positive evidence associating PC to numerous health benefits, evidence from human studies is not strong enough. Most epidemiological studies use food frequency questionnaires to determine the dietary intake, which results in imprecision (Picó et al. 2019).
Biological markers of PC exposure consist of one or more biochemical moieties being measured in an accessible fluid or tissue to provide a semiquantitative index of the exposure to individual food constituents (Spencer et al. 2008). Biomarkers in epidemiological studies have to be indicators of exposure and must be robust, sensitive to changes, specific to the dietary source, and biologically relevant (Spencer et al. 2008). Before a particular PC or its metabolite can be used as a sensitive and accurate biomarker, a number of factors must be taken in account. Firstly, a full understanding of the metabolism of PC in human subjects is required in order to select credible biomarkers. Secondly, it is important to understand the time–response relationship between PC intake and the appearance of the biomarker in biological fluids. Thirdly, the precise dose–response relationship between the intake of a specific PC and the appearance of its biomarker is essential. Finally, one must also have an understanding of the extent to which certain physiological and environmental factors affect the rate of PC metabolism in human subjects.
The relationship between dietary intake of PC and resulting concentrations of PC biomarkers in body fluids is highly complex. Usually, the bioavailability of PC is low due to their poor solubility and rapid transformations. Moreover, most PC are metabolized by human gut microbiota generating products that may be absorbed in the gut and display a beneficial role in several organs (Augusti et al. 2021). To date, the concentration of some PC (daidzein, genistein, glycitein, and hydroxytyrosol) in urine showed high correlation with the intake dose, revealing good sensitivity and robustness as biomarkers of intake throughout different studies. On the other hand, weaker correlations of urinary anthocyanins with the intake dose suggest that they are currently less suitable as biomarkers of PC intake (Pérez-Jiménez et al. 2010).
This chapter summarizes current evidence regarding the relationship between the concentration of PC or their metabolites in body fluids (plasma and urine) and the intake of PC. The biotransformation of PC during digestive process and their kinetics following exposure, as well as the methods used for estimating dietary intake of PC were also discussed.
Classification and Structure of Major Dietary PC
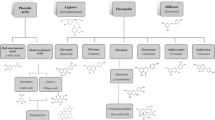
PC are products of the secondary metabolism of plants, providing essential functions for their survival (Augusti et al. 2021). Structurally, PC comprise a benzene ring bearing one or more hydroxyl substituents, and range from simple molecules to complex polymers (Tsao, 2010). According to their chemical structure, they can be divided into several classes that may differ significantly in stability, bioavailability, and bioactivity (Tsao 2010): phenolic acids, tannins, lignans, flavonoids, stilbenes, coumarins, and curcuminoids (Fig. 1). PC can also be divided into free, esterified, and insoluble-bound forms, depending on whether they occur in the free form or are covalently bound to other molecules such as fatty acids (soluble esters) or insoluble macromolecules (insoluble-bound PC). Most insoluble-bound PC are bound to cell wall substances including pectin, cellulose, arabionoxylan, and structural proteins and account for relatively large amount (20–60% in vegetable, fruits, and legume/seeds) compared to the soluble PC in foods. Given that the usual analytical methods for the measurement of the total contents of PC do not consider the insoluble-bound forms, the content of PC in foods should be higher than those described in nutritional tables (Shahidi and Yeo 2016).
Phenolic Acids
Phenolic acids are the simplest class of PC and their basic structure contain one phenolic ring and a carboxylic acid function. According to their carbon skeleton, they can be subdivided into hydroxybenzoic acids (derived from benzoic acid) or hydroxycinnamic acids (derived from cinnamic acid), the latter one comprising the most common compounds, such as caffeic and ferulic acids (Albuquerque et al. 2021). Fruits and vegetables contain mostly free phenolic acids that are readily solubilized during digestion and in the solvents used for the quantitative analysis of PC in food sources. On the other hand, phenolic acids from grains and seeds are often found covalently bound to complex structural carbohydrates, which do not solubilize during digestion or in the hydroalcoholic solvents currently used for PC analysis (Tsao 2010).
Flavonoids
Flavonoids are a PC group mainly consisting of a benzopyrone ring bearing phenolic or polyphenolic groups at different positions. The structural complexity of flavonoids has led to their subclassification into flavonols, flavones, flavanones, flavan-3-ols (including their oligomeric and polymeric forms, proanthocyanidins), isoflavones, and anthocyanins. This classification considers their chemical structure, the degree of unsaturation, and the oxidation of carbon ring (Cassidy and Minihane 2017). In plants, most flavonoids exist as glycosides, although their basic structures are aglycones. The biological activities of these compounds, including their antioxidant activity, depend on the structural difference and the glycosylation patterns. Also, the number of hydroxyl groups, configuration, and substitution are responsible for their antioxidant capacity (Albuquerque et al. 2021).
Tannins
Tannins are a unique group of PC with molecular weights between 500 and 30,000 Da widely distributed in almost all plant foods and beverages. Condensed and hydrolysable tannins are the two major groups of these bioactive compounds, but complex tannins contain structural elements of both groups (Serrano et al. 2009). Hydrolysable tannins are formed by simple phenols, e.g., ellagic and gallic acids, bound to carbohydrate moieties, while condensed tannins are formed by condensing two or more than 200 monomers of flavan-3-ol units.
Stilbenes
Stilbenes represent a class of compounds with a common 1,2-diphenylethylene backbone that have shown extraordinary potential in the biomedical field. As the most well-known example, resveratrol proved to have antiaging and other important health effects (Andrei et al. 2019). Stilbenes are characterized by two benzene rings connected by a double bond, which depending on its configuration, divide the class into the isomers Z and E that usually have different biological activities.
Lignans
Lignans are a class of PC that usually contain a core scaffold formed by two or more phenylpropanoid units linked by the central carbons of the side chains (Cui et al. 2020). They are usually present as dimers, but some of them are trimers or tetramers. Most plant lignans are in the free state, while some of them can combine with sugar to form glycosides and other derivatives. The monomers forming lignans are cinnamic acid, cinnamyl alcohol, propenyl benzene, and allyl benzene. Lignans can be grouped in classical lignans when the molecular linkage of monomers occurs between positions β-β′ and in neolignans, when the main structural units are coupled in any other way (Cui et al. 2020).
Coumarins
Coumarins are 1,2-benzopyrones that consist of a benzene ring linked to a pyrone ring. Based on modifications of this core, coumarins can be classified into complex and simple coumarins. Complex coumarins are produced by the addition of heterocyclic compounds on the basic coumarin core and are further classified into furanocoumarins, pyranocoumarins, phenylcoumarins, dihydrofurocoumarins, and biscoumarins. Simple coumarins includes scopolin, scopoletin, esculin, esculetin, umbelliferone, fraxetin, and sideretin (Stringlis et al. 2019).
Curcuminoids
Curcuminoids are a class of PC found in turmeric (Curcuma longa), a common spice used in the preparation of curries in India and other Asian countries because of its flavor and color. Among curcuminoids, curcumin accounts for approximately 77%, while demethoxycurcumin accounts for 17% and bisdemethoxycurcumin accounts for 3–6% (Kotha and Luthria 2019), all belonging to the diarylheptanoid family. Chemically, curcumin consists of two similar aromatic rings each with o-methoxy phenolic groups, connected by linear carbon chain, having an α,ß-unsaturated ß-diketone moiety. While curcumin is the most studied curcuminoid, it has low bioavailability. Thus, structures without the methoxy group on the benzene ring of the parent structure, such as demethoxycurcumin, has greater stability and has been associated to health benefits (Hatamipour et al. 2019).
Biotransformation of PC During Digestive Process
Food digestion comprises a complex combination of chemical, biochemical, and mechanical processes that operate to disintegrate food into small particles and convert macromolecular nutrients into their basic units that will be available for absorption. The process starts in the mouth where chewing reduces the size of solid foods, which are mixed with saliva reaching pH values between 5 and 7. The pH of digesta rapidly drops out to values between 1 and 3, when it reaches the stomach, being subsequently neutralized to pH values between 6 and 7.5 at the small intestine (Sensoy 2021). Although human enzymes are responsible for the biotransformation of food constituents up to the small intestine, they do not directly act on PC. However, PC may be released upon food matrix disintegration due to the digestion of carbohydrates, proteins, and lipids. Moreover, pH changes along the gastrointestinal tract play a major role in the transformation of PC up to the small intestine (Quatrin et al. 2020; da Silva et al. 2021).
