Abstract
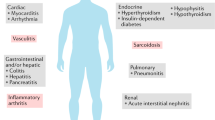
The development and approval of immune checkpoint inhibitors (ICIs) have dramatically improved the outcomes for many cancer patients. However, these agents have unique toxicities known as immune-related adverse events (irAEs), which result from unpredictable activation of the immune system against host organs. A wide myriad of musculoskeletal and rheumatologic irAEs have been reported, but inflammatory arthritis remains the most frequent. Here, we will highlight the current state of knowledge about the epidemiology, pathogenesis, salient clinical presentations, and treatment strategies of ICI-induced inflammatory arthritis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
18.1 Introduction
Cancer treatment has been transformed with the development and approval of immune checkpoint inhibitors (ICI). Several ICIs have been approved for the treatment of cancer, providing remarkable survival benefits in both metastatic and adjuvant settings. Immune checkpoint inhibitors are monoclonal antibodies that target the regulatory immune checkpoints which inhibit T cell activation [1]. Thus, ICIs take the brakes off of the immune system enhancing the host’s antitumor immune response. The approved ICIs are: (1) ipilimumab, and tremelimumab, which are antibodies against cytotoxic T lymphocyte-associated protein 4 (CTLA-4); (2) nivolumab, pembrolizumab, cemiplimab-rwlc, dostarlimab-gxly, and camrelizumab, which are antibodies against programmed death receptor-1 (PD-1); and (3) atezolizumab, durvalumab, and avelumab, which are antibodies against programmed death-ligand 1 (PD-L1). The use of combination therapy including 2 ICI with different mechanisms of action is becoming more common to address tumor resistance to treatment with a single agent.
Though effective in eliciting antitumor responses, ICIs often result in severe and occasionally fatal off-target inflammatory and autoimmune effects owing to unpredictable immune system activation against host organs [2]. While many of these immune-related adverse events (irAEs) are transient and resolve rapidly with immunosuppressive therapies and ICI discontinuation, some, especially, endocrinopathies, neurologic, and rheumatologic syndromes can have long-lasting effects and sequelae, significantly impacting patients’ function and activities of daily living (ADL).
18.2 Epidemiology
Arthralgia can occur in up to approximately 40% of patients receiving ICI. Definite arthritis with synovitis is less frequent and has been reported in up to 9% of patients treated with ICI. However, the true incidence and severity of arthritis-irAE are still undetermined as most studies are retrospective case series [3]. Arthritis-irAE seems to be underreported in oncology trials that use the Common Terminology Criteria for Adverse Events (CTCAE). The CTCAE version 5.0 defines arthritis as grade II if it limits instrumental ADL, which from a rheumatology perspective is considered sufficient for treatment as musculoskeletal functional limitations severely affect the quality of life. However, many trials report primarily irAE grades III–V, and arthritis may have been initially underreported. Prospective longitudinal studies including denominators with all patients treated with ICI are necessary to accurately estimate the true incidence rate, severity, and outcome of arthritis-irAE.
18.3 Risk Factors
Arthritis-irAE occurs more frequently in patients receiving combination ICI therapy. A higher proportion of irAEs, in general, have been reported in Caucasians compared to African Americans; however, no racial association with arthritis-irAE was identified [4]. Although the female gender was found in one study to be an independent risk factor for irAE, it does not seem to be a risk factor for developing arthritis-irAE. Age has varied widely at presentation and does not appear to be independently associated with developing arthritis-irAE. Of note, body mass index ≥25 kg/m2 was recently identified as a risk factor for irAE, including arthritis and other rheumatologic irAEs, in patients receiving anti-CTLA-4 or anti-PD-1/PD-L1 monotherapies, or ICI combination therapies [5]. As for cancer types, rheumatologic irAEs and arthritis-irAE may occur more frequently in patients with melanoma and genitourinary cancer receiving ICI [4]. Treatment with ICI combination therapy, glucocorticoid use within 1 year before ICI initiation, and history of preexisting autoimmune diseases are also associated with rheumatologic irAEs and arthritis-irAE [4]. Notably, patients with preexisting inflammatory arthritis (rheumatoid, psoriatic, and spondyloarthritis) can experience arthritis flares upon ICI initiation, and this risk is higher among patients with active symptoms and in those who discontinued immunosuppressant therapy at ICI initiation. Moreover, the presence of autoantibodies including antinuclear antibodies (ANA), rheumatoid factor (RF), antithyroglobulin, and antithyroid peroxidase before ICI initiation have been identified as risk factors for developing irAEs, in general, although these results were not specific to rheumatologic irAEs or arthritis-irAE [6]. Some patients with arthritis-irAE were found to have preexisting anti-citric citrullinated peptide (anti-CCP) antibodies, suggesting that a pre-RA status can manifest clinically after ICI initiation. So far, no new autoantibodies have been identified in association with de novo arthritis-irAE.
