Abstract
In aqueous acid solution and in mixed alcohol-water solvents ([H3O+] > 10–2 M), in the dark and in the absence of reductants, the spontaneous decomposition of aryldiazonium, ArN2+, salts proceeds through borderline SN1 (DN + AN) -SN2 mechanisms. The rate constant values depend strongly on the nature of the substituents attached to the aromatic ring of ArN2+ and, for those with electron-withdrawing substituents, on solution composition. The product distribution is proportional to the composition of the solvation shell of the ipso carbon, which reflects the composition of the water/cosolvent mixture. However, upon decreasing moderately the acidity, reactions involving the formation of diazohydroxides, ArN2OH, diazoethers, ArN2OR, and diazoates, ArN2O−, become competitive and may even be the main decomposition pathway. The stability of ArN2OH, ArN2OR, and ArN2O− species (which may coexist with ArN2+ in solution) is intimately related to the Z-E (syn-anti, cis-trans) isomerization of the O-adducts, so that they may undergo further reactions when they are components of a Lewis acid-base equilibrium, or undergo homolytic scission to produce homolytic reduction products. In this book chapter, we aim to provide the reader with a practical and (hopefully) useful view of the complex chemistry of ArN2+ in aqueous and mixed alcohol-water solutions, mainly covering the kinetics and mechanisms of the reactions. In a last section, we introduce some analytical methods for the determination of diazonium salts and their degradation products.
Dedicated to Prof. Laurence S. Romsted (Rutgers, the State University of New Jersey, USA) for introducing us to the very complex—yet fascinating—chemistry of aryldiazonium ions, for his continuous support, encouragement, extremely fruitful discussions, and, most importantly, for his friendship.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
1 Introduction
Aryldiazonium, Ar-N2+, and ions played a major role in organic chemistry since their discovery in 1858 by Peter Griess [1, 2]. From a historical perspective, the chemistry of aryldiazonium salts has been developed, in most instances, in aqueous media, and classical applications of arenediazonium salts include the Sandmeyer, Balz-Schieman, Gomberg-Bachman, Pschorr, and Meerwein reactions [3–5]. In the last decades, there has been renewed interest in their use as reactants in solvents other than water and alcohols, in catalytic cross-coupling reactions, [6, 7] in the chemical-biological field through modifications of amino acids, peptides, and proteins, [8] with functionalization of surfaces [9–12] and as chemical probes for probing interfacial concentrations of nucleophiles in a variety of colloidal systems [13, 14].
The utility of aryl diazonium salts in the solid-state may be, however, diminished by their potential instability, which is often governed by the nature of the counterion, [3, 15] and during the second half of the twentieth century, some chemists focused in developing synthetic methods to prepare more stable aryldiazonium salts, mostly in the form of tetrafluoroborates, hexafluorophosphates, tosylates, and disulfonamides (Chap. 2) [15, 16]. The tetrafluoroborates, in particular, can often be easily prepared and isolated as crystalline solids that can be stored in the dark, in the absence of water, at low temperatures for months to years without significant decomposition (Chap. 2) [3].
When in solution, aryldiazonium, ArN2+, ions may behave as Lewis acids, thus reacting with Lewis bases (nucleophiles Nu– or NuH followed by loss of a proton) to yield covalently bonded adducts, ArN2-Nu, at the electrophilic reactive center (the β-nitrogen of ArN2+) [3, 4]. Typical examples of the ArN2-Nu adducts are the azo dyes, which are formed through a C-coupling mechanism [3, 17]. The formation of azo dyes takes place when ArN2+ ions react with aromatic substrates containing strong electron donors such as hydroxy or amino groups [17]. Covalently bonded adducts can also be formed with other nucleophiles leading to O-, S-, N-, and P-adducts [3, 4, 18].
This book chapter will focus exclusively on the reactivity of aryldiazonium ions in aqueous solution, with particular emphasis on the formation of O-coupling adducts of the type ArN = NO-Nu because H2O and alcohol/water mixtures, together with H2O/CH3CN mixtures (in CH3CN, the corresponding acetamides are formed [19]) are, by far, the most important solvents for diazonium salts, and where a variety of nucleophiles can be present or dissolved depending on experimental conditions [3]. Details on reactions in other solvents can be found elsewhere [3, 18]. The synthesis of ArN2+, their classical reactions such as the Sandmeyer reaction, and their recent applications will not be covered as this material can be found in other chapters of the present book and in specialized books or reviews [3, 4, 17, 20, 21]. In addition, the chemistry of alkanediazonium, RN2+, ions will not be covered here because they are of much less practical application than the aromatic ones because of their much lower stability [20, 22].
Furthermore, the present book chapter is not intended to be exhaustive in covering all literature but is rather aimed to provide the reader a broad view of the complex chemistry of ArN2+ in aqueous solution, with special emphasis on the kinetics and mechanism of the reactions involved. Indeed, this solution chemistry has important consequences in the grafting of diazonium salts. In the next sections, we will discuss the various mechanisms in some detail.
2 A Brief Survey of the Chemistry of Arenediazonium Ions in Aqueous Solutions
One of the most important—but mechanistically complicated—reaction is the deceptively spontaneous decomposition of ground state Ar-N2+ in aqueous solution and in alcohol/water mixtures. Part of the complexity of the reaction arises from the fact that water and aliphatic and aromatic alcohols (ROH, ArOH) may also act as a neutral nucleophiles in addition to act as a solvents, and, depending on the pH, can even ionize so that species such as OH− and ArO− may be present in solution and act as potential nucleophiles [18, 23–25].
