Abstract
The synovial membrane lines the cavity of articular joints and supports joint health. Synovial functions include the production of synovial fluid components, the movement of nutrients and metabolites to and from the joint space, and the prevention of pathologic inflammation to limit joint tissue pathology. The cellular components and structure of the synovial membrane directly relate to these critical functions. Synovial lining fibroblasts produce key components of synovial fluid that provide lubrication during normal movement. Synovial resident macrophages may be important in preventing or limiting pathologic inflammation in the joint. The sublining vasculature allows diffusion of nutrients and metabolites into and out of the joint space to maintain articular health. In many rheumatic diseases, pathologic synovitis develops which can compromise normal synovial functions. In this chapter, we will review important historical studies that provide a foundation for our understanding of synovial structure, along with recent investigations that are shedding light on the complexity of cellular functions in the synovium that contribute to joint health and disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The synovium is a connective tissue that lines the cavity of articular joints. It lies just beneath the fibrous joint capsule, and extends to the bone-cartilage interface without encroaching on articular cartilage (Fig. 2.1a). The tissue of the synovium is generally separated into two regions: a superficial lining layer (intima) which is one to three cell layers thick and faces the joint space, and a sublining layer (subintima) which contains blood vessels, lymphatics, and nerves (Fig. 2.1b), and a variable amount of adipose tissue [3]. The subintima eventually transitions to the denser, more fibrous joint capsule. The appearance of the subintimal layer can vary even within the same joint; three basic patterns have been identified: areolar, fibrous, and fatty [4]. Areolar synovium is characterized by loose subintimal connective tissue and is highly vascular; fibrous synovium is denser and poorly vascularized, and fatty synovium has a higher proportion of adipocytes in the sublining.
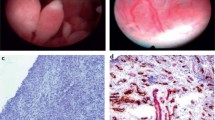
(a) A normal human interphalangeal joint, in sagittal section, as an example of a synovial joint. The location of the synovium, facing the joint space and attaching to the inner surface of the joint capsule and the outer surface of the bone up to the bone/cartilage junction, is shown. (Reused with permission from Sokoloff and Bland [1]. Copyright 1975 the Williams & Wilkins Co, Baltimore.) (b) Photomicrograph of a thin section of normal human synovial tissue (H&E, 10X). The thin (1–2 cell layer) synovial lining layer is at the top of the section, which lines the loose connective tissue of the sublining layer below it. Varying degrees of adipose (present in the lower part of the frame) can be seen within the sublining layer. (Reused with permission from Gravallese, et al. [2]. Copyright 2021 Elsevier, Philadelphia)
One of the unique features of the synovium is that the lining and sublining layers are not separated by an organized basement membrane as in other barrier tissues throughout the body (i.e., epithelial linings such as pleura). Although many of the molecular components of basement membranes are still found in the synovial extracellular matrix (including perlecan, fibronectin, and laminin) [5], it is more loosely organized. This lack of a structured, basement membrane makes the lining semipermeable to many molecular species, allowing for filtration of plasma components which comprise a portion of the synovial fluid that bathes the joint space. Still, the lining and sublining layers have distinct functions [4]. Cells of the intima (synoviocytes) are responsible for production of synovial fluid that provides nutrition for the articular cartilage and lubricates the articular surfaces. In addition, intimal cells protect the joint from inflammatory damage by providing a barrier that prevents cellular extravasation, and by clearing debris. The vasculature and lymphatics of the subintima allow trafficking of substances into and out of the joint space [3]. These functions are critical for the maintenance of joint health throughout our lifespan. This chapter will provide an overview of the structure and function of synovial membrane and its cellular components, and review pathologic changes to the synovium seen in common rheumatologic diseases that compromise its normal function.
Synovial Intima
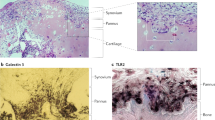
Synoviocytes that comprise the intimal layer are of two main types: type A synoviocytes which are macrophages of hematopoietic origin, and type B synoviocytes which are fibroblasts of mesenchymal origin (Fig. 2.2a). Early electron microscopy studies of the synovium [8] led to the identification of these cell types and demonstrated that type A synoviocytes contain vacuoles, a prominent Golgi apparatus, and filopodia, but they have little rough endoplasmic reticulum. In contrast, type B synoviocytes contain fewer vacuoles and abundant rough endoplasmic reticulum, suggesting an important function in protein synthesis. Type A cells express typical macrophage markers, including nonspecific esterase and CD68, while type B cells express high levels of uridine diphosphoglucose dehydrogenase (UDPGD, an enzyme involved in hyaluronic acid synthesis) and CD55. In the normal, healthy state, type B (fibroblast-like) cells predominate while type A cells make up about 10–20% of the lining cells.