Most dietary PC (90% of intake) will not be solubilized and absorbed up to the small intestine and will therefore reach the colon (Kawabata, Yoshioka and Terao 2019), where gut microbiota triggers the deconjugation of glycoside, glucuronide, and organic acid moieties. Then, PC-derived aglycones are released and subsequently cleaved by fission of heterocyclic and aromatic rings, and undergo dihydroxylation, decarboxylation, demethylation, reduction, and isomerization of alkene moieties (Cortés-Martín et al., 2020). Some of these biotransformation pathways have been elucidated (Fig. 2). This extensive transformation by gut microbiota generates small molecular weight compounds (Fig. 2) that usually have higher absorption rate and therefore can reach higher plasma levels than their parent PC. In addition, many of these microbial-derived PC metabolites have bioactive effects and are actually the major responsible for the systemic biological effects of dietary PC (Cortés-Martín et al. 2020).
The other face of the interplay between PC and gut microbiota is the reshape of the former one by dietary phenolics in a prebiotic-like effect (Cortés-Martín et al. 2020). These changes in microbiota composition may affect its catabolic activity, inducing further changes in the bioavailability and profile of microbial-derived PC metabolites, which in turn affects the bioactivity of dietary PC (Mena et al. 2019). In addition, as the composition of gut microbiota is remarkably affected by diet composition, fecal bacteria have been recently suggested as biomarkers for predicting specific foods and food group intake (Shinn et al. 2021).
This section will address the potential of microbial-derived PC metabolites to be used as biomarkers of PC intake. The great structural diversity of PC leads to distinct biotransformation pathways during digestion, which are being unveiled by recent advances in metabolomic analysis and will be further addressed according to the class of PC.
Anthocyanins and Other Flavonoids
Anthocyanins are quite stable at the acidic gastric pH but are readily converted into hemiketal, chalcone, and quinoidal forms at the intestinal pH values (Quatrin et al. 2020). Delayed neutralization of gastric chyme by intestinal fluid seems to increase anthocyanin stability and bioavailability (da Silva et al. 2021). The absorption of anthocyanins in forms that preserve their aglycone moieties is estimated to be approximately 1% of total ingested amount (Zhong et al. 2017). Most anthocyanins will reach the colon where they are deglycosylated, and undergo opening of the C-ring to form phenolic acids and aldehydes (Catalkaya et al. 2020). Cyanidin glucosides are primarily transformed into protocatechuic acid (also known as 3,4-dihydroxybenzoic acid) (Vitaglione et al. 2007). In fact, protocatechuic acid and other hydroxybenzoic acids are also the major microbial metabolites of other anthocyanins (pelargonidin, peonidin, malvidin, delphinidin, and petunidin glycosides) and even of non-anthocyanin flavonoids (Catalkaya et al. 2020; Cortés-Martín et al. 2020). After human intake of cranberry juice, plasma levels of protocatechuic acid have been shown to be higher than its parent PC (McKay et al. 2015). Interestingly, protocatechuic acid has been demonstrated to be the actual responsible for some biological effects of anthocyanins, such as the antiatherogenic effects of dietary cyanidin-3-glucoside in a mice model of atherosclerosis (Wang et al. 2012).
As anthocyanins , most of other dietary flavonoids usually occur as O- or C-glycosides, which will also be deconjugated by gut colonic bacteria (Cortés-Martín et al. 2020). The aglycone moieties released share a common heterocyclic pyrone C-ring that links benzene rings A and B. The C-ring can be opened through demethylation and dihydroxylation reactions generating simpler PC derived from rings A and B with hydroxyl or methyl ester radicals. Flavonols, flavanones, and flavones share some common transformation products. The flavanone hesperidin is converted into its aglycone form, hesperitin, that can be further catabolized into phenylpropionic, phenylacetic, and hippuric acids. Hydroxyphenylpropionic acid has been described as the major fermentation product of naringenin and kaempferol, and the final product of eriodictyol and quercetin (Catalkaya et al. 2020).
Hydrolyzable Tannins
Gut bacteria can hydrolyze the ester bonds of gallo- and ellagitannins (Cortés-Martín et al. 2020). Gallic acid can undergo decarboxylation and dihydroxylation reactions. Urolithins are major metabolites of ellagic acid–related PC (Kawabata, Yoshioka and Terao 2019; Quatrin et al. 2020), being formed upon dihydroxylation and intramolecular condensation. Urolithin A (dihydroxy-urolithin) and urolithin B (hydroxy-urolithin) are produced by most population, while type C and D urolithin production is less frequent (Catalkaya et al. 2020). There is also a group of population that do not produce urolithins, likely due to the absence of some Gordonibacter bacteria (Catalkaya et al. 2020). The distinct profile in the production of urolithins is remarkably affected by aging and allowed the identification of three ellagic acid metabotypes in the population. Metabotypes are described as specific microbial-metabolic phenotypes that result in qualitative differences among individuals (metabolite producers vs. nonproducers) rather than a metabolite production gradient in the population (Cortés-Martín et al. 2020). Metabotype A is characterized by the production of only urolithin A, whereas metabotype B produces urolithin B and isourolithin A in addition to urolithin A, and metabotype 0 are those individuals that do not produce urolithins (Cortés-Martín et al. 2020). Despite the variability in the metabolic profile between individuals, urinary urolithin A, along with the phase II metabolite di-methyl ellagic acid, have been recently used as biomarkers of ellagitannin intake from black raspberry food products (Roberts et al. 2020).
Flavan-3-Ols
Different from other flavonoids, flavan-3-ols , which is the major class of native flavonoids in human diet, usually do not occur as glycosides but may occur as monomeric or polymeric compounds (Zamora-Ros et al. 2013, 2016). Studies in ileostomized humans indicate that more than 70% of flavan-3-ols will reach the colon after the intake of green tea (Stalmach et al. 2010).
Polymeric flavan-3-ols, such as proanthocyanidins, which are also known as condensed tannins, are first converted into their monomeric units (catechins). Subsequent microbial catabolism converts catechins into hydroxyphenyl-γ-valerolactones, which are thereafter sequentially converted into the following phenolic acids: hydroxyphenylvaleric, hydroxyphenylpropionic, hydroxyphenylacetic, hydroxybenzoic, and hippuric acids (Appeldoorn et al. 2009). Although many catabolic routes involved in these transformations remain to be elucidated, it is clear that the major gut metabolites of flavan-3-ols are hydroxyphenyl-γ-valerolactones and, to a lesser extent, their derived hydroxyphenyl valeric acids (Mena et al. 2019). These compounds could be used as potential biomarkers of flavan-3-ols intake because they have high absorption rate and reach relevant circulating concentrations (Ottaviani et al. 2018, 2019; Mena et al. 2019). Nevertheless, methodological difficulties in the accurate chromatographic quantification of polymeric flavan-3-ols in food matrices poses a hindrance to establish the relationship between flavan-3-ols intake and the biological levels of their metabolites (Mena et al. 2019). In addition, the lag time between the intake of flavan-3-ols and the assessment of hydroxyphenyl-γ-valerolactones or their derived hydroxyphenyl valeric acids should also be considered. These flavan-3-ol metabolites are intermediates that will be further catabolized into other phenolic acids, which can be also generated from the catabolism of many other PC (Cortés-Martín et al. 2020).
All these microbial-derived flavan-3-ol metabolites can be absorbed and metabolized by phase II enzymes producing conjugated derivatives that are subsequently eliminated in the urine (Mena et al. 2019). The lack of commercial analytical standards of microbial-derived flavan-3-ols metabolites, especially for the conjugated compounds, limits their assessment in biological fluids to tentative identification and quantification.
Isoflavones
Isoflavones such as daidzein is metabolized by gut microbiota generating equol and O-desmethylangolensin (ODMA), whereas genistein generates 5-hydroxy-equol and 6′-hydroxy-ODMA (Cortés-Martín et al. 2020). Two metabotypes have been already identified for daidzein, the equol- and ODMA-producer individuals (Mayo, Vázquez and Flórez 2019). These metabotypes are independent from each other and their population distribution has been shown to be associated to dietary patterns, sociodemographic characteristics, race, and ethnicity (Cortés-Martín et al. 2020).
Phenolic Acids
Most phenolic acids escape absorption in the small intestine and will reach the colon intact. Simple hydroxybenzoic acids, such as gallic acid, ellagic acid, protocatechuic acid, and 4-hydroxybenzoic acids, are usually found at low concentrations in foods, but they may appear as constituents of complex PC, such as the hydrolysable tannins or as metabolites of hydrolysable tannins and flavonoids (Cortés-Martín et al. 2020). Their metabolic fate during digestion has been already discussed in previous sections.
Hydroxycinnamic acids, such as p-coumaric acid, caffeic acid, ferulic acid, and sinapic acid, are commonly found in foods in the free form or esterifying sugars and organic acids (quinic, tartaric, and malic). After hydrolysis by bacterial esterases, hydroxycinnamic acids are released and metabolized to phenylpropionic acids and subsequently to hydroxybenzoic acids (Cortés-Martín et al. 2020).