The reaction of ArN2+ with water was first reported by Griess in the late nineteenth century and its “apparent” complexity made it to be the origin of some scientific controversy between E. Bamberger and A. Hantzsch for almost two decades (1894–1912) because of the structures of the products [3, 4, 26, 27]. In aqueous acid solution (pH < 2), in the absence of reductants, and in the dark, the mechanism of the spontaneous decomposition of ArN2+ is still a matter of debate, as there is not yet clear evidence if the reaction proceeds through SN1 (DN + AN) or SN2 mechanisms, Scheme 1, leading to the formation of heterolytic products [19, 28–30]. A decrease in the acidity of the solution modifies the mechanism of their spontaneous decomposition so that between pH ~ 4 and ~9, diazohydroxides, ArN2OH, and diazoethers, ArN = N-OR, are formed from the reaction of ArN2+ with H2O and ROH, respectively, which in this case act as a nucleophiles) [3, 18, 26, 27]. When phenoxide ArO− ions are present, ArN2+ may also react with them and diazoethers Ar-N = N-OAr´ may be formed [18]. Both ArN2OH and ArN = N-OR or (Ar-N = N-OAr´) may undergo homolytic decomposition, leading to the formation of reduction products and, in some instances, tarry materials [4].
a Ionic or heterolytic SN1 (DN + AN) dediazoniation mechanism in the presence of various nucleophiles illustrating the formation of an intimate ion•molecule or a solvent separated ion-molecule pair that traps all nucleophiles available in their solvation shell b SN2 dediazoniation mechanism. In both cases, heterolytic products are formed. The selectivity of ArN2+ against nucleophiles is very low, an argument commonly employed as an evidence for the SN1 mechanism against the SN2 one [3, 31, 32].
In aqueous alkaline solutions (pH > 9), ArN2+ function as Lewis acids, reacting at the terminal nitrogen with OH−, and diazohydroxides ArN2OH are formed which can further react with OH− to finally form diazoates ArN2O−, which are quite stable and can be isolated [3, 18].
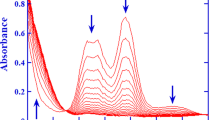
Spontaneous dediazoniations, thus, may involve a variety of mechanisms and the following example should be sufficient to illustrate how apparently minor modifications in the experimental conditions may lead to major changes in the dediazoniation kinetics and in the product distributions. In aqueous acid solution, in the dark and in the absence of reductants, dediazoniation of 4-nitrobenzenediazonium (PNBD) follows the SN1 mechanism depicted in Scheme 1, with a half-life t1/2 = 1383 min [33]. However, addition of small amounts of MeOH leads to non-first-order kinetics compatible with a radical mechanism, so that t1/2 ≈ 6 min at 10% (v:v) MeOH, Fig. 1. Interestingly enough, a further increase in [MeOH] makes the reaction to progressively revert to a first-order behavior, slowing down the rate of dediazoniation so that at percentages of MeOH higher than 90%, clean first-order kinetics are obtained with t1/2 ≈ 77 min. In the absence of MeOH, 4-nitrophenol (product from heterolytic cleavage) is formed in quantitative yield; however, at only 20% MeOH, the reduction product nitrobenzene (product from homolytic cleavage) is obtained in more than 90% and the yield of 4-nitrophenol is less than 10%. Upon increasing the MeOH content of the solution, the formation of 4-nitroanisole (obtained from the heterolytic reaction between PNBD and MeOH, Scheme 1) becomes competitive and, at 99% MeOH, both nitrobenzene (60%) and 4-nitroanisole (40%) are obtained [33]. The reduction product 4,4-dinitrobiphenyl (obtained from the coupling reaction of two Ar. radicals) is detected only at MeOH percentages in the range 0.5–15%.
Variations of the half-life (left) and of the product distribution (right) for the spontaneous decomposition of 4-nitrobenzenediazonium as a function of the percentage of MeOH (v:v) in solution. Up to four products can be detected depending on experimental conditions, 4-nitrophenol (ArOH), 4-nitroanisole (ArOMe), nitrobenzene (ArH), and 4,4-dinitrobiphenyl (only detected in the range 0.5–15%). Adapted with permission from ref. [33],
A major feature that needs to be considered when dealing with reactions where ArN2ONu adducts are formed is the geometrical Z-E (syn-anti) isomerism , Scheme 2, leading to the Z-(anti) and E-(syn) forms. Isomerization is possible in reactions of ArN2+ with nucleophiles Nu− where the nitrogen-nitrogen double-bond is retained, i.e., R–N = N-R’ (R and R’ can be alkyl or aryl residues). Their formation was recognized experimentally as early as 1938 by Hartley [34] after Hantzsch and Werner [35] put forward the hypothesis of their existence [3] and was investigated by employing a variety of nucleophiles such as Nu = O− (diazoates), Nu = RO− (diazoethers), Nu = SO3− (sulfonates), Nu = −SO2-Ar (sulfones), Nu = CONH2 (carboxamides) and Nu = S-R, S-Ar (diazothioethers), Nu = CN− (cyanides) and Nu = R1-NH-R2 (triazenes). ArN2OH adducts are components of a Lewis acid-base equilibrium and react with OH− ions to give diazoates, ArN2O−.
Z (syn)-E (anti) isomerism following the formation of Ar-N = N-Nu adducts which may undergo homolytic scission. The competitive spontaneous decomposition of ArN2+ through the formation of a highly reactive aryl cation (SN1 mechanism, Scheme 1) is also shown for illustrative purposes. Dediazoniations that follow homolytic mechanisms are always (as far as they are known today) faster than the heterolytic ones. Adapted with permission from ref. [18],
In most instances, the Z-isomer is the kinetically controlled product, but the E-isomers are thermodynamically more stable. Z-isomers are formed in reactions with OH−, CN−, RO−, CN− and SO32−, whereas E-compounds are formed with reactants such as phenols and tertiary amines. Further details on the Z-E isomerism can be found elsewhere [3, 4, 18].