(a) Transmission electron microscopy of monkey synovial lining cells. “A” indicates a Type A synoviocyte (macrophage) on the lining surface with many processes, vacuoles, and dense bodies. “B” indicates a deeper type B synoviocyte (fibroblast) which has more rough endoplasmic reticulum. “C” indicates collagen bundles in the matrix. A small superficial vessel occupies the bottom of the Fig. 9000X. (Reused with permission from Schumacher [6]. Copyright 1975 Association for Clinical Scientists.) (b) Origin and phenotypic characteristics of synovial lining macrophages. Resident CX3CR1+ lining macrophages and RELMα+ sublining macrophages are replenished throughout adulthood by a resident interstitial macrophage that resides in the sublining. (Reused with permission from Culemann, et al. [7]. Copyright 2019 Elsevier, Philadelphia)
Type B Synoviocytes
The developmental origin of the synovium, and specifically fibroblast-like synoviocytes, remained a mystery until recently, when several groups demonstrated that synovial tissue develops early during embryogenesis from the same mesenchymal precursor cells that give rise to other joint tissues including cartilage, bone, meniscus, and ligaments [9,10,11]. These cells, identified by expression of growth differentiation factor-5 (GDF-5) condense to form a lining layer. This is facilitated by expression of cadherin-11, a key adhesion molecule that mediates adherens junction formation and regulates the formation of the synovial lining during development [12, 13]. As mentioned above, these cells are rich in endoplasmic reticulum suggesting a synthetic function, and they produce synovial extracellular matrix components including collagen type 1, fibronectin, and proteoglycans. In addition, type B lining cells express UDPGD, hyaluronate synthetase 1 (HAS1), and proteoglycan 4 (PRG4) which encodes lubricin. They are the main producers of synovial fluid lubricin and hyaluronic acid which together provide boundary lubrication of cartilage [14] and shock absorption [15], allowing friction-free and pain-free motion of the joint during activity. Lubricin also prevents pathologic deposition of protein onto the surface of articular cartilage.
In addition to these important synthetic functions, type B synoviocytes have phagocytic capacity and the ability to present antigens, and thus may act as “immune sentinels” poised to activate immune responses when needed. They express decay-accelerating factor (CD55) [16] which inactivates intermediates of the complement cascade, suggesting an immunoregulatory function. However, under inflammatory conditions, these cells can produce large amounts of metalloproteinases, inflammatory cytokines, and molecules involved in activation of osteoclasts (i.e., RANKL), contributing to pathology throughout the joint. Heterogeneity in synovial fibroblast cadherin-11 expression can contribute to the invasiveness of the synovium at the pannus-cartilage interface in rheumatoid arthritis [12, 17]. Croft et al. [18] showed that CD90− synovial lining fibroblasts expressed FAPα (fibroblast activation protein-α), and through adoptive transfer demonstrated that these cells mediated bone and cartilage destruction in a murine model of inflammatory arthritis. Epigenetic changes to synovial fibroblasts have been implicated in modulating a change from an immunoregulatory role in health, to an active pathogenic role in chronic arthritis.
Type A Synoviocytes
Synovial intimal macrophages express high levels of the fractalkine receptor CX3CR1 [19], and scavenger receptors such as CD163 and MERTK [20] which contribute to phagocytic capacity. This feature allows them to participate in clearing debris and dying cells from the joint space. Although the synovial lining is not a true epithelial barrier, synovial lining macrophages have unique features which allow them to maintain a barrier between the joint space and the sublining capillaries. Specifically, they attach to neighboring cells through desmosomes and tight junctions [19], allowing the lining to maintain the joint space as an immune-privileged site in the healthy state by limiting cellular movement into the joint. In joint disease, this barrier function can become compromised, allowing an influx of inflammatory leukocytes into the joint space and synovial fluid that compromises the integrity of other joint tissues.