Hippuric acid, which can be formed from the conversion of the quinic acid moiety of PC by gut microbiota but also from the hepatic metabolism of benzoic acids, is the most common and abundant urinary and plasma metabolite detected after the intake of various foods rich in PC (Catalkaya et al. 2020). Although hippuric acid is also formed during the metabolism of aromatic amino acids, its urinary levels have been shown to be consistently increased after the intake of tea (Clifford et al. 2000; Daykin et al. 2005), cocoa (Rios et al. 2003), vegetables, and fruits (Krupp et al. 2012). Thus, urinary levels of hippuric acid, along with 3,4-dihydroxyphenylacetic acid and simple hydroxycinnamic acids, have been proposed as candidate biomarkers for the intake of a PC-rich diet in a cross-sectional study of free-living individuals (Yang et al. 2019).
Lignans
Only 7% of the ingested lignans have been shown to be absorbed in the small intestine after the intake of a cereal source containing secoisolaricinol, matairesinol, lariciresinol, pinoresinol, syringaresinol, and medioresinol by catheterized pigs that were used as a model for humans (Bolvig et al. 2016). Colonic fermentation plays a key role in the bioavailability and bioactivity of lignans that are catabolized by gut microbiota into enterodiol and enterolactone, also known as enterolignans. In fact, about 40% of the ingested cereal lignans were excreted as enterolignans in the urine (Bolvig et al. 2016). Diverse bacterial species are involved in the complex metabolic pathway that starts with the deglycosylation of lignans and is followed by reduction, demethylation, dihydroxylation, and lactonization reactions to generate the final metabolites, enterolignans (Cortés-Martín et al. 2020).
Kinetics of PC After Dietary Intake
Absorption
Dietary PC are usually chemical structures of high complexity with ester bonds that may appear as polymerized and glycosylated forms, which prevent their direct absorption. The absorption of some PC seems to start in the oral cavity after the aglycone release by the action of glycosidases from buccal microbiota (Hussain et al. 2019). Anthocyanins have been detected in the plasma 5 min after the contact with oral tissues (Kamonpatana et al. 2014). Around 83% of the total amount of anthocyanins and 46% of non-anthocyanin PC from jaboticaba peel (Myrciaria trunciflora ) powder were solubilized during the gastric phase of digestion (Quatrin et al. 2020). Phenolic acids and anthocyanins can be absorbed in their free forms in the stomach, via passive diffusion or monocarboxylic acid transporter (MCT)-mediated transport system and bilitranslocase proteins (Bohn 2014). However, most PC undergo hydrolysis and deconjugation reactions in the gastric compartment or remain intact until reaching the intestine (Williamson and Clifford 2017).
The small intestine and colon are considered the main sites for PC absorption (Matuschek et al. 2006). Both the chemical structure of the phenolic moiety and any attached chemical groups define whether the PC is absorbed in the small intestine or colon (Williamson and Clifford 2017). In the small intestine, PCs are subjected to the action of digestive enzymes such as cytosolic β-glucosidase (CBG), lactase-phlorizin hydrolase (LPH), and esterases, which promote the cleavage and release of aglycones from the remaining food matrix (Bohn 2014). Phenolic acids and aglycones of low molecular weight are absorbed by the enterocytes through passive diffusion. Conversely, compounds of high molecular weight and complex chemical structure use cotransporters mediated by sodium-glucose-linked transporters 1 (SGLT1) and MCT (Hussain et al. 2019). However, the physicochemical characteristics of the intestine can contribute for the decomposition of anthocyanins, even before their absorption takes place (Grgić et al. 2020). For instance, only 10% of anthocyanins from jaboticaba peel were recovered after the intestinal phase of digestion, while recovery indexes between 52% and 134% were found for non-anthocyanin compounds (Quatrin et al. 2020). The intestinal pH around 7 favors the transformation of anthocyanins into a chalcone pseudo-basis that can be cleaved at the C-ring and originates phenolic acids (Bohn 2014). Possibly, this fact contributes to the low bioavailability of anthocyanins in plasma. Furthermore, intestinal microbiota also plays a prominent role in the colonic absorption of PC. Anthocyanins and other polymeric PC are subject to the action of microbial esterases that are not produced by mammalian cell. Such esterases convert polymeric PC into simple phenolic acids and phenolic metabolites, capable of being absorbed (Williamson and Clifford 2017).
Distribution
Most data on the tissue distribution of PC come from animal studies. The distribution of PC and their metabolites depends on the dose administered, solubility, need for tissue-specific carriers, and tissue functionality (Carecho et al. 2020). Once absorbed, the PC and their metabolites depart from the small intestine to the liver, via the portal circulation, to undergo a series of phase I and II biotransformation reactions and are distributed into the bloodstream mostly bound to proteins, especially albumin (Cao et al. 2019). Due to their low absorption, only a small portion of PC and their metabolites are deposited in tissues (Bohn 2014), mainly in those tissues where they have been metabolized. However, they can also reach specific target tissues such as pancreas, brain, heart, and spleen (Velderrain-Rodríguez et al. 2014).
Anthocyanins appear to be more abundant in gastrointestinal tissues such as the stomach, jejunum, ileum, and colon in rats (Kalt 2019). Even so, anthocyanin content was greatest in the kidneys, liver, heart, lungs, and brain of rodents (Sandoval-Ramírez et al. 2018). Curiously, the treatment time seems to influence in the bioavailability profiles of anthocyanins in animal tissues. Anthocyanin metabolites are predominant in short-term experiments, while the parent anthocyanin in long-term experiments (Sandoval-Ramírez et al. 2018). However, this does not seem like a definitive rule. A study where rats consumed a single dose of 400 mg of the bilberry extract Bilberon/kg of weight body demonstrated major amounts of parent anthocyanins in tissues and fluids (e.g., urine, feces, and bile) when compared to anthocyanin metabolites (Ichiyanagi et al. 2006).
Liver tissue was the second largest site of deposition of phase II metabolites of catechin and epicatechin and the first for naringenin-glucuronides and sulfates or even products of hydroxylation/methylation of anthocyanins (Margalef et al. 2015; Lin et al. 2014; Fornasaro et al. 2016). These data are expected since the main flavonoid metabolism occurs in this tissue (Manach et al. 2004). The incorporation of hydrophilic conjugates allows these compounds to be mainly eliminated in the urine. Thus, it is not surprising that phase II metabolites of catechin and quercetin were found at the highest amount in the kidneys (Margalef et al. 2015; Yang et al. 2016).
The distribution to the brain is very small, as not all PC or metabolites can cross the blood-brain barrier (BBB) (Margalef et al. 2015). Factors such as chemical structure, polarity, metabolism by phase I and phase II enzymes, intestinal microbiota, and pathways used to access the brain can affect the permeability of the BBB (Carecho, Carregosa and dos Santos, 2020). Nevertheless, animal studies revealed that intact forms of anthocyanins (cyanidin-3-glucoside) (Fornasaro et al. 2016) and metabolites such as naringenin-sulfates, quercetin-3-O-β-glucuronide, or methylated derivatives of catechin and epicatechin (Lin et al. 2014; Yang et al. 2016; Margalef et al. 2015) were found in brain tissue or cerebrospinal fluid. Moreover, dietary PC have recently been found in the cerebrospinal fluid of patients with neurological disorders, showing that dietary compounds can also cross the BBB in humans (Grabska-Kobylecka et al. 2020).
Metabolization
Following absorption of aglycones and the formation of their conjugated derivatives via phase II reactions in the small intestine, PC metabolites rapidly reach the liver by transferring via portal blood (Donovan et al. 2006). In the liver, both conjugated and unmetabolized aglycones can undergo phase I and phase II metabolism (Velderrain-Rodríguez et al. 2014), with additional conversions and enterohepatic recirculation which may result in the re-excretion of compounds into gut lumen through biliary route (Crozier et al. 2010; Donovan et al. 2006).
Concerning hepatic phase I metabolism, flavonoids may be biotransformed via oxidation or O-demethylation reactions by cytochrome P450 monooxygenases. However, phase I metabolism contributes to minor metabolites of most flavonoids and other PC, as these compounds undergo conjugation reactions faster than oxidation (Manach et al. 2004).
Intestinal and hepatic phase II reactions of PC consist in the conjugation of methyl, sulfate, and/or glucuronic acid moieties to the hydroxyl groups of PC. These structural modifications detoxify and facilitates the biliary and urinary elimination of PC metabolites (Manach et al. 2004). However, this biotransformation decreases the bioefficacy of PC in relation to their parent aglycones (Scalbert and Williamson 2000).
