What You Will Learn in This Chapter
Why optical sectioning is required to image thick fluorescent specimens
How microscopists manage fluorescence blurring
What we mean by ‘optical sectioning’
Why the confocal microscope is used to obtain blur-free images by optical sectioning
How the confocal microscope works
When to select the confocal microscope to image fluorescent specimens
How to operate a single beam-scanning confocal microscope
How to improve signal-to-noise ratios (SNR) in your images
What alternatives are available to using the confocal microscope for optical sectioning
What to consider when using the confocal microscope as a quantitative instrument
How to keep your confocal microscope functioning optimally
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optical sectioning
- Confocal
- Laser-scanning
- LSCM
- Fluorescent blurring
- Deconvolution
- Pinhole
- Pixel re-assignment
Why optical sectioning is required to image thick fluorescent specimens
How microscopists manage fluorescence blurring
What we mean by ‘optical sectioning’
Why the confocal microscope is used to obtain blur-free images by optical sectioning
How the confocal microscope works
When to select the confocal microscope to image fluorescent specimens
How to operate a single beam-scanning confocal microscope
How to improve signal-to-noise ratios (SNR) in your images
What alternatives are available to using the confocal microscope for optical sectioning
What to consider when using the confocal microscope as a quantitative instrument
How to keep your confocal microscope functioning optimally
5.1 Introduction
The confocal microscope is key to successfully viewing and analysing thick fluorescent specimens. Whilst fluorescence is a very versatile contrast enhancement technique that—under the right conditions—is capable of producing very high contrast images, it suffers from three inherent disadvantages: bleaching, bleed-through and blurring. The scientific worker and microscopist must be aware of these three phenomena and manage them to acquire scientifically rigorous images. The first is bleaching, which has been covered in the chap. 3 of this volume on fluorescence microscopy. Light and free oxygen radicals bleach fluorescent molecules so that they don’t forever emit light when illuminated. Think of bleaching as an ‘ageing’ process. The second disadvantage of fluorescence is bleed-through. It occurs when a sample is labelled with more than one fluorophore. The third disadvantage is blurring, and this necessitates using ‘optical sectioning’ in one form or another to acquire a blur-free image. The multi-photon microscope is used to image very thick tissue samples, discussed elsewhere in this book. In this chapter we discuss optical sectioning stratagems, in particular the function and operation of the single beam-scanning confocal microscope to obviate fluorescent blurring.
5.2 Fluorescence Blurring
5.2.1 The Advantages and Disadvantages of Fluorescence
Fluorophores have been used for many years to label cells and tissues [1]. Biological systems are highly dynamic environments with molecules diffusing around the cell or undergoing active transport along protein or nucleic acid tracks. Even when fixed, cells and tissues still represent complex environments worthy of study. Inorganic fluorophores, quantum dots, conjugate tags and fluorescent proteins are suited to marking and tracking proteins and other cell moieties [2,3,4] allowing us to see cells, and parts of cells, that would not be possible to see otherwise. Reverting to fundamentals, a microscope must provide:
-
1.
Resolving power to carry fine detail in the specimen to the image, with
-
2.
sufficient contrast to show differences between image features and the background, at
-
3.
sufficient magnification to present the resolved detail to the eye or digital detector.
Whilst the primary function of the microscope is to resolve fine detail, this cannot be achieved satisfactorily unless sufficient contrast is present in the image. It is more difficult to resolve details in a pellucid image arising from an unstained object than one that is stained. Resolving power and contrast are therefore linked [5]. Of all the contrast enhancing techniques, if properly applied, fluorescent markers give the highest contrast with a dark background and also high signal-to-noise ratio. Fluorescent labels are advantageous in that they are highly sensitive even at low concentration and, generally, non-destructive to the target cell or tissue.
To summarise, fluorescent markers offer the following advantages to marking cells and tissues: fluorophores are self-luminous and under the right conditions exhibit very high contrast; they are very sensitive markers, detecting small concentrations of proteins and cell moieties; they are very specific markers. With proper control of background and blocking of non-specific signal, fluorescent markers generally give good discrimination of the tissues, cells, organelles and proteins they are employed to mark. A wide range of fluorescent probes are available, and these can be used simultaneously to label multiple targets in cells and tissues. Endogenous fluorescent proteins are very versatile and can also be used to label multiple cell components. Finally, fluorophores are relatively cheap and easy to apply and the high quality CCD, CMOS and PMT detectors available are able to collect weak signals easily. These are the advantages afforded by using fluorescent probes.
Against these important advantages there are three disadvantages that must be considered and managed in order to achieve meaningful and scientifically rigorous data with high signal-to-noise ratios. These are: bleaching, blurring and bleed-through. Unless the specimen is very thin, blurring in the image is probably the most serious of the three disadvantages that must be addressed. At present, we shall discuss blurring, for bleaching is addressed in the chapter on fluorescence and we shall briefly address bleed-through when covering the operation of the confocal microscope.
5.2.2 Why Blurring Occurs
Fluorophores are self-luminous; when illuminated with light of sufficient energy they emit light (for an excellent review, see [6]). The entire thickness of the fluorescently-labelled cell or tissue fluoresces, as shown in Fig. 5.1. Moreover, the depth of field of the high numerical aperture microscope objectives used to acquire the image is very small. The depth of field is the axial depth of the space on both sides of the object plane within which the object can be moved without detectable loss of sharpness in the image, and within which features of the object appear acceptably sharp in the image while the position of the image plane is maintained.
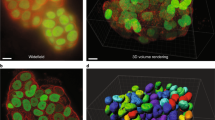
Typically, the objective depth of field is less than 1 μm, and values of 200–400 nm are common. Even flattened adherent cells grown onto a coverslip are much thicker than this, approximately 3–5 μm [7, 8]. The result will be a low-contrast image, composed of a bright but very blurred background upon which is superimposed the much dimmer in-focus information (Fig. 5.1). That part of the image which is in focus will be degraded by light that is emitted or scattered by the tissue outside the narrow plane of focus.
Since the entire fluorescently-stained tissue, section or cell emits signal when illuminated with high energy light, and because the thickness of the fluorescently-stained sample is greater than the depth of field of the microscope objective (a) blurring will occur. The effect is shown in (b) with two different samples: kidney (upper panels) and cilia in the node of a mouse embryo (lower panels). The left-hand panels (upper and lower) show the fluorescent blurring that occurs, whilst the right-hand panels show the improvement from acquiring a single optical section with the confocal microscope. Compare this figure with Fig. 5.11 showing a maximum intensity projection of an entire z-stack. Mouse images courtesy of Dr. J Keynton. Reproduced from Understanding Light Microscopy with permission from John Wiley & Sons Ltd. Image copyright, author: J Sanderson
If an adherent cell is, say, 4 μm thick and the depth of field of the microscope objective is 400 nm, then 90% of the image seen will be blurred because most of the light is contributed by regions that are not exactly in focus. The contribution of the blurred background light from the out-of-focus regions is superimposed over the weaker (less-intense) in-focus image. This will reduce the signal-to-background ratio, and thus the contrast, of the image. This out-of-focus haze will also reduce the resolution of detail in the image. Signal-to-noise ratios (SNR) and signal-to-background ratios (SBR) are different. The SBR is a measure of contrast in an image whereas SNR describes the variability in photon intensity in time at a single sampling point, or pixel, in the image [9]. A confocal microscope generates good SBRs, but is still inherently noisy, for reasons we shall discuss later.
For non-adherent cells (cells generally round up when undergoing division) and tissues or tissue slices, the situation is worse. With cells and tissues of a thickness greater than about 10 μm, scattering of light from the refractive index differences between the cell, its constituents and the local environment also begin to degrade image quality [10, 11]. This scattering occurs because curved surfaces of moieties in the cell act as microlenses scattering and reflecting light randomly. Light may be scattered whilst propagating down both the illumination and imaging pathways [12]. The reason the multi-photon microscope (Chap. 9) is successful at viewing very thick tissues (those that are too thick to be imaged by the confocal microscope) is because non-linear multi-photon microscopy is insensitive to scattering of the emitted signal; the longer-wavelength (red; infrared) illumination scatters minimally; the optical train is simpler, does not require descanning and the excitation volume at the plane of focus is exceedingly small [13, 14].
In order to acquire a thin blur-free section, or an in-focus blur-free z-stack of images, we must employ the so-called optical sectioning in one form or another. If the cells or tissue section that you are investigating is suitably thin, then use a widefield fluorescence microscope to acquire your images. The flow chart in Fig. 5.2 will guide you in the choice of which microscope to use.
5.2.3 Optical Sectioning
The confocal approach to optical sectioning scans the illumination across the image sequentially, the single-beam point-scanning confocal being the most widespread and popular of all the optical sectioning fluorescence microscopes. Several designs are available on the market, but they all do one thing: optical sectioning is achieved by point illumination and point detection. By restricting the illumination to a pinhole, rather than illuminating the entire field of view at once it becomes possible to build up a blur-free image in three dimensions (Fig. 5.3). The entire image is built up point by point in each frame as the diffraction-limited spot of the illuminating beam rasters across the frame, like reading words in lines along a page of script in a book. To take the analogy further, each two-dimensional section is like a page, and a 3-D so-called z-stack (a series of multiple images taken at different lateral focal planes to provide a composite image with a greater depth of field) is equivalent to a chapter or entire book. The single-beam laser-scanning microscope does not form a real image, as a widefield microscope does, but builds it up pointwise.