Synovial Subintima
Lining vs. Sublining Macrophages
Both the lining and sublining regions contain resident populations of macrophages that are critical to maintaining joint health. The op/op osteopetrotic mouse, which is deficient in macrophages because of an absence of macrophage colony-stimulating factor, also lacks synovial lining macrophages [21], suggesting that type A cells share a common lineage with other tissue macrophages. For many years it was assumed that both lining and sublining macrophages were derived from monocyte precursors from the bone marrow and were replenished from the circulation throughout adulthood. But several new findings have advanced our understanding of synovial macrophage origins and function. In 2019, two reports demonstrated that CX3CR1+ embryonic macrophages (ESMs) begin to populate the developing synovium in the mouse early, between day E12.5 and E15 [19, 22]. In contrast, bone-marrow-derived synovial macrophages (BMSMs), characterized by expression of CD11b and Ly6c, are not observed until later, after day 19. Most synovial resident macrophages in the adult mouse appear to be of embryonic origin and express the cytokines IL-4 and IL-10 consistent with an immune regulatory role, while BMSMs preferentially express M1 pro-inflammatory type cytokines such as IL-1β and TNFα [22]. Resident macrophages of the lining (type A synoviocytes) are continuously replenished throughout adulthood from a pool of proliferating MHCII+ sublining resident macrophages, and not from circulating bone-marrow-derived monocytes. The same MHCII+ sublining precursors that give rise to the lining macrophages (type A synoviocytes) also give rise to a population of CX3CR1− resident sublining macrophages (Fig. 2.2b). These resident macrophages express resistin-like molecule-α (RELMα) and CD163, associated with alternatively activated (M2) macrophages, and they may be important in limiting pathologic synovial inflammation. The function of synovial macrophages is influenced both by their origin (embryonic vs. bone-marrow/circulation) and their spatial location within the synovium (lining vs. sublining). The specific mechanisms that regulate the differentiation and function of macrophage populations in human disease need further investigation, to determine if specific subgroups can be targeted for therapy.
Subintimal Stromal Cells
The sublining stroma is a highly variable loose connective tissue characterized by a loose collagenous matrix, and populated by fibroblasts which produce the matrix. Mizoguchi et al. [17] identified three distinct populations of human synovial fibroblasts, using cell-surface markers and transcriptomics. In addition to the CD90−CD34− lining fibroblasts previously described, they found a population of sublining CD90+CD34− cells that surround blood vessels and capillaries. This population highly expressed RANKL, and promoted osteoclastogenesis in vitro suggesting a role in synovial bone erosion. A third CD34+ fibroblast subset was found to be distributed in the lining and sublining, and in contrast to CD34− cells, secreted IL-6, CXCL12, and CCL2 in response to TNFα. Thus, this population may promote cellular recruitment driving synovitis. These distinct populations have relevance to OA as well as inflammatory arthritis, as OA lining fibroblasts (CD90−CD34−) correlated with synovial macrophage content, and sublining (CD90+CD34+) fibroblasts correlated with synovial T-cell content [23]. Clearly, there is phenotypic heterogeneity of synovial fibroblasts, and single-cell sequencing showed that there was a continuum of fibroblast phenotypes between the synovial sublining and lining [24] suggesting a plasticity in phenotype that needs further elucidation.
Consistent with the common origin of synovium and other joint tissues [9, 11], the synovial stroma is a rich source of mesenchymal stem cells (MSCs) which can give rise to different cell lineages, including chondrocytes, osteoblasts, and adipocytes. Synovial-derived MSCs have a high chondrogenic potential compared to other tissue sources of MSCs [10], consistent with older reports of the importance of synovium in cartilage repair [25]. Bone-marrow (BM) and adipose-derived MSCs have been shown to have potent immunosuppressive effects in vitro on T-lymphocyte responses, and augment T regulatory cell development [26]. The effects on T cells may be due to both cytokine production [27] and mechanisms requiring cell-contact [28], but more work is needed evaluating mechanisms specifically in synovial-derived MSCs. MSCs in synovium increase in arthritis [29, 30], and animal models of joint tissue injury, due to both infiltration from BM sources and local proliferation of resident populations [31]. CD271+ MSCs expanded in arthritis may become pathogenic and lose their immunosuppressive functions [29, 32]. Whether the immunomodulatory function of synovial MSCs can be harnessed for cell-based arthritis therapeutics is under investigation by several groups (reviewed in [33]).
Subintimal Vasculature
The synovial subintimal contains a rich network of vessels and lymphatics which allows for movement of molecules, nutrients, and metabolites into and out of the joint to maintain the health of the avascular articular cartilage. The vasculature of the synovial sublining is most dense closest to the lining layer, and contains highly fenestrated capillaries and venules [8, 34]. In healthy joints, low molecular weight substances diffuse across their concentration gradients. Plasma components and nutrients diffuse through fenestrated capillaries and into the synovial fluid, while low-molecular-weight metabolites produced within the joint are taken up by sublining venules and cleared. Larger molecular weight substances (such as Hyaluronic acid, a key component of synovial fluid) are generally retained in the joint space or cleared much more slowly by the synovial lymphatics [35]. In addition, synovial lymphatics are an important conduit to clear cells from the synovial tissue and fluid that accumulate during chronic inflammation.
Synovial Inflammation in Disease
Synovial Tissue Pathology
As discussed above, tissue macrophages are the most numerous resident leukocytes of the synovium. A small population of resident mast cells are also present in the normal synovium [36] as well as scattered perivascular T lymphocytes [3], but B lymphocytes are rare to nonexistent [37]. The pattern of inflammatory cell content in the sublining region can change drastically in rheumatic diseases, with infiltration by both myeloid and lymphoid cells. Moreover, synovial lining hyperplasia occurs, with increased cell layers, formation of synovial villi, and deposition of fibrin on the lining surface [38]. The sublining vascular density can be either increased or decreased, and the subintima can become fibrotic, particularly in chronic synovitis. Although there is significant overlap in the features of synovial membrane pathology between common rheumatic diseases [39], there are also some features more typical of certain diseases; these are summarized in Table 2.1.