Catechol-O-methyl transferase activity produces O-methylated metabolites by transferring a methyl group from S-adenosyl-L-methionine to the 3′ position (or to a lesser extent to the 4′ position) of PC having a catechol moiety. 3-O-methylated derivatives have been reported for quercetin, catechin, caffeic acid, luteolin, and cyanidin (Manach et al. 2004). A substantial amount of a 4’-O-methylated product was reported for 4′-methylepigallocatechin in human plasma following ingestion of tea (Lee et al. 2002; Meng et al. 2001).
Sulphotransferases generate O-sulfates mainly in the liver by catalyzing the transfer of a sulfate moiety from 3′-phosphoadenosine-5′-phosphosulfate to a hydroxyl group on PC, which can contain multiple possible conjugation site depending on the compound and enzyme isoform. Phenolic acids such as caffeic and dihydrocaffeic acids are both sulfated in 3-OH and 4-OH whereas the flavonoids daidzein and genistein are sulfated in 7-OH and 4’-OH and resveratrol in 3 and 4’positions (Wu et al. 2011).
UDP-glucuronosyl transferases localized in the endoplasmic reticulum is highly expressed in intestine, liver, and kidney and produce O-glucuronides by catalyzing the transfer of a glucuronic acid from UDP-glucuronic acid to PC. Interestingly, glucuronidation of PC first occurs in the enterocytes before further conjugation in the liver (Crespy et al. 2001; Manach et al. 2004).
In general, the profile of phase II metabolites of PC is complex and the preference for the type of conjugation reaction may vary even among compounds within the same chemical class (Velderrain-Rodríguez et al. 2014). For hydroxycinnamic acids, it was shown that methylation, glucuronidation, and sulfation were the preferential pathways for caffeic acid metabolism, whereas ferulic acid was only glucuronidated and chlorogenic acid showed null metabolism in HepG2 cells (Mateos et al. 2006). The ingested dose must also be considered, since a shift from sulfation toward glucuronidation occurs when the ingested dose of PC increases (Manach et al. 2004). Conversely, quercetin metabolism in HepG2 cells shifts from glucuronidation toward sulfation when methylation reaction is inhibited (O’Leary et al. 2003). Several other factors also influence on the balance between sulfation and glucuronidation, such as stereochemistry, polymorphism of phase II enzymes, metabolism site, species, sex, and food deprivation (Velderrain-Rodríguez et al. 2014). Saturation of the conjugation reactions can also occur, as observed in rats exposed to high doses and rats given an acute supply of PC by gavage (Piskula and Terao 1998).
Regardless of the type of phase II reactions, the production of conjugated metabolites from PC is a highly efficient process so that absorbed PC circulate mainly on the conjugated forms. Accordingly, aglycones are either not found in blood or are presented in low concentrations following consumption of nutritional doses (Velderrain-Rodríguez et al. 2014) except for anthocyanins and tea catechins (Lee et al. 2002). Unfortunately, circulating conjugated metabolites have been identified for only a few PC. 3-O-glucuronide, 3’-O-methylquercetin 3-O-glucuronide, and quercetin 3’-O-sulfate were the major conjugated metabolites from quercetin in human plasma following consumption of onions containing quercetin glucosides (Day et al. 2001), even though the presence of sulfated quercetin was not further corroborated (Wittig et al. 2001).
Excretion
Unmetabolized PC, their phase II metabolites, and bacterial derivatives are excreted through urine and feces. Most phase II metabolites undergo first-pass metabolism and are transferred to the large intestine where they will be subjected to colonic microbiota metabolism (Velderrain-Rodríguez et al. 2014). For large, extensively conjugated metabolites, the biliary route is preferred. On the other hand, urinary excretion is the mainly excretion pathway for phase II metabolites (especially small conjugates), since conjugation reactions convert PC into more hydrophilic compounds (Manach et al. 2004).
Methods Used for Estimation of Dietary Intake of PC
It is difficult to quantitatively measure the benefits of consuming PC due to many variables: (1) a great diversity of PC in the diet; (2) limited data on the PC composition and content in the food databases; (3) limited understanding about the absorption and metabolism of PC; and (4) limitations in the methods for analyzing PC metabolites in biological fluids (Spencer et al. 2008; Zamora-Ros et al. 2012; Pinto and Santos 2017).
Therefore, the study of biological markers of exposure is a tool that has been used in many studies with PC (Manach et al. 2005; Mennen et al. 2006; Pérez-Jiménez et al. 2010; Clarke et al. 2021). Thus, we could have an index of consumption of PC in body fluids that could estimate the exposure of the individual food. However, this relationship between food intake and the concentration of biomarkers in body fluids is extremely complex. In this topic, we will approach the main methods used to estimate the food consumption of PC.
Dietary Assessment Methods
In nutritional studies, the intake of nutrient and non-nutrient food components is generally performed using diet assessment methods, such as diet histories, food frequency questionaries (FFQ), or diet diaries (Zamora-Ros et al. 2012; Burkholder-Cooley et al. 2017; Pinto and Santos 2017). Subsequently, these intake data are transformed into quantitative information using food composition databases. However, several recent studies have shown the difficulty in comparing results of dietary assessment methods among different studies (Pinto and Santos 2017). This occurs due to several factors, such as the difficulties found with the participants’ answers, as they are reflecting the foods they consume without underreported or overreported information. The lack of global food composition databases that comprise full composition of PC is another relevant issue. Food composition databases have scarce data on regional or local foods, on the variations in the content and composition of PC in foods grown in different regions, on the differences in the food processing and storage (Zamora-Ros et al. 2012; Burkholder-Cooley et al. 2017; Pinto and Santos 2017). Additionally, the composition and content of matrix-bound PC, which are not soluble in the conventional extractive solvents used for PC analysis, is missing from food composition databases. Finally, there are many variables to be considered when using diet assessment methods, which limits its accuracy to reflect the situation of the population at the study site and its usefulness to express the world situation.
The main methods used to assess dietary data are 24-hour diet recalls and semiquantitative FFQ (Zamora-Ros et al. 2012; Pinto and Santos 2017). The difficulty in comparing the results and the variability of results among different studies are mainly due to differences in the methods used and the use of different databases to calculate the consumption of PC. Therefore, the choice of method for collecting dietary data determines the accuracy of results.
FFQ are very useful and used by studies using a large number of individuals because they are easier and simpler, although they only reflect information on the frequency of consumption of the food in question. If we need information about the portion size, these questionnaires need adaptations and validations before use (Zamora-Ros et al. 2012; Pinto and Santos 2017). In such case, an additional problem may arise: How to standardize this portion measure? Portion sizes can be made by household measurements, which can increase the chance of errors, or they can be reported in units such as grams or even accompanied by portion size photographs. These points must be well explained and standardized in the questionnaire (Pinto and Santos 2017; Clarke et al. 2020, 2021).
The 24-hour diet recall provides more detailed information about all foods and beverages consumed during the day (Burkholder-Cooley et al. 2017). Despite being more accurate than FFQ methods, only a 24-hour recall does not represent the individual’s habitual consumption. Ideally, more than one 24-hour recall should be performed on nonconsecutive days, and these recalls should be held throughout the year, because eating habits can vary a lot during the weekdays and some foods rich in PC compounds are seasonal and suffer great changes in consumption along the year (Pinto and Santos 2017).
Biomarkers
As most studies on PC intake use data from food questionnaires, biomarkers could be used to obtain more accurate measurements of tissue exposure to PC, which consider interindividual variability in the absorption and metabolism of these compounds.
A biomarker should be quantitatively associated with the ingestion of the compound in question, that is, it must reflect differences in its consumption. Accordingly, several studies associate the ingestion of PC with biomarkers in blood and urine (Manach et al. 2005; Spencer et al. 2008; Pérez-Jiménez et al. 2010; Zamora-Ros et al. 2012; Clarke et al. 2021). Given that PC metabolism is fast, urine biomarkers are more frequently used because they have gone through all stages of metabolization. Pharmacokinetic studies revealed that most PC have half-lives between 1 and 12 h in plasma (short-term biomarkers) and between 1 and 5 days in urine (medium-term biomarkers); and long-term intake (weeks or months) markers are still lacking (Zamora-Ros et al. 2012).
Additionally, the chemical form of the PC present in food (glycosylated compounds, e.g.) and the food composition can influence the metabolism and, therefore, the time of appearance and concentration of metabolites in plasma and urine (Spencer et al. 2008).