18.4 Pathogenesis
Our understanding of the pathogenic mechanisms of irAEs, including arthritis-irAE, is still limited. Several mechanisms have been proposed [7]: (1) breach of self-tolerance and enhanced preexisting autoimmunity result from generalized immune activation induced by ICIs. In arthritis-irAE, immunoprofiling of the synovial fluid showed expanded CD38hi CD8 T cells and Th17 cells; (2) release of cytokines and chemokines from immune cells causing damage in tissues with an anatomic predisposition. Indeed, circulating cytokines such as the colony-stimulating factors (G-CSF and GM-CSF), chemokines (fractalkine), growth factors (FGF-2) , interferons (IFNα2 and IFNγ), and interleukins (IL12p70, IL1α, IL1β, IL1RA, IL2, IL6, IL13, and IL-17) have been linked to irAEs development [6]. In arthritis-irAE, the successful use of antitumor necrosis factor (anti-TNF) and anti-IL-6 receptor (anti-IL-6R) antibodies for arthritis management suggest a role of these cytokines in pathogenesis [8, 9]; (3) cross-antigen reactivity against tumor-specific antigens and self-antigens released from healthy tissues located within and around the tumor milieu; (4) off-target effect of ICI therapy leading to damage in nonhematopoietic cells that express the target ligand; (5) genetic predisposition must play a role in irAEs susceptibility. Germline genetic features identified through small pilot studies suggested shared biological pathways between irAEs development and autoimmune diseases [6]. Of note, carriers with interferon-gamma (IFNG)—1616T > C single nucleotide polymorphism homozygous variant were found to have an increased risk for rheumatologic irAEs, and the presence of human leukocyte antigen (HLA) DRB1 shared epitope alleles (a known risk factor for rheumatoid arthritis) was found to be higher in patients with arthritis-irAE compared with healthy controls; and (6) apart from the usual factors, gut microbiota, primarily Bacteroides intestinalis were associated with irAEs development in ICI combination therapy-treated patients [6].
18.5 Clinical Features
Arthritis-irAE can present anytime after initiation of ICI therapy; immediately after receiving the first dose or as a late adverse event (AE) occurring 44 months posttreatment, and it may persist even after ICI discontinuation. Some patients may initially present with arthralgia, with or without joint stiffness, but develop overt synovitis over time. Most patients developing arthritis-irAE have an undifferentiated clinical presentation that does not always fulfill diagnostic criteria for primary autoimmune inflammatory arthritis. We describe below the most common patterns (Table 18.1):
Undifferentiated inflammatory arthritis: Patients may present with oligoarthritis or polyarthritis of large joints such as knees, ankles, or wrists, which can be symmetric or asymmetric in distribution [10]. Sometimes patients present with monoarthritis. These patients are negative for RF and anti-CCP, although some may be ANA positive. They generally have normal radiographs at presentation; however, they can have persistent inflammation resulting in erosive disease [11, 12].
Rheumatoid arthritis (RA)-like: Patients may present with symmetrical polyarthritis predominantly involving small joints of the hands and wrists, along with positive RF and/or anti-CCP antibodies in their sera, fulfilling the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) diagnostic criteria for rheumatoid arthritis (RA) [10]. While not commonly reported, this pattern is potentially erosive and may lead to permanent joint damage.
