Abstract
Plants are constantly exposed to various kinds of biotic and abiotic stresses, with cold stress being common. Cold- or low-temperature stress is divided into chilling and freezing. The degree and duration of the low temperature are key factors that affect the degree of damage to the plant. The responses of plants to low-temperature stress include changes in plant morphology, physiology, and biochemistry, as well as growth and developmental characteristics. Cold stress destroys the plant membrane system and reduces photosynthesis and general respiration. Applications of the appropriate selenium and nano-selenium concentrations alleviate the adverse changes in plant photosynthetic parameters under chilling stress and increase both antioxidant enzyme activity, and the resulting antioxidant substance, levels. These applications also promote the metabolism of photosynthetic system-associated proteins. Exogenous selenium enhances ascorbate–glutathione cycle-related enzyme activities that regulate plant metabolism under chilling-stress conditions. Selenium enhances the tolerance of crops to low-temperature stress by regulating the expression of sulfur-related genes. Applications of selenium induce proteins involved in photosynthesis and ATP synthesis, which propel energy-driven metabolic processes toward plant growth, development, and stress acclimation, resulting in a significant increase in the cold-stress tolerance of plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
9.1.1 Low-Temperature Stress
Low temperature is an abiotic environmental stress often encountered in plant growth that leads to plant metabolic disorders. It not only reduces plant yields, but it can also cause plant death in serious cases. Based on the different degrees of plant damage, low-temperature stress can be divided into two categories: chilling stress when the environmental temperature is above 0 °C and freezing stress when the environmental temperature is less than 0 °C (Yamamoto et al. 2012). For crops of tropical and subtropical origin, such as rice, corn, and soybean, a temperature less than 10 °C or 12 °C will cause low-temperature damage. Additionally, temperatures of less than 15 °C and 23 °C stop vegetative growth and pollen abortion, respectively, resulting in grain-filling failure and yield reduction or extinction (Keshavkant and Naithani 2001; Song et al. 2012). Even for winter crops of temperate and northern subtropical regions, such as barley, wheat, grape, and strawberry, chilling stress has adverse effects, especially at low temperatures between 0 °C and 5 °C or less than 0 °C in the spring season, sometimes causing serious losses (Huang et al. 2016). Therefore, low-temperature stress is a concerning environmental factor in agricultural production.
The degree of plant damage resulting from a low temperature is affected by two factors. The degree of the low temperature (how cold the low temperature becomes) and the duration (how long the plant is exposed to the low temperature). The lower the temperature and the longer the duration, the greater the plant damage.
9.1.2 Selenium and Nano-Selenium
Selenium is located in the VIA group of the fourth cycle of the periodic table of chemical elements. It is in the same group as oxygen and sulfur, and its physical and chemical properties are similar to those of sulfur. It is an essential nutrient element for humans and animals, and it is a beneficial nutrient element for plants (Ellis and Salt 2003). Selenium can be divided into its physical and chemical forms in accordance with its nature and state. The physical form includes ordinary and nano-selenium. The chemical form is divided into inorganic and organic selenium. In nature, selenium exists in the inorganic chemical form and as plant-active selenium. Inorganic selenium refers to sodium selenite and sodium selenate; plant-active selenium is formed by combining selenium with amino acids through biological transformation. It mainly exists in the form of selenomethionine. An appropriate concentration of selenium plays an important role in plant growth, the antioxidant system, and the photosynthetic system, as well as in producing osmotic adjustment substances (Gupta and Gupta 2017).
Under low-temperature stress conditions, plant growth status and physiological and biochemical indicators are affected, as is photosynthetic respiration. In addition, cell structure damage occurs and antioxidant system activity is reduced. Appropriate amounts of selenium and nano-selenium improve plant performances under low-temperature stress conditions. The chlorophyll and proline contents increase, the malondialdehyde (MDA) content decreases, and the level of active oxygen is adjusted, thereby alleviating the damage to plants caused by low-temperature exposure (Zhang et al. 2013).
9.2 The Responses of Plants to Low-Temperature Stress
9.2.1 Impacts on Plant Morphology and Growth
9.2.1.1 Changes in Plant Morphology
Under low-temperature stress conditions, the most intuitive effect is the changes in plant biological characteristics. The external morphology of plant bodies revealed that the tissues are soft, part or all of the leaves are water-stained, and the leaf or fruit surfaces appear brown, yellow, spotted, and chlorotic. In extreme cases, withering or shedding and death occur (Gao et al. 2011; Tan et al. 2012).
9.2.1.2 Changes in Plant Growth and Development
Low-temperature stress causes adverse effects during the entire growth and development process of plants, including seed germination, seedling and plant growth, and flowering and fruiting, as well as quality-related trait formation. The degree of chilling damage to the plant body depends not only on the degree of the low temperature, but also on the length of the low-temperature exposure. The colder the low temperature and the longer the exposure, the lower the viability of cell protoplasm and the greater the inhibition of plant growth. If the low temperature lasts too long or the temperature is too cold, then it will delay plant growth and development, which will cause a decline in the yield, and may even lead to plant death in severe cases. However, rice and tomato crops undergo low-temperature acclimation after 48- or 72-h chilling treatments, which leads to increases in chilling tolerance (Kato-Noguchi 2007; Zhou et al. 2012).
9.2.2 Impacts on Plant Membrane System
The plant membrane system includes the cell membrane, cell nuclear membrane, chloroplast, and mitochondrial membrane. They work closely together during plant growth and development, cooperate with each other, and undertake various physiological functions. In addition, they complete necessary physiological and morphological processes, such as photosynthesis, respiration, cell division, flowering, and fruiting. The membrane system is the plant component most vulnerable to low temperature- and drought-related injury (Duan et al. 2012; Huang et al. 2020).