Plasma
There is limited data regarding the precise half-lives of PC in plasma. Data suggest that these half-lives are about 2 h for anthocyanins and flavanones and 2–3 h for flavanols (Spencer et al. 2008; Zamora-Ros et al. 2012). The rapid excretion of PC is facilitated by the conjugation of aglycones or O-methylated forms to sulphate or glucuronic acid. Therefore, many studies to date have analyzed plasma PC levels 1–6 h post-intake (Manach et al. 2005; Spencer et al. 2008). The main limitation of the use of biomarkers in plasma is the wide variation in the half-life of PC when considering intestinal absorption. For example, the plasma half-life of PC absorbed in the small intestine usually ranged between 1 and 12 h. However, if we consider metabolites from colonic microbiota (e.g., equol, a colonic metabolite of daidzein), the half-life increases to >2 days (Spencer et al. 2008; Zamora-Ros et al. 2012).
According to Manach et al. (2005), the plasma concentration of total metabolites ranges from 0 to 4 μmol/L after the intake of 50 mg of aglycone equivalents. The PC that are most well absorbed in humans are isoflavones and gallic acid, followed by catechins, flavanones, quercetin glucosides and proanthocyanidins (galloylated tea catechins), and anthocyanins. Another important point is that some PC have high affinity for proteins, such as plasma albumin, which increases their elimination time. An example of this are flavonols such as quercetin, which has an elimination time of 11–28 h due to its high affinity for albumin (Spencer et al. 2008).
Thus, studies on plasma biomarkers should ideally be carried out with multiple blood samples over a 24-hour period. However, as blood collection is an invasive method, usually one collection of fasting blood is obtained, which can lead to errors and absence of the biomarker due to the metabolization process (Manach et al. 2005).
Urine
Urine biomarkers have been suggested to be more suitable than plasma to assess PC intake since absorbed metabolites can be rapidly removed from the circulation, both by enteric tissues and by urine excretion (Spencer et al. 2008). However, the time of appearance of metabolites in urine varies widely (Mennen et al. 2008; Pérez-Jiménez et al. 2010). Thus, urine is useful to assess the intake of PC that have short to medium half-lives. Another advantage is that urine collection is a noninvasive method. However, the number of metabolites excreted via urine may not accurately reflect the quantities consumed. The urinary half-life of individual PC metabolites is highly variable and ranges from approximately 1 h to just over 1 day and can be influenced by the type and structure of foods consumed (Spencer et al. 2008; Zamora-Ros et al. 2012). Thus, most research has focused on 24-hour urine samples, whereas few studies used one-spot urine sample (Mennen et al. 2008; Pérez-Jiménez et al. 2010; Pinto and Santos 2017). Researchers should consider that 24-hour urine sampling is viable and applicable for studies with a limited number of subjects. On the other hand, for epidemiological studies involving a large number of people, 24-hour urine sampling can become a problem, and the collection of a one-spot urine sample is more coherent (Pinto and Santos 2017).
In fact, some studies have found good correlations between 24-hour and one-spot urine samples collected during a same day, indicating that a spot sample may be used to assay potential biomarkers of PC intake (Mennen et al. 2008). Additionally, studies found that many metabolites estimated in urine are strongly correlated with the consumption of PC (Mennen et al. 2008; Pérez-Jiménez et al. 2010).
Pérez-Jiménez and collaborators (Pérez-Jiménez et al. 2010) conclude that some metabolites are more promising as biomarkers because they show a great recovery in urine and a good correlation with the ingested dose: daidzein, genistein, formononetin, and glycitein. Another study shows that chlorogenic acid, m-coumaric acid, gallic acid, quercetin, isorhamnetin, kaempferol, hesperitin, naringenin, enterolactone, and enterodiol measured in spot urine samples are potentially useful biomarkers for PC intake (Mennen et al. 2008).
A meta-analysis has shown that daidzein, glycitein, enterolactone, and hydroxytyrosol had both a high recovery yield in urine and a high correlation with the intake of isoflavones, flaxseed, and olive oil, respectively. Other studies suggested that dihydrocaffeic acid-3′-O-sulphate and feruloylglycine were considered suitable urine biomarkers for coffee ingestion; conjugates of the flavanones hesperidin and naringenin for orange juice; (epi)gallocatechin, (epi)catechin, and methyl-(epi) catechin metabolites for flavan-3-ols, whereas free and conjugated forms of urolithins may be used as markers of ellagitannins (Pinto and Santos 2017).
Finally, some factors can influence the accuracy of PC metabolite analyses, such as: the stability of metabolites in biological fluids, sample preparation methods, and, essentially, the analytical method, instrument sensitivity, and availability of standards for identification (Spencer et al. 2008; Pinto and Santos 2017). In conclusion, biomarkers should not be used as the sole measure of dietary PC intake but can be used in combination with dietary assessment methods to provide more complete information about what people are consuming and the bioavailability of bioactive PC forms.
Analytical Techniques Used to Identify and Quantify PC Biomarkers
Concerning metabolomic analysis for the search of biomarkers, two analytical platforms highlight among the wide variety of techniques currently available in the scientific scenario. The analysis of several kinds of metabolites is commonly carried out by nuclear magnetic resonance spectroscopy (NMR) or mass spectrometry analyzers (MS), these last commonly coupled to a separative technique such as liquid chromatography (LC) or gas chromatography (GC) (Scalbert et al. 2014; Gibbons et al. 2015). These analytical methodologies differ in their sensitivity, sample processing requirements, and metabolome coverage. Among these techniques, NMR is a robust and fast detection method for high abundance metabolites characterized by a high reproducibility and the potential for the identification of compounds with identical molecular weights, which could not be uniquely elucidated by other techniques. Indeed, NMR highlighted for the study of unknown compounds structures (Markley et al. 2017). NMR is an unbiased technique based on the detection of protonated compounds in the sample despite their differential physical properties. Moreover, recent advances in the field have been resulted in cryogenically cooled probes, microcoil probes, high-performance radio frequency coils, and high-field-strength superconducting magnets. All these improvements are translated into an enhanced sensitivity of NMR by several folds, which is critical for metabolomics applications (Rolin 2012; Putri et al. 2013). Furthermore, NMR is commonly known by its high interlaboratory reproducibility, which is very appreciated in broad-based analyses of high cohort metabolomic studies (Pan and Raftery 2007). Additionally, NMR via stable isotopic labeling techniques could provide a deep understanding of the mechanisms and dynamics related to various metabolic pathways (Nagana Gowda and Raftery 2015). This technique is also capable of detecting compounds that could not be ionized in MS with little or no sample preparation (Ulaszewska et al. 2019). Moreover, one of the main advantages in metabolomic studies is that NMR is a nondestructive technique, so the biological sample used for the analysis could be conserved and reanalyzed in different batches. This fact is of great importance due to the difficulty of sample collection of several biological fluids for their scarcity and invasive procedures needed. Another positive characteristic is that site-specific NMR imaging approach could be effective in the research of metabolic events in living organs (Fan and Lane 2016), whereas high-resolution magic angle spinning (HRMAS) could be used for the analysis of intact tissues (Beckonert et al. 2010). Therefore, all these aspects make NMR a good choice for being the selected analytical technique in varied multitude metabolomic studied. On the other side, NMR requires higher quantities of biological sample for their analysis and possesses lower sensitivity compared to MS (Wishart 2008).
Nevertheless, despite all the advantages above detailed of NMR, in the case of phytochemical biomarkers, MS coupled to LC or GC is extensively used in metabolomic applications. In this sense, GC-MS is suitable for the analysis of different metabolites found in vegetable or biological samples, such as amino acids, sugars, organic acids, steroids, fatty acids, and volatile metabolites analysis. The major negative side of GC-MS analysis is the hard labor of sample treatment, consisting in a time-consuming derivatization step which could complicate quantitative approaches.
However, for the analysis of PC and their metabolites in plants and biological samples in metabolomic studies, the ideal analytical platform is LC-MS. MS is extremely sensitive, and its coupling with high-performance LC allows the separation and detection of almost 10,000 features and the identification of a wide range of chemical species. It has been reported that this platform could determine between 400 and 1500 substances depending on the metabolomic approach, targeted versus untargeted (Cajka et al. 2017). Compound identification can be made by combining retention times information with m/z along with additional analyses, such as fragmentation patterns from tandem MS, to compare against standards and spectral libraries (Sparkman et al. 2011). Additionally, their different modalities cover a wide range of compounds nature, from more polar compounds analyzed by hydrophilic interaction chromatography to neutral and nonpolar metabolites with reversed-phase chromatography. This reverse mode, using polar mobile phase (acidified water with organic solvents like methanol or acetonitrile) combined with nonpolar stationary phase (such as C-18 columns), is applied for the analysis of PC and their metabolites. Its limitation to fully characterize the complete metabolome resides in more than one single LC-MS methodology is needed. This fact, together with lower reproducibility, laborious sample treatment and certain lack of information for an undoubted spectral assignments and compounds elucidation to a high confidence level, summarizes the major bottleneck for its application in PC biomarkers discovery (Ulaszewska et al. 2019), even though some of these limitations are currently overriding (Cajka et al. 2017).