The ray path of a single-beam, laser-scanning confocal microscope, showing how (a) only the in-focus signal passes through the pinhole to the PMT detector. The versatility of the confocal microscope allows data to be collected in up to five dimensions (x, y, z, t and λ) as shown in (b). Panel (a) reproduced with permission from Springer. Panel (b) reproduced from Understanding Light Microscopy with permission from John Wiley & Sons Ltd
Marvin Minsky originated the concept of optical sectioning in 1955 to produce blur-free images, motivated by the need to see and understand how neural networks were connected. He reasoned that the blurring and scattering ‘would be gone if we could only illuminate one specimen point at a time’. In a delightful memoir well worth reading [15] he goes on to say ‘the price of single-point illumination is being able to measure only one point at a time. This is why the confocal microscope must scan the specimen point by point and that can take a long time’. Minsky also commented ‘In retrospect, it occurs to me that this concern for real time speed may have been what delayed the use of that scheme [confocal microscopy] for almost thirty years’. Figure 3 of Minsky’s patent application in 1961 is remarkably similar to the configuration of the modern single-beam point-scanning confocal (see also Fig. 1 in [16]). Practical implementation of Minsky’s design was impeded firstly by the lack of sufficiently bright illumination, secondly from a means to display and capture the image and thirdly because scanning was implemented by moving the stage rather than the illuminating beam. Stage-scanning confocal microscopes are mainly used now for inspection in the microchip industry and in materials science where specimens are very much larger and flatter than biological tissues. Beam scanning does not vibrate or insult delicate biological tissues, is not limited in the raster scan by the weight of the stage, does not suffer loss of resolving power from mechanical vibration or geometrical distortion in the image and is potentially much faster.
Mojmír Petráň developed the reflected-light tandem scanning confocal microscope in 1967, a pocket-sized instrument with multiple pinholes that was notoriously difficult to align, but the image could be seen directly in real time. The Nipkow disc of Petráň’s design attenuated the illumination and signal to such an extent that only the brightest-stained samples could be viewed, and the advantage of its fast frame rate was lost. In 1981, Wilson and Sheppard (for a historical summary, see [17]) proposed a theoretical solution to combine the resolving power and depth discrimination of a conventional fluorescence microscope. The first practical single-beam laser-scanning design of confocal microscope, in which the illumination was scanned rather than moving the stage, was developed by William ‘Brad’ Amos, John White, Mick Fordham and Richard Durbin at the Medical Research Council laboratory in Cambridge in 1986 [18, 19] followed very shortly thereafter by a Swedish group (Carlssen and Aslund 1987). The breakthrough [20] making this possible was not only due to bringing together suitable lasers, galvanometer mirrors and computers to display the image, but achieving accurate raster scanning at high speed. When considering the development of practical confocal microscopy, the important development of the dichromatic beam-splitter by JS ‘Bas’ Ploem [21] is almost always overlooked.
The confocal microscope is so-called because the projection of the illumination pinhole, the plane of focus within the specimen and the back-projection of the detector pinhole are all situated at conjugate focal planes [22]. Every laser-scanning confocal (a generic design is shown in Fig. 5.4) has the following features in common:
-
One or more gas or diode laser(s) providing illumination of specific wavelengths
-
Fluorescence filters and a dichroic mirror as a filter set to direct the illumination onto the sample and direct the emitted fluorescence of specific bandwidth towards the detector
-
A mirror and galvanometer-based raster scanning mechanism
-
One, or more, pinhole apertures
-
Photomultiplier tube (PMT) detectors for each channel
Because the confocal microscope depends upon point-scanning and a pinhole aperture to exclude out-of-focus light, lasers are used because they have sufficient collimation and power to provide the necessary illumination flux. Lasers produce very intense, coherent beams of very narrow wavelength that are ideal for illumination in a microscope where 90% or more of the emitted signal may (intentionally, due to the pinhole) not reach the detector. It is essential to be able to adjust the laser intensity so that fluorophores are not saturated and bleached. Lasers are generally used at fractions of a per cent of their maximum output power unless, of course, they are intentionally used to bleach the sample [23]. The lasers can be switched very rapidly, and adjusted in intensity, by the use of an Acousto-Optic Tuneable Filter (AOTF) or neutral density filters. Generally, AOTFs are used; they are more versatile and also allow specific regions of interest to be scanned in the field of view.
The beam is raster scanned using two oscillating mirrors driven electromagnetically by a mechanism similar to a moving-coil galvanometer. As such they are colloquially referred to as ‘x- and y-galvos’. The sawtooth duty cycle is demanding on power, with the x-galvo working harder than the y-galvo. Higher frame rates can be achieved by driving the mirrors at resonant frequency. Although confocal microscopes have been made without mirrors, they endure because of their achromatic behaviour.
Photomultiplier tubes (PMTs) are used as detectors in point-scanning confocal microscopes because these enhance the weak signal and can also very rapidly collect single points of light emitted as signal. Using a CMOS or CCD detector would be far too slow. The PMT does not ‘see’ the entire image as a CCD detector does, but very rapidly produces a voltage equivalent to the photon intensity emitted at each sampling point in the object, which is then digitised to form a corresponding pixel in the image.
With only a single point illuminated (rather than the entire field of view as in ‘widefield’ mode) the illumination intensity rapidly falls off above and below the plane of focus as the beam converges to a point and then diverges. This reduces excitation of fluorescence of these objects situated out of the focal plane, improving depth discrimination. The pinhole in front of the PMT detector excludes out-of-focus light giving a sharp, blur-free image. The confocal microscope differs from widefield fluorescence microscopy in that the microscope configuration for widefield (both brightfield and fluorescence) microscopy is designed to be used with Köhler illumination where the excitation illumination is maximally out of focus at the specimen plane (i.e. the lamp filament is not imaged at the specimen plane, but a different conjugate plane: the entire raison d’être of Köhler illumination). In the confocal microscope the illumination is focused onto the specimen plane as a diffraction-limited point. For an explanation of Khler illumination, see Chap. 9 in Sanderson [24].
5.2.4 Structural Illumination Microscopy (SIM)
The confocal microscope remains the most popular optical sectioning instrument, but there are two other approaches: structural illumination microscopy and deconvolution. If we regard confocal microscopy as the optical solution to optical sectioning, then deconvolution is a purely mathematical approach, sometimes called computational optical sectioning microscopy (COSM) whilst SIM is a mixture of the two. In COSM a 3-D dataset is collected as a z-stack of a series of 2-D images, each with the microscope focused at a different plane through the specimen.
In SIM a grid pattern is superimposed upon the specimen at the focal plane, and subsequently shifted to allow the out-of-focus signal to be subtracted from the in-focus information to yield an optical section. The Ronchi grid pattern introduces an artificial high frequency spatial modulation which rapidly attenuates either side of the focal plane. The optical sectioning efficiency depends upon the pitch and contrast of the grating; in practice, different gratings are used with particular objectives. The grating is held in a custom-built slider which is inserted into a slot in the microscope body conjugate with the field diaphragm, to project the grid image onto the plane of focus. A plane-parallel plate is automatically moved and tilted, by piezoelectric motor, to shift the image of the grid above and below focus. The microscope hardware and software then acquires at least three images: one in-focus and two out of focus, to generate an optical section. This method has been called ‘the poor man’s confocal’ but this does no justice to the elegance of the technique. The advantage of deconvolution is that it is software-based and can be used on any microscope, whereas SIM requires a specialist upgrade or third-party add-on to the microscope. The advantage of SIM is that raster scanning of the sample by intense laser illumination is not required.
Optical sectioning SIM typically uses coarse gratings and incoherent light. High resolution SIM, which can improve resolving power by a factor of two over the Abbe limit, uses finer gratings, superimposing a moiré pattern onto the sample [25,26,27]. SIM is significantly faster than conventional point-scanning microscopic techniques, which are inherently limited in speed by illumination intensity, fluorophore saturation, and raster scanning. With SIM one needs to acquire several frames for a single reconstruction; thus, the speed of the method scales with the availability of fast camera technology.
The aperture-correlation microscope [28] uses a similar technique, but has a higher frame rate akin to the spinning disc microscope. In aperture-correlation microscopy, the final image is calculated in three steps: first, the two images have to be extracted from the side-by-side view and one image is mirrored to match the image orientations. The second step involves a registration of both images to ensure that the overlay is precise on a pixel-by-pixel basis. In the registration step, distortions as mapped in a previous calibration step are corrected between the two imaging beam paths. The third step is the actual calculation of the optical section itself. A scaled subtraction of both images will yield the optical section. Both SIM and aperture correlation microscopy are well suited for thin to medium thickness specimens, where the SBR is low. Sometimes artifactual stripes occur [29] due to absorption and scattering in the illumination path but these can be overcome [30, 31].
5.2.5 Deconvolution
We will only consider the basic principles of deconvolution here. A short and excellent primer which I recommend to my students is Shaw [32]; another good explanation of deconvolution is Biggs [33]. All imaging systems are imperfect. When any image is formed—or convolved—from an object it suffers degradation. Convolution is a formal mathematical operation, just like multiplication, addition and integration—which in our case is applied to image formation. A convolution operation takes two signals and produces a third signal, in our case the input signal (from the object) is convolved with the second signal (the PSF) to produce the output signal (the image), see [34].
If a lens behaved perfectly, points in the object would not be smeared into a point spread function (PSF) and a defocused image of beads would appear black. As it is, a sub-resolution fluorescent bead forms the image seen in Fig. 5.5. A bright point is seen at the focal plane; either side of the focal plane, the focal spot is transformed into a disc that becomes both larger and dimmer in intensity. Ideally, the defocused image should be the same either side of the focal plane, but again this is usually not the case—compare the calculated theoretical PSF with the empirical PSF collected from a sub-resolution fluorescent bead. In three dimensions this image is seen as an hour-glass shape and describes the PSF characteristic of that particular microscope. The point spread function is the signature of the microscope. The confocal PSF is smaller than the widefield PSF (Fig. 5.8), as explained in Sect. 5.3.2 below.