The cell membrane system is the first plant part damaged by chilling and (or) freezing stress. Low temperatures cause the breakdown of thylakoid structures in plant cells, reduce the selectivity of plant membranes and cause the intracellular solutions to extravasate. Under strong freezing-stress conditions, extracellular and intracellular freezing occurs. The intracellular water flows out through the plasma membrane, and the ice crystals formed outside the cell pierce the cell wall or cell membrane, which causes cell rupture, leading to severe cellular dehydration and to structural and functional membrane damage (Gurme et al. 2021).
Impacts on membrane components: Exposure to low-temperature conditions alters membrane lipid components. To improve cold resistance, the contents and proportions of unsaturated fatty acids must be increased. When plants are under low-temperature conditions, the numbers of carbon atoms and carbon–carbon double bonds in the lipid membrane will change, which is conducive to directly controlling the membrane’s structure and function to adapt to the low-temperature environment. As the temperature decreases, fatty acid desaturases are activated, the fatty acid composition changes greatly, and more unsaturated fatty acids are metabolized to produce more unsaturated fatty acids. Unsaturated fatty acids bind to the cell surface, preventing the permeation of water molecules and regulating the phase-transition temperature of the plasma membrane. The increase in their content increases the plant’s ability to resist chilling and freezing damage (Erdal 2012).
Impacts on the membrane lipid phase: When plants encounter a low-temperature stress, a series of physiological, biochemical, and physical changes occur in the cell, which reduce the fluidity of the membrane, and the membrane lipid undergoes a phase change, changing from the original disordered state to an ordered state, and from the liquid crystal state to the gel state. Some areas in the membrane lipid bilayer enter a solid phase, separate from the membrane lipid. Thus, the structure of the membrane lipid is changed, which destroys the structural and functional unity (Sun et al. 2011). As the unsaturated fatty acid content in the lipid component of the cell membrane increases, which increases the membrane lipid unsaturation, the morphology of the protoplasts also changes accordingly to maintain the active liquid crystal state and maintain the normal life activities of the plant (Hassanein et al. 2021).
Impacts on the protective enzyme systems: Owing to low-temperature stress, the active oxygen metabolic balance in the cell is destroyed, resulting in the production of reactive oxygen species (ROS), including active oxygen and superoxide free radicals, and the reduction of antioxidant enzyme activity levels (Huang et al. 2016, 2018). The accumulation of ROS under low-temperature stress induces membrane lipid peroxidation in the plant cell membrane system, destroys the membrane structure, and causes the substances in the membrane to leak out, thereby inflicting low-temperature damage (Li et al. 2013). The penetration of the cell membrane causes a large amount of external calcium to enter the cytoplasmic matrix. The combination of calcium ions and calmodulin activates phospholipase A2, resulting in the release of unsaturated fatty acids, which are under the action of lipoxygenase. Consequently, unsaturated fatty acids form hydroxyperoxides, which can spontaneously decompose MDA. Its presence causes membrane damage and leakage, which allows more calcium ions to enter the cell, and this eventually results in phospholipid degradation. Then, the cell membrane collapses (Ruelland and Collin 2012).
9.2.3 Impacts on Photosynthesis and Respiration
9.2.3.1 Impacts on Photosynthesis
Low-temperature stress leads to a decline in the photosynthetic intensity of a plant. It has significant impacts on a series of important physiological and biochemical parameters, such as plant photosynthetic pigment content and chloroplast submicroscopic structure, and processes, such as photosynthetic energy metabolism and photosynthesis system II (PSII) activity (Zhou et al. 2012).
Under low-temperature stress conditions, the chlorophyll fluorescence and photosynthetic quantum efficiency are rapidly inhibited, and the inhibition increases with a greater degree of low-temperature stress. Analyses of weakening chloroplast fluorescence under low-temperature stress revealed that the site of low-temperature damage may be PSII in the transmission chain. Under low-temperature stress conditions, the abilities of plants to quench excitation energy are inhibited. A low-light intensity also causes an excess of excitation energy, which affects the transmission of light energy and causes peroxidation, resulting in an impaired PSII activity level (Zhou et al. 2012; Huang et al. 2016).
Low temperatures lead to plant photoinhibition and even photooxidation, which may result from the relatively high plant chloroplast-encoded D1 protein synthesis rate under normal temperature conditions, whereas under low-temperature stress, the chloroplast-encoded D1 protein synthesis rate is relatively low. Low temperatures inhibit PS repair by inhibiting protein degrading enzyme activity levels and DI protein synthesis, leading to increased photoinhibition (Huang et al. 2016). Chilling damage inhibits chlorophyll synthesis, resulting in the destruction of chloroplast structures, a reduced photosynthesis rate, and abnormal respiration.
Chen (1998) reported that the photosynthetic rate of bananas decreases rapidly under low-temperature stress. The photosynthetic rates are 27%, 16.2%, and 13.4% of the control at 2, 5, and 8 d, respectively, after a 10 °C low-temperature treatment compared with control 25 °C normal conditions. In mulberry, low-temperature treatments (10–12 °C, 3 d) significantly decrease the net photosynthetic rate by 22.1% (Liu et al. 2019). Low-temperature treatments significantly reduce the net photosynthetic rate of Wenzhou mandarin orange leaves at a saturated CO2 concentration, implying that the ribulose 1,5-diphosphate carboxylase regeneration rate in the leaves is affected, and the decrease in the initial slope of the CO2 concentration curve and net photosynthetic rate indicate a decrease in ribulose 1,5-diphosphate carboxylase activity. The chlorophyll fluorescence parameter Fv/Fm indicates the original light energy conversion efficiency of PSII. Under low-temperature stress, the Fv/Fm of Wenzhou mandarin orange significantly decreases (Guo et al. 1998).