Conclusion
Current knowledge does not allow to identify a single biomarker of total PC intake. Due to the great structural diversity of PC, various candidate biomarkers are being pursued for the different classes or structure-related compounds within each PC class. The bioavailability of parent PC, which is usually low, has driven biomarker search to PC metabolites which are formed by human digestive enzymes, phase I and II enzymes, and colonic microbial enzymes.
Remarkable advances in analytical tools allowed to increase the sensitivity for the assessment of parent PC and their metabolites in biological samples but the lack of analytical standards remains a bottleneck for the accurate quantification of many PC metabolites. Some useful biomarkers have been identified for the intake of specific PC. Despite the growing interest on microbial-derived PC metabolites, the lack of specificity and the great variability in the gut microbiome/metabolic profile among population groups (Pérez-Jiménez et al. 2010) remains a relevant issue for their use as biomarkers of PC intake.
Applications to Other Diseases or Conditions
In this study, we review some useful biomarkers that have been identified for the intake of specific PC. However, the available literature does not allow pointing a single biomarker of total intake of PC. Except for studies on phytoestrogens, the available literature provides only few reports in which biomarker measurements of PC were applied. Thus, in a near future, the knowledge of biomarkers of PC intake may be useful in the risk prediction for some diseases, such as cancer, diabetes, and cardiovascular diseases.
Mini-Dictionary of Terms
-
Phenolic acids: Class of PC containing one phenolic ring and a carboxylic acid function. The most common compounds of this class are caffeic and ferulic acids.
-
Flavonoids: Class of PC subclassified into flavonols, flavones, flavanones, flavan-3-ols (including proanthocyanidins), isoflavones, and anthocyanins. Most flavonoids exist in plant as glycosides.
-
Tannins: Class of PC with high molecular weight. They are divided into hydrolysable (formed by simple phenols bound to carbohydrate moieties) and condensed (two or more than 200 monomers of flavan-3-ol units condensed) tannins.
-
Stilbenes: Class of PC characterized by two benzene rings connected by a double bond with resveratrol as its most known compound.
-
Lignans: Class of PC with a core scaffold formed by two or more phenylpropanoid units linked by the central carbons of the side chains. The monomers forming lignans are cinnamic acid, cinnamyl alcohol, propenyl benzene, and allyl benzene.
-
Coumarins: Class of PC with fragrant properties consisting of a benzene ring linked to the pyrone ring. This class can be divided into simple and complex coumarins.
-
Curcuminoids: Class of PC found in turmeric (Curcuma longa). Curcumina is the main compound and consists of two similar aromatic rings each with o-methoxy phenolic groups, connected by linear carbon chain.
Key Facts
Key Facts of PC Digestion and Metabolism
-
PC may suffer transformations during digestive processes generating new compounds.
-
Most of dietary PC will not be absorbed up to the small intestine and will reach the colon, where gut microbiota triggers the catabolism of PC.
-
The half-lives of PC usually range from 1 to 12 h in plasma and 1–5 days in urine.
Key Factors of Biomarkers of PC Intake
-
The main methods used to assess dietary data are 24-hour diet recalls and semiquantitative food frequency questionnaires.
-
Food frequency questionnaires are very useful and used by studies using a large number of individuals because they are easier and simpler, although they only reflect information on the frequency of consumption of the food in question.
-
The 24-hour diet recall provides more detailed information about all foods and beverages consumed during the day, although only a 24-hour recall does not represent an individual’s habitual consumption.
-
The chemical form of the PC influences the metabolism and, therefore, the time of appearance and concentration of metabolites in plasma and urine.
Summary Points
-
PC are associated to the protective effect of a high intake of fruits and vegetables against chronic diseases.
-
PC have low bioavailability and suffer catabolism by gut microbiota.
-
Food frequency questionnaires are incomplete to determine the role of PC against diseases.
-
The concentration of PC or their metabolites in body fluids (plasma and urine) may be a valuable tool to determine the actual beneficial role of these compounds.
Abbreviations
- BBB:
-
Blood-brain barrier
- CBG:
-
Cytosolic β-glucosidase
- FFQ:
-
Food frequency questionaries
- GC:
-
Gas chromatography
- HepG2:
-
Hepatocellular carcinoma cell line
- HRMAS:
-
High-resolution magic angle spinning
- LC:
-
Liquid chromatography
- LPH:
-
Lactase-phlorizin hydrolase
- MCT:
-
Monocarboxylic acid transporter
- MS:
-
Mass spectrometry analyzer
- NMR:
-
Nuclear magnetic resonance spectroscopy
- ODMA:
-
O-desmethylangolensin
- PC:
-
Phenolic compounds
- SGLT1:
-
Sodium-glucose-linked transporter 1
- UDP:
-
Uridine diphosphate
References
Albuquerque BR, Heleno SA, MBPP O, et al. Phenolic compounds: current industrial applications, limitations and future challenges. Food Funct. 2021;12:14–29. https://doi.org/10.1039/d0fo02324h.
Andrei V, Copolovici D, Munteanu FD, et al. Detection of biomedically relevant stilbenes from wines by mass spectrometry. Adv Exp Med Biol. 2019;1140:665–84. https://doi.org/10.1007/978-3-030-15950-4_40.
Appeldoorn MM, Vincken JP, Aura AM, et al. Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-γ-valerolactone as the major metabolites. J Agric Food Chem. 2009;57:1084–92. https://doi.org/10.1021/jf803059z.
Augusti PR, Conterato GMM, Denardin CC, et al. Bioactivity, bioavailability, and gut microbiota transformations of dietary phenolic compounds: implications for covid-19. J Nutr Biochem. 2021;97:108787. https://doi.org/10.1016/j.jnutbio.2021.108787.
Beckonert O, Coen M, Keun HC, et al. High-resolution magic-angle-spinning NMR spectroscopy for metabolic profiling of intact tissues. Nat Protoc. 2010;5:1019–32. https://doi.org/10.1038/nprot.2010.45.
Bohn T. Dietary factors affecting polyphenol bioavailability. Nutr Rev. 2014;72:429–52. https://doi.org/10.1111/nure.12114.
Bolvig AK, Adlercreutz H, Theil PK, et al. Absorption of plant lignans from cereals in an experimental pig model. Br J Nutr. 2016;115:1711–20. https://doi.org/10.1017/S0007114516000829.
Burkholder-Cooley NM, Rajaram SS, Haddad EH, et al. Validating polyphenol intake estimates from a food-frequency questionnaire by using repeated 24-h dietary recalls and a unique method-of-triads approach with 2 biomarkers. Am J Clin Nutr. 2017;105:685–94. https://doi.org/10.3945/ajcn.116.137174.
Cajka T, Smilowitz JT, Fiehn O. Validating quantitative untargeted lipidomics across nine liquid chromatography-high-resolution mass spectrometry platforms. Anal Chem. 2017;89:12360–8. https://doi.org/10.1021/acs.analchem.7b03404.
Cao H, Liu X, Ulrih NP, et al. Plasma protein binding of dietary polyphenols to human serum albumin: a high performance affinity chromatography approach. Food Chem. 2019;270:257–63. https://doi.org/10.1016/j.foodchem.2018.07.111.
Carecho R, Carregosa D, Dos Santos CN. Low molecular weight (poly)phenol metabolites across the blood-brain barrier: the underexplored journey. Brain Plast. 2020;6:193–214. https://doi.org/10.3233/bpl-200099.
Cassidy A, Minihane AM. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am J Clin Nutr. 2017;105:10–22. https://doi.org/10.3945/ajcn.116.136051.
Catalkaya G, Venema K, Lucini L, et al. Interaction of dietary polyphenols and gut microbiota: microbial metabolism of polyphenols, influence on the gut microbiota, and implications on host health. Food Front. 2020;1:109–33. https://doi.org/10.1002/FFT2.25.
Clarke ED, Rollo ME, Collins CE, et al. The relationship between dietary polyphenol intakes and urinary polyphenol concentrations in adults prescribed a high vegetable and fruit diet. Nutrients. 2020;12:1–16. https://doi.org/10.3390/nu12113431.
Clarke ED, Collins CE, Rollo ME, et al. The relationship between urinary polyphenol metabolites and dietary polyphenol intakes in young adults. Br J Nutr. 2021;1–10 https://doi.org/10.1017/S0007114521001343.
Clifford MN, Copeland EL, Bloxsidge JP, Mitchell LA. Hippuric acid as a major excretion product associated with black tea consumption. Xenobiotica. 2000;30:317–26. https://doi.org/10.1080/004982500237703.