Seronegative spondyloarthropathy (SPA)-like: In addition to oligo/polyarthritis, some patients present with axial disease (inflammatory back pain or cervical pain) and enthesopathy (pain/tenderness in connective tissues between bones and tendons or ligaments such as the heel or iliac crest) [12]. The facet, costovertebral, and sacroiliac joints are involved [12, 13], but unlike primary spondyloarthritis, the few reported patients tested negative for Human Leukocyte Antigen (HLA)-B27 alleles. Moreover, psoriatic arthritis with and without skin changes also presents following ICI initiation; all reported patients were seronegative and none had a prior history of psoriasis [10]. The triad of reactive arthritis (arthritis, conjunctivitis, and sterile urethritis) has been observed in some patients, especially after receiving ICI combination therapy [10, 12].
Polymyalgia rheumatica (PMR)-like: Some patients present with morning stiffness and pain of both shoulders and hips, with elevated inflammatory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP), and negative RF and anti-CCP antibodies, fulfilling the 2012 EULAR/ACR classification criteria for polymyalgia rheumatica (PMR) [14]. In these patients, joint swelling is not typical, but some develop effusions in shoulders/hips, subdeltoid bursitis, or biceps tenosynovitis, which can be seen by ultrasound or MRI.
PMR/arthritis overlap: Some patients have atypical PMR features with inflammatory arthritis involving other joints most commonly the knees, followed by the small joints of the hands and elbows. A few patients have been reported presenting with normal inflammatory markers. Patients with PMR have normal muscle strength and creatine kinase (CK) levels within normal limits. Unlike primary PMR, patients with PMR-irAE may require higher doses of glucocorticoids, exceeding 20 mg daily of prednisone.
Concomitant giant cell arteritis (GCA) has been occasionally reported, with patients presenting with jaw claudication, temporal headache, scalp tenderness , and vision loss.
Remitting seronegative symmetrical synovitis with pitting edema has been reported following ICI therapy, while all reported patients had elevated ESR and CRP and were negative for autoantibodies [10, 15].
Tenosynovitis: Some patients develop tenosynovitis in the hands, forearms, shoulders, and/or knees. Tenosynovitis may occur either alone or associated with arthritis. A few patients were found to be ANA positive [16].
Further studies are required to understand why patients present with such different clinical patterns. For some presentations such as RA-like, or psoriatic arthritis, it is possible that patients may have a preexisting subclinical disease or predisposing genotypes, and that therapy with ICI triggers the subsequent clinical disease. Some patients who developed RA-like arthritis were found to have RF and/or anti-CCP antibodies in serum before they received ICI [6]. Some patients with cutaneous psoriasis also developed psoriatic arthritis after ICI treatment. One study has reported variations in clinical presentation and outcomes in patients receiving anti-PD1 alone compared to those receiving ICI combination [12]. Patients receiving single-agent anti-PD1 were more likely to develop arthritis-irAE as the only toxicity, presenting with small joints arthritis. On the other hand, those who received ICI combination were more likely to develop multiple other irAEs and to present with knee arthritis [12]. Persistence of arthritis-irAE after ICI discontinuation was reported in more than 50% of the patients in this cohort [17] and was associated with having received ICI combination, and with a longer duration of ICI treatment.
18.6 Diagnosis
The ACR generally recommends five measures for RA disease activity including (1) clinical disease activity index (CDAI); (2) simple disease activity index (SDAI); (3) disease activity score-28-ESR (DAS28 ESR); (4) disease activity score-28-CRP (DAS-28 CRP), and routine assessment of patient index data 3 (RAPID 3) and three measures for RA functional assessment including (1) health assessment questionnaire-II (HAQ-II); (2) patient activity scale II (PAS II); and patient-reported outcomes measurement information system short form—physical function 10a (PROMIS PF10a) for use in clinical practice. While few of these measures have been utilized for evaluating patients with arthritis-irAE, they still need to be validated in this setting.