O-adducts may also be formed when reacting with nucleophiles Nu− other than OH−, but their formation, identification, and isolation may be very difficult because their stability depends on the leaving ability of the nucleophile involved in the reaction. If the reactant Nu− is a poor leaving group (even if it is a good nucleophile), such as ascorbate or gallate—3,4,5-trihydroxybenzoate- ions, some stabilization may occur by conversion to the thermodynamically stable isomer (Z-E isomerization) [36, 37]. If Nu− is a good nucleophile and good leaving groups, such as halide or acetate ions, the equilibrium lies largely on the side of reactants so that formation of O-adduct is negligible and ArN2+ undergoes spontaneous decomposition through the heterolytic mechanism [3, 23, 38]. In some instances, isomerization is not possible and the adduct splits homolytically to finally give reduction products [33, 39, 40].
3 Kinetics and Mechanisms of Spontaneous Decomposition of ArN2+ in Aqueous Solution and in Alcohol-Water Mixtures
3.1 Dediazoniations Under Acidic (pH < 2) Conditions
It is generally accepted that in aqueous acid (pH < 3) solution, in the absence of reductants and in the dark, the spontaneous hydrolysis of aryldiazonium salts afford phenols through bordeline SN1-SN2 mechanisms, Scheme 1 [3, 18, 26, 31]. A variety of studies including N2 incorporation and rearrangement substituent effects, nucleophile and solvent effects have led to the widely accepted mechanism involving the rate-determining loss of N2 and formation of an extremely reactive aryl cation intermediate, Ar+, that reacts with nucleophiles in its solvation shell in a time faster than that required for cation diffusion out of the solvent cage [3, 13, 19, 26, 28]. However, recent reports, mostly based on theoretical calculations, suggest that the formation of a free phenyl cation is not an obligatory intermediate and that a borderline SN1-SN2 mechanism is compatible with experimental results [19, 28, 29].
Table 1 collects some values for the half-lives for the spontaneous decomposition of various arenediazonium ions in different aqueous solvents. As shown, ArN2+ decomposes spontaneously in aqueous acid and alcohol/water mixtures ([H3O+] < 10–2 M), with have-lives ranging from minutes to hours, depending on the substituents in the aromatic ring. Solvolytic studies in alcohol-water (ROH/H2O) or acetonitrile-water (ACN/H2O) mixtures show that, in aqueous acid solution, aryldiazonium ions undergo preferential solvation by water, [23, 40, 41] results confirmed later by classical molecular dynamics calculations of solvent distribution around ArN2+ for water/solvent mixtures [19]. The bulk product distribution reflects the relative nucleophile distribution in the solvation shell and the relative concentration of nucleophiles in solution [19, 23, 33, 40, 42].
3.2 Kinetics and Mechanisms of Dediazoniation at Moderate Acidities (pH 3-8)
The apparently “simple” decrease of the acidity of the solution (while keeping other experimental conditions constant) leads to a completely different—and much more complex—kinetic behavior than that observed under acidic conditions, deserving a specific section for the description of the mechanistic and practical consequences of the reactions involved. Production of higher amounts of homolytic products in alcohols than in water would be anticipated based on the nucleophilicity of the solvents. However, this is not the case. Dediazoniations in alcohols usually yield three or four products (including reduction ones) and diazo tars seldom form [4]. However, in weakly acidic or alkaline aqueous solutions, tarry materials may be formed and dediazoniations produce a complex mixture of other products [3, 4, 27].
At moderate acidities, the nucleophilic character of the solvent is reinforced, and the reactions of ArN2+ with H2O and ROH (as nucleophiles) lead to the formation of diazohydroxides, ArN2OH, and diazoethers, ArN2OR, becoming competitive so that the observed rates of the reactions are much faster than under acidic (pH < 2) conditions and lead to the formation of a variety of products, including reduction products that in many instances are difficult to isolate or identify, suggesting that mechanisms other than the described SN1-SN2 mechanisms (Scheme 1) are operating and becoming increasingly important.
The formation of diazohydroxides ArN2OH in aqueous solution was demonstrated on the basis of the variations of the peak currents and peak potentials (Ep) of the first reduction peak of a number of aryldiazonium ions with acidity, Fig. 2 [39, 46–51]. The dependence of Ep on pH is typical of acid-base systems and results were interpreted according to Scheme 3 [52].

Figures reproduced with permission from reference [53]. Copyright 2014 J. Wiley & Sons
Left. Illustrative variation of the first (irreversible) reduction peak of benzenediazonium ions with pHs. Right, variation of peak potential (Ep) of the first reduction peak of 4-methylbenzenediazonium ion with pH. Details on calculations can be found elsewhere [53].
Some values for the equilibrium constants KR corresponding to the formation of hydroxides are shown in Table 2, indicating that effects of the nature of the substituents in the aromatic ring of ArN2+ are very small, suggesting an increase in the electron density on the atom adjacent to the ring during the reaction, neutralizing the positive charge on the diazonium group [54].
A large body of solvolytic studies shows that the formation of O-diazo adducts with neutral nucleophiles (including the solvent) is quite common [3, 18, 33, 40, 45]. The studies permitted the recognition of the dual role of H2O and ROH molecules as nucleophiles. On one side, H2O and ROH act as solvents solvating the diazonium ions and allowing them to undergo thermal heterolytic decomposition, Scheme 1. On the other hand, H2O and ROH molecules can act as nucleophiles reacting directly with arenediazonium ions to yield O-coupling adducts, Scheme 3, in the highly unstable Z configuration, i.e., Z-diazoethers. The Z-diazoethers then undergo either homolytic fragmentation, initiating radical processes leading to the formation of quantitative amounts of the reduction product, or isomerize to finally yield the E-isomer [18].