A number of histopathologic grading systems have been developed to characterize pathologic features of the synovium observed in rheumatic diseases. The most commonly applied histopathology score is that developed by Krenn and colleagues [38, 47], which can discriminate highly inflammatory (i.e., rheumatoid arthritis, Fig. 2.3a) synovium from less inflammatory (i.e. osteoarthritis, Fig. 2.3b) synovial pathology. However, there is considerable overlap, and even low-grade synovitis in OA has clinical relevance as it is associated with severity of symptoms and progression of disease (reviewed in [48]). Routine histopathology has been used to describe distinct “pathotypes” of rheumatoid arthritis synovitis (lympho-myeloid, diffuse myeloid, and pauci-immune), and this classification has shown some promise for predicting responses to available treatments [49]. However, as discussed in the preceding sections, advanced techniques such as single-cell sequencing are revealing distinct patterns of synovial cellular infiltration and activation that may have important implications for prognosis, and predicting responses to therapy. These studies will likely impact clinical trial design for RA treatments in the near future [50], but currently synovial biopsy and pathologic evaluation has limited utility in the clinical setting.
Synovial pathology in (a) rheumatoid arthritis and (b) osteoarthritis. Varying degrees of synovial lining hyperplasia (black pointers), perivascular lymphocytic infiltrates (white arrows) are shown in both tissues, with increased vascularity exhibited in (a). Hematoxylin and eosin stain, 10X. (Images courtesy of E. DiCarlo, MD, Professor of Clinical Pathology, Weill Cornell Medical College and Attending Pathologist, Hospital for Special Surgery)
Mechanisms of Cell Homing in Synovitis
The factors that instigate synovitis vary in different rheumatic diseases (i.e., crystals in gouty arthritis, tissue injury in osteoarthritis), and are still not entirely clear in all contexts. What is becoming clear is that the recruitment and retention of inflammatory cells into the synovium is driven by chemokines and adhesion molecules, many of which are produced by activated synovial macrophages, fibroblasts, and endothelial cells (Fig. 2.4). This has been best studied in rheumatoid arthritis. CCL2 (also known as Monocyte Chemotactic Protein-1, or MCP-1) is an important chemokine that attracts peripheral blood monocytes into the synovium, as well as CD4+ T lymphocytes [51, 52]. CCL5 (Regulated upon Activation, Normal T Cell Expressed and Secreted, or RANTES), and CXCL12 (Stromal Cell Derived Factor-1, or SDF-1) may also drive T-cell recruitment [53,54,55], and may promote angiogenesis and bone destruction [56]. CCL3 and CCL4 recruit and retain the Th1 T-lymphocyte subset, while CCL20 may support recruitment of Th17 cells. The chemokines CXCL13 and CCL21 drive recruitment and retention of B-lymphocytes and plasma cells, and play an important role in the formation of lymphoid aggregate structures that are observed in up to 20% of patients with RA [57]. Endothelial cells and stromal cells within inflamed synovium express adhesion molecules, including integrins and selectins, which help recruit and retain leukocytes in the tissue [58,59,60]. Neutrophils make up a large proportion of cells in RA synovial fluid but are not necessarily retained in the tissue. Macrophages and fibroblasts produce neutrophil chemotactic factors such as IL-8 and ENA-78, which are found in high quantities in RA SF [52].
Schematic of the development of synovial inflammation, and its impact on other joint tissues in rheumatic diseases. Molecular mediators in bold type are targets of common biologic therapies used to treat different types of arthritis. (Figure created with BioRender.com. Image of lymphocytic aggregate courtesy of E. DiCarlo, MD, Professor of Clinical Pathology, Weill Cornell Medical College and Attending Pathologist, Hospital for Special Surgery)
Once chronic synovitis is established, the resident and infiltrating cells produce a wide variety of cytokines, growth factors, and enzymes that contribute to joint damage. Many of these have become well-known targets of arthritis therapy (Fig. 2.4). Infiltrating synovial monocytes and macrophages produce cytokines such as IL-1β, TNFα, and IL-6, which activate synovial fibroblasts, endothelial cells, and lymphocytes to perpetuate synovitis. Successful treatments targeting these “monokines” (cytokines produced primarily by monocytes and macrophages) are in common use for rheumatoid arthritis, spondyloarthritis and gout [61,62,63]. Synovial myeloid cells produce IL-12 and 23, which support the differentiation and survival of Th1 and Th17 cell types, respectively. Blockade of IL-12/23 [64], as well as IL-17 [65], has proven successful in treating psoriatic arthritis and spondyloarthritis. In addition, targeting both T lymphocytes (via CTLA4-Ig) and B lymphocytes (via anti-CD20) directly has proven useful in the treatment of inflammatory arthritis. TGF-β, a growth factor produced by synovial macrophages, is linked to osteoblast activation and osteophyte formation in models of osteoarthritis [66]. It is not yet clear whether pharmacologic targeting of this molecule for therapy will be possible. However, inflammatory matrix metalloproteinases produced by synovial macrophages and fibroblasts, including ADAMTS-5, MMP13, and Cathepsin K, are responsible for cartilage matrix damage in OA. Agents selectively targeting ADAMTS5 [67] and Cathepsin K [68] are under development for treatment of OA.
