Abstract
Peritoneal dialysis (PD) remains a lifesaving procedure for advanced stages of acute kidney injury in children. This is more relevant in the low- and middle-income countries (LMICs) where there are limited resources and technical support for other dialytic modalities. This chapter provides details on the indications for PD, how to adapt PD at the bedside using available resources like feeding tubes as PD catheters and reconstitution intravenous fluids as a dialysate.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Case Example
A five-year-old female presents to your hospital in Cameron after a four-day history of fever, malaise, and passage of dark brown urine. The patient had presented at two other health facilities before arriving. She has been diagnosed with malaria and prescribed appropriate treatment. On arrival, she is acutely ill-appearing with generalized edema. She is noted to have decreased urine output and has been passing “Cola” colored urine. She is afebrile. Her initial laboratory results show a hemoglobin of 7 gm/dl, a serum creatinine of 9.9 mg/dl, a blood urea nitrogen of 312 mg/dl, and a serum potassium of 4.8 mmol/l. You diagnose acute kidney injury secondary to malaria and recognize that peritoneal dialysis is necessary, but your surgeon does not have a commercial peritoneal dialysis catheter available and hemodialysis is not feasible given the intensive care needs and equipment. Commercial peritoneal dialysis fluid is not available.
2 Introduction
Acute kidney injury (AKI) is a relatively common clinical condition in the critically ill child [1, 2]. It is often associated with poorer outcomes in low-resource settings compared to high-income settings [3]. Factors that contribute to poor outcomes include lack of access to dialysis due to prohibitively expensive cost of consumables, non-availability of consumables, and technical requirement for some kidney replacement therapy such as hemodialysis [3].
Although there are different kidney replacement modalities, peritoneal dialysis (PD) appears to be one of the most feasible and accessible forms of kidney replacement therapy in low-middle-income countries (LMICs) [4]. PD requires the least technical requirements among the various modalities of treatment, which makes it possible (even for patients) to administer the physician prescriptions at home [5]. Additionally, PD is the preferred modality in neonates and infants, in hemodynamically unstable patients, and patients who cannot tolerate anticoagulation. PD provides a slower clearance rate, which limits the risk for dialysis disequilibrium syndrome, a syndrome that is often associated with hemodialysis. All of these advantages make PD a modality of choice for renal replacement therapy in LMICs.
The attainment of zero preventable death from AKI by 2025 (0 by 2025), proposed by the International Society of Nephrology, can only be achieved through scale-up of access to dialysis in LMICs [6, 7]. Access to dialysis can be improved through the use of adaptable methods in low-resource settings [5, 8]. This chapter describes indication for dialysis and options for a modified peritoneal catheter. This section also describes the PD procedure, including options for dialysates and complications for low-resource settings.
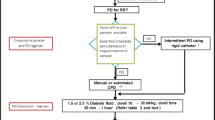
3 Indications
The indications for kidney replacement therapy (KRT) can be categorized into clinical and laboratory indications (Table 1).
4 Contraindications
The contraindications include known allergy to any of the PD fluid constituent, previous major recent abdominal surgery, acute abdomen, intraabdominal abscess, and peritoneal membrane failure.
5 Supplies/Equipment
5.1 PD Catheter Options
Peritoneal dialysis catheters are tubes or rods with an open-end port and side fenestrations. They come in various shapes and tensile strengths. They may either be flexible or rigid. Over the years, the flexible catheters have become the most preferred, but the reusable rigid catheters may be useful where the former is not available. In terms of shapes; catheters may be straight (typical of the adaptable/improvised catheters), pigtail curled, and swan-neck (Fig. 1).
They may be of various lengths and may have cuffs for retention in the anterior abdominal wall. A typical example includes Tenckhoff™ and Dacron™ catheters. In resource-limited settings, the commercial catheters are often not available and where they are available, they are not affordable ($40). In place of the commercial catheter, other tubes/catheters can be adapted and modified for use as a PD catheter. Commonly adapted equipment includes use of feeding tubes of various sizes depending on the patients’ age and weight (Table 2). Similar sized urethral catheters could also be improvised in place of feeding tubes with similar efficacy.
