Abstract
Chinese PCNA (C-PCNA) refers to the pollination constant and non-astringent (PCNA)-type persimmon that originated in China. C-PCNA type mainly includes “Luotian-tianshi”, “Eshi 1”, “Xiaoguo-tianshi”, “Baogai-tianshi”, and “Sifang-tianshi”, etc., which are mainly distributed in the Dabie Mountain area at the junction of Hubei, Henan, and Anhui Provinces in central China. Like Japanese PCNA (J-PCNA), C-PCNA has smaller tannin cells and lost their astringency during fruit development, but is later than that of J-PCNA. The mechanism of natural de-astringency in J-PCNA is mainly a result of the dilution of PAs due to the cessation of PA accumulation in the early stage of fruit development. However, this “dilution effect” was not sufficient to explain the loss of astringency in C-PCNA fruit, and the insolubilization of soluble PAs could be the other important effect for its natural de-astringency process. At present, the molecular marker linked to the natural de-astringency trait of C-PCNA has been developed and had made significant progress in identification and characterization of key genes involved in natural de-astringency in C-PCNA. Using C-PCNA cultivar as male parent and crossing with the main cultivar of J-PCNA or non-PCNA, and do early selection of the PCNA candidates assisted by molecular marker could improve the efficiency of PCNA breeding. In addition, with the deciphering of genome information of persimmon, identifying the key genes for natural de-astringency of C-PCNA, and using molecular breeding techniques such as genome editing and genetic transformation will become the major approach for PCNA germplasm innovation in the future.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
Persimmon (Diospyros kaki Thunb., 2n = 6x = 90) is native to China and has been cultivated for more than 2,000 years (Luo and Wang 2008). Pollination constant and non-astringent (PCNA) is a mutant-type persimmon that has lost the ability to accumulate high levels of PAs in the fruit, and naturally lose its astringency during fruit development, whereas non-PCNA-type fruit is astringent and inedible without any artificial treatment. The non-PCNA type is further classified into pollination-variant and non-astringent (PVNA) types, pollination-variant and astringent (PVA) types, and pollination constant and astringent (PCA) types according to whether or not loss of astringency is depending on the formation and accumulation of volatile compounds in flesh (Sugiura 1983). PCNA-type persimmon was thought to uniquely originate in Japan until a new PCNA type “Luotian-tianshi” was found in Luotian Country in Hubei Province (Wang 1983). This genotype has been confirmed to be a PCNA persimmon after it was introduced in the Institute of Fruit Tree and Tea Science, NARO, Japan (Yamada et al. 1993). Furthermore, it had a different genetic background from the Japanese PCNA (J-PCNA) type, since it appeared that also the astringent type of offspring is produced when crossing with J-PCNA type (Ikeda et al. 1985; Yamada and Sato 2002).
According to the records of Luotian County Annals, Chinese PCNA (C-PCNA) has been planted in 1,032, about 180 years earlier than the oldest PVNA cultivar “Zenji-maru” in Japan (Wang 1983). The survey of C-PCNA types in China began in 1958 (Pan et al. 2002). In 1990–1994, the Hubei Academy of Forestry Research and Luotian Institute of Forestry Research cooperated to investigate the resources of “Luotian-tianshi” and others, in order to ascertain its distribution and cultivation status and screened out ten excellent strains and five variation types of C-PCNA (Pan et al. 1994). In recent years, some androecious germplasms and one PVNA type “90-1-10” were found in the Dabie Mountains in central China (Luo et al. 2005), and the studies on their reproductive characteristics and genetic relationship (Xu 2008; Zhang et al. 2009), suggested that the germplasms obtained by survey may be the variation of seedlings of local C-PCNA.
It is generally believed that PCNA is the mutation of non-PNCA type, and these mutant individuals gradually become the current cultivar group in repeated natural hybridization with other genotypes (Yamada et al. 1993). In comparison with the astringency traits and tannin cell characteristics of C-PCNA and non-PCNA fruit, Wang (1983) speculated that “Luotian-tianshi” was originated from the persimmon germplasm with lower tannin (belong to condensed tannin, also known as proanthocyanidin, PA) content and high ethanol dehydrogenase activity in the Dabie Mountain. Using random amplified polymorphic DNA (RAPD) markers, Luo et al. (1999) showed a close relationship among “Luotian-tianshi” and persimmon resources in the Dabie Mountain, but a distant relationship was observed between “Luotian-tianshi” and the PCNA cultivars of Japanese origin. Amplified fragment length polymorphism (AFLP) analysis also showed that the PCNA cultivars of Chinese origin were distant from those of Japanese origin (Kanzaki et al. 2000). Using sequence-related amplified polymorphism (SRAP) (Guo and Luo 2006), inter-retrotransposon amplified polymorphism (IRAP), and retrotransposon–microsatellite amplified polymorphism (REMAP) markers (Guo et al. 2006) also showed that C-PCNA cultivars were distant from those of J-CPNCA cultivars, but cannot separate C-PCNA cultivars from non-PCNA. A similar result was also obtained by other markers, including mitochondrial DNA (mtDNA) noncoding region (Hu and Luo 2006) and chloroplast DNA (cpDNA) RFLP (Hu et al. 2008), sequence-specific amplification polymorphism (SSAP) (Du et al. 2009a), and inverse sequence-tagged repeats (ISTR) (Du et al. 2009b). These results indicated that C-PCNA may be derived from the variation of non-PCNA, and other new C-PCNA types, such as “Eshi 1” and “Baogai-tianshi” that are found in the Dabie Mountain may be derived from the offspring of “Mopanshi” and “Luotian-tianshi”. Kanzaki (2016) indicated that D. louts, D. oleifera, and D. glandulosa may be the diploid ancestor of persimmon, and evolved to non-PCNA type after polyploidization from an unknown Diospyros species in China, finally evolved to C-PCNA and J-PCNA independently in China and Japan, respectively.