Laboratory testing should include: (1) ESR and CRP, which are typically elevated in patients with active inflammatory symptoms although not seen in all patients with arthritis-irAE; (2) ANA, RF, and anti-CCP, most patients predominantly those with undifferentiated arthritis are typically negative; (3) HLA-B27 primarily in patients presented with seronegative spondyloarthropathy-like arthritis; (4) Muscle enzymes in patients presenting with PMR-like arthritis, though are typically normal; (5) Joint aspiration and synovial fluid analysis, which typically reveals inflammation with neutrophilic predominance; and (6) hepatitis B and C, human immunodeficiency virus (HIV), and tuberculosis primarily if patients will require immunosuppressive therapies as this may result in reactivation of latent infection. Of note, several biomarkers (blood-based, immunogenetic, and microbial) were suggested as predictors for irAEs development, including endocrine toxicities, colitis, dermatitis, and pneumonitis [6]. However, predictive biomarkers for arthritis-irAE have not been suggested yet. Future studies with a prospective standardized collection of biospecimens (blood and synovial fluid) are important to identify predictive biomarkers for arthritis-irAE.
Imaging can be employed to confirm diagnosis early on and to exclude other possible causes of arthritis. Plain radiography of the affected joints can detect joint erosions and joint space narrowing. Whereas ultrasound and magnetic resonance (MRI) images can detect synovitis, inflammatory signals, tendinitis, enthesopathy, and erosions [11]. Importantly, in patients presenting with PMR-like along with features suggestive of GCA, urgent ophthalmological exam and temporal artery biopsy should be considered due to the known risk of permanent blindness with this type of vasculitis. Also, MRI, electromyography, or muscle biopsy may be considered to exclude muscle inflammation or myopathy in case of diagnostic uncertainty. Close monitoring is required for patients with arthritis-irAE with periodic clinical evaluations including joint examination and serial testing of inflammatory markers (ESR and/or CRP) every 4–6 weeks to monitor the therapeutic response until symptoms improve. Imaging should also be repeated to follow up for structural damage.
18.7 Differential Diagnosis
At presentation, clinicians typically confirm that symptoms started after initiation of ICI and exclude other conditions that may cause similar symptoms including preexisting autoimmune disease, osteoarthritis, or crystal arthritis as few patients have been reported with worsening or recurrent symptoms following ICI initiation. Paraneoplastic arthritis should also be excluded especially if symptoms started around the time of cancer diagnosis. Additionally, metastatic disease within the adjacent bone or joint structures, and septic arthritis should be excluded primarily in patients with monoarthritis.
18.8 Management
The guidelines for the management of irAEs have been published by several key oncology and rheumatology societies, based on the severity of presentation as per CTCAE grades [8, 9]. For arthritis-irAE, a prompt rheumatology consult is recommended if there is joint pain for more than 4 weeks, joint swelling, arthritis ≥ grade 2, or unable to taper corticosteroids to <10 mg/day within 4 weeks, and to identify signs of joint damage early on. Afterward, an assessment should be made for the need for arthrocentesis/intra-articular corticosteroid injection, and initiation/optimal dosing of disease-modifying anti-rheumatic drugs (DMARDs) . The decision to initiate ICI therapy in patients with preexisting inflammatory arthritis requires a rheumatology-oncology multidisciplinary approach to carefully weigh the benefit/risk ratio while considering the severity of the underlying autoimmune disease, the prognosis of cancer, alternative therapies, and patients’ preferences. For these patients, it is important to keep their baseline immunosuppressive regimen at the lowest efficient dose before ICI initiation as these patients are at high risk of arthritis flare after therapy initiation. Generally, the existing guidelines define three treatment escalations for arthritis-irAE, which are summarized in Table 18.2 [8, 9].
Mild arthritis-irAE (grade 1 per CTCAE): Patients with mild arthritis with no functional impact on their ADL are managed with acetaminophen or non-steroidal anti-inflammatory drugs (NSAIDs) if there is no contraindication, and/or intra-articular corticosteroid injection. Continuation of ICI therapy is recommended. However, if arthritis does not improve within 4 weeks, treatment should be escalated to the next step.
Moderate arthritis-irAE (grade 2 per CTCAE): Patients with moderate arthritis with functionally impacted ADL, but not interfering with self-care are managed with 10–20 mg/day of oral prednisone or equivalent for 4 weeks, and intra-articular corticosteroid injection if ≤2 large joints are involved. ICI therapy should be placed on temporary hold. If arthritis improves, oral prednisone should be tapered slowly over 4–6 weeks, and ICI therapy should be resumed when prednisone is ≤10 mg/day. However, if arthritis does not improve within 4 weeks, or if unable to taper prednisone to ≤10 mg/day after 6–8 weeks, treatment should be escalated to the next step and initiation of DMARDs is recommended.