9.2.3.2 Impacts on Respiration
Under low-temperature stress conditions, within a certain temperature range, plant respiration decreases along with the temperature. The respiration increases during the initial stage of low-temperature stress and then decreases. Additionally, the mitochondrial structures are damaged to varying degrees (Hu et al. 2006).
At a low temperature of 4 ± 1 °C, the breath intensity of rubber continues to decrease as the low-temperature treatment time increases (Li et al. 1994)., Microscopic observations of corn mitochondria under low-temperature stress conditions have revealed that the inner and outer double membranes are damaged. The ridge structure is blurred, the nuclear mimicking disappears, the mitochondrial profile is in a disordered state, the cytoplasmic membrane is broken or the double-layered membrane structure is blurred, the plasmodesmata are enlarged and deformed, and even the connection with the plasma membrane is broken and rough (Zhang et al. 1995). The endoplasmic reticulum curls into a hollow circle and the starch grains are broken, deformed, or fused together.
9.2.4 Impacts on Plant Endogenous Hormones
The changes in plant endogenous hormones under low-temperature stress are closely related to cold resistance and tolerance. Both ABA and GA play important roles in the cold resistance of plants. Maintaining a high ABA level and ABA/GA ratio under low-temperature stress conditions is of great significance for resisting related damage.
Higher ABA levels, lower GA levels, and higher ABA/GA ratios are the inherent reasons for the cold resistance of grafted cucumber seedlings. Additionally, the GA content is the same after exposure to different treatments. Under low-temperature and weak-light conditions, resulting in greenhouse tomatoes experiencing low-temperature stress, the ABA content increases, and the IAA and GA contents decrease. The contents undergo different changes in different varieties. Thus, the changes in some endogenous hormones are active adaptive processes in plants responding to low temperature-related adversity (Gonzalez-Villagra et al. 2021).
9.2.5 Effects on Plant Soluble Sugars
Soluble sugars accumulate and protect plants against cold damage under low-temperature stress (Chen et al. 2014). As an osmotic protective substance, soluble sugars increase the cell sap concentration and the non-icing water in the cell water-holding tissues, and they reduce the cytoplasmic water content. The increased soluble sugars decrease the freezing point and buffer the excessive dehydration of the cytoplasm, protect the cytoplasmic colloid from freezing and solidification, and generate energy and protective substances through metabolism. There is a positive correlation between the soluble sugar content and the cold resistance of plants.
As the treatment temperature decreases, the soluble sugar content in tomatoes gradually increases, and the increase in cold-tolerant varieties is greater than that of non-cold-tolerant varieties. A selenium treatment increases the soluble sugar contents of Jatropha curcas (Wang et al. 2018).
9.2.6 Effects on Amino Acids in Plants
Under normal conditions, the free amino acid contents in plants are very low, but under low-temperature stress conditions, the free amino acid contents rise rapidly, which increases the cell sap concentration, providing a collateral protective effect on the cells. In addition, free amino acids have a strong affinity, which affects the water retention capacity and colloidal stability of the protoplasm. Among the free amino acids, the relationship between proline and plant cold resistance has received widespread attention. Proline contains imino groups, and its hydrophobic pyrrolidine ring can bind to the hydrophobic region of the protein, whereas the hydrophilic groups are distributed on the surface. This increases the protein’s hydrophilic surface and improves its solubility, thereby increasing the soluble protein content and maintaining the enzyme’s conformation at a low temperature (Tan et al. 2012).
9.2.7 Effects on Plant Soluble Proteins
In plants, there is a close relationship between the soluble protein content and cold resistance. The hydrocolloids of soluble proteins are strong, which significantly enhances the cell’s water-holding capacity. Under low-temperature stress, the soluble protein content of plants increases, which allows plants to retain more water and reduce their probability of death owing to protoplasm icing (Gao et al. 2011; Hassanein et al. 2021).
The treatment of Dendrobium officinale seedlings with low-concentration Na2SeO3 solutions significantly increases the soluble protein, free amino acid, and soluble sugar contents (Zhang et al. 2013). An analysis of rice thylakoid membrane protein components showed that the increases in the soluble protein contents at low temperatures may result from a decrease in the degradation rate or an enhancement of synthesis (He et al. 1999).
9.3 The Influence of Selenium and Nano-Selenium on Sulfur Metabolism
Although selenium is not an essential nutrient element for plants, it effectively participates in plant metabolism and has a significant alleviation effect when plants are under low-temperature stress (Feng et al. 2013; Huang et al. 2018). Nano-selenium is a nano-scale single particle that is stable and has high biological activity (Ghasemian et al. 2021). Compared with inorganic selenium, such as selenite, at the same concentration, nano-selenium is less toxic, In addition, nano-selenium can be more effectively used by organisms than inorganic or organic selenium (Hu et al. 2012).
Selenium and sulfur are elements of the same group. Sulfate and selenate have similar chemical properties. Sulfur is an important factor that affects the plant root absorption of selenium (Gupta and Gupta 2017).
At low levels, sulfur has an antagonistic effect on selenate, whereas sulfur has little effect on the absorption of selenite or selenomethionine (Djanaguiraman et al. 2010). Selenium can replace the sulfur in the sulfhydryl group of three seleno-sulfur-containing amino acids to participate in protein synthesis, thereby affecting the nitrogen, sulfur, and amino acid metabolism of plants (Feng et al. 2013; Huang et al. 2018).