Cortés-Martín A, Selma MV, Tomás-Barberán FA, et al. Where to look into the puzzle of polyphenols and health? The Postbiotics and gut microbiota associated with human Metabotypes. Mol Nutr Food Res. 2020;64:1–17. https://doi.org/10.1002/mnfr.201900952.
Crespy V, Morand C, Besson C, et al. Comparison of the intestinal absorption of quercetin, phloretin and their glucosides in rats. J Nutr. 2001;131:2109–14. https://doi.org/10.1093/jn/131.8.2109.
Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–43. https://doi.org/10.1039/b802662a.
Crozier A, Del Rio D, Clifford MN. Bioavailability of dietary flavonoids and phenolic compounds. Mol Asp Med. 2010;6:446–67. https://doi.org/10.1016/j.mam.2010.09.007.
Cui Q, Du R, Liu M, Rong L. Lignans and their derivatives from plants as antivirals. Molecules. 2020;25:1–17. https://doi.org/10.3390/molecules25010183.
da Silva DT, Smaniotto FA, Costa IF, et al. Natural deep eutectic solvent (NADES): a strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021;364:130370. https://doi.org/10.1016/j.foodchem.2021.130370.
Day AJ, Mellon F, Barron D, et al. Human metabolism of dietary flavonoids: identification of plasma metabolites of quercetin. Free Radic Res. 2001;35:941–52. https://doi.org/10.1080/10715760100301441.
Daykin CA, Van Duynhoven JPM, Groenewegen A, et al. Nuclear magnetic resonance spectroscopic based studies of the metabolism of black tea polyphenols in humans. J Agric Food Chem. 2005;53:1428–34. https://doi.org/10.1021/jf048439o.
Donovan JL, Manach C, Faulks RM, et al. Absorption and metabolism of dietary secondary metabolites. In: Crozier A, Clifford MN, Ashihara H, editors. Plant secondary metabolites. Occurrence, structure and role in the human diet. Oxford, New York: Blackwell Publishing; 2006. p. 303–51.
Fan TWM, Lane AN. Applications of NMR spectroscopy to systems biochemistry. Prog Nucl Magn Reson Spectrosc. 2016;92–93:18–53. https://doi.org/10.1016/j.pnmrs.2016.01.005.
Fornasaro S, Ziberna L, Gasperotti M, et al. Determination of cyanidin 3-glucoside in rat brain, liver and kidneys by UPLC/MS-MS and its application to a short-term pharmacokinetic study. Sci Rep. 2016;6:1–11. https://doi.org/10.1038/srep228152.
Gibbons H, O’Gorman A, Brennan L. Metabolomics as a tool in nutritional research. Curr Opin Lipidol. 2015;26:30–4. https://doi.org/10.1097/MOL.0000000000000140.
Grabska-Kobylecka I, Kaczmarek-Bak J, Figlus M, et al. The presence of caffeic acid in cerebrospinal fluid: evidence that dietary polyphenols can cross the blood-brain barrier in humans. Nutrients. 2020;12:1–15. https://doi.org/10.3390/nu12051531.
Grgić J, Šelo G, Planinić M, et al. Role of the encapsulation in bioavailability of phenolic compounds. Antioxidant. 2020;9:1–35. https://doi.org/10.3390/antiox9100923.
Hatamipour M, Ramezani M, Tabassi SAS, et al. Demethoxycurcumin: a naturally occurring curcumin analogue for treating non-cancerous diseases. J Cell Physiol. 2019;234:19320–30. https://doi.org/10.1002/jcp.28626.
Hussain MB, Hassan S, Waheed M, et al. Bioavailability and metabolic pathway of phenolic compounds. In: Plant physiological aspects of phenolic compounds. IntechOpen; 2019. p. 1–18. https://doi.org/10.5772/intechopen.84745.
Ichiyanagi T, Shida Y, Rahman MM, et al. Bioavailability and tissue distribution of anthocyanins in bilberry (Vaccinium myrtillus L.) extract in rats. J Agric Food Chem. 2006;54:6578–87. https://doi.org/10.1021/jf0602370.
Kalt W. Anthocyanins and their c6-c3-c6 metabolites in humans and animals. Molecules. 2019;24:1–18. https://doi.org/10.3390/molecules24224024.
Kamonpatana K, Failla ML, Kumar SP, et al. Anthocyanin structure determines susceptibility to microbial degradation and bioavailability to the buccal mucosa. J Agric Food Chem. 2014;62:6903–10. https://doi.org/10.1021/jf405180k.
Kawabata K, Yoshioka Y, Terao J. Role of intestinal microbiota in the bioavailability and physiological functions of dietary polyphenols. Molecules. 2019;24:370. https://doi.org/10.3390/molecules24020370.
Kotha RR, Luthria DL. Curcumin: biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules. 2019;24:2930. https://doi.org/10.3390/molecules24162930.
Krupp D, Doberstein N, Shi L, Remer T. Hippuric acid in 24-hour urine collections is a potential biomarker for fruit and vegetable consumption in healthy children and adolescents. J Nutr. 2012;142:1314–20. https://doi.org/10.3945/jn.112.159319.
Lee MJ, Maliakal P, Chen L, et al. Pharmacokinetics of tea catechins after ingestion of green tea and (_)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomark Prev. 2002;11:1025–32.
Lin SP, Hou YC, Tsai SY, et al. Tissue distribution of naringenin conjugated metabolites following repeated dosing of naringin to rats. Biomedicine. 2014;4:1–6. https://doi.org/10.7603/s40681-014-0016-z.
Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr. 2004;79:727–47. https://doi.org/10.1093/ajcn/79.5.727.
Manach C, Williamson G, Morand C, et al. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230–42. https://doi.org/10.1093/ajcn/81.1.230s.
Margalef M, Pons Z, Bravo FI, et al. Tissue distribution of rat flavanol metabolites at different doses. J Nutr Biochem. 2015;26:987–95. https://doi.org/10.1016/j.jnutbio.2015.04.006.
Markley JL, Brüschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS. The future of NMR-based metabolomics. Curr Opin Biotechnol. 2017;43:34–40. https://doi.org/10.1016/j.copbio.2016.08.001.
Mateos R, Goya L, Bravo L. Uptake and metabolism of hydroxycinnamic acids (chlorogenic, caffeic, and ferulic acids) by HepG2 cells as a model of the human liver. J Agric Food Chem. 2006;54:8724–32. https://doi.org/10.1021/jf061664g.
Matuschek MC, Hendriks WH, McGhie TK, et al. The jejunum is the main site of absorption for anthocyanins in mice. J Nutr Biochem. 2006;17:31–6. https://doi.org/10.1016/j.jnutbio.2005.04.005.
Mayo B, Vázquez L, Flórez AB. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients. 2019;11:2231. https://doi.org/10.3390/nu11092231.
McKay DL, Chen CYO, Zampariello CA, Blumberg JB. Flavonoids and phenolic acids from cranberry juice are bioavailable and bioactive in healthy older adults. Food Chem. 2015;168:233–40. https://doi.org/10.1016/j.foodchem.2014.07.062.
Mena P, Bresciani L, Brindani N, et al. Phenyl-γ-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity. Nat Prod Rep. 2019;36:714–52. https://doi.org/10.1039/c8np00062j.
Meng X, Lee MJ, Li C, et al. Formation and identification of 4’-Omethyl-( ) epigallocatechin in humans. Drug Metab Dispos. 2001;29:789–93.
Mennen LI, Sapinho D, Ito H, et al. Urinary flavonoids and phenolic acids as biomarkers of intake for polyphenol-rich foods. Br J Nutr. 2006;96:191–8. https://doi.org/10.1079/bjn20061808.
Mennen LI, Sapinho D, Ito H, et al. Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects – variability and possible use as biomarkers of polyphenol intake. Eur J Clin Nutr. 2008;62:519–25. https://doi.org/10.1038/sj.ejcn.1602744.
Nagana Gowda GA, Raftery D. Can NMR solve some significant challenges in metabolomics? J Magn Reson. 2015;260:144–60. https://doi.org/10.1016/j.jmr.2015.07.014.
O’Leary KA, Day AJ, Needs PW, et al. Metabolism of quercetin-7- and quercetin-3-glucuronides by an in vitro hepatic model: the role of human beta-glucuronidase, sulfotransferase, catechol-O-methyltransferaseandmulti-resistant protein2 (MRP2) in flavonoid metabolism. Biochem Pharmacol. 2003;65:479–91. https://doi.org/10.1016/s0006-2952(02)01510-1.
Ottaviani J, Fong R, Ottaviani JI, et al. Evaluation at scale of microbiome-derived metabolites as biomarker of flavan-3-ol intake in epidemiological studies. Sci Rep. 2018;8 https://doi.org/10.1038/S41598-018-28333-W.