In solution, ArN2+, ArN2OH, and/or ArN2OR adducts may coexist in moderate acid solutions. The variation of the relative concentrations of these species as a function of acidity is shown in Fig. 3.
Variations of the percentages of ArN2+, diazohydroxides ArN2OH (left), and ArN2OR (right) in aqueous solution and in ethanol/water mixtures, respectively, upon changing the solution acidity. Average values of pKR = 5.6 and pKDE = 3.6 were employed in calculations. The large difference between pKR and pKDE values makes that the formation of ArN2OH in alcohol/water mixtures to be negligible compared to that of ArN2OR
A product and kinetic analyses of dediazoniations in alcohol/water mixtures show that sigmoidal variations in both the observed rate constant kobs and product yields with acidity for a number of arenediazonium ions [33, 40, 43, 45] as illustrated in Fig. 4. At relatively high acidities (–log([HCl] < 2), only products from ionic mechanisms (phenols, ArOH, ethers, ArOEt, halo derivatives, ArCl) are detected. But, upon decreasing the acidity, the homolytic product ArH becomes predominant at the expense of the heterolytic ones [40].

Reproduced with permission from reference [18] Copyright 2010 John Wiley & Sons
Left: Effects of acidity on kobs for the solvolytic decomposition of 4-methylbenzenediazonium, 4-Me-ArN2+ in 35 and 70% EtOH-H2O mixtures. Right: Effects of acidity on the dediazoniation product distribution obtained in a 70% EtOH-H2O mixture. The main solvolytic products were cresol, ArOH, methylphenetole, ArOEt, and toluene, ArH. Note the quantitative formation of the homolytic (reduction) product ArH at moderate acidities (pH = 3 −5). [4-Me-ArN2+] ~1 × 10–4 M, T = 60 ℃.
Sigmoidal variations of kobs with acidity are usually observed in reactions of acid-base pairs where both forms are attainable and show different reactivities. Thus, in aqueous solutions or in alcohol/water mixtures at moderate acidities, two competing mechanisms are operating, Scheme 4, and Eq. 1 can be derived.
Two limiting situations can be considered, (i) When [H+] ≫ KDE[ROH], kobs ≈ kHET, i.e., the reaction proceeds wholly through the ionic DN + AN mechanism, and only heterolytic products are obtained. On the other hand, when [H+] ≪ KDE[ROH], kobs ≈ kHOM, i.e., the reaction proceeds wholly through the O-diazo ether and formation of homolytic reduction products is favored. Some representative values for the average pK value, as well as those for kHET and kHOM are listed in Table 2.
Results in the presence of molecules bearing OH groups in their molecular structure such as ascorbic acid (vitamin C, VC) [24, 36, 56–59] are also consistent with the formation of O-adducts, which were detected experimentally [36, 58, 59] and unambiguously identified by 1H and 13C NMR spectroscopy as 3-O-arenediazo ascorbic acids. [56] O-adducts are also formed in reactions of ArN2+ with trihydric phenols such as gallic acid (GA, 3,4,5-trihydroxybenzoic acid) and methyl gallate (MG, methyl 3,4,5-trihydroxybenzoate) and catecholics such as hydroxytyrosol [25, 60–63]. For these reactions, a mechanism comprising consecutive, reversible steps, have been proposed. The first one involves a fast, bimolecular step, in which ArN2+ ions react with GA2− to form a Z-diazoether complex, which is subsequently isomerized to the ionized form of the much more stable E-diazoether in a second, slow, unimolecular step (Scheme 5) [25, 60, 61].
Proposed reaction mechanism between 3-methylbenzenediazonium ions and methyl gallate, MG. The reaction takes place through the ionized form of MG (pKa = 8.03) and only tiny amounts of the ionized polyphenol are necessary for the reaction to proceed through the formation of the diazo ether at rates (kHOM) much higher than those of the spontaneous decomposition (kHET) [61].
3.3 Dediazoniations in Alkaline (pH > 9) Aqueous Solutions
Aromatic diazonium ions behave as Lewis acids, as they can react with OH− ions to form diazohydroxides, ArN2OH. The diazohydroxides then became Brönsted acids and may lose a proton to yield diazoates, Scheme 6.
Equilibria showing the formation of diazoates [18]
Reports by Wittwer and Zollinger [64] showed that the variation of the degree of neutralization with acidity is different from that expected for a dibasic acid because the equilibrium constant for the formation of the diazoate is higher than that for the formation of the diazohydroxide, that is, K2 > K1. This behavior is in contrast with that obtained for typical dibasic acids, where K1 > K2. Wittwer and Zollinger [64] estimated, from neutralization curves, that K2 > 103 K1 and for this reason, it was not possible to separate, at that time, the individual values but only could be determined their product K1K2. Individual values of K1 and K2 were first determined by Lewis and Suhr [65] by taking advantage of the fact that rate constant for the addition of OH− ions to ArN2+ ions (k3 in Scheme 6) is slower than the rate of deprotonation of the diazohydroxide (k4). The general conclusion that can be drawn is that K2 is greater than K1 by 3–5 orders of magnitude, depending on the nature of the substituent in the aromatic ring.
By using the corresponding equilibrium constants, Eq. 2 has been derived, which shows that (pK1 + pK2)/2 = pHm equals the pH of the solution in which [ArN2+] = [ArN2O−]. The ratio [ArN2+] / [ArN2O−] changes 100 fold when the pH is changed by one [1] unit so that at pH = pHm + 1, the reaction mixture only contains 1% of ArN2+ and 99% diazotate. Values for the rate constants, pK1, pK2 for a series of arenediazonium ions, and the variations of the relative amounts of ArN2+, ArN2OH, and ArN2O− can be found elsewhere [3, 18].
The relative concentrations of the diazonium and the diazoate ions (the major equilibrium forms) are shown in Fig. 5. The equilibrium concentrations of the diazohydroxide and the diazoanhydride are usually very small at all pH values, with a maximum at pH = pKm.