The effects of exogenous sulfur on the absorption and accumulation of selenium in tobacco are dependent on the growth stage when applied, the organs exposed and the selenium and sulfur concentrations in the plants. In the early stages of tobacco growth, selenium accumulation in the whole plant and above-ground part occurs under low sulfur (75 μg⋅g−1) conditions. The accumulation of exogenous selenium in tobacco decreases as the sulfur application increases, and it has an antagonistic effect. When no exogenous selenium is applied, sulfur applications reduce the selenium content of mature tobacco leaves, revealing a selenium–sulfur antagonistic effect. When selenium is applied, the selenium content of mature tobacco leaves increases along with the sulfur application, revealing a selenium–sulfur synergistic effect (Ma et al. 2001).
When using a low-sulfur nutrient solution, selenium applications promote the absorption of sulfate ions by barley and rice seedlings, whereas sulfur starvation promotes the absorption and transportation of selenium by tomato roots. Thus, there is a relationship between selenium and sulfur that is both synergistic and mutually antagonistic (Ellis et al. 2003).
Compared with inorganic selenium, nano-selenium is more easily absorbed and used in plant metabolism, and it plays an important role in alleviating abiotic stress (Ghasemian et al. 2021).
9.4 The Mitigation Effects of Selenium and Nano-Selenium on Low-Temperature Stress
9.4.1 Regulating the Proline and MDA Contents
Under short-term low-temperature stress conditions, selenium applications can significantly increase the proline content and reduce the MDA content, thereby alleviating low-temperature stress-related damage. Proline metabolism is a typical biochemical adaptive mechanism of plants under stress conditions. Proline may function to improve plant tolerance by protecting the mitochondrial electron transport chilling chains in plants, as well as inducing the production of protective proteins and antioxidant enzymes. It aids in increasing the contents of protective substances, such as ubiquitin and dehydrin, and the activation of the corresponding anti-stress metabolic pathways (Zhang et al. 2013). Selenium applications increase the proline content and reduce the MDA content in plants, because the appropriate amount of selenium enhances glutathione peroxidase system (GSH-Px) activity and effectively reduces lipid peroxidation (Huang et al. 2018).
Appropriate selenium applications on sweet potato significantly increase the biomass, peroxidase activity, and net photosynthetic rates of the plants when under drought stress, whereas they significantly decrease the MDA level (Huang et al. 2020).
9.4.2 Promoting Protein Metabolism
It is generally believed that selenium promotes protein synthesis and metabolism in two ways. One is that inorganic selenium partially replaces the sulfur in the sulfhydryl (-SH) groups, resulting in three seleno-sulfur-containing amino acids (selenomethionine after entering the plant body, selenocysteine, and selenocysteine) that are involved in protein synthesis, thereby reducing the cysteine and methionine contents in the free amino acids. Second, selenium may be a necessary component of a tRNA ribonucleic acid chain in plants. tRNA containing selenocysteine residues exists in plants, and its main physiological function is to transport amino acids for use in proteins synthesis.
After a 10-day 75Se4+ solution treatment on wheat and three kinds of pastures, 60–80% of selenium was involved in protein functions, whereas 20–30% was contained in various selenium-containing amino acids (Shang et al. 1998). In soybeans exposed to different selenium levels, 42.6–62.6% of the selenium is bound to water-soluble proteins, and protein is the main selenium-enriched component (Xie et al. 1995). Thus, selenium participates in protein synthesis and metabolism in plants.
9.4.3 Regulating Photosynthesis
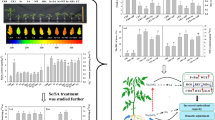
Many studies show that the application of appropriate concentration selenium or nano-selenium solution can alleviate the adverse impacts when plants are exposed to low-temperature environments (Feng et al. 2013; Zhang et al. 2013). It was reported that exposure of strawberry plants to chilling stress decreased the net photosynthetic rate (Pn) (Huang et al. 2018). However, this effect could be removed by applying exogenous Se. Seedling leaves receiving different concentrations 2.5, 5, and 10 mg⋅L−1 of exogenous Se demonstrated significant increases in the Pn of 66.07%, 102.60%, and 96.18%, respectively, for Akihime, and 13.90%, 52.70%, and 6.42%, respectively, for Benihoppe, at the beginning of chilling stress (0 h) when compared with CK. With extended low-temperature exposure, the Pn decreased gradually and the application of Se retarded this decline. After 6 h of chilling stress, the Pn values of Akihime leaves in treatments of 2.5 and 5 mg⋅L−1 were significantly greater than that of the control group, increasing by 32.93% and 30.87%, respectively. After the 12-h treatment, 2.5 and 5 mg⋅L−1 increased by 28.32% and 35.27%, respectively, compared with CK. The detailed information is shown in Fig. 9.1.
Effects of different exogenous Se treatments (C0, 0 mg⋅L−1; C1, 2.5 mg⋅L−1; C2, 5 mg⋅L−1; C3, 10 mg⋅L−1) on net photosynthetic rate (Pn) (a, b), stomatal conductance (Gs) (c, d) and intercellular CO2 concentration (Ci) (e, f) in leaves of two strawberry varieties under chilling stress. Bars with different letters are significantly different at the 0.05 level (LSD test). (Huang et al. 2018)
The stomatal conductance (Gs) and intercellular CO2 concentration (Ci) are closely related Pn. The Gs of strawberry leaves decreased as the exposure time to low temperature increased from 0 to 12 h. Foliar sprays containing different Se concentrations could alleviate the decline of Gs in strawberry seedlings’ leaves under chilling stress. The Ci increased as the exposure time to low temperature increased from 0 to 12 h. Exposure to exogenous Se resulted in a significant decrease in Ci values compared with the control. For Akihime treated at 0 °C for 6 h, the Ci values of exogenous Se-treated (2.5, 5, and 10 mg⋅L−1) strawberry leaves decreased by 8.40%, 13.77%, and 8.03%, respectively, when compared with CK. As the treatment time extended to 12 h, the leaf Ci values increased further. Applications of exogenous Se had significant stimulatory effects on Ci values. The 2.5 and 5 mg⋅L−1 concentrations had greater effects on both strawberry varieties than the 10 mg⋅L−1 concentration.