5.1.1 Supplies Required for PD Catheter Placement (Fig. 2)
-
Bedside table
-
Surgical masks (2)
-
Sterile towels* (2)
-
Small sterile bowl*
-
Sterile forceps*
-
Sterilizable stylet or bicycle spoke
-
Scissors
-
Artery forceps
-
Sterile cotton swabs* (2)
-
Squares sterile gauze* (4)
-
Sterile 4×4 gauze*
-
Lactated Ringer (LR) – ordered amount*
-
D50W – ordered amount*
-
Large syringe with needle
-
Infusion Set – may be reused for 24 h if sterility is maintained
-
Urinary catheter bag*
-
Container with a lid containing 90% alcohol (change weekly)
-
3-way stopcock (in open-all-ways position in 90% alcohol)
-
4–5 cm section of suction tubing (in 90% alcohol)
-
Sterile gloves* (2 pairs)
-
Non-sterile kidney basin or bowl for discarded fluid
-
Bucket for drained dialysate
*indicates items that are NOT reused, they are NEW with every exchange
5.2 Technique
5.2.1 How to Make or Obtain PD Fluid and PD Fluid Sustainability
PD fluid is referred to as dialysate. This fluid is composed of water, physiologic concentrations of electrolytes, and an osmotic agent. Commonly glucose is the osmotic agent, which pulls toxins including waste products, excess body water, and electrolytes from the body. The PD fluid composition is shown in Table 3. PD fluid can either be commercially prepared or locally constituted by the healthcare provider. Usually, glucose is added to intravenous fluid such as ringer lactate or saline at various concentrations. Higher dextrose concentration would generate more osmotic pressure and drag to pull in excess body water in a hypervolemic state. The efficacy and safety of the locally prepared fluid are well documented in the literature [9].
-
(a)
Commercial PD Fluid
The various available commercial PD fluids include a 1.5% or 2.3% glucose strength. The choice of the glucose concentration is determined by the patient’s fluid status. For instance, where there is significant fluid overload/retention, a higher (2.3%) glucose concentration should be selected as the fluid of choice (Table 3).
-
(b)
Locally Prepared PD Fluid
When commercial PD fluids are not available, intravenous fluids with a physiologic constituent of electrolyte should be prepared by the team. Strict aseptic technique is important during the entire process of the fluid constitution in order to minimize the risk of bacterial contamination and subsequent peritonitis; a common complication of PD.
The fluid composition that is prepared should be as close as possible to the electrolyte composition of commercial PD fluid. Using locally prepared solutions is advantageous as the needed solutions can be readily made available in the concentration needed. Typically, Ringer’s lactate or Hartman’s solution is used but normal saline may be used if necessary (Table 3). Dextrose 50% is added to the primary solution to produce the desired concentration of PD solution. Antibiotics may be added to the PD solution to reduce the incidence of peritonitis or for treatment depending on the risk factors and concern level for infection. Heparin may also be added to the PD fluid to prevent fibrin clots from blocking the PD catheter. If the patient is hypokalemic, potassium chloride may be added to the constituted fluid as well.
-
(c)
Adaptation for Sustainability – PD Fluid Revolving Fund Model
A major barrier to PD is the availability of PD fluid. Where commercial PD fluid is available, a model that has been proven to be efficient in making commercial PD fluid use possible is the use of a “PD revolving fund’. This model followed a key strategy for health care financing “the drug revolving fund model”. A revolving fund is a fund that is always available to finance an organizations continuing operations – it is replenished by the organization anytime funds are used and does not have fiscal year limitations. This model, when applied to PD fluid, ensures PD fluid is stocked and made available and accessible when needed. The seed funding for this model may be obtained from a donor for the acquisition of the initial stock of PD fluids. Fluids are then replenished at restocking cost to always have a constant available level. A marginal profit may be added to the cost to ensure sustainability and to account for the care of indigent patients who may not be able to pay for cost recovery. Adoption of this model has been shown to improve commercial dialysis access rate to well above 90% and demonstrated a low infection rate of 0.5% in a single-center experience in Nigeria. This model is recommended where infection control and prevention are of major concern since the risk of contamination, a key challenge with manually constituted fluids, is avoided.