Reproduced with permission from ref. [3] Copyright 1994 J. Wiley & Sons
Variation of the relative concentrations of various species in equilibrium with ArN2+ as a function of acidity.
4 Analytical Chemistry of Diazonium Salts and Their Degradation Products
Regarding to analytical chemistry of these compounds and the chemistry around them, the identification and the quantification of the ArN2+ itself and all by-products generated in dediazoniation reactions will be the main challenge to get sufficient valuable data on these processes and to offer a convincing choice between the mechanistic alternatives, in particular during surface reactions. The compounds to be identified and quantified are aromatic derivatives (typically, phenolics, haloarenes, benzene derivatives, biaryls, aromatic ethers, etc.), as well as the possible transient and stables intermediates, depending on the structural nature of ArN2+ and the reaction media composition.
As a starting point, some of the analytical techniques are able to monitor only one component at a time, and some others are limited to narrow ranges of useful experimental conditions, once the adjustable parameters to describe the measured property in any particular solution composition have been optimized [66]. As experts in the field, the readers know well that there is not only one technique to be used. The combination of several techniques is necessary in order to get insight into complete knowledge of what is going on in the dediazoniation reactions, how is the disappearance of the ion and its rate, which are the products formed and their reaction rates or the stable/transient intermediates that can be detected and monitored. On these bases, the possible degradation pathway in any matrix or composition of the reaction media (aqueous or non-aqueous solutions, colloidal and macromolecular systems, presence of different electrolytes, etc.) can be proposed. To achieve these goals, valuable and quality data that help to work out a real mechanism interpretation of dediazoniation reaction will be needed. Therefore, it is easy to understand that the use of several techniques that complement each other to solve the problem is the best way to validate the results obtained. Furthermore, the analytical signal of the measured property must be correlated with the concentration of the analyte (calibration) to obtain minimum uncertainty in concentration level, and then, high-quality kinetic data can be achieved. Keep in mind that this section is not intended to be exhaustive. The proposal is to address a brief description of most popular instrumental analytical techniques used for monitoring ArN2+ in kinetics studies up to date, with some references as representatives.
The UV–Vis spectra of ions and molecules in solution were the first instrumental methodology used to obtain both qualitative and quantitative chemical information and were widely applied to obtain kinetic data [23, 24, 33, 34, 38, 57, 58, 67, 68]. This technique is simple to use and relatively rapid, limited to transparent media and by the cutoff point of solvents. UV–Vis spectrophotometers with stopped-flow devices for monitoring very fast reactions are extremely valuable [69]. However, the UV region is not selective enough and most of the products involved in the dediazoniation reaction absorb in the same region, being impossible to monitor the reactive loss and/or products formation at the same time. For solving this situation, the absorption band can be shifted to the visible region, more selective, by a derivatization reaction that quantitatively transforms the unreacted ArN2+ into a compound with highly conjugated double bonds and heteroatoms. The coupling reaction with naphthols and naphthylamines to form azo dyes based on Griess reaction [7] provide the essential tool to monitor the degradation of ArN2+ by indirect monitoring the azo dye formed, but the experimental conditions must be chosen to ensure that azo dye formation is much faster than dediazoniation [17, 70]. In contrast with UV–Vis spectrophotometric technique, scant attention has been paid to the use of fluorescence for detection and monitoring dediazoniation. The ArN2+ derivatives with one aromatic ring system show a poor fluorescence, but enough for dediazoniations following up [70]. This technique is more sensitive (at least by two orders of magnitude) for polyaromatic molecules with rigid planar structures as naphthalene N2+ derivatives and much more selective than UV–Vis spectrophotometry.
Nuclear magnetic resonance, NMR, and Fourier transformed infrared, FT-IR, spectroscopies, together with Mass spectrometry by electrospray ionization, EIS-MS, are techniques that support all kinetics experiments, giving information about purity of standards, screening quantification, and structural elucidation and confirmation. They determine whether a synthetic reactant or product is the compound expected or planned. NMR and MS are remarkably close to each other in relation to the information obtained, but the advantage of MS is that it only needs small amount of sample (ng contrary to µg in NMR) [19, 30]. MS disadvantages are to be a destructive analytical technique and limited in solving structural isomerism, where NMR is still needed. The 13C NMR or 1H NMR techniques are additionally used in chemical analysis of dediazoniations by monitoring the chemical shift of the products in the spectra [71, 72]. Electron paramagnetic resonance, EPR, or Electron spin resonance, ESR, spectroscopy is another spectroscopic technique used to study radical chemical species in dediazoniation reactions, providing a valuable measure of how well the homolytic mechanism is going on and which are the radical intermediates found [70]. The principles of EPR are similar those to NMR, but give higher time resolution and sensitivity than NMR.
The most effective solution to prevent interferences is to use a separation technique previously to the detection. High-pressure liquid chromatography, HPLC, is a versatile technique widely used in kinetic studies of ArN2+ for obtaining kinetic data of products formation, choosing the chromatographic mode by reverse–phase and UV or diode array detectors [23, 24, 33, 40, 57, 58, 73]. However, this technique is time-consuming, tedious, and reactive samples cannot be obviously injected. The derivatization protocol mentioned above takes advantage of quenching instantaneously the dediazoniation reaction and preventing side reactions of highly reactive ArN2+ or its intermediates with the solvents of the mobile phase or other parts of the chromatographic system. The latter would ultimately lead to erroneous identification and quantification of dediazoniation products. At that point, the coupling reactions provide the best performance in the HPLC system and HPLC column and avoid pitfalls that can impact in the HPLC analysis [74]. Gas chromatography with flame-ionization detector, GC-FID, is one of the fastest and most useful separation techniques available [37, 72, 75], but limited to organic compounds that are volatile and not thermally labile. This is why HPLC has been gaining followers up to date. The most sophisticated detection system for both qualitative and sensitive quantitative analysis in GC or HPLC systems is Mass Spectrometry, MS, which provides retention-time data and peak area measurements, but structural information obtained from the fragmentation patterns can be used to positively identify the mixture components and to determine the products of the reactions with higher sensitivity and selectivity [76, 77]. However, this methodology requires several steps for sample preparation, expensive instrumentation, and trained professionals to operate the equipment.