A point spread function shown laterally in x, y dimensions in panels (a) and (c) with the corresponding z-stack projected in x, z in panels (b, d) respectively. A calculated PSF is shown in the upper pair of panels, while an experimentally-collected PSF from a fluorescent bead sample is shown in the lower two panels. The objective used to acquire these data-sets was a 63× NA 1.4 plan-apochromat with a prepared sub-resolution 0.1 μm bead slide, having a refractive index of 1.518—as close to homogeneous immersion as possible. The calculated PSF was generated with the PSF Generator plugin for Imagej/Fiji developed by the Biomedical Imaging Group at the École Polytechnique Fédérale de Lausanne (EPFL). Image copyright, author: J Sanderson
Image degradation occurs not only because of fluorescence blurring (see Sect. 5.2 above), but also from the effects of diffraction and aberrations of the objective. This is inescapable. Because (from an image formation perspective) self-luminous fluorophores and self-luminous stars are similar, image restoration and sharpening by deconvolution is a useful technique in microscopy that was originally borrowed from astronomy [35].
Mathematically, convolution involves replacing each point in the specimen during the process of image formation to form a (blurred) point in the image. This direct convolution operation may appear complex, and indeed it is very computationally time-consuming. However the mathematical operations described above in ‘real space’ can be simplified by computation in the frequency domain, or ‘Fourier space’ using Fourier transforms, which describe images mathematically in terms of their sine and cosine components. The convolution operation reduces to: FTmicroscope image = FTobject × FTPSF Knowing the PSF, deconvolution can be performed in reverse (FTobject = FTmicroscope image/FTPSF) to sharpen the image as a more faithful representation of the object.
There are two main types of deconvolution algorithm: deblurring and restorative (Fig. 5.6). Restoration algorithms are more accurate and can be used for quantitative purposes because they keep the relative intensity relationships seen in the data forming the original image. Most restoration algorithms are iterative. With iterative deconvolution, the ideal ‘model’ image is compared with the results of computation successively allowing for noise and signal integration. Blind deconvolution (more properly referred to as adaptive blind deconvolution) is an alternative restorative method that extracts the PSF directly from the image data. This may seem counter-intuitive, since the algorithm is trying to compute a solution for both the image and the PSF, but it is quick and proponents argue that the calculated PSF best fits the data. For those wishing to use freeware rather than a commercial software package, try DeconvolutionLab2 ([36]; EPFL Lab, Lausanne) in Fiji/ImageJ.
Image improvement with deconvolution, showing the use of deblurring and restorative (constrained iterative) algorithms on a tissue sample. A widefield image and a maximum intensity projection (MIP) taken with a confocal are also shown for comparison. Further details may be found on page 9 of the Carl Zeiss Technical Note ‘How to Get Better Images With Your Widefield Microscope’ (Stickler et al., March 2020). Reproduced with the kind permission of Carl Zeiss and René Buschow, Max Planck Institute for Molecular Genetics, Berlin. Image: Copyright ZEISS Microscopy
In order to acquire a meaningful scientifically rigorous deconvolved dataset, the correct algorithm must be selected for the task or application in hand and the correct point spread function, whether calculated or experimentally acquired, must also be used. Without the most appropriate algorithm or correct PSF, there is little, or no, benefit to post-processing the image, and the old computer adage holds true: ‘garbage in equals garbage out’. Clearly, a PSF generated from sub-resolution fluorescent beads give more precise results than a calculated PSF, but acquiring datasets from the former is more labour intensive and a calculated PSF may be all that is needed. Deconvolution will not make bad data good; it will only make good data better. The key to successful deconvolution is acquiring an accurate PSF. Cannell et al. [37] report that measured PSFs are usually 20% larger than calculated PSFs and nearly always are non-symmetrical due to the presence of spherical aberration. Practical details for collecting PSFs from sub-resolution fluorescent beads are given in [38]. Most deconvolution software has dialogue boxes to plug in values to generate a calculated PSF.
Deconvolution should improve the contrast and SBR with reduced background haze [39]. The edges of objects should be sharper, and the intensity of features in the image enhanced. The artifactual elongation of features in the z-axis should also be reduced. Although fibre optics make this artifact less common, flickering lamps and a change in illumination intensity during acquisition can lead to stripes being seen in the image. If the deconvolution algorithm is unsuitable or else applied too aggressively, artifactual rings and points can appear in the image. Rings may occur from sampling irregularities; it is important to sample according to the Nyquist-Shannon sampling criterion. Clearly, if the incorrect PSF is applied, the image will probably be skewed or asymmetric in some form. This may also occur if refractive index mismatches occur in or between the specimen and its environment. Indeed, where these mismatches occur and—for an immersion objective—homogeneous immersion generally does not occur, then the firmly-held idea that optical sectioning occurs because light is excluded by the pinhole is also upset. You should be asking yourself whether the confocal plane of focus is ‘an actual geometrical plane resembling a mechanical section, or merely the surface described by an array of points at which the rastered laser beam happens to reach best focus’ [40]. If the specimen is too thick, deconvolution algorithms will fail to deblur the image or properly to reassign the out-of-focus light. Deconvolution is no longer useful when the image contrast between the signal and the background noise falls to <1% as seen through the entire thickness of the cell or tissue [41].
Should deconvolution be used with confocal data? Some people reason that the pinhole has already ‘sampled’ the data, rendering post-acquisition deconvolution less important than with widefield microscopy. Nevertheless, there are very sound reasons why datasets from confocal and other optical sectioning microscopes should be deconvolved, as Jim Pawley consistently advocated [42]. A laser scanning confocal microscope (LSCM) is inefficient at collecting light because not only is it noisier than widefield microscopes, it throws away out-of-focus light rather than reassigning it as a deconvolution system does. Low intensity signals contain a lot of high frequency Poisson, or shot, noise which can be erroneously sampled and digitised into non-existent single-pixel ‘features’ that could never have been resolved by the microscope. Secondly, deconvolution averages the signal over the many voxels in the image needed to sample the signal from a single point in the object sampled at Nyquist frequency. This is a very effective way of reducing the overall noise and boosting the SNR. To achieve the same result with the confocal microscope would require Kalman averaging the signal anywhere between 80 and 130 times. This is not feasible and would bleach the specimen. Thirdly, the spread of the image dataset in the z-axis is reduced. Fourthly, the Nyquist-Shannon sampling theorem stipulates that the bandwidth of the (output) display device must be equal to that of the (input) digitising device. Deconvolution is the correct way to implement this (often forgotten) condition of Nyquist sampling. Each objective has an optimal lateral and axial sampling rate. The values given by Biggs [33] are dependent upon NA, wavelength and refractive index: dx,y = 0.25λ/NA and dz = 0.5λ/(RI −√[RI2 − NA2]). When you deconvolve confocal data, the contrast drops and the image doesn’t look so sharp. Those ‘sharp’ objects were noise artifact and the contrast can always be raised to match the characteristics of the display monitor.
5.3 The Confocal Microscope
5.3.1 How the Confocal Microscope Works
The modern single beam-scanning laser confocal is offered by the big four microscope manufacturers, and also independent companies, as a turn-key system. This makes the instrument easy to use. The generic design and configuration has already been described above. In this section we consider practical functional aspects of the lasers, the pinhole and PMT detectors.
Traditionally the lasers in a confocal microscope were Argon-Krypton and Helium-Neon gas lasers, which offered a range of discrete wavelengths. These are still used, but dye lasers, diode and diode-pumped solid state lasers are increasingly common due to their size, low heat dissipation, convenience of use and longer operational lifetimes and supercontinuum white-light lasers [43] are now available. The air-cooled argon-ion laser is still widely employed as a light source for confocal microscopy because of its brightness level, small size, excellent beam geometry and the suitability of its spectral lines for green fluorophores. Typical laser line values are as follows: 351, 364, 405, 430, 458, 477, 488, 497, 514, 532, 543, 561, 568, 594 and 633 nm, the bandwidth being 1 nm. Not all these lines will be present in one instrument, a typical combination of lasers on a high specification instrument being: 405, 458, 488, 514, 561 and 633 nm. For further information, see Table 18.1 in Sanderson [24].
Different laser lines emanate from different power lasers, from 1–2 mW up to 10–15 mW. A typical working output value (usually selected automatically in software) will be 2%. The brightness of the illumination cannot be increased indefinitely. In fact, there is a very small window of opportunity regarding laser power. Irrespective of photo-damage to living cells and tissues, the photophysics of fluorescence limits the useful intensity of illumination that may be used. Above a certain threshold the fluorophore saturates and molecules remain in the excitation orbital rather than returning to the dark ground state to absorb further photons. The result is fluorescence signal intensity falling with further increases in laser power.
During normal operation of single point-scanning confocal microscopes, the beam dwells on each pixel for 1–20 μs. Averaged over 4 μs the laser intensity fluctuates by 2–3%, but fluctuations up to 10–15% are observed with sub-microsecond dwell times. This introduces significant noise [9], which may drown out weakly-fluorescent structures; it is not due to poor resolving power. When averaged over a much longer time (ms to s), the amplitude of the fluctuation is small, in the range of 0.5–1%.
Confocal microscopes are set up to receive laser light via a single-mode fibre connector. Coupling these is extremely challenging, requiring adjustments in six degrees of freedom (three rotational and three linear parameters) to control the alignment of the laser beam into the objective. This procedure can take hours to optimise and, for virtually all microscope users, it is simply not worth the effort. It is always advisable to maintain instruments under a service contract, and alignment is best left to the service engineer.