Severe arthritis-irAE (grade 3 and 4 per CTCAE): Patients with severe arthritis impacting self-care and ADL are managed with 0.5–1 mg/kg/day of oral prednisone or equivalent. ICI therapy should be placed on temporary hold. If arthritis improves, oral prednisone should be tapered slowly over 4–6 weeks, and treatment with ICI should be resumed when prednisone is ≤10 mg/day. However, if arthritis does not improve within 2 weeks or if worsening of symptoms is noted, initiation of conventional synthetic DMARDs (cs-DMARDs) is recommended; methotrexate, hydroxychloroquine, sulfasalazine, or leflunomide (alone or in combination) are the most common at doses used to treat RA. In case of severe or refractory arthritis, the use of certain biological DMARDs (b-DMARDs) such as anti-TNF or anti-IL-6R antibodies is recommended. One should keep in mind that persistent inflammation, as well as treatment with corticosteroids (prednisone of ≥2.5 mg/day or equivalent for ≥3 months), increase the risk of osteoporosis, and therefore patients with arthritis-irAE should be encouraged to maintain a healthy lifestyle with adequate nutrition and weight-bearing exercises and may require pharmacological therapy [18]. While on DMARDs, these patients also need close monitoring of neutrophil and platelet counts, serum lipids, liver transaminases, and serum creatinine. If arthritis improves to grade 1, ICI therapy should be resumed but should be discontinued permanently if there is no improvement after 4–6 weeks of treatment.
Finally, it is worth mentioning that the occurrence of irAEs has been suggested as a surrogate for effective antitumor immune response; patients with any grade irAEs were found to have higher objective response rate, disease control rate, and overall survival, and those with grade 2 or higher irAEs or multiple irAEs had better progression-free survival and overall survival [19]. Similarly, patients who develop rheumatic and musculoskeletal irAEs per se were found to have better tumor response [20]. Therefore, future studies should focus on investigating how we can effectively manage irAEs without hindering the antitumor immune response to ICI therapy. While published guidelines endorse corticosteroids as first-line therapy for irAEs, targeted therapies could be safer and preferable to corticosteroids especially for arthritis-irAE, which may likely require prolonged therapy. Studies from melanoma and non-small cell lung cancer patients treated with ICIs have shown that the use of prednisone ≥10 mg/day led to detrimental cancer outcomes and worsen survival [21, 22]. Also, the timing of treatment initiation was found to affect the response to ICI therapy; patients treated with corticosteroids within the first 2 months after ICI initiation had shorter progression-free survival and overall survival as compared to those who received corticosteroids later [23]. With regard to corticosteroid-sparing agents, one study showed that the use of cs-DMARDs or b-DMARDs for arthritis-irAE did not impact the tumor response to ICI [17]. However, another study showed that the use of hydroxychloroquine led to decreasing the efficacy of anti-PD-1 agents [24]. Players in the autoimmune pathways can be targeted, such as TNF-alpha, but, given its role in antitumor immunity, concerns remain regarding the safety of prolonged anti-TNF therapy and its impact on survival [25]. On the other hand, anti-IL-6R antibody shows promising results for irAE management; a recent systematic review of the literature provided data on 91 patients, where the use of tocilizumab resulted in clinical benefit and none of them were reported with tumor progression [26]. Another study that combined translational, preclinical, and clinical analyses, identified that targeting IL-6 could be an effective approach for irAE management while maintaining and possibly boosting tumor immunity [27]. However, anti-IL-6R antibody might not be suitable for patients who also had colitis-irAE or preexisting inflammatory bowel disease due to the potential risk of intestinal perforation, although isolated case reports have not shown complications in these patients [28, 29]. To date, there is only one published case reporting the use of tofacitinib; JAK inhibitor, for treatment of arthritis-irAE in a patient with metastatic lung adenocarcinoma who achieved remission of arthritis and cancer [30]. However, the FDA has recently announced a black box warning on tofacitinib use due to concerns about the increasing risk of serious cardiovascular adverse events, thrombosis, cancer, and death. To our knowledge, 15 clinical trials are currently investigating the use of therapies for prevention and management of irAEs in patients receiving ICI therapy (Table 18.3).