C-PCNA is mainly distributed in the Dabie Mountains (115° 06′–115° 46′ E, 30° 35′–31° 16′ N) at the junction of Hubei, Henan, and Anhui provinces (Pan et al. 1994), and no natural distribution in other regions has been reported so far. Luotian County in Hubei Province has the largest number of trees and the most abundant variations of C-PCNA and can find over 200 years old trees, while rarely find 100 years old trees in Shangcheng, Henan Province, and Jinzhai, Anhui Province.
The C-PCNA are distributed in almost all towns in Luotian County, Hubei Province, mostly in the west and north. The trees over 100 years old are mainly distributed in the town of Hepu and Sanlifan, and a 350-year-old tree was found in Tangjiashan Village, Hepu town (Fig. 10.1) (Yan and Zhao 2006). From the perspective of vertical distribution, C-PCNA cultivars are distributed between 100 and 700 m altitude, with the most in the range of 300–500 m, and only a few Diospyros species were found above 700 m (Pan et al. 1994).
C-PCNA in the Dabie Mountains are usually growing together with non-PCNA, chestnuts (Castanea mollissima), tung trees (Vernicia fordii), and Chinese tallow tree (Sapium sebiferum), and most of them freely grow around the house and field. Moreover, the existing adult persimmon trees in China are mostly grafted, and graft unions are clearly identifiable. Before the 1980s, persimmon was usually grafted on high rootstocks of wild persimmon (D. kaki Thunb. var. silvestris) or oil persimmon (D. oleifera Cheng) with a diameter of 3–4 cm with cleft grafting. After that, wild persimmon or lotus (D. lotus L.) were gradually used for seedbed sowing and stock seedling breeding and produced grafted seedlings with cleft grafting or single bud cutting–grafting in the following spring (Pan et al. 1994).
There are more than 20 genotypes of C-PCNA were reported, mainly including “Luotian-tianshi”, “Eshi 1” (original name is Yinyangshi or Qiuyan-tianshi), “Baogai-tianshi”, “Sifang-tianshi”, “Xiaoguo-tianshi”, and “Yesheng-tianshi” (Fig. 10.2) (Pan et al. 1994). In addition, there are other genotypes, including “Qiuhongyu” and “Qiuji” (Li 2003), “Baohua-tianshi” (Li 2004), “Tianbaogai” and “Baozhu-tianshi” (Li et al. 2006), “Wuhe-tianshi”, “Longzhuashi”, “Jinqiu”, and “Mitangshi” (Yan and Zhao 2006). Among them, there may exist homonym or synonym phenomena. For example, “Tianbaogai” and “Baohua-tianshi” may be the other name of “Baogai-tianshi”, and “Tianminshi” may be a type of “Xiaoguo-tianshi”.
10.2 Natural De-astringency Characteristics of C-PCNA
The genetic behavior of the PCNA trait of C-PCNA differs from that of J-PCNA (Ikegami et al. 2004, 2006), its origin and evolution pathway are also different from those of J-PCNA (Luo et al. 1999; Kanzaki et al. 2000; Du et al. 2009a, b). All offspring from crosses among J-PCNA cultivars yield only PCNA phenotypes, and almost all of the offspring from crosses between J-PCNA and non-PCNA were non-PCNA types. Notably, about 50% of the offspring from crosses between “Luotian-tianshi” and non-PCNA cultivars had the PCNA phenotype, indicating that the PCNA trait of “Luotian-tianshi” is genetically dominant to the non-PCNA traits (Ikegami et al. 2006). Moreover, “Luotian-tianshi” does not have any ast alleles (Akagi et al. 2010a) and J-PCNA cultivars do not have any dominant C-PCNA alleles, since no PCNA offspring yield from crosses between J-PCNA and non-PCNA (Ikeda et al. 1985). These results indicate that C-PCNA is not closely related to J-PCNA, they are different PCNA traits.
10.2.1 The Development of Tannin Cells
Tannin cells are a kind of special cells that accumulate PAs in vacuoles. The stop of tannin cell augmentation in the number and the size at an early stage of fruit development is the main cause of the loss of astringency in J-PCNA-type fruits (Yonemori and Matsushima 1985, 1987). The tannin cell size is smaller in PCNA fruit rather than those in non-PCNA fruit, and large in C-PCNA type than those in J-PCNA type, due to the stopped development later in C-PCNA type (Ikegami et al. 2006). The area of tannin cells was less than 33 × 103 μm2 in the PCNA-type fruits, whereas it ranged from 55 × 103 to 140 × 103 μm2 in non-PCNA-type fruits (Ikegami et al. 2004). The tannin cell size of “Qiyan-tianshi” (“Eshi 1”, C-PCNA type) is 43.0 × 103 μm2, which is a little bit large than other PCNA cultivars and had a less astringent taste, but the area is much smaller than non-PNCA, and also thought to be PCNA type (Yonemori et al. 2005). Yonemori et al. (1985) found that PAs in J-PCNA-type fruits hardly coagulated, although at the time those in PVNA-type fruits completely changed into insoluble complexes. However, the coagulated tannin cell can be found in C-PCNA-type fruits. So, the development of the tannin cell in C-PCNA fruit differs from those in J-PCNA type.