Ottaviani JI, Fong R, Kimball J, et al. Evaluation of(−)-epicatechin metabolites as recovery biomarker of dietary flavan-3-ol intake. Sci Rep. 2019;9 https://doi.org/10.1038/S41598-019-49702-Z.
Pan Z, Raftery D. Comparing and combining NMR spectroscopy and mass spectrometry in metabolomics. Anal Bioanal Chem. 2007;387:525–7. https://doi.org/10.1007/s00216-006-0687-8.
Pérez-Jiménez J, Hubert J, Hooper L, et al. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. Am J Clin Nutr. 2010;92:801–9. https://doi.org/10.3945/ajcn.2010.29924.
Picó C, Serra F, Rodríguez AM, et al. Biomarkers of nutrition and health: new tools for new approaches. Nutrients. 2019;11:1–30. https://doi.org/10.3390/nu11051092.
Pinto P, Santos CN. Worldwide (poly)phenol intake: assessment methods and identified gaps. Eur J Nutr. 2017;56:1393–408. https://doi.org/10.1007/s00394-016-1354-2.
Piskula MK, Terao J. Accumulation of (_)-epicatechin metabolites in rat plasma after oral administration and distribution of conjugation enzymes in rat tissues. J Nutr. 1998;128:1172–8. https://doi.org/10.1093/jn/128.7.1172.
Putri SP, Yamamoto S, Tsugawa H, et al. Current metabolomics: technological advances. J Biosci Bioeng. 2013;116:9–16. https://doi.org/10.1016/j.jbiosc.2013.01.004.
Quatrin A, Rampelotto C, Pauletto R, et al. Bioaccessibility and catabolism of phenolic compounds from Jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J Funct Foods. 2020;65 https://doi.org/10.1016/j.jff.2019.103714.
Rios LY, Gonthier M-P, Rémésy C, et al. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr. 2003;77:912–8. https://doi.org/10.1093/ajcn/77.4.912.
Roberts K, Grainger E, Thomas-Ahner J, et al. Dose-dependent increases in ELLAGITANNIN metabolites as biomarkers of intake in humans consuming standardized black raspberry food products designed for clinical trials. Mol Nutr Food Res. 2020;64:1900800. https://doi.org/10.1002/MNFR.201900800.
Rolin D. Metabolomics coming of age with its technological diversity, vol. 67. Oxford, UK: Academic Press; 2012.
Sandoval-Ramírez BA, Catalán Ú, Fernández-Castillejo S, et al. Anthocyanin tissue bioavailability in animals: possible implications for human health. A systematic review. J Agric Food Chem. 2018;66:11531–43. https://doi.org/10.1021/acs.jafc.8b04014.
Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. J Nutr. 2000;130:2073S–85S. https://doi.org/10.1093/jn/130.8.2073S.
Scalbert A, Brennan L, Manach C, et al. The food metabolome: a window over dietary exposure. Am J Clin Nutr. 2014;99:1286–308. https://doi.org/10.3945/ajcn.113.076133.
Sensoy I. A review on the food digestion in the digestive tract and the used in vitro models. Curr Res Food Sci. 2021;4:308–19.
Serrano J, Puupponen-Pimiä R, Dauer A, et al. Tannins: current knowledge of food sources, intake, bioavailability and biological effects. Mol Nutr Food Res. 2009;53:S310–29. https://doi.org/10.1002/mnfr.200900039.
Shahidi F, Yeo J. Insoluble-bound phenolics in food. Molecules. 2016;21:1216. https://doi.org/10.3390/molecules21091216.
Shinn LM, Li Y, Mansharamani A, et al. Fecal bacteria as biomarkers for predicting food intake in healthy adults. J Nutr. 2021;151:423–33. https://doi.org/10.1093/jn/nxaa285.
Sparkman OD, Penton Z, Kitson F. Gas chromatography and mass spectrometry: a practical guide. 2nd ed. Oxford, UK: Academic Press Elsevier; 2011.
Spencer JPE, Abd El Mohsen MM, Minihane A-M, Mathers JC. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br J Nutr. 2008;99:12–22. https://doi.org/10.1017/S0007114507798938.
Stalmach A, Mullen W, Steiling H, et al. Absorption, metabolism, and excretion of green tea flavan-3-ols in humans with an ileostomy. Mol Nutr Food Res. 2010;54:323–34. https://doi.org/10.1002/mnfr.200900194.
Stringlis IA, De Jonge R, Pieterse CMJ. The age of Coumarins in plant-microbe interactions. Plant Cell Physiol. 2019;60:1405–19. https://doi.org/10.1093/pcp/pcz076.
Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–46. https://doi.org/10.3390/nu2121231.
Ulaszewska MM, Weinert CH, Trimigno A, et al. Nutrimetabolomics: an integrative action for metabolomic analyses in human nutritional studies. Mol Nutr Food Res. 2019;63:1–86. https://doi.org/10.1002/mnfr.201800384.
Velderrain-Rodríguez GR, Palafox-Carlos H, Wall-Medrano A, et al. Phenolic compounds: their journey after intake. Food Funct. 2014;5:189–97. https://doi.org/10.1039/c3fo60361j.
Vitaglione P, Donnarumma G, Napolitano A, et al. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J Nutr. 2007;137:2043–8. https://doi.org/10.1093/jn/137.9.2043.
Wang D, Xia M, Yan X, et al. Gut microbiota metabolism of anthocyanin promotes reverse cholesterol transport in mice via repressing miRNA-10b. Circ Res. 2012;111:967–81. https://doi.org/10.1161/CIRCRESAHA.112.266502.
Williamson G, Clifford MN. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem Pharmacol. 2017;139:24–39. https://doi.org/10.1016/j.bcp.2017.03.012.
Wishart DS. Metabolomics: applications to food science and nutrition research. Trends Food Sci Technol. 2008;19:482–93. https://doi.org/10.1016/j.tifs.2008.03.003.
Wittig J, Herderich M, Graefe EU, et al. Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography– tandem mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;753:237–43. https://doi.org/10.1016/s0378-4347(00)00549-1.
Wu B, Basu S, Meng S, et al. Regioselective sulfation and glucuronidation of phenolics: insights into the structural basis. Curr Drug Metab. 2011;12:900–16. https://doi.org/10.2174/138920011797470100.
Yang L, Xiao N, Li XW, et al. Pharmacokinetic comparison between quercetin and quercetin 3-O-β-glucuronide in rats by UHPLC-MS/MS. Sci Rep. 2016;6:1–9. https://doi.org/10.1038/srep35460.
Yang R, Xiu H, Zhou Q, et al. Application of urinary polyphenol biomarkers measured by liquid chromatography tandem mass spectrometry to assess polyphenol intake and their association with overweight and obesity in free-living healthy subjects. Oxidative Med Cell Longev. 2019;2019 https://doi.org/10.1155/2019/4809836.
Zamora-Ros R, Rabassa M, Llorach R, et al. Application of dietary phenolic biomarkers in epidemiology: past, present, and future. J Agric Food Chem. 2012;60:6648–57. https://doi.org/10.1021/jf204742e.
Zamora-Ros R, Knase V, Luján-Barroso L, et al. Differences in dietary intakes, food sources and determinants of total flavonoids between Mediterranean and non-Mediterranean countries participating in the European prospective investigation into cancer and nutrition (EPIC) study. Br J Nutr. 2013;109:1498–507. https://doi.org/10.1017/S0007114512003273.
Zamora-Ros R, Knase V, Rothwell JA, et al. Dietary polyphenol intake in Europe: the European prospective investigation into cancer and nutrition (EPIC) study. Eur J Nutr. 2016;55:1359–75. https://doi.org/10.1007/S00394-015-0950-X.
Zhong S, Sandhu A, Edirisinghe I, Burton-Freeman B. Characterization of wild blueberry polyphenols bioavailability and kinetic profile in plasma over 24-h period in human subjects. Mol Nutr Food Res. 2017;61:1700405. https://doi.org/10.1002/MNFR.201700405.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Nature Switzerland AG
About this entry
Cite this entry
Augusti, P.R. et al. (2022). Biological Markers of Plant Phenolic Compounds Intake. In: Patel, V.B., Preedy, V.R. (eds) Biomarkers in Nutrition . Biomarkers in Disease: Methods, Discoveries and Applications. Springer, Cham. https://doi.org/10.1007/978-3-031-07389-2_60
Download citation
DOI: https://doi.org/10.1007/978-3-031-07389-2_60
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07388-5
Online ISBN: 978-3-031-07389-2
eBook Packages: Biomedical and Life SciencesReference Module Biomedical and Life Sciences