The investigation of dediazoniations by electroanalysis showed that a variety of polarographic and voltammetric peaks can be chosen to obtain kinetic information of ArN2+ [46–48, 51, 78]. In this context, the selective detection is possible through the electroreduction on drop mercury electrode, DME, of the ArN2+ and products formed by differential pulse polarography, DPP, and the electrooxidation on carbon electrodes of some products (i.e., phenolics) by DP voltammetry [39, 47]. An interesting statement of these techniques, in comparison with all others reported herein, is that the reaction of 4-hexadecylbenzenediazonium ions, 16-ArN2+, is used as probe to better understand the colloidal interfaces and the distribution of antioxidants in very complex matrix as emulsions, can be directly monitored by linear sweep voltammetry, LSV [78, 79]. The advantages of these techniques rely on short analysis time, low cost, and simple sample preparation steps (unique dilution in electrolyte). Moreover, electroanalysis stands out for being useful for simultaneously detecting ArN2+ loss, transient intermediates as diazoethers [36], and products formed by dediazoniation with high sensitivity (detection limits lower than those obtained by the aforementioned techniques, except MS) and with similar selectivity to HPLC techniques in some dediazoniations [39, 46]. Furthermore, the electroanalysis can be applied not only in transparent solutions but also in both opaque solutions and suspensions.
5 Conclusions and Future Perspectives
The unique multimodal reactivity of ArN2+, while offering multiple options for the preparation of new compounds (Pd-catalyzed reactions, thermal or photosensitized radical chain reactions, and azo-coupling reactions) and materials, can also be fine-tuned via the appropriate choice of experimental conditions and reaction partners. This overview shows a great potential for the formation of a wide variety of azo compounds and their use in chemistry. However, it should also be mentioned that protocols involving the use of arenediazonium salts have the drawbacks of ArN2+ being unstable in solution, highlighting the need for a careful control of experimental conditions, since apparently minor changes may lead to substantial modifications in the kinetics and mechanisms of their spontaneous decomposition, leading to large modifications in their effective concentrations in solution.
In this review, we focused on the main kinetic and mechanistic consequences of changing experimental conditions in the spontaneous decomposition of aryldiazonium salts to draw the attention of scientists interested in future applications of ArN2+ in solution. Indeed, the various applications of aryldiazonium salts, which are readily obtained from inexpensive anilines, will continue to attract attention of chemists, but the spontaneous reaction of diazonium salts in various solvents will need to be considered anyway as it may be competitive with the other processes involved.
References
Griess JP (1864) Philos Trans R Soc. London. https://doi.org/10.1098/rstl.1864.0018
Griess P (1858) Liebigs Ann Chem 106:123
Zollinger H (1994) Diazo chemistry I: aromatic and heteroaromatic compounds
Saunders KH, Allen RLM (1985) Aromatic Diazo compounds. Baltimore, MD, USA, E Arnold
Mo F, Qiu D, Zhang Y, Wang J (2018) Acc Chem Res 51:496–506. https://doi.org/10.1021/acs.accounts.7b00566
Roglans A, Pla-Quintana A, Moreno-Mañas M (2006) Chem Rev 106:4622–4643. https://doi.org/10.1021/cr0509861
Kostas ID (2018) Suzuki–Miyaura cross—coupling reaction and potential applications. MDPI AG
Sengupta S, Chandrasekaran S (2019) Org Biomol Chem 17:8308–8329. https://doi.org/10.1039/C9OB01471C
Mohamed AA, Salmi Z, Dahoumane SA, Mekki A, Carbonnier B, Chehimi MM (2015) Adv Coll Interface Sci 225:16–36. https://doi.org/10.1016/j.cis.2015.07.011
Chehimi MM (2012) Aryl diazonium salts: new coupling agents in polymer and surface science. Wiley
Granozzi G, Alonso-Vante N (2019) Electrochemical surface science: basics and applications. Mdpi AG
Hetemi D, Noël V, Pinson J (2020) Biosensors 10. https://doi.org/10.3390/bios10010004
Dar AA, Bravo-Diaz C, Nazir N, Romsted LS (2017) Curr Opin Colloid Interface Sci 32:84–93. https://doi.org/10.1016/j.cocis.2017.09.001
Bravo-Díaz C, Romsted LS, Liu C, Losada-Barreiro S, Pastoriza-Gallego MJ, Gao X, Gu Q, Krishnan G, Sánchez-Paz V, Zhang Y, Ahmad-Dar A (2015) Langmuir 31:8961–8979. https://doi.org/10.1021/acs.langmuir.5b00112
Firth JD, Fairlamb IJS (2020) Org Lett 22:7057–7059. https://doi.org/10.1021/acs.orglett.0c02685