10.2.2 PA Composition in Persimmon Fruit
The persimmon PA composition is complex and is thought to consist of two types of flavan-3-ol monomers: cis-flavan-3-ol and trans-flavan-3-ol. Including catechin (C), gallocatechin (GC), epicatechin (EC), and epigallocatechin (EGC), and their gallate ester forms, C-3-O-gallate (CG) and GC-3-O-gallate (GCG), EC-3-O-gallate (ECG) and EGC-3-O-gallate (EGCG) (Matsuo and Ito 1978; Akagi et al. 2009a, 2010b). In the fruit development stage, the main components of persimmon PAs include C, GC, EC, EGC, ECG, and EGCG at the developmental stage (Akagi et al. 2009a, 2010b). Most PA units were suggested to be either EGC or its gallate ester form (EGCG) in PCNA and non-PCNA types, and the composition was varied among fruit developmental (Akagi et al. 2009a, 2010b). In addition, EC and its gallate ester form (ECG) were detectable during fruit development; C and GC were detected at an early stage of fruit developmental but were barely detectable after the middle stage of fruit development (Akagi et al. 2009a). However, there are few studies on the PA composition of C-PCNA cultivars. Fei et al. (1999) found that the “Luotian-tianshi” has a higher content of C and gallic acid (GA), and lower content of GC. These results indicated that the tannin composition of C-PCNA is different from those of J-CPNCA, which may be related to the unique natural de-astringency characteristics of C-PCNA, but further studies were needed to clarify.
10.2.3 PA Accumulation Patterns
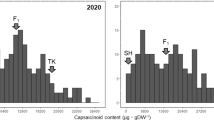
The PA content was decreased gradually with fruit development in both PCNA and non-PCNA fruits. The soluble PA levels were reduced to a much lower level and astringency was lost in the early stage of fruit development (earlier than 10 weeks after bloom, WAB) in J-PCNA, while still maintained at a higher level in C-PCNA until 20 WAB (Fig. 10.3) (Mo et al. 2016; Chen et al. 2017, 2021). A quick reduction of soluble PA in C-PCNA was observed from 20 WAB and almost lose their astringent at 25 WAB (soluble PA content < 0.2%), but non-PNCA cannot lose their astringency. Notably, the dilution of PAs in C-PCNA fruit is not sufficient to explain the loss of astringency. An increase in insoluble PAs was also observed during this period in C-PCNA fruit, which may be due to the insolubilization of soluble PAs at the later stage of fruit development (Guan et al. 2016, 2017; Mo et al. 2016; Chen et al. 2017, 2021).
Dynamic changes of soluble (left panel) and insoluble (right panel) proanthocyanidin (PA) levels among stages of fruit development. Error bars indicate SD (n = 3) (FW, fresh weight) (Chen et al. 2021)
10.2.4 Response to Artificial De-astringency Treatment
Removing astringency from persimmon fruits can be achieved by different methods, such as keeping the fruit under anaerobic conditions or being exposed to products that enhance anaerobic respiration (Pesis et al. 1986; Ben-Arie and Sonego 1993). The acetaldehyde produced by the fruit under these conditions is believed to cause the polymerization of tannin which leads to loss of astringency (Matsuo and Itoo 1982; Tanaka et al. 1994). However, treatment of young astringent fruits of J-PCNA with either ethanol or acetaldehyde was ineffective for the removal of their astringency, the tannins do not coagulate (Sugiura 1983; Zhang et al. 2013). Similar to non-PCNA fruit, the ethanol (Zhang et al. 2013; Luo et al. 2014), warm water (Guan et al. 2015; Chen et al. 2017) treatment can easily remove its astringency. This phenomenon is indicating that the molecular events of natural de-astringency in C-PCNA different from J-PCNA fruit but close to non-PNCA, which may cause by its specific PAs composition, but further studies are needed to be done to clarify this molecular basis.
10.3 Identification and Characterization of Natural De-astringency Associated Genes in C-PCNA Fruit
The PCNA trait in the J-PCNA type is controlled by a single recessive gene, while that in the C-PCNA type is controlled by a single dominant gene (Yonemori et al. 2000; Ikegami et al. 2006). The mechanism of natural de-astringency in J-PCNA is mainly a result of the dilution of PAs due to the cessation of PA accumulation in the early stage of fruit growth. Conversely, PA accumulation continues in non-PCNA persimmons until the late stages of fruit development. This process is mainly controlled by an MYB transcription factor, DkMyb4. A reduction in the expression of DkMyb4 in J-PCNA fruit leads to the downregulation of PA biosynthesis during the early stages of fruit development and results in the non-astringency trait (Akagi et al. 2009b). Notably, the dilution of PAs in C-PCNA fruit is not sufficient to explain the loss of astringency. The content of insoluble PAs increased, while that of soluble PAs decreased during the late stages of C-PCNA fruit development (Fig. 10.3). In recent years, a series of analyses, including genetic, transcriptome, and proteome were performed to identify the genes associated with nature astringency in C-PCNA.