-
(d)
PD Catheter Insertion Procedure
PD catheter insertion should be performed by an experienced member of the team who has previously been trained to perform this procedure. Trainees must always be supervised by experienced trained providers. It is beyond the scope of this book to teach the entire PD catheter placement procedure, what is described here is to show modifications of what can be used for an experienced provider to place the PD catheter.
The procedure should follow a strict aseptic pre-condition in line with global standard practice for infection prevention and control policy. Sterile materials from the central sterilizing unit should be available for the procedure. All eligible patients are catheterized to ensure the bladder is empty and for monitoring of urine output.
The catheter insertion can be done in a dedicated room or at the patient’s bedside. About 30 min before PD catheter insertion an intravenous 2 mg/kg of intravenous ethamsylate should be administered to the patient for maintenance of good homeostasis. The peritoneal cavity should be filled with 10 ml/kg of normal saline through a sterile needle inserted into the left iliac fossa so the abdominal organs could be freely displaced during catheter insertion. A video of the procedure is accessible through this link below:
-
https://www.youtube.com/watch?v=-pB7kPT4gfc&t=22s&ab_channel=DennisPalmer: PD Catheter Placement Video used with permission from Dr. Dennis Palmer
In brief, the anterior abdomen should be cleaned with an antiseptic (povidone-iodine), and the surgical site infiltrated with 0.5% lidocaine at 4 mg/kg (Fig. 3). Thereafter, a 0.5 cm incision should be made about 2.5 cm below the umbilicus with a size 15 surgical blade deep through to the fascia.
If a sterile, reusable stylet is not available, an adapted, sterile bicycle spoke can be inserted into a fenestrated size 10 feeding tube for resilience through the skin nick with sustained pressure until a feel of some “give” is experienced. The adapted stylet should be removed, and the tube should be gently advanced into the peritoneal cavity (Fig. 3). A deep subcutaneous purse-string suture should be applied if the catheter moves loosely through the incision as shown in Fig. 3. The improvised PD catheter can be connected to a 3-way tap (Fig. 2), with the second end connected to the PD fluid tubing, while the third limb is connected to a urinary bag (waste bag) to drain the effluent. The PD fluid may have 100 IU/L of heparin and 4 mg/L of gentamycin added as indicated.
6 Instructions for Use
6.1 Basics on How to Do PD
The PD should be carried out in a dedicated room in the ward where the patients requiring dialysis are located. However, where this is not available, a dedicated part of the ward may be screened for this purpose.
6.2 PD Cycling
There are three phases in one cycle of peritoneal dialysis (PD), they are: Fill, Dwell, Drain. During each exchange, the first phase is draining from the previous cycle and then filling to begin the next cycle.
To start with, a 5–10 mL/kg PD fluid is instilled to the peritoneal cavity for the first three cycles without a dwell time to ensure smooth ruining of the circuit. The PD cycling volume may be increased to 20 and up to 40 mL/kg depending on patients’ tolerability and absence of respiratory distress. Cycles of PD should be performed until patients regained full consciousness, urinary output improves ≥1.5 mL/kg/h and patients become ambulant. However, in the event of recurrence of oliguric, anuric, or rising azotemia or worsening encephalopathy, dialysis should be re-commenced. If the patient’s serum potassium falls below 4 mmol/l, a potassium supplement of 4 mmol/l should be added to 1 l of the PD fluid. Patients’ vital signs are to be closely monitored throughout the procedures. The presence of abdominal pain, tenderness, guarding, and cloud effluent of PD fluid should be suggestive of peritonitis.