18.9 Conclusion
Immune checkpoint inhibition has transformed cancer treatment. However, ICIs often result in off-target inflammatory and autoimmune effects. The chances of irAEs are higher in those with preexisting autoantibodies and rheumatic diseases. Thus, the decision to initiate ICIs in such patients should be taken in consultation with a rheumatologist. Treatment of arthritis irAEs involves temporary withdrawal of the ICI and the administration of NSAIDs, glucocorticoids, and/or DMARDs depending upon the severity of the irAE. Rare cases may however require permanent discontinuation of ICI therapy.
References
Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–86.
Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse events associated with immune checkpoint blockade in patients with cancer: a systematic review of case reports. PLoS One. 2016;11(7):e0160221.
Abdel-Wahab N, Suarez-Almazor ME. Frequency and distribution of various rheumatic disorders associated with checkpoint inhibitor therapy. Rheumatology (Oxford, England). 2019;58(Suppl 7):vii40–8.
Cunningham-Bussel A, Wang J, Prisco LC, Martin LW, Vanni KMM, Zaccardelli A, et al. Predictors of rheumatic immune-related adverse events and de novo inflammatory arthritis after immune checkpoint inhibitor treatment for cancer. Arthritis Rheumatol. 2022;74(3):527–40. https://doi.org/10.1002/art.41949. Epub ahead of print.
Guzman-Prado Y, Ben Shimol J, Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunol Immunother. 2021;70(1):89–100.
Hommes JW, Verheijden RJ, Suijkerbuijk KPM, Hamann D. Biomarkers of checkpoint inhibitor induced immune-related adverse events—a comprehensive review. Front Oncol. 2021;10:585311.
Esfahani K, Elkrief A, Calabrese C, Lapointe R, Hudson M, Routy B, et al. Moving towards personalized treatments of immune-related adverse events. Nat Rev Clin Oncol. 2020;17(8):504–15.
Haanen J, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv264–6.
Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–126. https://doi.org/10.1200/JCO.21.01440. Epub ahead of print.
Pundole X, Abdel-Wahab N, Suarez-Almazor ME. Arthritis risk with immune checkpoint inhibitor therapy for cancer. Curr Opin Rheumatol. 2019;31(3):293–9.
Albayda J, Dein E, Shah AA, Bingham CO 3rd, Cappelli L. Sonographic findings in inflammatory arthritis secondary to immune checkpoint inhibition: a case series. ACR Open Rheumatol. 2019;1(5):303–7.
Cappelli LC, Brahmer JR, Forde PM, Le DT, Lipson EJ, Naidoo J, et al. Clinical presentation of immune checkpoint inhibitor-induced inflammatory arthritis differs by immunotherapy regimen. Semin Arthritis Rheum. 2018;48(3):553–7.
Feist J, Murray A, Skapenko A, Schulze-Koops H. A rare side effect of checkpoint inhibitor therapy-nivolumab-induced axial polyarthritis of the facet and costovertebral joints. Arthritis Rheumatol. 2019;71(11):1823.
Calabrese C, Cappelli LC, Kostine M, Kirchner E, Braaten T, Calabrese L. Polymyalgia rheumatica-like syndrome from checkpoint inhibitor therapy: case series and systematic review of the literature. RMD Open. 2019;5(1):e000906.
Yamamoto S, Fujita S, Mukai T, Sawachika H, Morita Y. Paraneoplastic remitting seronegative symmetrical synovitis with pitting edema syndrome should be treated with low-dose prednisolone during pembrolizumab therapy. Intern Med. 2020;59(4):597–8.
Murakami S, Nagano T, Nakata K, Onishi A, Umezawa K, Katsurada N, et al. Tenosynovitis induced by an immune checkpoint inhibitor: a case report and literature review. Intern Med. 2019;58(19):2839–43.