10.3.1 Structural Genes
Early steps in the biosynthesis of PA from phenylpropanoids and malonyl-CoA into anthocyanidins are catalyzed by chalcone synthase (CHS), chalcone isomerase (CHI), flavonoid 3′-hydroxylase (F3′H), flavonoid 3′5′-hydroxylase (F3′5′H), dihydroflavonol 4-reductase (DFR), and anthocyanidin synthase (ANS). Anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR) are specific to the PA branch of the pathway and produce flavan-3-ols, typically (−)-epicatechin and (+)-catechin, respectively (Abrahams et al. 2003; Xie et al. 2003). Finally, the PA precursors are transported to and stored into the vacuole.
In persimmon, the PA biosynthetic pathway has also been well defined through homology cloning and RNA-seq analysis (Akagi et al. 2011; Chen et al. 2017). The genes involved in PA biosynthesis, such as phenylalanine ammonia-lyase (PAL), CHS, CHI, F3H, F3′5′H, DFR, ANS, ANR, and LAR have been isolated (Ikegami et al. 2005a, 2007; Akagi et al. 2009a). The PA transportation and polymerization-related genes, DkMATE1 and DkLAC (homologs of TT12 and TT10, respectively) have also been isolated (Hu et al. 2013; Yang et al. 2016). In PCNA cultivars of Japanese origin, the PA biosynthetic-related genes were synchronously downregulated from 5 WAB and were almost below the detection limit after 7 WAB, and the accumulation of tannins was terminated in the early stage of fruit development, the dilution of PAs by fruit growth is the main reason for natural de-astringency (Yonemori and Matsushima 1985; Yonemori et al. 2003; Akagi et al. 2009b). However, the accumulation of soluble PA was terminated in the later development stage (20 WAB) of C-PCNA fruit (Mo et al. 2016), thus the “dilution effect” was not sufficient to cause C-PCNA fruit to lose its astringency. The expression pattern of genes related to PA biosynthesis between C-PCNA and J-PCNA fruits are quite different. Ikegami et al. (2005b) compared the expression patterns of PA biosynthesis genes in “Luotian-tianshi” (C-PCNA), “Suruga” (J-PCNA), and “Kuramitsu” (PCA, non-PCNA), and found that the C-PCNA cultivar behaves similar to PCA cultivar with regard to expression of the genes involved in PA biosynthesis except for DkSCPL1. Moreover, the PA branch-specific ANR was continuously expressed in C-PNCA after the termination of tannin cell development in J-PCNA cultivar (Ikegami et al. 2005b); another PA branch-specific gene LAR, whose expression in C-PCNA was also similar to the PCA cultivar (Wang et al. 2010). Besides the genes in the biosynthesis of PA precursors, the PA precursors transportation and polymerization associated genes DkMATE1 and DkLAC have been also isolated from C-PCNA fruit (Hu et al. 2013; Yang et al. 2016). Chen et al. (2017) found that the transcription level of PA biosynthesis genes was consistent with the accumulation pattern of PA in different astringent-type cultivars, most of them are show high expression in C-PCNA than J-PCNA cultivar, but lower than in non-PCNA cultivars.
Soluble PA is the main reason that causes fruit astringency, which can interact with acetaldehyde under artificially astringency removal treatment. Pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) are two key enzymes that catalyzed pyruvate and ethanol into acetaldehyde, respectively, in plants (Yamada et al. 2002; Pesis 2005; Botondi et al. 2012). Luo et al. (2014) performed a genome-wide transcriptome analysis of “Luotian-tianshi” after ethanol treatment to remove fruit astringency and found that PDC gene was upregulated, and aldehyde dehydrogenase family 2 (ALDH2) that reduce acetaldehyde accumulation by canalizing acetaldehyde to acetic acid was significantly downregulated after treatment. DkADH1 and DkPDC2 were suggested to be key genes involved in persimmon astringency removal under high CO2 treatment (Min et al. 2012), and may participate in natural de-astringency of C-PCNA fruit through a comprehensive analysis of gene expression patterns, seasonal change of tannins and acetaldehyde content of persimmon fruit flesh, and gene transient expression assay in persimmon leaves (Mo et al. 2016). Xu et al. (2017) cloned two ALDH2 genes DkALDH2a and DkALDH2b from C-PCNA fruit and found that DkALDH2a and DkALDH2b are negatively correlated with natural de-astringency in C-PCNA persimmon. Moreover, pyruvate kinases 1 and 8 (PK1 and PK8), the upstream genes of pyruvate synthesis were suggested to be involved in natural de-astringency in C-PCNA by upregulating the DkPDC and DkADH expression (Guan et al. 2016, 2017).
10.3.2 Transcription Factors
The ternary complex comprising of MYB transcription factor, basic helix–loop–helix (bHLH) protein, and WD40 protein (MBW complex) have been reported in regulates the expression of PA structural genes (Koes et al. 2005; Ramsay and Glover 2005). In Arabidopsis, the complex is formed by at least four MBW complexes, namely TT2-TT8/GL3/EGL3-TTG1, and MYB5-TT8-TTG1, and activates the genes DFR, ANS, and ANR, the products of which cooperate to regulate the specific accumulation of PAs in the innermost cell layer of the seed coat (i.e., endothelium, chalaza, and micropyle) (Nesi et al. 2001; Debeaujon et al. 2003; Baudry et al. 2004).