9.4.4 Regulating Respiratory Metabolism
Both the mitochondrial respiration rate and the chloroplast electron transfer rate are significantly related to the presence or absence of selenium and its level. Within a certain range (below 0.10 mg⋅L−1), selenium enhances the mitochondrial respiration rate and the chloroplast electron transfer rate. However, at a higher concentration (≥ 1 mg⋅L−1), these rates decrease, indicating that selenium may be involved in energy metabolic processes in plants (Wu et al. 2000).
A low concentration of sodium selenite has a certain stimulatory effect on the respiratory rate of barley, whereas a high concentration inhibits respiration. As the sodium selenite concentration increases, the inhibitory effect becomes more obvious. When the sodium selenite concentration reaches an upper limit, the structures of the cell membrane and mitochondria are destroyed, inhibiting enzyme activity and hindering the transmission of electrons in the respiration process. Thus, the cells are basically in a dead state, and the respiratory rate is reduced (Wang et al. 2002).
9.4.5 Promoting Chlorophyll Synthesis and Metabolism
Appropriate selenium concentrations effectively enhance plant photosynthesis and increase the chlorophyll content. This may be achieved by promoting the absorption of mineral elements (e.g., P, K, Ca, Mg) related to chlorophyll synthesis by plants. Additionally, selenium stimulates the respiratory rate and the electron flow of the respiratory chain, and it can protect chloroplast enzymes. Furthermore, selenium affects the interaction of 5-aminolevulinic acid dehydratase with -SH and porphobilinogen deaminase, which regulate chlorophyll synthesis (Gupta and Gupta 2017).
Selenium may be involved in energy metabolic processes in plants. Selenium and sulfur have similar chemical properties, and there may be selenoproteins, like thioredoxin and iron-sulfur protein, in plants that can be used in photosynthesis and respiration. Additionally, it may play a similar role in transmission (Zhang et al. 2013).
Low temperatures and weak light significantly reduce strawberry seedling growth and related parameters, such as leaf chlorophyll content and photosynthetic rate, and an appropriate amount of selenium enhances strawberry chlorophyll synthesis. Foliar applications of selenium also increase the chlorophyll contents in plants. For example, when the concentration of selenium sprayed on carrot leaves is lower than 10 mg⋅L−1, the chlorophyll content of the carrots is significantly higher than that of the control (Huang et al. 2018).
The chlorophyll content in leaves of Nicotiana tabacum seedlings was significantly positively correlated with the applied selenium concentration. In wheat, sodium selenite treatments revealed that selenium contributes to the accumulation of chlorophyll in the leaves and the formation of its precursor 5-aminolevulinic acid (Han et al. 2013). In soybean treated with relatively high selenium concentrations, selenium regulates the chlorophyll level through the interaction of 5-aminolevulinic acid dehydratase with -SH and porphobilinogen deaminase synthesis (Feng et al. 2013). Thus, selenium promotes and regulates the synthesis and metabolism of chlorophyll.
9.4.6 Promoting Plant Antioxidant Systems
The main biological function of selenium in animals and humans is as a component of the glutathione peroxidase system, which participates in redox reactions in the body, removes free radicals, such as lipid peroxides, and reduces peroxidative damage to the body caused by biofilms. A large number of free radicals are also produced during higher plant metabolic processes and in response to environmental stresses. These free radicals can be eliminated by corresponding enzyme systems, such as superoxide dismutase and catalase (CAT), and they can also be cleared by GSH-Px. GSH-Px has been detected in different tissues of different types of plants, and selenium applications enhance the GSH-Px activity levels in plant tissues, thereby confirming that selenium has antioxidant effects in plants (Huang et al. 2018).
In rice, sorghum, and wheat crops, selenium can increase the antioxidant capacity, improve resistance and anti-senescence abilities, and ensure normal plant growth (Peng et al. 1997; Djanaguiraman et al. 2010; Chu et al. 2010). In tomatoes grown under salt stress conditions, the application of selenium significantly increases the GSH-Px activity in plant leaves and significantly reduces the MDA content in tomato leaves (Diao et al. 2014). In sunflower seedlings, the content of the membrane peroxidation product MDA, the production rate of O2−, and the generation of ROS all decrease as the selenium concentration increases, within an appropriate range, indicating that selenium eliminates excessive free radicals in plants (Habibi 2017).
9.4.7 Mode of Action
9.4.7.1 Antioxidant Mechanisms
Selenium mainly regulates the antioxidant system through three modes. First, it promotes the spontaneous disproportionation of O2− into H2O2 without the catalysis of the superoxide dismutase enzyme. Second, it directly quenches O2− and OH−1 with selenium compounds, and third, it regulates the activities of antioxidant enzymes (Djanaguiraman et al. 2010).
Selenium is an essential component of the plant protective enzyme GSH-Px. The change in the GSH-Px activity caused by an exogenous selenium treatment affects the entire protective enzyme system in the plant. GSH-Px catalyzes the conversion of GSH into GSSH, thereby reducing toxic peroxides into non-toxic hydroxyl compounds. Additionally, it promotes the decomposition of H2O2 and the scavenging of lipid peroxides and other free radicals, thereby reducing the impact of ROS-associated superoxidation damage on biological membranes (Mroczek-Zdyrska et al. 2017).