Pathologic Changes to Synovial Vasculature and Lymphatics
Both vascularization of the subintima, as well as clearance of substances from the joint space, can be altered in arthritic diseases. Synovial vascularization can be increased in synovium from patients with OA, but is more marked in RA [34]. Increased synovial doppler signal by ultrasound, a surrogate marker of increased blood flow, is more common in active RA where it is a predictor of disease activity [69]. In RA synovium though, new capillary growth does not meet the needs of the increasing mass of inflamed synovium, contributing to tissue hypoxia and acidification [52]. Despite increased synovial lymphatic vessels [70], lymphatic clearance was shown to be decreased in both OA and RA [71]. An interesting recent report showed that synovial lymphatic function decreases during development of OA in a post-traumatic mouse model, and may be a target for treatment [72]. Whether interventions designed to modulate synovial vascularity or lymphatic function will translate to effective treatments for human disease characterized by synovial inflammation, remains to be seen. But research efforts to understand how to target synovial vasculature for drug delivery to treat synovitis and its consequences are being explored [73].
Summary and Conclusions
The synovial membrane plays several critical roles in the maintenance of joint homeostasis. It provides a permeable barrier between the vasculature and the joint space to allow nutrient and metabolite trafficking, which is central to maintaining the health of the avascular cartilage. Synovial intimal fibroblasts produce key molecular substances (lubricin and hyaluronic acid, among others) that contribute to the functional properties of synovial fluid. These substances maintain adequate shock absorption and lubrication of the joint required for smooth, friction-free articulation during movement. Synovial lining macrophages have the unique ability to form a barrier between the sublining vasculature and the joint space that prevents cellular egress into the joint space. Available evidence suggests that both lining and sublining resident macrophages in health are skewed toward phenotypes that might serve to limit pathologic inflammation in the synovial tissue. The sublining also contains fibroblasts and mesenchymal stem cells that may contribute to maintaining a non-inflammatory environment. However, in many rheumatic diseases, other leukocytes are recruited to the synovium under the influence of various chemotactic factors, which creates pathologic synovitis that can compromise the function of the synovium. The inflammatory mediators produced by infiltrating cells can influence the function of resident cells, and promote arthritic joint damage. There is a lot of overlap in histopathologic features of synovitis across the spectrum of arthritides. Still there are some features that are more common in certain types of arthritis (Table 2.1). In some diseases synovitis may be a primary cause of joint damage, while in others it may be a consequence of joint damage. Regardless, many of the cellular and molecular products of synovial inflammation have become targets for therapy in various arthritic conditions, while others are under investigation. Modern techniques such as single-cell RNA sequencing are revealing the complexity of cell phenotypes that contribute to the function of the synovium in health and disease, and are providing better insights into the clinical heterogeneity of patients with arthritis and response to arthritis therapies.
References
Sokoloff L, Bland JH. The musculoskeletal system. Baltimore: Williams & Wilkins; 1975.
Gravallese EM, Smolen JS, van der Heijde D, Weinblatt ME, Weisman MH, Hochberg MC. Rheumatology. 8th ed. Philadelphia: Elsevier; 2021. In press
Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100–6.
Veale DJ, Firestein GS. Chapter 2: synovium. In: Firestein GS, Budd RC, Gabriel SE, Korestsky GA, McInnes IB, O'Dell JR, editors. Firestein and Kelley’s textbook of rheumatology. 11th ed. Philadelphia, PA: Elsevier; 2021. p. 20–33.
Dodge GR, Boesler EW, Jimenez SA. Expression of the basement membrane heparan sulfate proteoglycan (perlecan) in human synovium and in cultured human synovial cells. Lab Investig. 1995;73(5):649–57.
Schumacher HR. Ultrastructure of the Synovial Membrane. Annals Clin & Lab Sci. 1975;5:489–99.
Culemann S, Gruneboom A, Kronke G. Origin and function of synovial macrophage subsets during inflammatory joint disease. Adv Immunol. 2019;143:75–98.