In persimmon, some MYB-like genes have been identified from J-PCNA or non-PCNA cultivars (Akagi et al. 2009b), among them DkMyb2 and DkMyb4 were suggested to regulate PA biosynthesis in J-PCNA (Akagi et al. 2009b, 2010c). Reduced expression of DkMyb4 leads to J-CPNA fruit-specific downregulation of PA biosynthesis at the early stage of fruit developmental and resultant non-astringent trait (Akagi et al. 2009b). DkbZIP5 was found to respond to the seasonal abscisic acid signal and act as a DkMyb4 regulator to modify PA accumulation in J-PCNA (Akagi et al. 2012). However, these factors are not the major factors that regulate PA accumulation in C-PNCA cultivars.
Su et al. (2012) cloned a bHLH factor, VvMYC1, the homolog of AtTT8 from C-PCNA cultivar, and the expression pattern of DkMYC1 was correlated with the PA accumulation in C-PCNA and non-PCNA cultivars. Guan et al. (2020) isolated two regulators of DkPK1 genes, DkWRY3 and DkWRY15 from C-PCNA using yeast one-hybrid screen strategy. Transient overexpression of DkWRKY3 and DkWRKY15 in persimmon leaves reduced the soluble PA accumulation by activating acetaldehyde synthesis-related gene expression.
Recently, Chen et al. (2021) reported an MYB transcription factor, DkMYB14, which regulates the accumulation of PA in C-PCNA fruit flesh, is a bifunctional transcription factor that acts as a repressor in PA biosynthesis but becomes an activator when involved in acetaldehyde biosynthesis. Notably, both functions contribute to the elimination of astringency by decreasing PA biosynthesis and promoting its insolubilization. Furthermore, the amino acid Gly39 in the R2 domain and the EAR-like motif in the C-terminal are essential for the activities of DkMYB14, namely repressing PA biosynthesis and promoting PA insolubilization. CPCNA may regulate natural de-astringency through regulating DkMYB14 expression, since the same sequence of DkMYB14 among different cultivars (Chen unpublished data), but the underlying mechanisms are not clear.
10.3.3 microRNA
microRNAs (miRNAs) are small non-coding RNAs, with an average of 22 nucleotides in length, that functions in RNA silencing and post-transcriptional regulation of gene expression (Bartel 2018). Luo et al. (2015) performed a high-throughput small RNA sequencing on C-PNCA “Eshi 1” fruits at stages of 15 and 20 WABs, and a total of 236 known miRNAs and 33 novel miRNAs were identified. Among them, miRNA156j-5p, miRNA858b, miRNA385p-3p, miRNA2911a were suggested to have an important role in the regulation of PA accumulation in persimmon. Yang et al. (2020) further conducted a functional analysis on miRNA858b and found that miRNA858b negatively regulating the PA accumulation by inhibiting the MYB transcription factors DkMYB19 and DkMYB20 expression.
In summary, the genes involved in acetaldehyde metabolism include DkADH1, DkPDC2, DkALDH2, DkPK1/8, and the regulators DkWRKY3/15 were thought to play an important role in natural de-astringency in C-PNCA fruits. In addition, miRNA858b and DkMYB14 regulate PA accumulation in C-PCNA fruits, but DkMYB14 plays an important role in natural de-astringency regulation in C-PCNA (Fig. 10.4).
10.4 Molecular Markers Related to CPCNA Trait
Ikegami et al. (2011) developed an effective SCAR marker, RO2, which specifically links to C-PCNA trait and can be used for selecting the C-PCNA type offspring in the early stage. Pei et al. (2013) verified the efficiency of RO2 marker in other F1 populations that yield from the crossing with C-PNCA cultivar. Notably, the androecious persimmon genotypes, namely Male strains No. 1, No. 2, No. 3, No. 6, No. 7, and No. 8 are also carrying the RO2 marker and some of the offspring generated from crossing combination “Huashi 1” (PVA) × Male strain No. 3 also find carrying RO2 marker. These results indicated that the androecious persimmon genotypes have CPCNA locus and can be used from PCNA breeding.
10.5 Future Prospects
PCNA is the most desirable type since the fruit is edible without any de-astringency treatment; therefore, breeding PCNA type is one of the most important goals for persimmon breeding. Since the very narrow genetic variability of J-PCNA-type cultivars, using only J-PCNA cultivars causes a serious problem for breeding new cultivars because of inbreeding depression in breeding population. However, the genetic behavior of the PCNA trait of C-PCNA differs from that of J-PCNA cultivar, and yields 50% of the PCNA offspring from a cross with C-PCNA cultivar. Using C-PCNA as male parent and crossing with the main cultivar of J-PCNA or non-PCNA, and early selection of the PCNA candidates assisted by molecular marker could improve the efficiency of PCNA breeding. In addition, with the release of genome information of persimmon, identification of the key genes for natural de-astringency of C-PCNA, and using molecular breeding techniques such as genome editing and genetic transformation will become the major approach for PCNA germplasm innovation in the future.