Ascorbate peroxidase (APX) is a signal substance that regulates ROS, whereas CAT (mainly located in peroxisomes) may be responsible for removing excess ROS. Under stress conditions, selenium greatly increases the activity levels of APX and CAT enzymes (Habibi 2017).
9.4.7.2 The Ascorbic Acid–Glutathione (ASA–GSH) Cycle
The ASA–GSH cycle, which mainly exists in the cytoplasm and chloroplasts, is an important mechanism for removing H2O2 in plants, and it plays important roles in the defense of plants against abiotic stresses, such as low temperature, that cause oxidative damage (Huang et al. 2018).
Under low-temperature stress conditions, the increase in the ROS contents of plants stimulates increases in the activity levels of the corresponding enzymes in the ASA defense system, which enhances the defensive abilities of the plants. The components of the ASA–GSH cycle include ASA, GSH, APX, and monodehydroascorbate reductase. Both ASA and GSH are key non-metabolic antioxidants in plants that protect cell components from oxidative damage and are directly involved in the elimination of O2.- and OH.. APX plays a key role in eliminating plant H2O2, and both ASA and APX jointly participate in the disproportionation of H2O2. H2O2 is reduced to H2O and O2 by the electrons of NADPH. GSH plays an intermediary role and is oxidized to GSSG, and ASA is oxidized to monodehydroascorbic acid, which can re-form ASA through monodehydroascorbate reductase action or non-enzymatic dismutation. Additionally, GSH-Px is reused in the ASA–GSH cycle. Selenium may partially replace the sulfur in GSH-Px, thereby promoting the ASA–GSH cycle, which then enhances the cold tolerance of plants (Gupta and Gupta 2017; Huang et al. 2018).
9.5 Selenium Regulates Chilling Injury, Low Temperature, and Gene Expression
GSH-Px is also known as selenium glutathione peroxidase because selenium is a component. Selenium regulates the stability of GSH-Px mRNA, and GSH-Px is involved in redox reactions in animals and plants. GSH-Px catalyzes GSH into GSSH, reduces toxic peroxides to non-toxic hydroxyl compounds, promotes the decomposition of H2O2, removes free radicals, such as lipid peroxides, and reduces the effects of peroxidation on biofilms. Its activity level indirectly reflects the plant’s ability to scavenge oxygen free radicals, and it plays an important plant protective role under low-temperature stress conditions (Qi et al. 2021).
GSH-Px has been detected in different types of plant tissues, and selenium increases the GSH-Px activity in plant tissues, thereby confirming the antioxidant effect of selenium in plants.
The antioxidant effect of selenium is closely related to GSH-Px activity and lipid peroxidation. A fluorescence quantitative PCR analysis of GSH-Px gene expression in potato leaves under different low-temperature stress conditions revealed that the expression level in the leaves of the selenium-sprayed plants was highest after a 3-h low-temperature stress, and as the low-temperature stress time, the expression level first increased and then decreased, while the control did not change significantly. It indicates that selenium may play a crucial role in the expression of GSH-Px genes (Kaur et al. 2016).
9.6 Progress in the Study of Selenium-Regulating Chilling Injury
9.6.1 Effects on Genomic Characteristics
Selenium applications significantly change the characteristics of genomics and proteomics. For tea roots treated with exogenous selenium and sulfur, 7877 differentially expressed unigenes have been screened, of which 4845 were upregulated and 3032 were downregulated. After a sulfur treatment, 2313 unigenes were differentially expressed, of which 1028 were upregulated and 1285 were downregulated. By comparing the transcriptomes after different treatments, a large number of selenium response-related genes, as well as metabolic pathway information, have been obtained. The metabolic pathways, such as cysteine and methionine metabolism, glutathione metabolism, and thiometabolism, are closely related to the selenium-based responses of tea plants. Some key genes of metabolic pathways, such as ATPS1/3, APR, SAT, GS, and CS, are upregulated (Hu 2016).
9.6.2 Effects on Proteomics Characteristics
Sodium selenite has physiological effects on peach and grape, with the soluble sugar, soluble protein, and chlorophyll contents in peach leaves increasing at the appropriate selenium concentration (20 mg⋅L−1). Proteomics analyses of peach leaves before and after selenium applications have been explored using sample phenol preparations, two-dimensional electrophoresis and mass spectrometry. A bioinformatics study on the differential proteins produced by selenium treatment has been performed. The results were as follows: (1) 1391 protein spots were detected in the two-dimensional electrophoresis results from normal peach leaves. A total of 1204 protein spots were detected in selenium-enriched leaves. After a selenium treatment, the strengths of 187 protein spots were reduced, and 33 protein spots showed significant qualitative and quantitative changes. Among them, 11 protein spots were upregulated, and 18 protein spots were downregulated. After a selenium treatment, 4 specific protein spots were missing from the leaves, and 23 proteins showing large expression differences and good dot types were selected for a mass spectrometry analysis. Complete peptide fingerprints were obtained. The peptide data were imported into the NCBI nr protein database, and 12 protein spots were identified. A bioinformatics analysis of the identified proteins revealed that most of their functional sites were in chloroplasts, with three protein spots belonging to the RuBisCO long-chain family. Protein spot 343 belongs to the oxygen-releasing complex of PSII, which could maintain its stability; protein spot 410 is lactyl glutathione lyase, and protein spot 853 is a dehydrated protein. The selenium treatment increased the expression levels of these proteins and promoted plant photosynthesis and stress resistance.