Schumacher HR Jr. Ultrastructure of the synovial membrane. Ann Clin Lab Sci. 1975;5(6):489–98.
Roelofs AJ, Zupan J, Riemen AHK, Kania K, Ansboro S, White N, et al. Joint morphogenetic cells in the adult mammalian synovium. Nat Commun. 2017;8:15040.
Decker RS, Um HB, Dyment NA, Cottingham N, Usami Y, Enomoto-Iwamoto M, et al. Cell origin, volume and arrangement are drivers of articular cartilage formation, morphogenesis and response to injury in mouse limbs. Dev Biol. 2017;426(1):56–68.
Shwartz Y, Viukov S, Krief S, Zelzer E. Joint development involves a continuous influx of Gdf5-positive cells. Cell Rep. 2016;15(12):2577–87.
Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GF, Chisaka O, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315(5814):1006–10.
Valencia X, Higgins JM, Kiener HP, Lee DM, Podrebarac TA, Dascher CC, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200(12):1673–9.
Abubacker S, Dorosz SG, Ponjevic D, Jay GD, Matyas JR, Schmidt TA. Full-length recombinant human proteoglycan 4 interacts with Hyaluronan to provide cartilage boundary lubrication. Ann Biomed Eng. 2016;44(4):1128–37.
Jay GD, Lane BP, Sokoloff L. Characterization of a bovine synovial fluid lubricating factor. III. The interaction with hyaluronic acid. Connect Tissue Res. 1992;28(4):245–55.
Stephenson W, Donlin LT, Butler A, Rozo C, Bracken B, Rashidfarrokhi A, et al. Single-cell RNA-seq of rheumatoid arthritis synovial tissue using low-cost microfluidic instrumentation. Nat Commun. 2018;9(1):791.
Mizoguchi F, Slowikowski K, Wei K, Marshall JL, Rao DA, Chang SK, et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat Commun. 2018;9(1):789.
Croft AP, Campos J, Jansen K, Turner JD, Marshall J, Attar M, et al. Distinct fibroblast subsets drive inflammation and damage in arthritis. Nature. 2019;570(7760):246–51.
Culemann S, Gruneboom A, Nicolas-Avila JA, Weidner D, Lammle KF, Rothe T, et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572(7771):670–5.
Kurowska-Stolarska M, Alivernini S. Synovial tissue macrophages: friend or foe? RMD Open. 2017;3(2):e000527.
Brown NJ, Hutcheson J, Bickel E, Scatizzi JC, Albee LD, Haines GK 3rd, et al. Fas death receptor signaling represses monocyte numbers and macrophage activation in vivo. J Immunol. 2004;173(12):7584–93.
Tu J, Hong W, Guo Y, Zhang P, Fang Y, Wang X, et al. Ontogeny of synovial macrophages and the roles of synovial macrophages from different origins in arthritis. Front Immunol. 2019;10:1146.
Labinsky H, Panipinto PM, Ly KA, Khuat DK, Madarampalli B, Mahajan V, et al. Multiparameter analysis identifies heterogeneity in knee osteoarthritis synovial responses. Arthritis Rheumatol. 2020;72(4):598–608.
Wei K, Korsunsky I, Marshall JL, Gao A, Watts GFM, Major T, et al. Notch signalling drives synovial fibroblast identity and arthritis pathology. Nature. 2020;582(7811):259–64.
Hunziker EB, Rosenberg LC. Repair of partial-thickness defects in articular cartilage: cell recruitment from the synovial membrane. J Bone Joint Surg Am. 1996;78(5):721–33.
De Bari C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res Ther. 2015;17:113.
Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24(2):386–98.
Luz-Crawford P, Hernandez J, Djouad F, Luque-Campos N, Caicedo A, Carrere-Kremer S, et al. Mesenchymal stem cell repression of Th17 cells is triggered by mitochondrial transfer. Stem Cell Res Ther. 2019;10(1):232.
Del Rey MJ, Fare R, Usategui A, Canete JD, Bravo B, Galindo M, et al. CD271(+) stromal cells expand in arthritic synovium and exhibit a proinflammatory phenotype. Arthritis Res Ther. 2016;18:66.
Sekiya I, Ojima M, Suzuki S, Yamaga M, Horie M, Koga H, et al. Human mesenchymal stem cells in synovial fluid increase in the knee with degenerated cartilage and osteoarthritis. J Orthop Res. 2012;30(6):943–9.
Sergijenko A, Roelofs AJ, Riemen AH, De Bari C. Bone marrow contribution to synovial hyperplasia following joint surface injury. Arthritis Res Ther. 2016;18:166.
Lee HJ, Lee WJ, Hwang SC, Choe Y, Kim S, Bok E, et al. Chronic inflammation-induced senescence impairs immunomodulatory properties of synovial fluid mesenchymal stem cells in rheumatoid arthritis. Stem Cell Res Ther. 2021;12(1):502.