References
Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR (2003) The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J 35:624–636
Akagi T, Ikegami A, Suzuki Y, Yoshida J, Yamada M, Sato A, Yonemori K (2009a) Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit. Planta 230:899–915
Akagi T, Ikegami A, Tsujimoto T, Kobayashi S, Sato A, Kono A, Yonemori K (2009b) DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 151:2028–2045
Akagi T, Takeda Y, Ikegami A, Kono A, Yamada M, Kanzaki S, Yonemori K (2010a) Quantitative genotyping for astringency locus in hexaploid persimmon cultivars using quantitative real-time PCR. J Am Soc Hortic Sci 135:59–66
Akagi T, Suzuki Y, Ikegami A, Kamitakahara H, Takano T, Nakatsubo F, Yonemori K (2010b) Condensed tannin composition analysis in persimmon (Diospyros kaki Thunb.) fruit by acid catalysis in the presence of excess phloroglucinol. J Jpn Soc Hortic Sci 79:275–281
Akagi T, Ikegami A, Yonemori K (2010c) DkMyb2 wound-induced transcription factor of persimmon (Diospyros kaki Thunb.), contributes to proanthocyanidin regulation. Planta 232:1045–1059
Akagi T, Katayama-Ikegami A, Yonemori K (2011) Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci Hortic 130:373–380
Akagi T, Katayama-Ikegami A, Kobayashi S, Sato A, Kono A, Yonemori K (2012) Seasonal abscisic acid signal and a basic leucine zipper transcription factor, DkbZIP5, regulate proanthocyanidin biosynthesis in persimmon fruit. Plant Physiol 158:1089–1102
Bartel DP (2018) Metazoan MicroRNAs. Cell 173:20–51
Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L (2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39:366–380
Ben-Arie R, Sonego L (1993) Temperature affects astringency removal and recurrence in persimmon. J Food Sci 58:1397–1400
Botondi R, Russo V, Mencarelli F (2012) Anaerobic metabolism during short and long term storage of kiwifruit. Postharvest Biol Technol 64:83–90
Chen WX, Xiong YL, Xu LQ, Zhang QL, Luo ZR (2017) An integrated analysis based on transcriptome and proteome reveals deastringency-related genes in CPCNA persimmon. Sci Rep 7:44671
Chen WX, Zheng QY, Li JW, Liu Y, Xu LQ, Zhang QL, Luo ZR (2021) DkMYB14 is a bifunctional transcription factor that regulates proanthocyanidin accumulation in persimmon fruit. Plant J 106:1708–1727
Debeaujon I, Nesi N, Perez P, Devic M, Grandjean O, Caboche M, Lepiniec L (2003) Proanthocyanidin-accumulating cells in Arabidopsis testa: regulation of differentiation and role in seed development. Plant Cell 15:2514–2531
Du XY, Zhang QL, Luo ZR (2009a) Development of retrotransposon primers and their utilization for germplasm identification in Diospyros spp. (Ebenaceae). Tree Genet Genomes 5:235–245
Du XY, Zhang QL, Luo ZR (2009b) Comparison of four molecular markers for genetic analysis in Diospyros L. (Ebenaceae). Plant Syst Evol 281:171–181
Fei XQ, Zhou LH, Gong BC (1999) Differences of the components of tannin among three types of persimmon fruits and characteristics of tannin from ‘Luotian Tianshi.’ J For Res 12:369–373 (in Chinese)
Guan CF, Chen L, Chen WX, Mo RL, Zhang QL, Du XY, Liu JH, Luo ZR (2015) SSAP analysis reveals candidate genes associated with de-astringency in persimmon (Diospyros kaki Thunb.) treated with 40 °C water. Tree Genet Genomes 11:20
Guan CF, Chen WX, Mo RL, Du XY, Zhang QL, Luo ZR (2016) Isolation and characterization of DkPK genes associated with natural de-astringency in C-PCNA persimmon. Front Plant Sci 7:156
Guan CF, Du XY, Zhang QL, Ma FW, Luo ZR, Yang YY (2017) DkPK genes promote natural de-astringency in C-PCNA persimmon by up-regulating DkPDC and DkADH expression. Front Plant Sci 8:149
Guan CF, Wang MK, Zhang YF, Ruan XF, Zhang QL, Luo ZR, Yang Y (2020) DkWRKY interacts with pyruvate kinase gene DkPK1 and promotes natural de-astringency in C-PCNA persimmon. Plant Sci 290:110285
Guo DL, Luo ZR (2006) Genetic relationships of some PCNA persimmons (Diospyros kaki Thunb.) from China and Japan revealed by SRAP analysis. Genet Resour Crop Evol 53:1597–1603
Guo DL, Zhang HQ, Luo ZR (2006) Genetic relationships of Diospyros kaki Thunb. and related species revealed by IRAP and REMAP analysis. Plant Sci 170:528–533
Hu DC, Luo ZR (2006) Polymorphisms of amplified mitochondrial DNA non-coding regions in Diospyros spp. Sci Hortic 109:275–281
Hu DC, Zhang QL, Luo ZR (2008) Phylogenetic analysis in some Diospyros spp. (Ebenaceae) and Japanese persimmon using chloroplast DNA PCR-RFLP markers. Sci Hortic 117:32–38
Hu QN, Luo C, Zhang QL, Luo ZR (2013) Isolation and characterization of a Laccase gene potentially involved in proanthocyanidin polymerization in oriental persimmon (Diospyros kaki Thunb.) fruit. Mol Biol Rep 40:2809–2820
Ikeda I, Yamada M, Kurihara A, Nishida T (1985) Inheritance of astringency in Japanese persimmon. J Jpn Soc Hortic Sci 54:39–45 (in Japanese with English summary)