Once the different fluorophores have been set up in software, they are usually assigned to individual ‘channels’ for sequential acquisition. Two commercially-available specimens suitable for training are the Fluocell slides from ThermoFisher. Slide #1 (F36924) comprises adherent bovine pulmonary arterial endothelial cells stained with DAPI (nuclei), MitoTracker Red CMXRos (mitochondria) in the live cells. Following fixation, the preparation is further stained with Alexa Fluor 488 phalloidin (F-actin). Slide #3 (F24630) is a 16 μm thick cryostat section of mouse kidney, ideal for demonstrating optical sectioning and z-stack acquisition. It is stained with two lectins, Alexa Fluor 488 wheat germ agglutinin (glomeruli; convoluted tubules) plus Alexa Fluor 568 phalloidin (F-actin in glomeruli & brush border) and DAPI. Using slide #1, each fluorophore is assigned to a channel: red, green and blue. Sometimes fluorophores in different channels, whose emission spectra do not overlap significantly, may be combined in a single ‘track’ to allow faster acquisition rates providing significant bleed-through does not occur. For example, the decision might be made to collect the signals from DAPI (channel 1) and MitoTracker Red (channel 3) simultaneously into one track.
The pinhole in front of each PMT detector associated with each channel or track is set to collect an optical section. Altering the pinhole size will alter the thickness of the optical section: a wider diameter, open pinhole collects a thicker optical section with brighter signal and a higher SNR, but consequently more blurring. Closing the pinhole will increase the SBR, increase the resolution slightly but attenuate the signal and make the SNR worse. There will be a ‘1 AU’ setting for the pinhole. This is a normalised value to allow the signal from the central Airy disc, excluding the diffraction rings, to enter the detector. Setting the pinhole to 1 Airy Unit isolates 80% of the photon intensity distribution for the point object from the central maximum to the first minimum (zero) either side of the PSF Gaussian intensity distribution: the central Airy disc. The Airy disc, named after Sir George Biddell Airy who studied diffraction patterns and image formation in stars, describes the central intensity maximum of the two-dimensional pattern of the point spread function, the PSF. If pinhole is opened above 1.35 AU, the increase in brightness is due to collecting unwanted extra-focal light—which was intended to be removed by investing in a confocal microscope! Below 0.6 AU a slight improvement in resolving power occurs from having a smaller PSF, but with a concomitant huge intensity loss.
The setting for the one Airy Unit value is dependent upon the wavelength of illumination and the numerical aperture of the objective. Clearly, the former can change by a factor of almost two, whilst the latter is constant. Most systems now have separate pinholes in front of each detector, and this will mean altering the pinhole diameter and normalised AU value to ensure optical sections of equivalent thickness are collected from each channel. Adjusting the pinhole to the optimum one Airy Unit reduces the background in the image from out-of-focus light by approximately 1000-fold relative to widefield microscopy [44]. Reducing the pinhole diameter below 1 AU will improve resolution, but at the expense of signal intensity. A recommendation is not to close the pinhole below 0.7 AU, conferring optimal axial resolving power.
The contrast and SBR are determined by the gain and offset settings of the PMT detectors. These should be adjusted so that the signal emitted from each fluorophore falls within the dynamic range of the PMT. Confocal images are usually displayed as 8-bit or 12-bit images in three-colour RGB format. The default is 8-bit (28 or 256 grey levels), but 12-bit (4096 grey levels) should be used when collecting images for quantitative analysis. Setting the offset (black level) and the gain (signal level) requires a false-colour look-up table to colourise undersaturated and oversaturated pixels in the image. This is because our eyes are approximately 6-bit devices (dynamic range of ≈ 64 grey levels) and we cannot discriminate the limits of the 8-bit or 12-bit dynamic range of the PMT detector. It is also worth mentioning that some users of confocal microscopes may be colour blind. For further information see Chap. 31 in Sanderson [24] and Note 14 in [1].
When sampling the analogue signal and converting the intensity value into individual pixel values, the detector must work extremely fast. The default raster on a confocal microscope is 512 × 512. That is, 512 horizontal lines, each composed of 512 individual sampling points, upon which the laser ‘dwells’ as it raster-scans across the specimen to produce an image of 5122, or 262,144 pixels. To acquire such an image normally takes 1–2 s, thus the dwell time for sampling each point in the sample to create the corresponding pixel in the image is approximately 4 μs. Although a CCD or CMOS detector is more efficient at sampling each photon of signal and creating photo-electrons to convert to a pixel intensity value, neither quantise the analogue signal fast enough. That is why a photomultiplier detector is used. The associated downside is that its quantum efficiency (QE) is much lower than either a CCD or CMOS camera, which is suitable for widefield microscopy. A high quality CCD camera will have a QE of 80–90%, converting nine out of ten photons to photo-electrons, but a PMT has a QE of about 15%. Gallium arsenide phosphide is a semi-conductor gallium alloy material with an extended sensitivity into the red end of the spectrum. Even the latest GaAsP, avalanche photo-diode and hybrid detectors have QE values of no more than 40%. This means that at low signal levels the gain control must be increased, and the resulting image will show high shot (Poisson) noise unless measures are taken to improve SNR. Figure 5.7 shows the differing image quality from a PMT detector and CCD camera.
Comparison of the quantum efficiency of PMT and CCD camera detectors. The widefield image is the left-hand panel, the single-scan confocal image on the right. The middle panel shows the confocal image taken with 16 average sans—the best possible. The sample is GFP-expressing Haemophilus influenzae bacteria and the scalebar is 2 μm. Reproduced from Understanding Light Microscopy with permission from John Wiley & Sons Ltd. Image copyright, author: J Sanderson
Because the single-beam laser-scanning confocal microscope rasters the laser across the sample with two galvanometer driven mirrors under computer control, this means that any scan zoom factor can be applied for image acquisition. All digital images are collected according to the Nyquist-Shannon sampling criterion [45, 46] such that an analogue photon signal must be sampled at least twice the highest frequency of the wave. In practice, the wave should be sampled at slightly higher than 2f because the image isn’t formed until the sampled information is reconstructed, which always takes place through a ‘filter’ (in our case, the eye or display monitor) limiting the image to lower frequencies. Since the microscope objective forms the image, an optimal zoom setting yields pixel dimensions—equivalent to just under half the resolving power of the objective—sufficiently small to satisfy the Nyquist criterion, but still large enough to avoid over-sampling. Be aware that as the scan zoom increases, the laser power is concentrated into a smaller area, so the specimen will bleach more readily.
5.3.2 Resolving Power of the Confocal Microscope
Although primarily used for an improvement in SBR and optical sectioning, the confocal microscope also offers slight improvement in lateral and axial resolving power over widefield fluorescence microscopy [47]. This condition assumes using a perfectly clean highly-corrected objective and well-stained samples. Because the confocal utilises point illumination and point detection, the total PSF is the product of both the excitation illumination (λex) PSF and the emitted signal (λem) PSF, improving resolving power by a factor of √2. This is seen in practice by the improved shape of the PSF in the confocal microscope as compared to a widefield PSF (Fig. 5.8).
Comparison of the PSF of a sub-resolution fluorescent bead taken with a widefield (a) and confocal (b) microscope respectively. The graph (c) represents the improvement in resolving power of a confocal over a widefield fluorescence microscope. Image copyright, author: J Sanderson. Graph redrawn by Gareth Clarke, MRC Harwell Institute
The classical equation for lateral resolving power becomes dx,y ≈ 0.44λ/NA, whilst in the axial direction dz ≈ 1.4λn/NA2. However, this assumes a pinhole diameter of much less than 0.5 AU, tending to zero, which is very rarely encountered because of rejection of most of the signal. Therefore for a pinhole of 1 AU, or greater, the following revised values apply: dx,y ≈ 0.51λex/NA and dz ≈ 0.88λex/[n − √(n2 − NA2)] for NA values greater than NA 0.5, whilst for NA values less than NA 0.5, dx,y ≈ 0.37λex/NA and dz ≈ 1.28λn/NA2 [48].
Since the minimum intensity at the first zero of the Airy pattern is hard to measure in practice, resolving power is usually calculated as the Full Width Half Maximum (FWHM) of the PSF of a sub-resolution fluorescent bead. From a z-stack, the FWHM is calculated from an intensity line profile of the image of the bead. In this case, the FWHMaxial = 0.64λex/[n − √(n2 − NA2)]. For a mathematical explanation of the improvement in resolving power by a factor of 1.4, see Diaspro et al. [14] and the references cited therein. Various experimental stratagems have been adopted to improve the resolving power of the confocal microscope (e.g. focal modulation microscopy; [49]) and divided-aperture microscopy [50] but unfortunately these applications are not widespread.