Haematococcus pluvialis has the ability to convert inorganic selenium into organic selenium, but its transformation mechanism and regulatory principles are not clear. To determine the physiological and biochemical effects of sodium selenite on H. pluvialis and the intracellular mechanism of selenium enrichment in H. pluvialis, protein expression mechanisms under sodium-selenite stress were analyzed using proteomics. H. pluvialis 192.80 was cultured for 10 d at different sodium-selenite concentrations. The growth of the algae was analyzed and the appropriate selenium-concentration gradient was determined.
The differences in protein expression in H. pluvialis after an exogenous selenium treatment were investigated. The main results were as follows: in the selenium and control groups, proteins were extracted using the TCA/acetone method for two-dimensional electrophoresis. A total of 40 significantly changed protein spots were obtained, of which 25 were upregulated and 15 were downregulated. The mass spectrometry revealed that 31 proteins were comparable with known proteins in the NCBI nr database, and 27 proteins were annotated to the database. The identified differential proteins are mainly involved in a series of cellular and molecular level regulatory processes that are involved in photosynthesis, antioxidation, amino acid metabolism, energy metabolism, ion transport, signal transmission, cell division, and cell repair.
In summary, selenium and nano-selenium play important roles in mediating cold-stress tolerance in crop plants. With the development of plant genomics, a deeper understanding of the mechanisms can be achieved, which will allow for better agricultural production practices.
References
Chen JZ, Xu CX (1998) Plant chilling injury and its cold resistance physiology. Fujian fruits 2:21–23. (In Chinese)
Chen D, Wang D, Sun L, Zhang YY, Huang CP (2014) Alleviating effects of exogenous salicylic acid on antioxidative physiological characteristics of Phalaenopsis leaves under low temperature stress. J Zhejiang Univ (Agric Life Sci) 40(3):266–274. (In Chinese)
Chu JZ, Yao XQ, Zhang ZN (2010) Responses of wheat seedlings to exogenous selenium supply under cold stress. Biol Trace Element Res 136:355–363
Diao M, Ma L, Wang JW, Cui JX, Fu AF, Liu HY (2014) Selenium promotes the growth and photosynthesis of tomato seedlings under salt stress by enhancing chloroplast antioxidant defense system. J Plant Growth Regul 33:671–682
Djanaguiraman M, Prasad PVV, Seppanen M (2010) Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol Biochem 48:999–1007
Duan M, Feng HL, Wang LY, Li D, Meng QW (2012) Overexpre-ssion of thylakoidal ascorbate peroxidase shows enhanced resistance to chilling stress in tomato. J Plant Physiol 169:867–877
Ellis DR, Salt DE (2003) Plant, selenium and human health. Curr Opin Plant Biol 6:273–279
Erdal S (2012) Androsterone-induced molecular and physiological changes in maize seedlings in response to chilling stress. Plant Physiol Biochem 57:1–7
Feng RW, Wei CY, Tu SX (2013) The roles of selenium in protecting plants against abiotic stresses. Environ Exp Bot 87:58–68
Gao DD, Tan YL, Ma GX, Zhou WJ, Huang CP (2011) Physi-ological responses of Phalaenopsis leaves to low temperature stress. J Zhejiang Univ (Agric Life Sci) 37(5):509–515. (In Chinese)
Ghasemian S, Masoudian N, Nematpour FS, Afshar AS (2021) Selenium nanoparticles stimulate growth, physiology, and gene expression to alleviate salt stress in Melissa officinalis. Biologia 76:2879–2888
Gonzalez-Villagra J, Figueroa C, Luengo-Escobar A, Morales M, Inostroza-Blancheteau C, Marjorie R-DM (2021) Abscisicacid and plant response under adverse environmental conditions. In: Husen A (ed) Plant performance under environmental stress. Springer, pp 17–47
Guo YP, Zhang LC, Shen YG (1998) Effects of chilling stress on photosynthesis of satsumamandarin Citrus unshiu Marc. Acta Horticul Sin 25(2):111–116. (In Chinese)
Gupta M, Gupta S (2017) An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci 7:2074
Gurme ST, Mundada PS, Ahire ML, Salunkhe SS (2021) Auxins and plant response to adverse environmental conditions. In: Husen A (ed) Plant performance under environmental stress. Springer, Cham, pp 49–69
Habibi G (2017) Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol Plant 39:213
Han D, Li XH, Xiong SL, Tu SX, Chen ZG, Li JP, Xie ZJ (2013) Selenium uptake, speciation and stressed response of Nicotiana tabacum L. Environ Exp Bot 95:6–14
Hassanein RA, Hussein OS, Abdelkader AF, Farag IA, Hassan YE, Ibrahim M (2021) Metabolic activities and molecular investigations of the ameliorative impact of some growth biostimulators on chilling stressed coriander (Coriandrumsativum L.) plant. BMC Plant Bio 21:361
He JX, Zeng NY, Yi J, Liang HG (1999) Effects of low temperature on photochemical function and levels of thylakoid proteins of chloroplasts in rice seedlings. Chin J Rice Sci 13(2):99–103
Hu YR (2016) Discovery and analysis of key genes of selenium absorption and metabolism in tea roots. Chin Acad Agricul Sci., Master’s Thesis: 1–7 (in Chinese)
Hu WH, Shi K, Song XS, Xia XJ, Zhou YH, Yu JQ (2006) Different effects of chilling on respiration in leaves and roots of cucumber (Cucumissativus). Plant Physiol Biochem 44:837–843