Ikegami A, Yonemori K, Sugiura A, Sato A, Yamada M (2004) Segregation of astringency in F1 progenies derived from crosses between pollination-constant, nonastringent persimmon cultivars. HortScience 39:371–374
Ikegami A, Kitajima A, Yonemori K (2005a) Inhibition of flavonoid biosynthetic gene expression coincides with loss of astringency in pollination-constant, non-astringent (PCNA)-type persimmon fruit. J Hortic Sci Biotechnol 80:225–228
Ikegami A, Sato A, Yamada M, Kitajima A, Yonemori K (2005b) Expression of genes involved in proanthocyanidin biosynthesis during fruit development in a Chinese pollination-constant, nonastringent (PCNA) persimmon, ‘Luo Tian Tian Shi’. J Am Soc Hortic Sci 130:830–835
Ikegami A, Eguchi S, Yonemori K, Yamada M, Sato A, Mitani N, Kitajima A (2006) Segregations of astringent progenies in the F1 populations derived from crosses between a Chinese pollination-constant nonastringent (PCNA) ‘Luo Tian Tian Shi’, and Japanese PCNA and pollination-constant astringent (PCA) cultivars of Japanese origin. HortScience 41:561–563
Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K (2007) Identification of genes involved in proanthocyanidin biosynthesis of persimmon (Diospyros kaki) fruit. Plant Sci 172:1037–1047
Ikegami A, Eguchi S, Akagi T, Sato A, Yamada M, Kanzaki S, Kitajima A, Yonemori K (2011) Development of molecular markers linked to the allele associated with the non-astringent trait of the Chinese persimmon (Diospyros kaki Thunb.). J Jpn Soc Hortic Sci 80:150–155
Kanzaki S (2016) The origin and cultivar development of Japanese persimmon (Diospyros kaki Thunb.). J Jpn Soc Food Sci 63:328–330
Kanzaki S, Yonemori K, Sato A, Yamada M, Sugiura A (2000) Analysis of the genetic relationships among pollination-constant and non-astringent (PCNA) cultivars of persimmon (Diospyros kaki Thunb.) from Japan and China using amplified fragment length polymorphism (AFLP). J Jpn Soc Hortic Sci 69:665–670
Koes R, Verweij W, Quattrocchio F (2005) Flavonoids: a colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci 10:236–242
Li XM (2003) Resources investigation of ‘Luo Tian Tian Shi.’ South China Fruits 32:69–70 (in Chinese)
Li XM (2004) ‘Baohua’—an early maturing variety of pollination constant non-astringent persimmon native to China. Guangxi Hortic 15:22–23 (in Chinese)
Li GC, Yang Y, Wang RZ (2006) Germplasm resources in persimmon coming of China. China Seed Ind 4:52–53 (in Chinese)
Luo ZR, Wang RZ (2008) Persimmon in China: domestication and traditional utilizations of genetic resources. Adv Hortic Sci 22:239–243
Luo ZR, Li FF, Cai LH (1999) Molecular systematics of China native non-astringent persimmon based on random amplified polymorphic DNA. Acta Hortic Sin 26:297–301 (in Chinese with English summary)
Luo ZR, Zhang QL, Guo DY, Gu QQ (2005) General situation on science and industry of persimmon in China mainland. Acta Hortic 685:29–36
Luo C, Zhang QL, Luo ZR (2014) Genome-wide transcriptome analysis of Chinese pollination constant nonastringent persimmon fruit treated with ethanol. BMC Genom 15:112
Luo YJ, Zhang XN, Luo ZR, Zhang QL, Liu JH (2015) Identification and characterization of microRNAs from Chinese pollination constant non-astringent persimmon using high-throughput sequencing. BMC Plant Biol 15:11
Matsuo T, Ito S (1978) The chemical structure of kaki-tannin from immature fruit of the persimmon (Diospyros kaki). Agric Biol Chem 42:1637–1643
Matsuo T, Itoo S (1982) A model experiment for de-astringency of persimmon fruit with high carbon dioxide treatment: in vitro gelation of kaki-tannin by reacting with acetaldehyde. Agric Biol Chem 46:683–689
Min T, Yin XR, Shi YN, Luo ZR, Yao YC, Grierson D, Ferguson IB, Chen KS (2012) Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J Exp Bot 63:6393–6405
Mo RL, Yang SC, Huang YM, Chen WX, Zhang QL, Luo ZR (2016) ADH and PDC genes involved in tannins coagulation leading to natural de-astringency in Chinese pollination constant non-astringency persimmon (Diospyros kaki Thunb.). Tree Genet Genomes 12:17
Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13:2099–2114
Pan DS, Ma YP, Yu QY, Yi ZW, Zhang SH (1994) Survey the resources of ‘Luotian-tianshi’ and selection of excellent strains. Hubei For Sci Technol 2:24–28 (in Chinese)
Pan DS, Ouyang SX, Wang XQ, Chen CF, Deng XZ (2002) Suggestions to rename ‘Luotian-tianshi’ to ‘China PCNA persimmon.’ Hubei For Sci Technol 2:27–28 (in Chinese)
Pei X, Zhang QL, Guo DY, Luo ZR (2013) Effectiveness of the RO2 marker for the identification of non-astringency trait in Chinese PCNA persimmon and its possible segregation ratio in hybrid F1 population. Sci Hortic 150:227–231