5.3.3 Advantages and Disadvantages of the Single-Beam Confocal Scanning Microscope
Advantages
-
Non-invasive optical sectioning—well-defined, sharp, optical sections
-
Good reduction of background—increased SBR
-
Laser switching allows good control of cross-excitation and bleed-through
-
Magnification zoom can be adjusted electronically with ease
-
PMT not limited by the matrix of a CCD detector—easy to draw regions of interest
-
Can image several fluorophore markers simultaneously
-
LSCM good for spectral unmixing fluorescent proteins with overlapping emission spectra
-
Smaller PSF—slightly better lateral and axial resolution than widefield
-
Can use deconvolution algorithms to further improve image quality
Disadvantages
-
Real-time collection difficult—need to wait to collect a low-noise signal
-
Use of laser illumination bleaches faster than widefield or spinning disc confocal
-
PMT detectors have a low quantum efficiency—samples must be well-stained
-
Scan speed and fluorescence saturation impose a frame-rate limit: slower than widefield or spinning disc
-
Laser power noise and fibre optic coupler leads to artifactual pixel-pixel fluctuations
-
Less sensitive than using the same objective on a fluorescence widefield microscope
-
Unlike multi-photon, a relatively large specimen volume is still illuminated
-
Light scattering/refractive index mismatches limits depth penetration to 100–200 μm
Despite the disadvantages listed, the single-beam laser-scanning confocal remains a versatile instrument with several significant advantages over alternative microscope options for collecting images from fluorescently-labelled cells and tissues. It is usually the optical sectioning microscope of choice and is ubiquitous in laboratories and research institutions worldwide. The majority of emitted photons are not detected: so a well-stained specimen, giving a low-noise signal, is required. The pixel dwell time cannot be too small (to increase acquisition speed) otherwise the light flux obtained from small volume of fluorophore contained within the focus of the scanned beam (about a cubic micron) is too small and affected by random fluctuations from shot noise. Image acquisition speeds can be increased using spinning-disc and line-scanning confocal techniques, but there will always be some loss of image resolution with these designs.
5.3.4 Line-Scanners and Array-Scanning Confocal Microscopes
The necessity to scan the illumination pointwise means confocal optical sectioning is not as fast as widefield fluorescence microscopy, but various stratagems are employed to increase image acquisition speed, by resonance scanning, programmable array designs or by line-scanning.
Line-scanning and programmable array microscopes use either slit apertures and by so doing sacrifice some confocality, or else use spatial grids or resonant scanning devices. In a single-beam point-scanning confocal, the x-galvanometer mirror works faster and is exposed to greater mechanical stress than the y-galvo because it must raster the point of the laser beam across the entire line scan, whereas the y-galvo merely drops the beam to the next line. If the mirror of the x-galvo is replaced by a slit, each line can be scanned at once. Furthermore a linear array CCD detector is used, with consequently greater quantum efficiency. Another arrangement is to sweep the illumination across the sample with a single galvanometer mirror, and the emitted signal is descanned using the reverse side of the same mirror. In another configuration the galvanometer mirror is replaced by an acousto-optical device which has no moving parts. The emitted signal cannot be descanned, as in the single-beam laser-scanning confocal, so is detected with a slit rather than a pinhole. With all these designs, the price paid for increased acquisition speed and frame-rate for live-imaging is partial loss of confocality in one direction.
An alternative method of increasing temporal resolution is to retain the single-beam point-scanning design and to increase the rate at which the galvanometer mirrors are driven. Unlike the spinning disc, swept-field or line-scanning confocals, resonant scanning single-beam point-scanning confocal microscopes are able to alter magnification without changing objectives by retaining the versatile confocal zoom functionality. The repetitive, shorter, exposures usually lead to brighter images, albeit with slightly worse signal-to-noise ratio but with much less bleaching per scan. The speed of the resonant scanner is fixed, usually at 8000 Hz, and it can be difficult to define regions of interest, except along horizontal lines.
5.4 Step by Step Protocol
5.4.1 Acquiring 2-D Sections and 3-D Z-Stack Images
This section gives guidance on how to view a specimen with a confocal microscope, set it up to collect in-focus optical sections and to acquire z-stacks. Like driving a car, once the basic principles are understood and have been learnt, it is possible to use different makes of confocal microscope and to obtain images of sufficient quality for publication. First and foremost, plan your experiment in advance. Don’t blindly follow a previous protocol (although this can help prevent re-inventing the wheel) but plan your own experiment and consider how the images collected will support your working hypothesis, because microscope images are not merely pretty pictures but rather photon intensity datasets which, if they have been properly acquired in the first place, have scientific merit.
Before imaging on the confocal, check the cells/tissue and fluorescent signal on a widefield microscope, so that you are familiar with the specimen, the density of cells or features in the tissue section, and will therefore spend a minimum of time locating the desired field of view and plane of focus in the confocal microscope. You will, in any case, first use the confocal in non-laser widefield mode to locate and focus the specimen before switching to laser-illuminated confocal mode. This is done using an LED, metal-halide, short-arc mercury or xenon light source. This may be obvious, but is worth stating nevertheless: you absolutely cannot use laser light to view the specimen by eye down the microscope. Most lasers used for microscopy are rated class 3B or class 4. You must be aware of the dangers posed by laser illumination, and of your legal responsibilities using laser-illuminated confocal microscopes. If in doubt, speak to the laser protection supervisor in your workplace (for further details on this particular topic, see Chap. 18 in [24]).
Think about what spatial resolution is required. Users seldom consider this, instead asking facility staff about magnification. Also consider the field of view required—a related issue. There is little point in using a high-NA immersion objective with small working distance if all you require is a dry objective of lower NA with a consequent longer working distance, for it is easier and quicker to use than a high-NA objective with a very small field of view. Try to image only the volume you require. Don’t scan large areas or depths, since this is time-consuming and bleaches tissue. Equally, image only for the minimum time required to collect the dataset needed. Use the lowest laser power possible.
For most tasks, plan-apochromat objectives are used, for they possess high numerical apertures [51]. The numerical aperture determines not only the resolving power but also the light gathering capacity of the microscope. When the NA is doubled, the light flux gathered is quadrupled—crucial for capturing fluorescent signal. Also as the NA increases, so also does the irradiance of the laser which means that the specimen will bleach more rapidly with a high-NA objective, particularly if a scan zoom is also applied when acquiring an image. Figure 5.9, reproduced from [52], compares three different types of objective. Some beginners assume—without checking with core facility staff first—that they can use a confocal to image cells in a multi-well plate. This is not the case unless a long working distance objective is used, in which case an inverted widefield fluorescence microscope is generally a better choice. Trying to image through a multi-well plate with a short working distance objective will mean that you won’t be able to focus on the specimen. Figure 5.9a shows that much useful confocal optical sectioning microscopy can be done with a reasonably low magnification objective with a high NA. In this case, the advantage lies in the good working distance and larger field of view than using the highest NA objective available. Therefore, select the objective you require for the job in hand; don’t waste photons.
Different objectives for confocal microscopy. For most work use the objective with the highest NA giving the shallowest depth of field for good optical sectioning. The 20×/NA 0.8 objective is good for low- to mid-magnification work (use a zoom value <1 to maintain light-gathering capacity and resolving power) whereas the 63×/NA 1.4 plan-apo is the workhorse of most confocal work. The long-working distance objective (32×/NA 0.4) is ideal for tissue culture, but with a low NA is not optimised for optical sectioning. The dry 20× objective still has a reasonable working distance, but has only approximately half the resolving power and a quarter of the light-gathering capacity of the oil-immersion of the 63× objective. To achieve this ultimate resolving power and high sensitivity, the working distance of the 63× objective is very small, restricting observation to very near the underside of the coverslip. As such it is good for collecting high resolution images of adherent cells. Reproduced with permission from Springer Nature AG
Although manufacturers design objectives such that spherical aberration is minimised, it is possible for these corrections to be upset by the microscopist, and thus reintroduce spherical aberration into the image. Spherical aberration manifests itself as a loss of signal intensity and unsharp images [53]. Using the wrong thickness of coverslip, or not allowing for the thickness of mounting medium if the specimen is mounted on the slide is one common cause. The other is refractive index mismatch [10] from preparing the specimen, and which may be unavoidable. Use a water-immersion objective for aqueous samples and if possible a multi-immersion objective for specimens contained in a glycerol-based mountant. Opening the confocal pinhole may help, as does also using a lower NA objective.
Most samples prepared for confocal microscopy will be sandwiched between a glass slide and coverslip. It is important to use the correct thickness of coverslip (No. 1.5H—high tolerance 0.17 mm) and also to take into account the thickness of any mounting medium if the sample is attached to the slide rather than the under-surface of the coverslip. Where living samples in culture medium are prepared for imaging, use petri-dishes with coverslips on the base (e.g. Ibidi, MatTek, Willco Wells). Proper fixation of excised tissue or dead cells is key; for other considerations regarding specimen preparation, see [52]).
Brief Generic Operating Protocol
Every point-scanning confocal microscope is operated in broadly the same fashion, differing only in minor points imposed by software design. For your particular confocal microscope, consult the manufacturer’s specific operating instructions. Further details are given in Sanderson [24].
-
1.
It is good practice—and essential for any form of quantitative analysis of fluorescence—to switch on the lasers at least 30 min beforehand in order for them to warm up sufficiently so as to put out a consistent illumination flux.
-
2.
Also check that the stage insert, holding the slide or glass-bottomed petri dish, is inserted correctly and the specimen is level. Trying to image with a loose stage insert is self-defeating.
-
3.
Image the brightest sample first—a positive control is ideal—to set a baseline for the other slides in the experiment and to ensure the gain settings chosen are sufficient to image all specimens at the same instrument settings.
-
4.
From a Smart Setup or Experiment Configuration dialogue box, select the required fluorophores, appropriate laser lines and filter combinations. Activate the acousto-optical tuneable filter (AOTF) and select the correct primary dichromatic mirror acousto-optical beam-splitter (AOBS) for directing the emitted signal to the PMT detectors.
-
5.
Selecting a configuration which assigns one PMT to collect each fluorophore independently in its own channel, sequentially from the longest to the shortest wavelength, will prevent bleed-through.
-
6.
Adjust the pinhole value in each channel or track to give the optical section required. If one fluorophore is much weaker in intensity, or photo-bleaches more than another, try where possible to assign the larger pinhole to the PMT collecting this weaker or more photo-labile fluorophore, and adjust the others accordingly.