Further details are shown in Appendix I (Peritoneal Dialysis Procedure – Mbingo Baptist Hospital).
7 Complications/Troubleshooting
-
1.
Peritonitis: The most common complication of PD is peritonitis, the tell-tale signs which include cloudy effluent, abdominal pain, and tenderness. Detailed attention should be paid to the color of effluent which should be normally clear. Where peritonitis is suspected, PD fluid sample and or PD catheter tip should be sent for culture and sensitivity test. The patient should be commenced on broad-spectrum antibiotics and revised with the culture result.
-
2.
Catheter malfunction: Partial or complete catheter malfunction can occur. This may be due to kinking or intraluminal obstruction by clots or tissues. In such an instance, the patient should be repositioned and the catheter can be re-adjusted. If no improvement with such manipulations a new catheter may be re-inserted.
-
3.
Other complications:
-
(a)
Hemorrhage from the surgical insertion site.
-
(b)
Hypokalemia. Close monitoring of electrolytes is essential and where hypokalemia ensure, potassium should be added to the dialysate.
-
(c)
Hypocalcemia. Hypocalcemia should be corrected with calcium gluconate.
-
(d)
Scrotal or abdominal wall edema. The scrotum should be elevated.
-
(e)
Hyperglycemia .
-
(a)
8 Case Resolution
A modified peritoneal dialysis catheter was inserted by the surgical team and PD was initiated. The child is commenced on PD using locally prepared fluid. Fifteen ml of 50% dextrose water was added to each 500 ml of ringer lactate solution to prepare an isotonic PD fluid of 1.5% strength. The PD was dosed at 10–30 m/kg per session of the dialysis with a dwell time of 45 min and let in and let out time of 15 min each. She received 36 sessions of peritoneal dialysis and was discharged home with improved renal function. At discharge, her serum creatinine was 7.0 mg/dl and the blood urea was 109 mg/dl.
At her one-month follow-up visit, her serum creatinine was 1.8 mg/dl and the blood urea was 41 mg/dl.
Abbreviations
- AKI:
-
Acute kidney injury
- HD:
-
Hemodialysis
- KRT:
-
Kidney replacement therapy
- LMICs:
-
Low-middle-income countries
- PD:
-
Peritoneal dialysis
References
Kellum JA, et al. Kidney disease: improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138.
Hessey E, et al. Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis. 2019;6:2054358119880188.
Olowu WA, et al. Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2016;4(4):e242–50.
Li PKT, Burdmann EA, Mehta RL. Acute kidney injury: global health alert. Arab J Nephrol Transplant. 2013;6(2):75–81.
Alao MA, et al. Acute pediatric peritoneal dialysis: impact of an opt-out model and adaptable methods in a hospital in Nigeria. Med J Indonesia. 2020;29(4):386–91.
Mehta RL, et al. International Society of Nephrology’s 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet. 2015;385(9987):2616–43.
Raimann JG, Riella MC, Levin NW. International Society of Nephrology’s 0by25 initiative (zero preventable deaths from acute kidney injury by 2025): focus on diagnosis of acute kidney injury in low-income countries. Clin Kidney J. 2018;11(1):12–9.
Alao MA, et al. Long-term survival of children following acute peritoneal dialysis in a resource-limited setting. Kidney Res Clin Pract. 2020;39(4):469.
Palmer D, et al. Peritoneal dialysis for AKI in Cameroon: commercial vs locally-made solutions. Perit Dial Int. 2018;38(4):246–50.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Alao, M.A., Palmer, D., Njini, N.N.N., Ibrahim, O.R. (2022). Peritoneal Dialysis in a Low-Resource Setting. In: Slusher, T.M., Bjorklund, A.R., Lauden, S.M. (eds) Pediatric Procedural Adaptations for Low-Resource Settings. Springer, Cham. https://doi.org/10.1007/978-3-030-99955-1_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-99955-1_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99954-4
Online ISBN: 978-3-030-99955-1
eBook Packages: MedicineMedicine (R0)