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210
Huang CP, Wang D, Sun L, Wei L (2016) Effects of exogenous salicylic acid on the physiological characteristics of Dendrobium officinale under chilling stress. Plant Growth Regul 79:199–208
Huang CP, Qin NN, Sun L, Yu MY, Hu WZ, Qi ZY (2018) Selenium improves physiological parameters and alleviates oxidative stress in strawberry seedlings under low-temperature stress. Int J Mol Sci 19:1913
Huang CP, Yu MY, Sun L, Qin NN, Wei L (2020) Physiological responses of sweet potato seedlings under drought-stress conditions with selenium applications. J Agri Crop Res 8(5):98–112
Kato-Noguchi H (2007) Low temperature acclimation to chilling tolerancein rice roots. Plant Growth Regul 51:171–175
Kaur S, Kaur N, Siddique KHM, Nayyar H (2016) Beneficial elements for agricultural crops and their functional relevance in defence against stresses. Arch Agron Soil Sci 62(7):905–920
Keshavkant S, Naithani SC (2001) Chilling-induced oxidative stress in young Sal (Shorearobusta) seedlings. APP 23:457–466
Li MX, Yang HJ (1994) Physiological responses of rubber trees to low temperature injury. Chin J Trop Crops 15(2):7–11. (In Chinese)
Li L, Dong CJ, Shang QM (2013) Role of endogenous salicylic acidin responding of cucumber leaf photosynthetic systems to low temperature stress. Acta Hortic Sin 40(3):487–497
Liu XJ, Xu N, Wu YN, Li JB, Zhong HX, Zhang HH (2019) Photosynthesis, chilling acclimation and the response of antioxidant enzymes to chilling stress in mulberry seedlings. J For Res 30(6):2021–2029
Ma YH, Ding RX, Zhang JZ, Zhu WM (2001) Interaction of Se and S on uptake of Se by flue-cured tobacco (Nicotinatabacum L.). J Nanjing Agri University 24(1):55–58. (In Chinese)
Mroczek-Zdyrska M, Strubinska J, Hanaka A (2017) Selenium im-proves physiological parameters and alleviates oxidative stress in shoots of lead-exposed Vicia faba L. minor plants grown under phosphorus-deficient conditions. J Plant Growth Regul 36:186–199
Peng K, Hong YH, Xia W (1997) Effect of selenium on the photo-synthesis and yield of early rice (Oryza sativa L.). J Hunan Agric Univ 23:432–434. (In Chinese)
Qi WY, Li Q, Chen H, Liu J, Xing SF, Xu M, Yan Z, Song C, Wang SG (2021) Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J Hazar Materi 417:125900
Ruelland E, Collin S (2012) Cilling stress. In: Shabala S (ed) Plant stress physiology. Springer, Hobart, pp 94–117
Shang QM, Li PL (1998) Physiological action of selenium in higher plant. Plant Physio Communi 34(4):284–287. (In Chinese)
Song YL, Gao ZC, Luan WJ (2012) Interaction between temperature and photoperiod in regulation off lowering time in rice. Sci China 55(3):241–249
Sun XL, Yang S, Wang LY, Zhang QY, Zhao SJ, Meng QW (2011) The unsaturation of phosphatidylglycerol in thylakoid membrane alleviates PSII photoinhibition under chilling stress. Plant Cell Rep 30:1939–1947
Tan YL, Zhang YY, Gao DD, Chen D, Shen WQ, Zhou JH, Huang CP (2012) Effects of chilling stress on ascorbate peroxidase activity, malondialdehyde and proline contents of Dendrobium officinale. J Zhejiang Univ (Agric Life Sci) 38:400–406. (In Chinese)
Wang ZJ, Jiang SL, LI S.J. (2002) Study on the riched-Se of barley and its biological character. J Yangzhou Univ (Agric Life Sci) 23(2):400–406. (In Chinese)
Wang HB, Gong M, Xin H, Tang LZ, Dai DQ, Gao Y, Liu C (2018) Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatrophacurcas seedlings. Physiol Mol Biol Plants 24(5):857–865
Wu YY, Lu XY, Peng ZK, Luo ZM (2000) Effect of Se on physiological and biochemical characters of paddy rice. Scientia Agri Sin 33(1):100–103. (In Chinese)
Xie SM, Wang ZJ, Song WP, Peng A (1995) Selenium distribution in soybean from different regions in China. Acta Nutri Sin 17(3):274–278. (In Chinese)
Yamamoto A, Shim IS, Fujihara S (2012) Chilling-stress responses by rice seedlings grown with different ammonium concentrations and its relationship to leaf spermidine content. J Plant Biol 55:191–197
Zhang Y, Dai JY, Su ZS (1995) Chilling injury of on maize kernel at grain filling stage. Acta Agro Sin 21(1):71–76. (In Chinese)
Zhang YY, Chen D, Tan YL, Wang D, Sheng WQ, Zhou JH, Huang CP (2013) Alleviating effects of exogenous selenium on Dendrobium officinale seedlings under low temperature stress and the change of its antioxidative physiology characteristics. Acta Bot Boreali-Occident Sin 33:747–754. (In Chinese)
Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ (2012) Hydrogenperoxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol Biochem 60:141–149
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chongping, H., Wenjie, H., Junlin, L. (2022). Selenium- and Nano-Selenium-Mediated Cold-Stress Tolerance in Crop Plants. In: Hossain, M.A., Ahammed, G.J., Kolbert, Z., El-Ramady, H., Islam, T., Schiavon, M. (eds) Selenium and Nano-Selenium in Environmental Stress Management and Crop Quality Improvement. Sustainable Plant Nutrition in a Changing World. Springer, Cham. https://doi.org/10.1007/978-3-031-07063-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-031-07063-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-07062-4
Online ISBN: 978-3-031-07063-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)