-
7.
Check that the correct raster scan is selected for optimum resolution. There may be a radio button in the software for this to set the Nyquist sampling limit. You can generally manage with under-sampling in the x-y direction, to allow priority for more important acquisition parameters which allow for better SNR and less photo-bleaching.
-
8.
Select each PMT in turn, switching any other PMT off. Using the continual ‘Fast Scan’ or ‘Live’, scan the sample continuously. Work quickly (to avoid unnecessary bleaching) but efficiently, to first adjust the black level via the ‘Offset’ control on the PMT, and then the signal level via the ‘Gain’ control. The key points are (1) not to bleach the sample and (2) not to over-saturate the signal, otherwise details in the specimen are not recorded in the image. The photomultiplier will only record tones of grey, not colour. The colour applied to the display is a false pseudo-colour to help us recognise each fluorophore.
-
9.
With all channels set with correct PMT offset and gain levels, a single image can be collected. Normally frame-by-frame collection is used, but if you are collecting moving objects collect line-by-line to minimise any blur in the captured image due to movement of the specimen.
-
10.
Optimise the laser intensity, often this is left at a fixed value. At fluorophore saturation, the signal intensity no longer increases in proportion to the laser power: avoid this happening.
-
11.
Image quality can be improved by either collecting a series of scans (averaging) to improve the signal-to-noise ratio or by reducing the scan speed so that the laser dwells on each sampling point for longer, allowing more emitted signal to be collected, but be aware (Fig. 5.10) of the potential for photo-bleaching.
-
12.
The SNR may also be improved by opening the pinhole, increasing the laser power and increasing the scan zoom, but these stratagems will also increase bleaching.
-
13.
To collect a z-stack, open the appropriate dialogue box, then manually focus through the sample and mark the first and last points in the stack. In most software, it is possible to perform a rapid x-z scan through the sample to help set these top and bottom limits. Take care, when setting these limits, to avoid collecting sections displaying no data and concomitantly bleaching the sample. Consider whether time may be saved by applying a region of interest to the z-stack (the ROI control can also be used to advantage when collecting single 2-D optical sections).
-
14.
Set the optical section thickness to satisfy the Nyquist sampling criterion. There will be a radio button in software to do this to the minimum value of 2× Nyquist. If required, manually select a thinner optical section to over-sample to some degree. This depends upon the sample.
-
15.
When collecting a very large z-stack and imaging deep into tissue, it may be necessary to either alter the laser power, gain or offset values to maintain signal intensity when focusing through tissue that scatters both the illumination and the emitted signal.
-
16.
The z-stack dataset contains a series of in-focus optical sections. This is normally viewed as an orthogonal presentation. The 3-D dataset can be rotated and saved as a movie, or it can be ‘collapsed’ into a 2-D dataset for publication (Fig. 5.11). There are several algorithms that will do this, but the most common is to render the image as a maximum intensity projection (sometimes referred to as an MIP). The brightest pixel is used for each location, regardless of which focal plane in the z-stack it originated from. An MIP is not suitable for colocalisation studies, because of loss of spatial information along the z-axis.
The effects of bleaching from over-exposure to light. Panel (a) shows that bleaching only destroys the fluorophore, not the tissue (photo-toxicity to living cells is a separate issue). Here a square raster has bleached the fluorescent marker leaving the kidney tissue intact. In panels (b) before, and (c) after, the rates of bleaching of different fluorophores is seen, following irradiation of the nucleus and cytoplasm in the circular region of interest. The results are seen in the graph (d). The nuclear DAPI marker is much more resistant to bleaching than either the Alexafluor 488 labelling the tubulin, or the Mitotracker Red labelling the mitochondria. Image copyright, author: J Sanderson. Graph kindly drawn by Derek Storey
Panel (a) shows an orthogonal view of a 3D z-stack of a 16 μm triple-labelled mouse kidney cryosection (ThermoFisher, Slide #3 F24630) which is useful for training. Panel (b) shows a 3-D rendering of the z-stack. Panel (c) shows the original blurred image of the entire tissue section seen in non-confocal ‘widefield’ mode whilst panel (d) shows the 2-D maximum intensity projection of the z-stack (compare this figure to Fig. 5.1). Panels (e, f) show two separate non-contiguous optical sections from the z-stack of mouse embryonic node, and (g) the maximum intensity projection. Image copyright, author: J Sanderson
The confocal microscope can be used to collect a transmission image using phase-contrast or Differential Interference Contrast (DIC) in non-confocal mode with laser illumination, using a dedicated transmission PMT detector. When setting up this detector, it may be necessary manually to switch the illumination path on the microscope. Failure to check and do this can catch even experienced users unawares.
What To Do If You Cannot See a Confocal Image
-
1.
Stop scanning—so that you don’t bleach the sample (do not panic and increase the laser illumination intensity in order to try and search for the specimen).
-
2.
Check that the sample is actually in focus by viewing through the microscope. Check that the cells or tissues have actually been stained, and check the quality of the staining, that the specimen is not bleached. The sample slide should not be placed incorrectly ‘upside-down’ on the stage.
-
3.
Check the laser interlocks are switched to scanning from observation mode, and that the laser bean is exiting from the objective.
-
4.
Check that the laser beam is actually exiting from the objective (maybe an incorrect filter or dichroic beam-splitter has been selected. This is a separate issue from the laser interlock).
-
5.
Check that the channel has not been switched off in the display software (easily overlooked).
-
6.
Check that sufficient PMT gain has been applied to form an image on the monitor.
-
7.
Open the pinhole to allow sufficient signal through to the detector.
(also refer to Chap. 18 in Sanderson [24] and Box 3 in [52])
5.4.2 Spectral Unmixing
If you have only one target to investigate, a single fluorophore marker is all that is required. The goal with multiple labelling is to choose each fluorescent marker with sufficient separation between the emission spectra so that the signal arising from each fluorophore is collected into its own individual detection channel. Bleed-through occurs when emission from a short wavelength fluorophore overlaps the excitation filter or laser line illuminating subsequent longer-wavelength fluorophores. The re-excited fluorescence is collected erroneously by the detector assigned to the second longer wavelength fluorophore, rather than the first. Fluorophore emission profiles increase sharply in intensity towards the peak value, but exhibit a longer profile towards the red end of the spectrum that tails off much less sharply, increasing the likelihood of bleed-through. Ideally, fluorophores would exhibit long Stokes shifts and also have both narrow excitation and emission spectra that did not overlap with other fluorophores on the spectrum, but this is rarely the case. With such a wide choice of fluorescent markers, it ought to be possible to select those with distinct spectra, and so avoid bleed-through. Sometimes it is not that simple. Your lab may possess a limited choice of conjugated fluorophores, you may be using fluorescent proteins whose spectra lie close together or overlap significantly, or else the available laser lines on the microscope restrict your choice of fluorophores. Effective separation of signals that would otherwise bleed-through is often a compromise between using narrow excitation and emission filters that discard signal and collecting sufficient signal from samples that are not intensely stained.
To be able to implement linear unmixing, a ‘lambda stack’ (x, y, λ) is collected using a spectral dispersion element, that is usually a prism or grating [54] or a filterset. Although used mainly on confocal microscopes, with their ability to switch rapidly between laser excitation wavelengths, a lambda scan can also be collected on a widefield microscope [55]. A region of interest (ROI) emitting the fluorophore signal of interest is scanned at various intervals along the wavelength axis to create a spectrum. Each fluorophore has a unique spectral signature that is independent of any overlap with other fluorophores. For example, a spectral stack may comprise a dataset across 10 nm bandwidths spanning the visible spectrum from 380 to 720 nm, represented as 32 separate measurements. This unique spectral profile enables reliable discrimination of one fluorophore from another (including separation of autofluorescence as an independent ‘hue’). A reference spectrum is taken; for accuracy, this is best taken from specimens containing only the signal of interest, under the same conditions as the imaging experiment. If no separately-stained sample is available, a reference spectrum can be taken from the multiply-labelled experimental sample, provided an ROI is selected containing only the pure signal that will provide the reference standard. The software unmixing algorithm assigns the spatial distribution of each fluorophore in the image, which can then be false-coloured for extra clarity.
Space precludes giving a protocol for spectral unmixing here, but these can be found in [56, 57]. ThermoFisher sell a custom-made test sample for checking spectral systems, the Focal Check slide #2 (F36913) can be used. Each slide has bead mixtures of dye pairs as well as individually-stained control bead populations. The theory and practical implementation of spectral unmixing is described in [58]. For practical guidance on how to calibrate confocal spectrophotometers, see [59] and also [60].
5.4.3 Quantitative Confocal Microscopy and Quality Control
The confocal microscope is used primarily for optical sectioning, but can also be used to collect data quantitatively, for digital images are datasets of photon intensity [61]. In order to use a confocal microscope quantitatively, it must first be aligned and calibrated [62]. It is therefore worth carrying out routine quality control performance checks on the instrument. Those recommended are:
-
Laser(s) power
-
Pinhole(s) and collimator alignments
-
Field of view intensity profile
-
PMT gain sensitivity check with standard reflective mirror
-
Objective alignment and chromatic correction—essential for colocalisation studies
-
Check and record any error logs
In recent years some excellent guides to quality control protocols for assessing confocal microscope performance have been published [63, 64]. Confocal check [65] with the updated app Intensity check [66] are excellent starting points. The former paper contains a comprehensive bibliography of earlier work in this field, particularly that of Bob Zucker. For testing field illumination, see [67] and, since confocal microscopes are inherently noisy, also see [68] for checking the performance of the PMT detectors and SNR of the microscope with NoiSee. If using a CCD detector on a slit scanner or spinning disc microscope, Lambert and Waters [69] provide guidance on assessing camera performance for quantitative microscopy, whilst in the same volume [70] introduce the subject of quantitative microscopy with a good section on control samples. This early work has recently been expanded and updated by a group of very experienced facility managers in an excellent paper [52]. It is easy to make qualitative comparisons from confocal datasets. Quantitative microscopy is much more challenging, making it all the more imperative to read the references cited here.