Trusova ME, Kutonova KV, Kurtukov VV, Filimonov VD, Postnikov PS (2016) Res-Efficient Technol 2:36–42. https://doi.org/10.1016/j.reffit.2016.01.001
Zollinger H (1991) Color chemistry. VCH
Bravo Díaz C (2011) Diazohydroxides, diazoethers and related species. In: Rappoport Z, Liebman JF (eds) The chemistry of hydroxylamines, oximes and hydroxamic acids. Wiley, Chichester, UK
Cruz GN, Lima FS, Dias LG, el Seoud OA, Horinek D, Chaimovich H, Cuccovia IM (2015) J Org Chem 80:8637–8642. https://doi.org/10.1021/acs.joc.5b01289
Zollinger H (1995) Diazo chemistry II. In: Aliphatic, inorganic and organometallic compounds. Weinheim, Germany, VCH
Zollinger H (1983) Dediazoniations of arenediazonium ions and related compounds. In: Patai S, Rappoport Z (eds) The chemistry of triple bonded functional groups. Wiley
Moss RA (1974) Acc Chem Res 7:421–427. https://doi.org/10.1021/ar50084a005
Pazo-Llorente R, Bravo-Díaz C, González-Romero E (2003) Eur J Org Chem 2003:3421. https://doi.org/10.1002/ejoc.200300183
Costas-Costas U, Bravo-Díaz C, González-Romero E (2003) Langmuir 19:5197–5203. https://doi.org/10.1021/la026922s
Losada-Barreiro S, Sánchez-Paz V, Pastoriza-Gallego MJ, Bravo-Diaz C (2008) Helv Chim Acta 91:21–34. https://doi.org/10.1002/hlca.200890009
Hegarty AF (1978) Kinetics and mechanisms of reactions involving diazonium and diazo groups. In: Patai S (ed) The chemistry of diazonium and diazo compounds. Wiley, NY
Galli C (1988) Chem Rev 88:765. https://doi.org/10.1021/cr00087a004
Cuccovia IM, da Silva MA, Ferraz HM, Pliego Jr JR, Riveros JM, Chaimovich H (2000) J Chem Soc Perkin Tans 2:1896. https://doi.org/10.1039/b003079l
García Martínez A, de la Moya Cerero S, Osío Barcina J, Moreno Jiménez F, Lora Maroto B (2013) Eur J Org Chem 6098–6107. https://doi.org/10.1002/ejoc.201300834
Ussing BR, Singleton DA (2005) J Am Chem Soc 127:2888. https://doi.org/10.1021/ja043918p
Bravo-Diaz C (2009) Mini-Rev Org Chem 6:105–113. https://doi.org/10.2174/157019309788167693
Bentley TW, Ryu ZH (1994) J Chem Soc Perkin Trans 2:761. https://doi.org/10.1039/P29940002531
Pazo-Llorente R, Maskill H, Bravo-Díaz C, González-Romero E (2006) Eur J Org Chem 2006:2201. https://doi.org/10.1002/ejoc.200500946
Hartley GS (1938) J Chem Soc 633. https://doi.org/10.1039/JR9380000633
Hantzsch A, Werner A (1890) Ber Dtsch Chem Ges 23:11. 443.webvpn.fjmu.edu.cn/https://doi.org/10.1007/978-3-642-99003-8_13
Costas-Costas U, Gonzalez-Romero E, Bravo-Díaz C (2001) Helv Chim Acta 84:632–648. https://doi.org/10.1002/1522-2675(20010321)84:3%3c632::AID-HLCA632%3e3.0.CO;2-0
Hanson P, Jones JR, Taylor AB, Walton PH, Timms AW (2002) J Chem Soc Perkin Trans 2:1135
Canning PSJ, Mccrudden K, Maskill H, Sexton B (1999) J Chem Soc, Perkin Trans 2(12):2735. https://doi.org/10.1039/A905567C
González-Romero E, Malvido-Hermelo B, Bravo-Díaz C (2002) Langmuir 18:46. https://doi.org/10.1021/la010938l
Pazo-Llorente R, Bravo-Díaz C, González-Romero E (2004) Eur J Org Chem 2004:3221. https://doi.org/10.1002/ejoc.200400170
Fernandez-Alonso A, Bravo-Diaz C (2010) J Phys Org Chem 23:938. https://doi.org/10.1002/poc.1730
Pazo-Llorente R, Bravo-Diaz C, Gonzalez-Romero E (2003) Langmuir 19:9142. https://doi.org/10.1021/la034879i
Fernández-Alonso A, Bravo-Diaz C (2010) Helv Chim Acta 93:877. https://doi.org/10.1002/hlca.200900322
Crossley ML, Kienle RH, Benbrook CH (1940) J Am Chem Soc 62:1400–1404. https://doi.org/10.1021/ja01863a019
Fernández-Alonso A, Bravo-Diaz C (2008) Org Biomol Chem 6:4004–4011. https://doi.org/10.1039/B809521C
González-Romero E, Fernández-Calvar MB, Bravo-Díaz C (2002) Langmuir 18:10311. https://doi.org/10.1021/la026312s
Bravo-Díaz C, González-Romero E (2003) Electroanalysis 15:303–311. 1040-0397/03/0402-0303
Bravo-Díaz C, González-Romero E (2003) Electrochemical behavior of arenediazonium ions. New trends and applications. In: Current Topics in Electrochemistry. Research Trends, Trivandrum, India
Pastoriza-Gallego MJ, Losada-Barreiro S, Bravo Díaz C (2012) J Phys Org Chem 25:908–915. https://doi.org/10.1002/poc.2949
Fry AJ (1978) Electrochemistry of the diazo and diazonium groups. In: Patai S (ed) The chemistry of Diazo and Diazonium Groups. Wiley, NY
Viertler H, Pardini VL, Vargas RR (1994) The electrochemistry of triple bond. In: Patai S (ed) The chemistry of triple-bonded functional groups, supplement C. Wiley, NY
Zuman P (1969) Physical organic polarography. In: Zuman P, Perrin CL (eds) Organic polarography. Wiley, NY
Sienkiewicz A, Szymulaa M, Narkiewicz-Michaleka J, Bravo-Díaz C (2014) J Phys Org Chem 27:284–289. https://doi.org/10.1002/poc.3194