Braaten TJ, Brahmer JR, Forde PM, Le D, Lipson EJ, Naidoo J, et al. Immune checkpoint inhibitor-induced inflammatory arthritis persists after immunotherapy cessation. Ann Rheum Dis. 2020;79(3):332–8.
Kobza AO, Herman D, Papaioannou A, Lau AN, Adachi JD. Understanding and managing corticosteroid-induced osteoporosis. Open Access Rheumatol. 2021;13:177–90.
Bai R, Li L, Chen X, Chen N, Song W, Zhang Y, et al. Correlation of peripheral blood parameters and immune-related adverse events with the efficacy of immune checkpoint inhibitors. J Oncol. 2021;2021:9935076.
Adda L, Batteux B, Saidak Z, Poulet C, Arnault JP, Chauffert B, et al. Rheumatic and musculoskeletal disorders induced by immune checkpoint inhibitors: consequences on overall survival. Joint Bone Spine. 2021;88(4):105168.
Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018;36(28):2872–8.
Faje AT, Lawrence D, Flaherty K, Freedman C, Fadden R, Rubin K, et al. High-dose glucocorticoids for the treatment of ipilimumab-induced hypophysitis is associated with reduced survival in patients with melanoma. Cancer. 2018;124(18):3706–14.
Maslov DV, Tawagi K, Kc M, Simenson V, Yuan H, Parent C, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J Immunother Cancer. 2021;9(7):e002261.
Krueger J, Santinon F, Kazanova A, Issa ME, Larrivee B, Kremer R, et al. Hydroxychloroquine (HCQ) decreases the benefit of anti-PD-1 immune checkpoint blockade in tumor immunotherapy. PLoS One. 2021;16(6):e0251731.
Verheijden RJ, May AM, Blank CU, Aarts MJB, van den Berkmortel F, van den Eertwegh AJM, et al. Association of anti-TNF with decreased survival in steroid refractory ipilimumab and anti-PD1-treated patients in the Dutch melanoma treatment registry. Clin Cancer Res. 2020;26(9):2268–74.
Campochiaro C, Farina N, Tomelleri A, Ferrara R, Lazzari C, De Luca G, et al. Tocilizumab for the treatment of immune-related adverse events: a systematic literature review and a multicentre case series. Eur J Intern Med. 2021;S0953-6205(21):00266–1.
Hailemichael Y, Johnson D, Abdel-Wahab N, Foo WC, Daher M, Haymaker C, et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell. 2022;9;40(5):509–23.e6. https://doi.org/10.1016/j.ccell.2022.04.004. PMID: 35537412. Epub 2022 May 9.
Kim ST, Tayar J, Trinh VA, Suarez-Almazor M, Garcia S, Hwu P, et al. Successful treatment of arthritis induced by checkpoint inhibitors with tocilizumab: a case series. Ann Rheum Dis. 2017;76(12):2061–4.
Uemura M, Trinh VA, Haymaker C, Jackson N, Kim DW, Allison JP, et al. Selective inhibition of autoimmune exacerbation while preserving the anti-tumor clinical benefit using IL-6 blockade in a patient with advanced melanoma and Crohn’s disease: a case report. J Hematol Oncol. 2016;9(1):81.
Murray K, Floudas A, Murray C, Fabre A, Crown J, Fearon U, et al. First use of tofacitinib to treat an immune checkpoint inhibitor-induced arthritis. BMJ Case Rep. 2021;14(2).
Acknowledgments
We thank Dr. Sirisha Yadugiri, the Senior Technical Writer in the Department of Melanoma Medical Oncology at The University of Texas MD Anderson Cancer Center for editorial support.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Abdel-Wahab, N., Suarez-Almazor, M.E. (2022). Arthritis Associated with Immune Checkpoint Inhibitors. In: Ravindran, V., Santhanam, S., Goyal, M. (eds) Rarer Arthropathies. Rare Diseases of the Immune System. Springer, Cham. https://doi.org/10.1007/978-3-031-05002-2_18
Download citation
DOI: https://doi.org/10.1007/978-3-031-05002-2_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-05001-5
Online ISBN: 978-3-031-05002-2
eBook Packages: MedicineMedicine (R0)