5.4.4 Towards Super-Resolution: Image-Scanning and Pixel Re-assignment
With improved photomultiplier tube detectors, a great deal of effort has gone into improving the SNR of confocal microscopes. Improving SNR is usually achieved at the expense of image acquisition speed and resolving power—the so-called iron triangle [24, 71].
The resolving power of a confocal microscope is marginally improved over that of a widefield microscope because the overall image PSF is the product of both illumination and detection PSFs. The image in a confocal is blurred by addition of information from neighbouring points, therefore resolving power is improved by closing down the pinhole—ultimately to zero—but at the expense of rejecting 95% of signal intensity at a setting of 0.2 AU. With this smaller pinhole, besides resolving power the contrast and SNR are also increased.
Many years ago Colin Sheppard [72] realised collecting this rejected light could be used as a form of structural illumination to improve resolving power. This approach works because a displaced pinhole produces an image about equal to the resolving power of a pinhole aligned to the optical axis, but of lower intensity. Since the PSF is the product of illumination and detection, this is true also of the PSF arising from a displaced pinhole.
A high-sensitivity multi-channel GaAsP detector is able to detect these offset signals. The problem is that, with respect to the detector axis, each individual image is recorded from a slightly different viewing angle and suffers from parallax. Combining these without further processing would lead to a blurred final image, so each individual image must first be shifted onto a common axis. Software then sums the signal, but first shifted back on-axis to avoid parallax error. Zeiss market this equipment as the Airyscan (Fig. 5.12; [73]) a detector like an insect’s compound eye consisting of 32 elements, each of them equivalent to a point detector sampled with a 0.2-AU pinhole. These elements are arranged in a circular geometry, such that the total detector area is equivalent to a 1.25-AU pinhole setting, collecting 50% more signal than at 1 AU. The spatial resolution of the final reconstructed image is defined by the sampling of the central pixel, comparable to images acquired with a conventional confocal at a 0.2 AU pinhole setting, while the total sensitivity of the system is equivalent to 1.25 AU. The Airyscan is a PMT detector, rather than a camera, to enable fast read-out. It scans the entire Airy disc, hence the name.
Simple pixel re-assignment is not the only method; slightly different image-scanning approaches may be taken, such as using digital micro-mirrors, re-scanning the image and shifting the phase of the Fourier image rather than shifting pixels directly in order to improve raster speed and axial resolution. Expanding the beam containing the emitted signal before descanning it [74] is the approach adopted by Olympus and Leica, since the Airyscan is patented by Zeiss. Deconvolving the images from each mini-detector ensures a 1.7× resolving power improvement is achieved, to give a lateral resolving power of 140 nm and an axial resolving power of 400 nm. Since the information still comes from a diffraction-limited pattern, it is ultimately limited, as with other structured illumination methods, to a two-fold increase in resolving power.
Take-Home Message
-
Thick fluorescent samples require optical sectioning to remove blurring
-
Single-beam laser-scanning confocal microscopes are the world’s workhorses for acquiring 3-D image datasets, but they are not the only option.
-
Confocal images are acquired by point illumination and point detection.
-
Single beam-scanning confocals are noisy and relatively slow.
-
Confocal images should be deconvolved.
References
Jacoby-Morris K, Patterson GH. Choosing fluorescent probes and labeling systems, Chapter 2. In: Brzostowski J, Sohn H, editors. Confocal microscopy: methods and protocols, methods in molecular biology, vol. 2304. Springer Nature US 2021; 2021.
Jensen EC. Use of fluorescent probes: their effect on cell biology and limitations. Anat Rec (Hoboken). 2012;295(12):2031–6.
Sahoo H. Fluorescent labelling techniques in biomolecules: a flashback. RSC Adv. 2012;2:7017–29.
Toseland CP. Fluorescent labeling and modification of proteins. J Chem Biol. 2013;6(3):85–95.
Stelzer EHK. Contrast, resolution, pixelation, dynamic range, and signal-to-noise ratio. J Microsc. 1998;189(1):15–24.
Lichtman JW, Conchello J-A. Fluorescence microscopy. Nat Methods. 2005;2(12):910–9.
Maric K, Wiesner B, Lorenz D, Klussmann E, Betz T, Rosenthal W. Cell volume kinetics of adherent epithelial cells measured by laser scanning reflection microscopy: determination of water permeability changes of renal principal cells. Biophys J. 2001;80(4):1783–90.
Model MA. Cell volume measurements by optical transmission microscopy. Curr Protoc Cytom. 2015;72:12.39.1–9. https://doi.org/10.1002/0471142956.cy1239s72.
Sheppard CJR, Gan X, Gu M, Roy M. Signal-to-noise ratio in confocal microscopes, Chapter 22. In: Pawley JB, editor. Handbook of confocal microscopy. 3rd ed. New York: Springer; 2006.
Hell S, Reiner G, Cremer C, Stelzer EHK. Aberrations in confocal fluorescence microscopy induced by mismatches in refractive index. J Microsc. 1993;169(3):391–405.
Jacques SL. Optical properties of biological tissues: a review. Phys Med Biol. 2013;58(11):R37–61.
Ntziachristos V. Going deeper than microscopy: the optical imaging frontier in biology. Nat Methods. 2010;7(8):603–14. https://doi.org/10.1038/nmeth.1483.
Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J. 1998;75(4):2015–24.
Diaspro A, et al. Fluorescence microscopy, Chapter 21. In: Hawkes PW, Spence JCH, editors. Springer handbook of microscopy. Cham: Springer; 2019. p. 1039–88. https://doi.org/10.1007/978-3-030-00069-1_21.
Minsky M. Memoir on inventing the confocal scanning microscope. Scanning. 1988;10:128–38.
Harvath L. Overview of fluorescence analysis with the confocal microscope, Chapter 20. In: Javois LC, editor. Methods in molecular biology, 115. Immunocytochemical methods and protocols. 2nd ed. Totowa, NJ: Humana Press; 1999. p. 149–58. https://doi.org/10.1385/1592592139.
Wilson T. Twenty-five years of confocal microscopy. Microsc Anal. 2012;2102:73–77. https://analyticalscience.wiley.com/do/10.1002/micro.265/full/iad081e22e52f50e3f3e52d62a56b2cd7.pdf
Amos WB, White JG. How the confocal laser scanning microscope entered biological research. Biol Cell. 2003;95(6):335–42. https://doi.org/10.1016/s0248-4900(03)00078-9.
White JG, Amos WB, Forham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J Cell Biol. 1987;105(1):41–8.
White JG, Amos WB. Confocal microscopy comes of age. Nature. 1987;328(6126):183–4.
Rusk N. Nature milestones, light microscopy: milestone 4 the fluorescence microscope, 2009. https://doi.org/10.1038/ncb1941. Also see commentary Piston DW (2009) The impact of technology on light microscopy. Nat Cell Biol 11:S23–24. https://doi.org/10.1038/ncb1936
Brakenhoff GJ, van der Voort HTM, van Spronsen EA, Linnemans WAM, Nanninga N. Three-dimensional chromatin distribution in neuroblastoma nuclei shown by confocal scanning laser microscopy. Nature. 1985;317(6039):748–9. https://doi.org/10.1038/317748a0.
Ishikawa-Ankerhold HC, Ankerhold R, Drummen GP. Advanced fluorescence microscopy techniques-FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17(4):4047–132. https://doi.org/10.3390/molecules17044047.
Sanderson J. Understanding light microscopy. Chichester: Wiley; 2019.
Demmerle J, Innocent C, North AJ, Ball G, Müller M, Miron E, Matsuda A, Dobbie IM, Markaki Y, Schermelleh L. Strategic and practical guidelines for successful structured illumination microscopy. Nat Protoc. 2017;12(5):988–1010.
Jost A, Heintzmann R. Superresolution multidimensional imaging with structured illumination microscopy. Annu Rev Mater. 2013;43:261–82. https://doi.org/10.1146/annurev-matsci-071312-121648.
Sheppard CJR. Structured illumination microscopy and image scanning microscopy: a review and comparison of imaging properties. Philos Trans A Math Phys Eng Sci. 2021;379(2199):20200154. https://doi.org/10.1098/rsta.2020.0154.
Neil MA, Wilson T, Juškaitis R. A light efficient optically sectioning microscope. J Microsc. 1998;189(2):114–7.
Schaeffer LH, Schuster D, Schaffer J. Structured illumination microscopy: artefact analysis and reduction utilizing a parameter optimization approach. J Microsc. 2004;216(2):165–74.
Johnson K, Hagan GM. Artifact-free whole-slide imaging with structured illumination microscopy and Bayesian image reconstruction. GigaScience. 2020;9(4):giaa035. https://doi.org/10.1093/gigascience/giaa035.
Karras C, Smedh M, Förster R, Deschout H, Fernandez-Rodriguez J, Heintzmann R. Successful optimization of reconstruction parameters in structured illumination microscopy – a practical guide. Opt Commun. 2018;436(2019):69–75.
Shaw P. Deconvolution in 3-D optical microscopy. Histochem J. 1994;26(9):687–94. https://doi.org/10.1007/BF00158201.