Lowry TH, Richardson KS (1987) Mechanism and theory in organic chemistry. Harper-Collins Pub, New York
Fernández-Alonso A, Pastoriza-Gallego MJ, Bravo-Diaz C (2010) Org Biomol Chem 8:5304–5312. https://doi.org/10.1039/c0ob00143k
Doyle MP, Nesloney CL, Shanklin MS, Marsh CA, Brown KC (1989) J Org Chem 54:3785–3789. https://doi.org/10.1021/jo00277a009
Costas Costas U, Bravo-Díaz C, González-Romero E (2005) Langmuir 21:10983–10991. https://doi.org/10.1021/la051564p
Costas-Costas U, Bravo-Díaz C, González-Romero E (2004) Langmuir 20:1631–1638
Pastoriza-Gallego MJ, Fernández-Alonso A, Losada-Barreiro S, Sánchez-Paz V, Bravo-Diaz C (2008) J Phys Org Chem 21:524–530. https://doi.org/10.1002/poc.1289
Losada-Barreiro S, Sánchez-Paz V, Bravo-Díaz C (2007) Helv Chim Acta 90:1559–1573. https://doi.org/10.1002/hlca.200790163
Losada-Barreiro S, Bravo-Diaz C (2009) Helv Chim Acta 92:2009–2023. https://doi.org/10.1002/hlca.200900080
Jaszczuk K, Dudzik A, Losada-Barreiro S, Szymula M, Narkiewicz-Michalek J, Bravo-Díaz C (2016) J Phys Org Chem 29:586–593
Dudzik A, Jaszczuk K, Losada-Barreiro S, Bravo-Díaz C (2017) New J Chem 41:2534–2542. https://doi.org/10.1039/C6NJ03670H
Wittwer R, Zollinger H (1954) Helv Chim Acta 37:1954. https://doi.org/10.1002/hlca.19540370707
Lewis ES, Surh H (1958) J Am Chem Soc 80:1367. https://doi.org/10.1021/ja01539a023
Brown KC, Doyle MP (1988) J Org Chem 53:3255–3261. https://doi.org/10.1021/jo00249a021
Zollinger H (2003) Color chemistry. In: Syntheses, properties, and applications of organic dyes and pigments, 3rd revised edn. Wiley-VCH Verlag, Zürich. https://doi.org/10.1002/anie.200385122 Is this book the vsame as ref 17
García-Meijide MC, Bravo-Díaz C, Romsted LS (1998) Int J Chem Kin 30:31–39. https://doi.org/10.1002/(SICI)1097-4601(1998)30:1<31::AID-KIN4>3.0.CO;2-V
Quintero B, Morales JJ, Quirós M, Martínez-Puentedura MI, Cabeza MC (2000) Free Radic Biol Med 29:464–479. https://doi.org/10.1016/s0891-5849(00)00321-x
Laali KK, Gettwert VJ (2001) J Fluor Chem 107:31–34. https://doi.org/10.1016/S0022-1139(00)00337-7
Chaudhuri A, Loughlin JA, Romsted LS, Yao J (1993) J Am Chem Soc 115:8351–8361. https://doi.org/10.1021/ja00071a050
Bravo-Díaz C, González-Romero E (2003) J Chromatogr A 989:221–229. https://doi.org/10.1016/S0021-9673(03)00170-5
Yasui S, Nakamura K, Ohno A (1984) J Org Chem 49:878–882. https://doi.org/10.1021/jo00179a024
Hanson P, Hammond RC, Goodacre PR, Purcell J, Timms AW (1994) J Chem Soc Perkin Trans 1(2):691–696. https://doi.org/10.1039/P2994000069
Pazo-Llorente R, Bravo-Díaz C, González-Romero E (2001) Fresenius J Anal Chem 369:582–586. https://doi.org/10.1007/s002160000694
Gunaseelan K, Romsted LS, González-Romero E, Bravo-Díaz C (2004) Langmuir 20:3047–3055. https://doi.org/10.1021/la0354279
Hanson P, Hammond RC, Goodacre PR, Purcell J, Timms AW (1994) J Chem Soc, Perkin Trans 1. 2:691–696. https://doi.org/10.1039/P2994000069
Gunaseelan K, Romsted LS, Pastoriza Gallego M-J, González-Romero E, Bravo-Díaz C (2006) Adv Colloid Interface Sci 123–126:303–311. https://doi.org/10.1016/j.cis.2006.05.007
Gunaseelan K, Romsted LS, González-Romero E, Bravo-Díaz C (2004) Langmuir 20:3047–3055. https://doi.org/10.1021/la0354279
Scaiano JC, Kim-Thuan N, Leigh WJ (1984) J Photochem 24:79–86. https://doi.org/10.1016/0047-2670(84)80009-X
Acknowledgments
This book chapter was prepared during a sabbatical leave of CBD supported by the University of Vigo. We thank all colleagues for helpful discussions and, especially, to all students who participated with enthusiasm for years in the aryldiazonium project, making important contributions to this work. Financial support from Ministerio de Ciencia e Innovación (Spain), Xunta de Galicia and Universidad de Vigo is also acknowledged.
Credit authorship contribution statement.
C. B-D. Sections 1–3 and 5: conceptualization, visualization, writing, review & editing.
E. G-R. Section 4: conceptualization, visualization, writing, review & editing.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Bravo-Díaz, C., González-Romero, E. (2022). Kinetics and Mechanisms of Aryldiazonium Ions in Aqueous Solutions. In: Chehimi, M.M., Pinson, J., Mousli, F. (eds) Aryl Diazonium Salts and Related Compounds. Physical Chemistry in Action. Springer, Cham. https://doi.org/10.1007/978-3-031-04398-7_3
Download citation
DOI: https://doi.org/10.1007/978-3-031-04398-7_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-04397-0
Online ISBN: 978-3-031-04398-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)