Pesis E (2005) The role of the anaerobic metabolites, acetaldehyde and ethanol, in fruit ripening, enhancement of fruit quality and fruit deterioration. Postharvest Biol Technol 37:1–19
Pesis E, Levi A, Ben-Arie R (1986) De-astringency of persimmon fruits by creating a modified atmosphere in polyethylene bags. J Food Sci 51:1014–1016
Ramsay NA, Glover BJ (2005) MYB-bHLH-WD40 protein complex and the evolution of cellular diversity. Trends Plant Sci 10:63–70
Su FY, Hu J, Zhang QL, Luo ZR (2012) Isolation and characterization of a basic helix-loop-helix transcription factor gene potentially involved in proanthocyanidin biosynthesis regulation in persimmon (Diospyros kaki Thunb.). Sci Hortic 136:115–121
Sugiura A (1983) Relationships of ethanol production by seeds of different types of Japanese persimmon and their tannin content. HortScience 8:319–321
Tanaka T, Takahashi R, Kouno I, Nonaka G-I (1994) Chemical evidence for the de-astringency (insolubilization of tannins) of persimmon fruit. J Chem Soc Perkin Trans 1:3013–3022
Wang RZ (1983) The origin of ‘Luo Tian Tian Shi.’ Chin Fruit Tree 2:16–19 (in Chinese)
Wang Y, Zhang QL, Luo ZR (2010) Isolation and expression of gene encoding leucoanthocyanidin reductase (LAR) from Japanese persimmon (Diospyros kaki Thunb.) during fruit development. Biol Plant 54:707–710
Xie DY, Sharma SB, Paiva NL, Ferreira D, Dixon RA (2003) Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis. Science 299:396–399
Xu LQ (2008) Studies on the biological characters and new germplasm creation of some staminate germplasm in Diospyros L. native to China. Ph.D. dissertation, Huazhong Agricultural University, Wuhan (in Chinese with English summary)
Xu JC, Ding JG, Gan JP, Mo RL, Xu LQ, Zhang QL, Luo ZR (2017) ALDH2 genes are negatively correlated with natural deastringency in Chinese PCNA persimmon (Diospyros kaki Thunb.). Tree Genet Genomes 13:122
Yamada M, Sato A (2002) Segregation for fruit astringency type in progenies derived from crosses of ‘Nishimura-wase’ and pollination constant non-astringent genotypes in oriental persimmon (Diospyros kaki Thunb.). Sci Hortic 92:107–111
Yamada M, Sato A, Yakushiji H, Yoshinaga K, Yamane H, Endo M (1993) Characteristics of ‘Luo Tian Tian Shi’, a non-astringent cultivar of oriental persimmon (Diospyros kaki Thunb.) of Chinese origin in relation to non-astringent cultivars of Japanese origin. Bull Fruit Tree Res Sta 25:19–32 (in Japanese with English summary)
Yamada M, Taira S, Ohtsuki M, Sato A, Iwanami H, Yakushiji H, Wang RZ, Yang Y, Li GC (2002) Varietal differences in the ease of astringency removal by carbon dioxide gas and ethanol vapor treatments among Oriental astringent persimmons of Japanese and Chinese origin. Sci Hortic 94:63–72
Yan HY, Zhao HQ (2006) Non-astringent persimmon. China Agriculture Press, Beijing (in Chinese)
Yang SC, Jiang Y, Xu LQ, Shiratake K, Luo ZR, Zhang QL (2016) Molecular cloning and functional characterization of DkMATE1 involved in proanthocyanidin precursor transport in persimmon (Diospyros kaki Thunb.) fruit. Plant Physiol Biochem 108:241–250
Yang SC, Zhang M, Xu LQ, Luo ZR, Zhang QL (2020) MiR858b inhibits proanthocyanidin accumulation by the repression of DkMYB19 and DkMYB20 in persimmon. Front Plant Sci 11:576378
Yonemori K, Matsushima J (1985) Property of development of the tannin cells from non-astringent and astringent type fruits of Japanese persimmon (Diospyros kaki) and its relationship to natural astringency. J Jpn Soc Hortic Sci 54:201–208 (in Japanese with English summary)
Yonemori K, Mastushima J (1987) Changes in tannin cell morphology with growth and development of Japanese persimmon fruit. J Am Soc Hortic Sci 112:818–821
Yonemori K, Yamada M, Sugiura A (2000) Persimmon genetics and breeding. Plant Breed Rev 19:191–225
Yonemori K, Ikegami A, Kanzaki S, Sugiura A (2003) Unique features of tannin cells in fruit of pollination constant non-astringent persimmons. Acta Hortic 601:31–35
Yonemori K, Ikegami A, Kitajima A, Luo ZR, Kanzaki S, Sato A, Yamada M, Yang Y, Wang RZ (2005) Existence of several pollination constant non-astringent type persimmons in China. Acta Hortic 685:77–84
Zhang QL, Guo DY, Luo ZR (2009) Identification and taxonomic status of Chinese Diospyros spp. (Ebenaceae) androecious germplasms. Acta Hortic 833:91-96F
Zhang QL, Chen DM, Luo ZR (2013) Natural astringency loss property of a pollination-constant non-astringent persimmon newly found in central China. Acta Hortic 996:207–211
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Chen, W., Luo, Z. (2022). Chinese PCNA. In: Tao, R., Luo, Z. (eds) The Persimmon Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-031-05584-3_10
Download citation
DOI: https://doi.org/10.1007/978-3-031-05584-3_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05583-6
Online ISBN: 978-3-031-05584-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)