Biggs DSC. 3D deconvolution microscopy. Curr Protoc Cytom. 2010;12:Unit 12.19.1-20. https://doi.org/10.1002/0471142956.cy1219s52.
Ellenberger SE, Young IT. Microscope image acquisition, Chapter 1. In: Baldock R, Graham J, editors. Image processing and analysis, the practical approach series. Oxford: Oxford University Press; 1999. p. 1–36.
Hiraoka Y, Sedat JW, Agard DA. Determination of three-dimensional imaging properties of a light microscope system: partial confocal behavior in epifluorescence microscopy. Biophys J. 1990;57(2):325–33.
Sage D, Donati L, Ferréol Soulez F, Fortun D, Schmit G, Seitz A, Romain Guiet R, Vonesch C, Unser M. DeconvolutionLab2: an open-source software for deconvolution microscopy. Methods. 2017;115:28–41.
Cannell MB, et al. Image enhancement by deconvolution. In: Pawley J, editor. Handbook of biological confocal microscopy. 3rd ed. New York: Springer; 2006. p. 488–500.
Cole RW, Jinadasa T, Brown CM. Measuring and interpreting point spread functions to determine confocal microscope resolution and ensure quality control. Nat Protocols. 2011;6(12):1929–41. https://doi.org/10.1038/nprot.2011.407.
Weigert M, Schmidt U, Boothe T, Müller A, Dibrov A, Jain A, Wilhelm B, Schmidt D, Broaddus C, Culley S, Rocha-Martins M, Segovia-Miranda F, Norden C, Henriques R, Zerial M, Solimena M, Rink J, Tomancak P, Royer L, Jug F, Myers EW. Content-aware image restoration: pushing the limits of fluorescence microscopy. Nat Methods. 2018;15(12):1090–7. https://doi.org/10.1038/s41592-018-0216-7.
Pawley JB. Limitations on optical sectioning in live-cell confocal microscopy. Scanning. 2002;24(5):241–6. https://doi.org/10.1002/sca.4950240504.
Murray JM, Appleton PL, Swedlow JR, Waters JC. Evaluating performance in three-dimensional fluorescence microscopy. J Microsc. 2007;228(3):390–405.
Pawley JB. Pawley, JB (2006) Points, pixels and gray levels: digitizing image data, Chapter 4. In: Pawley J, editor. Handbook of biological confocal microscopy. 3rd ed. New York: Springer; 2006. p. 59–79.
Granzow N. Supercontinuum white light lasers: a review on technology and applications. Proc SPIE. 2019;2019:1114408. https://doi.org/10.1117/12.2533094.
Sandison DR, Williams RM, Wells KS, Strickler J, Webb WW. Quantitative fluorescence confocal laser scanning microscopy, Chapter 3. In: Pawley J, editor. Handbook of biological confocal microscopy. 2nd ed. New York: Plenum; 1995. p. 39–53.
Oppenheim AV, Willsky AS, Young IT. Sampling, Chapter 8. In: Signals and systems. Hoboken, NJ: Prentice Hall; 1983. p. 519.
Webb RH, Dorey CK. The Pixelated Image, Chapter 4. In: Pawley J, editor. Handbook of biological confocal microscopy. 2nd ed. New York: Plenum; 1995. p. 41–51.
Cox G, Sheppard CJR. Practical limits of resolution in confocal and non-linear microscopy. Microsc Res Tech. 2004;63(1):18–22.
Wilhelm S, Gröbler B, Gluch M, Heinz H. Confocal laser scanning microscopy: principles. Jena: Carl Zeiss Microscopy GmbH; 2011.
Gong W, Si K, Sheppard CJR. Improved spatial resolution in fluorescence focal modulation microscopy. Opt Lett. 2009;34(22):3508–10.
Gong W, Si K, Sheppard CJR. Divided-aperture technique for fluorescence confocal microscopy through scattering media. Appl Opt. 2010;49(4):752–7.
Sanderson J. Fundamentals of microscopy. Curr Protoc Mouse Biol. 2020;10:e76. https://doi.org/10.1002/cpmo.76.
Jonkman J, Brown CM, Wright GD, Anderson KI, North AJ. Tutorial: guidance for quantitative confocal microscopy. Nat Protoc. 2020;15(5):1585–611. https://doi.org/10.1038/s41596-020-0313-9.
Diel EE, Lichtman JW, Richardson DS. Tutorial: avoiding and correcting sample-induced spherical aberration artifacts in 3D fluorescence microscopy. Nat Protoc. 2020;15(9):2773–84. https://doi.org/10.1038/s41596-020-0360-2.
Hiraoka Y, Shimi T, Haraguchi T. Multispectral imaging fluorescence microscopy for living cells. Cell Struct Funct. 2002;27(5):367–74. https://doi.org/10.1247/csf.27.367.
Garini Y, Young IT, McNamara G. Spectral Imaging: principles and applications. Cytometry A. 2006;69(8):735–47. https://doi.org/10.1002/cyto.a.20311.
Kraus B, Ziegler M, Wolff H. Linear fluorescence unmixing in cell biological research. Mod Res Educ Top Microsc. 2007;2:863–72.
Lerner JM, Gat N, Wachman E. Approaches to spectral imaging hardware. Curr Protoc Cytom. 2010;12:Unit 12.20. https://doi.org/10.1002/0471142956.cy1220s53.
Zimmermann T, Marrison J, Hogg K, O’Toole P. Clearing up the signal: spectral imaging and linear unmixing in fluorescence microscopy. Methods Mol Biol. 2014;1075:129–48. https://doi.org/10.1007/978-1-60761-847-8_5.
Zucker RM, Rigby P, Clements I, Salmon W, Chua M. Reliability of confocal microscopy spectral imaging systems: use of multispectral beads. Cytometry A. 2007;71(3):174–89. https://doi.org/10.1002/cyto.a.20371.
Cole RW, Thibault M, Bayles CJ, Eason B, Girard A-M, Jinadasa T, Opansky C, Schulz K, Brown CM. International test results for objective lens quality, resolution, spectral accuracy and spectral separation for confocal laser scanning microscopes. Microsc Microanal. 2013;19(6):1653–68. https://doi.org/10.1017/S1431927613013470.
Cromey DW. Digital images are data: and should be treated as such. Methods Mol Biol. 2013;931:1–27. https://doi.org/10.1007/978-1-62,703-056-4_1.
Murray JM. Practical aspects of quantitative confocal microscopy, Chapter 18. In: Sluder G, Wolf DE, editors. Digital microscopy. Methods in cell biology, vol. 114. 4th ed. New York: Elsevier; 2013. p. 427–40.
Montero Llopis P, Senft RA, Ross-Elliott TJ, Stephansky R, Keeley DP, Koshar P, Marqués G, Gao YS, Carlson BR, Pengo T, Sanders MA, Cameron LA, Itano MS. Best practices and tools for reporting reproducible fluorescence microscopy methods. Nat Methods. 2021;18(12):1463–76. https://doi.org/10.1038/s41592-021-01156-w.
Sasaki A. Recent advances in the standardization of fluorescence microscopy for quantitative image analysis. Biophys Rev. 2022;14(1):33–9. https://doi.org/10.1007/s12551-021-00871-0.
Hng K, Dormann D. Confocal check: a software tool for the automated monitoring of confocal microscope performance. PLoS One. 2013;8(11):e79879. https://doi.org/10.1371/journal.pone.0079879.
Dormann D. Intensity check – the light measuring app for microscope performance checks and consistent fluorescence imaging. PLoS One. 2019;14(3):e0214659. https://doi.org/10.1371/journal.pone.0214659.
Brown CM, Reilly A, Cole RW. A quantitative measure of field illumination. J Biomol Tech. 2015;26(2):37–44. https://doi.org/10.7171/jbt.15-2602-001.
Ferrand A, Schleicher KD, Ehrenfeuchter N, Heusermann W, Biehlmaier. Using the NoiSee workflow to measure signal-to-noise ratios of confocal microscopes. Sci Rep. 2019;9:1165. https://doi.org/10.1038/s41598-018-37,781-3.
Lambert TJ, Waters JC. Assessing camera performance for quantitative microscopy. Methods Cell Biol. 2014;123:35–53. https://doi.org/10.1016/B978-0-12-420,138-5.00003-3.
Jonkman J, Brown CM, Cole RW. Quantitative confocal microscopy: beyond a pretty picture, Chapter 7. In: Waters JC, Wittmann, editors. Quantitative imaging in cell biology. Methods in cell biology, vol. 123. New York: Elsevier; 2014. p. 113–34. https://doi.org/10.1016/B978-0-12-420138-5.00007-0.
Weisshart K. The basic principle of Airyscanning. Zeiss Technology Note, 2014.
Sheppard CJR. Super-resolution in confocal imaging. Optik. 1988;80(2):53–4.
Huff J, Bergter A, Berkenbeil J, Kleppe I, Engelmann R, Krzic U. The new 2D Superresolution mode for Zeiss Airyscan. Nat Methods. 2017;14:1223. https://doi.org/10.1038/nmeth.f.404.
Lam F, Cladière D, Guillaume C, Wassmann K, Bolte S. Super-resolution for every-body: an image processing workflow to obtain high-resolution images with a standard confocal microscope. Methods. 2017;115:17–27.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Sanderson, J. (2022). Confocal Microscopy. In: Nechyporuk-Zloy, V. (eds) Principles of Light Microscopy: From Basic to Advanced . Springer, Cham. https://doi.org/10.1007/978-3-031-04477-9_5
Download citation
DOI: https://doi.org/10.1007/978-3-031-04477-9_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04476-2
Online ISBN: 978-3-031-04477-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)