Lopez-Santalla M, Bueren JA, Garin MI. Mesenchymal stem/stromal cell-based therapy for the treatment of rheumatoid arthritis: an update on preclinical studies. EBioMedicine. 2021;69:103427.
Haywood L, Walsh DA. Vasculature of the normal and arthritic synovial joint. Histol Histopathol. 2001;16(1):277–84.
Doan TN, Bernard FC, McKinney JM, Dixon JB, Willett NJ. Endothelin-1 inhibits size dependent lymphatic clearance of PEG-based conjugates after intra-articular injection into the rat knee. Acta Biomater. 2019;93:270–81.
Dean G, Hoyland JA, Denton J, Donn RP, Freemont AJ. Mast cells in the synovium and synovial fluid in osteoarthritis. Br J Rheumatol. 1993;32(8):671–5.
Singh JA, Arayssi T, Duray P, Schumacher HR. Immunohistochemistry of normal human knee synovium: a quantitative study. Ann Rheum Dis. 2004;63(7):785–90.
Krenn V, Morawietz L, Haupl T, Neidel J, Petersen I, Konig A. Grading of chronic synovitis--a histopathological grading system for molecular and diagnostic pathology. Pathol Res Pract. 2002;198(5):317–25.
Goldenberg DL, Cohen AS. Synovial membrane histopathology in the differential diagnosis of rheumatoid arthritis, gout, pseudogout, systemic lupus erythematosus, infectious arthritis and degenerative joint disease. Medicine (Baltimore). 1978;57(3):239–52.
Della Beffa C, Slansky E, Pommerenke C, Klawonn F, Li J, Dai L, et al. The relative composition of the inflammatory infiltrate as an additional tool for synovial tissue classification. PLoS One. 2013;8(8):e72494.
Baeten D, Demetter P, Cuvelier C, Van Den Bosch F, Kruithof E, Van Damme N, et al. Comparative study of the synovial histology in rheumatoid arthritis, spondyloarthropathy, and osteoarthritis: influence of disease duration and activity. Ann Rheum Dis. 2000;59(12):945–53.
Krenn V, Perino G, Ruther W, Krenn VT, Huber M, Hugle T, et al. 15 years of the histopathological synovitis score, further development and review: a diagnostic score for rheumatology and orthopaedics. Pathol Res Pract. 2017;213(8):874–81.
Schumacher HR. Pathology of the synovial membrane in gout. Light and electron microscopic studies. Interpretation of crystals in electron micrographs. Arthritis Rheum. 1975;18(6 Suppl):771–82.
Towiwat P, Chhana A, Dalbeth N. The anatomical pathology of gout: a systematic literature review. BMC Musculoskelet Disord. 2019;20(1):140.
Canete JD, Celis R, Noordenbos T, Moll C, Gomez-Puerta JA, Pizcueta P, et al. Distinct synovial immunopathology in Behcet disease and psoriatic arthritis. Arthritis Res Ther. 2009;11(1):R17.
van de Sande MG, Baeten DL. Immunopathology of synovitis: from histology to molecular pathways. Rheumatology (Oxford). 2016;55(4):599–606.
Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49(4):358–64.
Scanzello CR. Role of low-grade inflammation in osteoarthritis. Curr Opin Rheumatol. 2017;29(1):79–85.
Nerviani A, Di Cicco M, Mahto A, Lliso-Ribera G, Rivellese F, Thorborn G, et al. A Pauci-immune synovial Pathotype predicts inadequate response to TNFalpha-blockade in rheumatoid arthritis patients. Front Immunol. 2020;11:845.
Lakhanpal A, Smith MH, Donlin LT. Rheumatology in the era of precision medicine: synovial tissue molecular patterns and treatment response in rheumatoid arthritis. Curr Opin Rheumatol. 2021;33(1):58–63.
Moadab F, Khorramdelazad H, Abbasifard M. Role of CCL2/CCR2 axis in the immunopathogenesis of rheumatoid arthritis: latest evidence and therapeutic approaches. Life Sci. 2021;269:119034.
Firestein GS. Chapter 75: Pathogenesis of rheumatoid arthritis. In: Firestein GS, Budd RC, Gabriel SE, Korestsky GA, IB MI, O'Dell JR, editors. Firestein and kelley’s textbook of rheumatology. 11th ed. Philadelphia, PA: Elsevier; 2021. p. 1200–35.
Stanczyk J, Kowalski ML, Grzegorczyk J, Szkudlinska B, Jarzebska M, Marciniak M, et al. RANTES and chemotactic activity in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Mediat Inflamm. 2005;2005(6):343–8.
Kanbe K, Chiba J, Inoue Y, Taguchi M, Yabuki A. SDF-1 and CXCR4 in synovium are associated with disease activity and bone and joint destruction in patients with rheumatoid arthritis treated with golimumab. Mod Rheumatol. 2016;26(1):46–50.
Nagafuchi Y, Shoda H, Sumitomo S, Nakachi S, Kato R, Tsuchida Y, et al. Immunophenotyping of rheumatoid arthritis reveals a linkage between HLA-DRB1 genotype, CXCR4 expression on memory CD4(+) T cells, and disease activity. Sci Rep. 2016;6:29338.
Pablos JL, Santiago B, Galindo M, Torres C, Brehmer MT, Blanco FJ, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003;170(4):2147–52.
Manzo A, Paoletti S, Carulli M, Blades MC, Barone F, Yanni G, et al. Systematic microanatomical analysis of CXCL13 and CCL21 in situ production and progressive lymphoid organization in rheumatoid synovitis. Eur J Immunol. 2005;35(5):1347–59.
Klimiuk PA, Sierakowski S, Latosiewicz R, Cylwik JP, Cylwik B, Skowronski J, et al. Soluble adhesion molecules (ICAM-1, VCAM-1, and E-selectin) and vascular endothelial growth factor (VEGF) in patients with distinct variants of rheumatoid synovitis. Ann Rheum Dis. 2002;61(9):804–9.
Salmi M, Rajala P, Jalkanen S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997;99(9):2165–72.
Kriegsmann J, Keyszer GM, Geiler T, Lagoo AS, Lagoo-Deenadayalan S, Gay RE, et al. Expression of E-selectin messenger RNA and protein in rheumatoid arthritis. Arthritis Rheum. 1995;38(6):750–4.
Mysler E, Caubet M, Lizarraga A. Current and emerging DMARDs for the treatment of rheumatoid arthritis. Open Access Rheumatol. 2021;13:139–52.
Sepriano A, Ramiro S, van der Heijde D, Landewe R. Biological DMARDs and disease modification in axial spondyloarthritis: a review through the lens of causal inference. RMD Open. 2021;7(2).
Schlesinger N. Canakinumab in gout. Expert Opin Biol Ther. 2012;12(9):1265–75.
Ritchlin C, Rahman P, Kavanaugh A, McInnes IB, Puig L, Li S, et al. Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis. 2014;73(6):990–9.
Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci. 2018;19(2).
Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169(1):507–14.
Brebion F, Gosmini R, Deprez P, Varin M, Peixoto C, Alvey L, et al. Discovery of GLPG1972/S201086, a potent, selective, and orally bioavailable ADAMTS-5 inhibitor for the treatment of osteoarthritis. J Med Chem. 2021;64(6):2937–52.
Conaghan PG, Bowes MA, Kingsbury SR, Brett A, Guillard G, Rizoska B, et al. Disease-modifying effects of a novel Cathepsin K inhibitor in osteoarthritis: a randomized controlled trial. Ann Intern Med. 2020;172(2):86–95.
Naredo E, Collado P, Cruz A, Palop MJ, Cabero F, Richi P, et al. Longitudinal power Doppler ultrasonographic assessment of joint inflammatory activity in early rheumatoid arthritis: predictive value in disease activity and radiologic progression. Arthritis Rheum. 2007;57(1):116–24.
Xu H, Edwards J, Banerji S, Prevo R, Jackson DG, Athanasou NA. Distribution of lymphatic vessels in normal and arthritic human synovial tissues. Ann Rheum Dis. 2003;62(12):1227–9.
Bell RD, Rahimi H, Kenney HM, Lieberman AA, Wood RW, Schwarz EM, et al. Altered lymphatic vessel anatomy and markedly diminished lymph clearance in affected hands of patients with active rheumatoid arthritis. Arthritis Rheumatol. 2020;72(9):1447–55.
Wang W, Lin X, Xu H, Sun W, Bouta EM, Zuscik MJ, et al. Attenuated joint tissue damage associated with improved synovial lymphatic function following treatment with Bortezomib in a mouse model of experimental posttraumatic osteoarthritis. Arthritis Rheumatol. 2019;71(2):244–57.
Yang YH, Rajaiah R, Ruoslahti E, Moudgil KD. Peptides targeting inflamed synovial vasculature attenuate autoimmune arthritis. Proc Natl Acad Sci U S A. 2011;108(31):12857–62.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Scanzello, C.R. (2022). Synovial Structure and Physiology in Health and Disease. In: Mandell, B.F. (eds) Synovial Fluid Analysis and The Evaluation of Patients With Arthritis. Springer, Cham. https://doi.org/10.1007/978-3-030-99612-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-99612-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-99611-6
Online ISBN: 978-3-030-99612-3
eBook Packages: MedicineMedicine (R0)